Soil Enzymatic Response to Nicosulfuron: A Preliminary Study in a Chernozem Typical to the Banat Plain, Western Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbicide
2.2. Soil Sampling and Treatment
2.3. Biochemical Analyses
2.4. Physicochemical Properties of the Soil
2.5. In Silico Characterization of Nicosulfuron Stability and Toxicity
2.6. Statistical Analysis
3. Results
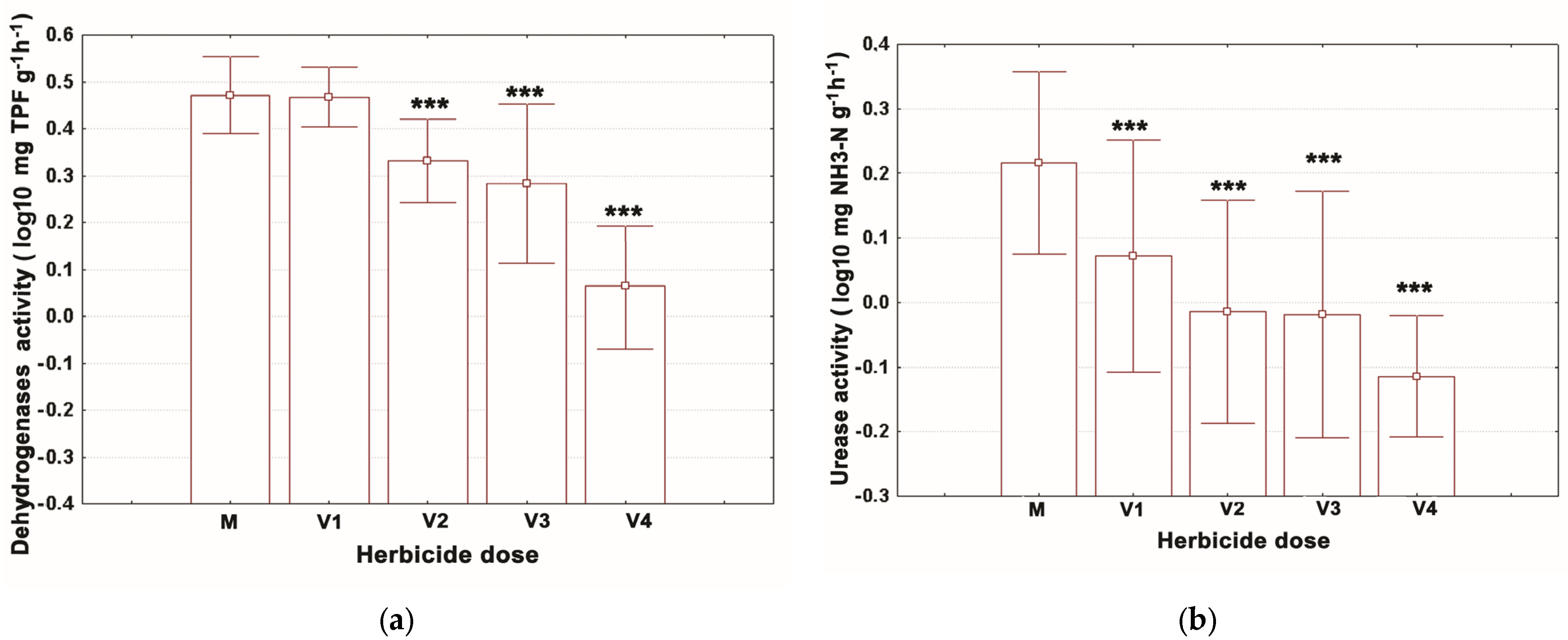
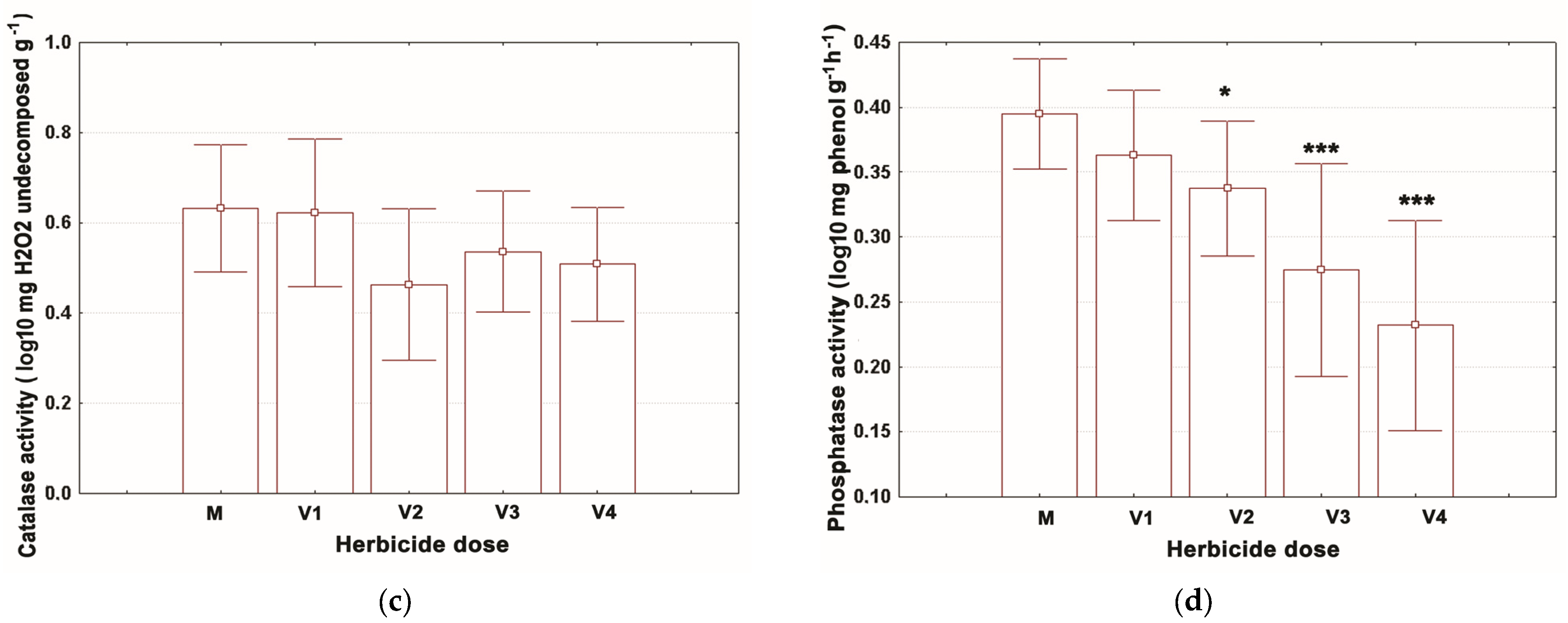
3.1. Assessment of the Enzymatic Activities
3.2. Correlations between the Enzymatic Activities and Physicochemical Properties of the Soil
3.3. The Results of in Silico Characterization of Nicosulfuron Stability and Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arabet, D.; Tempel, S.; Fons, M.; Denis, Y.; Jourlin-Castelli, C.; Armitano, J.; Redelberger, D.; Iobbi-Nivol, C.; Boulahrouf, A.; Méjean, V. Effects of a sulfonylurea herbicide on the soil bacterial community. Environ. Sci. Pollut. Res. 2014, 21, 5619–5627. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticide-a review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Filimon, M.N.; Borozan, A.B.; Bordean, D.M.; Popescu, R.; Gotia, S.R.; Verdes, D.; Sinitean, A. Sulphonylureic herbicidal risk in the detection of soil fungi communities. Afr. J. Microbiol. Res. 2011, 5, 5507–5511. [Google Scholar]
- Thayer, C.A.; Gilley, J.E.; Durso, L.M.; Marx, D.B. Run off nutrient loads as affected by residue cover, manureapplication rate, and flow rate. Trans. ASABE 2012, 55, 249–258. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Tomkiel, M.; Kucharski, J. Response of fungi, β-glucosidase and arylsulfatase to soil contamination by Alister Grande 190 OD, Fuego 500 SC and Lumax 357.5 SE herbicides. Pol. J. Environ. Stud. 2014, 23, 19–25. [Google Scholar]
- Filimon, M.N.; Voia, O.S.; Vlădoiu, D.L.; Isvoran, A.; Ostafe, V. Temperature dependent effect of difenoconazole on enzymatic activity from the soil. J. Serb. Chem. Soc. 2015, 80, 1127–1137. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, J.; Liu, S.; Zhou, X. Kinetics study of nicosulfurondegradation by a Pseudomonas nitroreducens strain NSA02. Biodegradation 2018, 29, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Jianfeng Zhong, J.; Wu, S.; Chen, W.-J.; Huang, Y.; Lei, Q.; Mishra, S.; Bhatt, P.; Chen, S. Current insights into the microbial degradation of nicosulfuron: Strains, metabolic pathways, and molecular mechanisms. Chemosphere 2023, 326, 138390. [Google Scholar] [CrossRef]
- Xu, N.; Wu, Z.; Li, X.; Yang, M.; Han, J.; Lu, B.; Wang, J. Effects of nicosulfuron on plant growth and sugar metabolism in sweet maize (Zea mays L.). PLoS ONE 2022, 17, e0276606. [Google Scholar] [CrossRef]
- Available online: https://www.marketresearchreports.com/lpi/global-nicosulfuron-market-growth-2023-2029 (accessed on 9 October 2023).
- Zhao, R.; Zhang, X.; Chen, F.; Man, X.; Jiang, W. Study on Electrochemical Degradation of Nicosulfuron by IrO2-Based DSA Electrodes: Performance, Kinetics, and Degradation Mechanism. Int. J. Environ. Res. Public Health 2019, 16, 343. [Google Scholar] [CrossRef]
- Sondhia, S.; Waseem, U.; Varma, R.K. Fungal degradation of an acetolactate synthase (ALS) inhibitor pyrazosulfuron-ethyl in soil. Chemosphere 2013, 93, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, D.; Si, H.; Wang, J.; Parales, R.E.; Zhang, J. Biotransformation of the herbicide nicosulfuron residues in soil and seven sulfonylurea herbicides by Bacillus subtilis YB1: A climate chamber study. Environ. Pollut. 2020, 263, 114492. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.R.; Shahbazi, S.; Diyanat, M. Analysis of nicosulfuron residues in maize field soil by high-performance liquid chromatography. Qual. Assur. Saf. Crop. 2017, 9, 229–235. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, X.; Xu, Y.; Han, L. Dissipation and residues of nicosulfuron in corn and soil under field conditions. Bul. Environ. Contam. Toxicol. 2010, 85, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.; Mizukami, A.M.; Lira, K.T.G.; Ferretti, M.Q.; Dias, P.M.; Ferreira, V.C. Physicochemical and toxicological characteristics of nicosulfuron and its environmental implications. Rev. Intertox Toxicol. Risco Ambient. E Soc. 2022, 15, 33–41. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, O.S.; Popescu, R.; Bordean, D.M.; Vladoiu, D.-L.; Mituletu, M.; Ostafe, V. The effect of chlorsulfurone and MCPB-Na on the enzymatic activity of microorganisms. J. Serb. Chem. 2014, 79, 1075–1084. [Google Scholar] [CrossRef]
- Filimon, M.N.; Vlad, D.C.; Verdes, D.; Dumitrascu, V.; Popescu, R. Enzymatic and biological assessment of sulphonylurea herbicide impact on soil bacterial communities. Afr. J. Agric. Res. 2015, 10, 170–1708. [Google Scholar]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar]
- Utobo, E.B.; Tewari, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2015, 13, 147–169. [Google Scholar]
- Vladoiu, D.-L.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Effects of herbicides and fungicides on the soil chitinolytic activity. A molecular docking approach. Ecol. Chem. Eng. S. 2015, 22, 439–450. [Google Scholar] [CrossRef]
- Moretto, J.A.S.; Stehling, E.G.; Andreote, F.D.; Altarugio, L.M.; Andrade, P.A.; Fachin, A.L. Changes in bacterial community after application of three different herbicides. FEMS Microbiol. Lett. 2017, 364, fnx113. [Google Scholar] [CrossRef] [PubMed]
- Filimon, M.N.; Popescu, R.; Verdes, D.; Dumitrescu, G.; Voia, O.S.; Ahmadi, M.; Dronca, D. The Effects of Difenoconazole Treatment on Microorganism from Soil. Communities 2018, 69, 1129–1133. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Matica, M.A.; Baerle, V.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Assessment of the effects of triticonazole on the soil and on the human health. Molecules 2022, 27, 6554. [Google Scholar] [CrossRef] [PubMed]
- Bending, G.D.; Rodríguez-Cruz, M.S.; Lincoln, S.L. Fungicide impacts on microbial communities in soils with contrasting management histories. Chemosphere 2007, 69, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, J.; Jose, S. Agroforestry for soil health. Agrofor. Syst. 2018, 92, 213–219. [Google Scholar] [CrossRef]
- Radivojević, L.; Gašić, S.; Šantrić, L.; Umiljendić, J.G.; Marisavljević, D. Short-time effects of the herbicide nicosulfuron on the biochemical activity of Chernozem soil. J. Serb. Chem. 2012, 77, 845–855. [Google Scholar] [CrossRef]
- Karpouzas, D.; Papadopoulou, E.; Ipsilantis, I.; Friedel, I.; Petric, I.; Udikovic-Kolic, N.; Djuric, S.; Kandeler, E.; Menkissoglu-Spiroudi, U.; Martin-Laurent, F. Effects of nicosulfuron on the abundance and diversity of arbuscular mycorrhizal fungi used as indicators of pesticide soil microbial toxicity. Ecol. Indic. 2014, 39, 44–53. [Google Scholar] [CrossRef]
- Petric, I.; Karpouzas, D.G.; Bru, D.; Udikovic-Kolic, N.; Kandeler, E.; Djuric, S.; Martin-Laurent, F. Nicosulfuron application in agricultural soils drives the selection towards NS-tolerant microorganisms harboring various levels of sensitivity to nicosulfuron. Environ. Sci. Pollut. Res. 2016, 23, 4320–4333. [Google Scholar] [CrossRef]
- Šantric, L.J.; Radivojevic, L.J.; Gajic-Umiljendic, J.; Saric-Krsmanovic, M.; Ðurovic-Pejcev, R. The Effects of Nicosulfuron and Glyphosate on Microbial Activity of Different Soils. Planta Daninha 2018, 36, e018159989. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, C.; Xu, L.; Zhao, C.; Liang, H.; Qiu, L. Biodegradation of nicosulfuron by a novel Alcaligenes faecalis strain ZWS11. J. Environ. Sci. 2015, 35, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Donnadieu, F.; Batisson, I.; Besse-Hoggan, P. Biodegradation and toxicity of a maize herbicide mixture: Mesotrione, nicosulfuron and S-metolachlor. J. Hazard. Mater. 2018, 354, 42–53. [Google Scholar] [CrossRef]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Batisson, I.; Besse-Hoggan, P. Identification of sulfonylurea biodegradation pathways enabled by a novel nicosulfuron-transforming strain Pseudomonas fluorescens SG-1: Toxicity assessment and effect of formulation. J. Hazard. Mater. 2017, 324, 184–193. [Google Scholar] [CrossRef]
- Bottaro, M.; Frascarolo, P.; Gosetti, F.; Mazzucco, E.; Giatiotti, V.; Polati, S.; Pollici, E.; Piacentini, L.; Pavese, G.; Gennaro, M.C. Hydrolytic and photoinduced degradation of tribenuron methyl studied by HPLC-DAD-MS/MS. J. Am. Soc. Mass Spectrom. 2008, 19, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nicosulfuron (accessed on 11 April 2024).
- European Food Safety Authority. Conclusion regarding the peer review of the pesticide risk assessment of the active substance nicosulfuron. EFSA Sci. Rep. 2007, 120, 1–91. [Google Scholar]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996; p. 241. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; p. 316. [Google Scholar]
- Filimon, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the Effect of Application of the Herbicide S-Metolachlor on the Activity of Some Enzymes Found in Soil. Agriculture 2021, 11, 469. [Google Scholar] [CrossRef]
- Filimon, M.N.; Roman, D.L.; Bordean, D.; Isvoran, A. Impact of the herbicide oxyfluorfen on the activities of some enzymes found in soil and on populations of soil microorganisms. Agronomy 2021, 11, 1702. [Google Scholar] [CrossRef]
- Dragan-Bularda, M. Microbiologie Generala-Lucrari Practice; Editura Universitatii Babes-Bolyai: Cluj-Napoca, Romania, 2000; pp. 175–176. (In Romanian) [Google Scholar]
- Filimon, M.N.; Voia, O.S.; Popescu, R.; Dumitrescu, G.; Petculescu Ciochina, L.; Mituletu, M.; Vlad, D.C. The effect of some insecticides on soil microorganisms based on enzymatic and bacteriological analyses. Rom. Biotech. Lett. 2015, 20, 10439–10447. [Google Scholar]
- Bordean, D.M.; Borozan, A.B.; Cojocariu, L.; Moigradean, D.; Cojocariu, A.; Nica, D.; Pirvulescu, L.; Alda, S.; Horablaga, M. Seasonal variation in nutrient content of some leafy vegetables from Banat County, Romania. Rev. Agric. Rural. Dev. 2013, 2, 170–174. [Google Scholar]
- Gergen, I. Analiza Produselor Agroalimentare; Editura Eurostamp: Timisoara, Romania, 2004; p. 316. [Google Scholar]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun. Soil. Sci. Plant. Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Amponsah, D.; Etsey, G.; Nagai, H. Determination of amount of phosphate and sulphate in soil samples from university of Cape Coast Farm. Int. J. Sci. Technol. Res. 2014, 3, 211–215. [Google Scholar]
- Jin, K.; Sleutel, S.; Buchan, D.; De Neve, S.; Cai, D.X.; Gabriels, D.; Jin, J.Y. Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Till Res. 2009, 104, 115–120. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Borba, J.V.B.; Alves, V.M.; Braga, R.C.; Korn, D.R.; Overdahl, K.; Silva, A.C.; Hall, S.U.S.; Overdahl, E.; Kleinstreuer, N.; Strickland, J.; et al. STopTox: An in Silico Alternative to Animal Testing for Acute Systemic and Topical Toxicity. Environ. Health. Perspect. 2022, 130, 027012. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Filho, J.T.; Braga, R.C.; Lemos, J.M.; Alves, V.M.; Borba, J.V.; Costa, W.S.; Kleinstreuer, N.; Muratov, E.N.; Andrade, C.H.; Neves, B.J. BeetoxAI: An artificial intelligence-based web app to assess the acute toxicity of chemicals to honey bees. Artif. Intell. Life Sci. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Uwah, E.I.; Abah, J.; Ndahi, N.P.; Ogugbuaja, V.O. Concentration levels of nitrate and nitrite in soils and some leafy vegetables obtained in Maiduguri, Nigeria. J. Appl. Sci. Environ. Sanit. 2009, 4, 233–244. [Google Scholar]
- Gruia, A.T.; Suciu, M.; Barbu-Tudoran, L.; Azghadi, S.M.R.; Cristea, M.I.; Nica, D.V.; Vaduva, A.; Muntean, D.; Mic, A.A.; Mic, F.A. Mesenchymal stromal cells differentiating to adipocytes accumulate autophagic vesicles instead of functional lipid droplets. J. Cell. Physiol. 2016, 231, 863–875. [Google Scholar] [CrossRef]
- Rumsey, D.J. How to interpret a correlation coefficient R. In Statistics for Dummies, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2003; Volume 286, ISBN 978-0470537039. [Google Scholar]
- Menon, P.; Gopal, M.; Parsad, R. Effects of chlorpyrifos and quinalphos on dehydrogenase activities and reduction of Fe3? in the soils of two semi-arid fields of tropical India. Agric. Ecosyst. Environ. 2005, 108, 73–83. [Google Scholar] [CrossRef]
- Guo, H.; Chen, G.-F.; Lu, Z.-P.; Zhao, H.; Yang, H. Alteration of microbial properties and community structure in soils exposed to napropamide. J. Environ. Sci. 2009, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Reshi, Z.A. Effect of the fungicide Mancozeb at different application rates on enzyme activities in a silt loam soil of the Kashmir Himalaya, India. Trop. Ecol. 2010, 51, 199–205. [Google Scholar]
- Xiong, D.; Gao, Z.; Fu, B.; Sun, H.; Tian, S.; Xiao, Y.; Qin, Z. Effect of pyrimorph on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2013, 56, 44–48. [Google Scholar] [CrossRef]
- Baćmaga, M.; Borowik, A.; Kucharski, J.; Tomkiel, M.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron-methyl + iodosulfuron-methyl-sodium. Environ. Sci. Pollut. Res. Int. 2015, 22, 643–656. [Google Scholar] [CrossRef]
- Furtak, K.; Gajda, A.M. Activity of dehydrogenases as an indicator of soil environment quality. Pol. J. Soil Sci. 2017, 50, 33. [Google Scholar] [CrossRef]
- Das, S.K.; Mukherjee, I.; Das, S.K. Metsulfuron-methyl herbicide on dehydrogenase and acid phosphatase enzyme activity on three different soils. Int. J. Bio-Resour. Stress Manag. 2017, 8, 236–241. [Google Scholar] [CrossRef]
- Radivojević, L.; Jovičić, J.D.; Šantrić, L.; Gašić, S.; Gajić, J.U. Effects of metsulfuron-methyl on soil microbial acitvity. Arhiv Za Tehničke Nauke. Arch. Tech. Sci. 2014, 1, 77–82. [Google Scholar] [CrossRef]
- Gołombieski, J.I.; Jonas Sutili, F.; Salbego, J.; Seben, D.; Tourem Gressler, L.; Arrudada Cunha, J.; Gressler, L.T.; Zanella, R.; Vaucher, R.d.A.; Marchesan, E.; et al. Imazapyr + imazapic herbicide determines acute toxicity in silver catfish Rhamdia quelen. Ecotox. Environ. Safe 2016, 128, 91–99. [Google Scholar] [CrossRef]
- Caraba, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the Effects of the Herbicide Aclonifen and Its Soil Metabolites on Soil and Aquatic Environments. Agriculture 2023, 13, 1226. [Google Scholar] [CrossRef]
- Baćmaga, M.; Boros, E.; Kucharski, J.; Wyszkowska, J. Enzymatic activity in soil contaminated with the Aurora 40 WG herbicide. Environ. Prot. Eng. 2012, 38, 9–102. [Google Scholar]
- Tejada, M. Evolution of soil biological properties after addition of glyphosate, diflufenican and glyphosate plus diflufenican herbicides. Chemosphere 2009, 76, 365–373. [Google Scholar] [CrossRef]
- Vlad, D.C.; Filimon, M.N.; Popescu, R.; Gurban, C.; Dumitrascu, V. The Influence of Sulphonylureic Herbicid On Soil Bacterial Enzyme Activities. Ann. West Univ. Timis. Ser. Chem. 2012, 20, 87–95. [Google Scholar]
- Ataikiru, T.L.; Okpokwasili, G.S.C.; Okerentugba, P.O. Impact of Pesticides on Microbial Diversity and Enzymes in Soil. S. Asian J. Res. Microbiol. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Yan, H.; Wang, D.D.; Dong, B.; Tang, F.F.; Wang, B.C.; Fang, H.; Yu, Y.L. Dissipation of carbendazim and chloramphenicol alone and in combination and their effects on soil fungal:bacterial ratios and soil enzyme activities. Chemosphere 2011, 84, 634–641. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S. Ecotoxicological implications of residual pesticides to beneficial soil bacteria: A review. Pestic. Biochem. Physiol. 2022, 188, 105272. [Google Scholar] [CrossRef]
- Li, A.; Yao, Y.; Sun, S.Q.; Jiang, L.Y.; Liu, X.; Gao, Z.G. Impact of Herbicide Atrazine and Nicosulfuron on the Soil Respiration and Enzyme Activities. Adv. Mat. Res. 2014, 1010–1012, 484–488. [Google Scholar] [CrossRef]
- Kumari, J.A.; Rao, P.C.; Madhavi, M.; Padmaja, G. Effect of herbicides on the activity of soil enzymes urease in maize crop. Indian J. Agric. Res. 2018, 52, 300–304. [Google Scholar] [CrossRef]
- Chabot, M.; Morales, E.; Cummings, J.; Rios, N.; Giatpaiboon, S.; Mogul, R. Simple kinetics, assay, and trends for soil microbial catalases. Anal. Biochem. 2020, 610, 113901. [Google Scholar] [CrossRef]
- Medo, J.; Hricáková, N.; Maková, J.; Medová, J.; Omelka, R.; Javoreková, S. Effects of sulfonylurea herbicides chlorsulfuron and sulfosulfuron on enzymatic activities and microbial communities in two agricultural soils. Environ. Sci. Pollut. Res. 2020, 27, 41265–41278. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Wołejko, E.; Kaczyński, P.; Łozowicka, B.; Wydro, U.; Borusiewicz, A.; Hrynko, I.; Konecki, R.; Snarska, K.; Dec, D.; Malinowski, P. Dissipation of S-metolachlor in plant and soil and effect on enzymatic activities. Environ. Monit. Assess. 2017, 189, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.; Wang, J.; Xie, H.; Wang, J.; Wang, F.; Sun, F. Effects of fomesafen on soil enzyme activity, microbial population, and bacterial community composition. Environ. Monit. Assess. 2014, 186, 2801–2812. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Si, Y.; Yang, W.; Zhu, S.; Ni, W. Variations in eco-enzymatic stoichiometric and microbial characteristics in paddy soil as affected by long-term integrated organic-inorganic fertilization. PLoS ONE 2017, 12, e0189908. [Google Scholar] [CrossRef] [PubMed]




| Treatment Abbreviation | Dose | Explanation |
|---|---|---|
| M | 0 | 0 μg nicosulfuron g−1 soil |
| V1 | D/2 | 0.2 μg nicosulfuron g−1 soil |
| V2 | D | 0.4 μg nicosulfuron g−1 soil |
| V3 | 2xD | 0.8 μg nicosulfuron g−1 soil |
| V4 | 3xD | 1.2 μg nicosulfuron g−1 soil |
| Time | Dose | Deh Activity (mg TPF g−1 Soil) | Ure Activity (mg NH3-N g−1 h−1 Soil) | Cat Activity (mg H2O2 Undecomposed g−1 Soil) | Alp Activity (mg Phenol g−1 Soil) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 7 days | M | 3.82 ± | 0.26 | 1.26 ± | 0.09 | 6.23 ± | 0.30 | 2.63 ± | 0.04 |
| 7 days | V1 | 3.35 ± | 0.27 | 0.68 ± | 0.11 *** | 6.15 ± | 0.52 | 2.33 ± | 0.41 |
| 7 days | V2 | 2.47 ± | 0.36 ** | 0.65 ± | 0.17 *** | 5.74 ± | 0.08 | 2.44 ± | 0.39 |
| 7 days | V3 | 2.40 ± | 0.29 *** | 0.62 ± | 0.16 *** | 4.45 ± | 0.15 | 2.06 ± | 0.35 |
| 7 days | V4 | 1.24 ± | 0.08 *** | 0.59 ± | 0.18 *** | 4.41 ± | 0.40 | 1.82 ± | 0.25 ** |
| 14 days | M | 3.17 ± | 0.23 | 1.18 ± | 0.12 | 5.36 ± | 0.47 | 2.72 ± | 0.13 |
| 14 days | V1 | 3.22 ± | 0.23 | 1.07 ± | 0.04 | 5.78 ± | 0.39 | 2.54 ± | 0.29 |
| 14 days | V2 | 1.61 ± | 0.22 *** | 0.81 ± | 0.06 ** | 4.91 ± | 0.24 | 2.10 ± | 0.20 |
| 14 days | V3 | 1.04 ± | 0.17 *** | 0.87 ± | 0.07 * | 4.67 ± | 0.14 | 2.16 ± | 0.04 |
| 14 days | V4 | 0.31 ± | 0.07 *** | 0.78 ± | 0.10 ** | 3.93 ± | 0.06 | 1.96 ± | 0.05 * |
| 21 days | M | 2.44 ± | 0.13 | 2.33 ± | 0.11 | 3.52 ± | 0.06 | 2.48 ± | 0.03 |
| 21 days | V1 | 2.60 ± | 0.44 | 1.39 ± | 0.11 *** | 3.22 ± | 0.05 | 2.30 ± | 0.06 |
| 21 days | V2 | 2.44 ± | 0.23 | 1.03 ± | 0.10 *** | 3.08 ± | 0.03 | 2.26 ± | 0.12 |
| 21 days | V3 | 2.52 ± | 0.09 | 0.85 ± | 0.03 *** | 2.93 ± | 0.05 | 1.52 ± | 0.33 *** |
| 21 days | V4 | 2.30 ± | 0.09 | 0.79 ± | 0.03 *** | 2.91 ± | 0.01 | 1.33 ± | 0.23 *** |
| 28 days | M | 2.62 ± | 0.07 | 2.14 ± | 0.01 | 2.90 ± | 0.06 | 2.14 ± | 0.01 |
| 28 days | V1 | 2.70 ± | 0.05 | 1.94 ± | 0.03 | 2.71 ± | 0.05 | 2.11 ± | 0.05 |
| 28 days | V2 | 2.23 ± | 0.08 | 1.69 ± | 0.47 | 2.32 ± | 0.02 | 1.96 ± | 0.02 |
| 28 days | V3 | 2.18 ± | 0.08 | 1.88 ± | 0.01 | 2.29 ± | 0.01 | 1.91 ± | 0.01 |
| 28 days | V4 | 2.13 ± | 0.01 | 0.98 ± | 0.00 *** | 2.17 ± | 0.04 | 1.82 ± | 0.02 |
| Physicochemical Parameters/ Enzymatic Activity | pH | EC (µS cm−1) | Water Content (mg g−1) | OM (mg g−1) | N-NH4 (mg kg−1) | N-NO3 (mg g−1) | Phosphate (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Deh | 0.37 * | −0.02 | 0.01 | 0.69 *** | 0.37 * | 0.24 | 0.45 ** |
| Ure | 0.03 | 0.20 | 0.03 | −0.03 | −0.28 | 0.07 | 0.08 |
| Cat | 0.59 *** | 0.20 | −0.08 | 0.84 *** | 0.54 *** | 0.30 * | 0.57 *** |
| Alp | 0.61 *** | 0.37 * | −0.02 | 0.69 *** | 0.18 | 0.51 *** | 0.60 *** |
| pKCSM | ||
|---|---|---|
| Parameters | Predicted | Descriptions |
| AMES toxicity | No | AMES mutagenicity test indicates that it may act as a carcinogen |
| Max. human tolerated dose | 0.602 | Dose (log mg/kg/day); Toxic effect > 0.477 log mg kg−1 day−1 |
| hERG I inhibitor | No | hERG I/II inhibitors could cause the development of the acquired long QT syndrome, which leads to fatal ventricular arrhythmia |
| hERG II inhibitor | No | |
| Oral Rat Acute Toxicity | 2.272 | LD50 (mol kg−1) |
| Hepatotoxicity | Yes | Categorical (Yes/No) |
| Skin Sensitisation | No | Categorical (Yes/No) |
| T.Pyriformis toxicity | 0.279 | log μg L−1; If value is >−0.5 log μg L−1 is considered to be toxic |
| Minnow toxicity | 3.690 | log mM; If values is <−0.3 indicate high acute toxicity |
| STOPTox | ||
| Endpoint | Prediction/Confidence | Predicted fragment contribution |
| Acute Inhalation Toxicity (* RF: MACCS fingerprints) | Non-Toxic (−) 90.0% |  |
| Acute Oral Toxicity (* RF: MACCS fingerprints) | Non-Toxic (−) 95.0% |  |
| Acute Dermal Toxicity (* RF: MACCS fingerprints) | Non-Toxic (−) 90.0% | 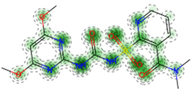 |
| Eye Irritation and Corrosion (* RF: MACCS fingerprints) | Toxic (+) 88.0% |  |
| Skin Sensitization (* RF: Morgan EFCP4) | Non-Sensitizer (−) 70.0% |  |
| Skin Irritation and Corrosion (* RF: Morgan EFCP4) | Negative (−) 90.0% | 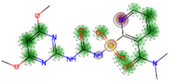 |
| BeeTOX | ||
| Endpoint | Prediction/Confidence | |
| Acute oral Toxicity (* RF: MACCS fingerprints) # Honey bee (Apis mellifera) | Non-Toxic (−) 54.0% |  |
| Acute Contact Toxicity (* SVM: FeatMorgan FCFP2) # Honey bee (Apis mellifera) | Non-Toxic (−) 95.0% | 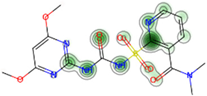 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caraba, M.N.; Caraba, I.V.; Pet, E.; Pet, I.; Crisan, L.; Sinitean, A.; Hutanu, D. Soil Enzymatic Response to Nicosulfuron: A Preliminary Study in a Chernozem Typical to the Banat Plain, Western Romania. Agriculture 2024, 14, 1380. https://doi.org/10.3390/agriculture14081380
Caraba MN, Caraba IV, Pet E, Pet I, Crisan L, Sinitean A, Hutanu D. Soil Enzymatic Response to Nicosulfuron: A Preliminary Study in a Chernozem Typical to the Banat Plain, Western Romania. Agriculture. 2024; 14(8):1380. https://doi.org/10.3390/agriculture14081380
Chicago/Turabian StyleCaraba, Marioara Nicoleta, Ion Valeriu Caraba, Elena Pet, Ioan Pet, Luminita Crisan, Adrian Sinitean, and Delia Hutanu. 2024. "Soil Enzymatic Response to Nicosulfuron: A Preliminary Study in a Chernozem Typical to the Banat Plain, Western Romania" Agriculture 14, no. 8: 1380. https://doi.org/10.3390/agriculture14081380
APA StyleCaraba, M. N., Caraba, I. V., Pet, E., Pet, I., Crisan, L., Sinitean, A., & Hutanu, D. (2024). Soil Enzymatic Response to Nicosulfuron: A Preliminary Study in a Chernozem Typical to the Banat Plain, Western Romania. Agriculture, 14(8), 1380. https://doi.org/10.3390/agriculture14081380









