Biochar Application in Combination with No Tillage Enhanced Yield and Grain Quality of Ratoon Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Details
2.2. Measurement of Grain Yield, Yield Components
2.3. Measurement of Grain Quality
2.4. Measurement of Antioxidant Activities
2.5. Statistical Analysis
3. Results
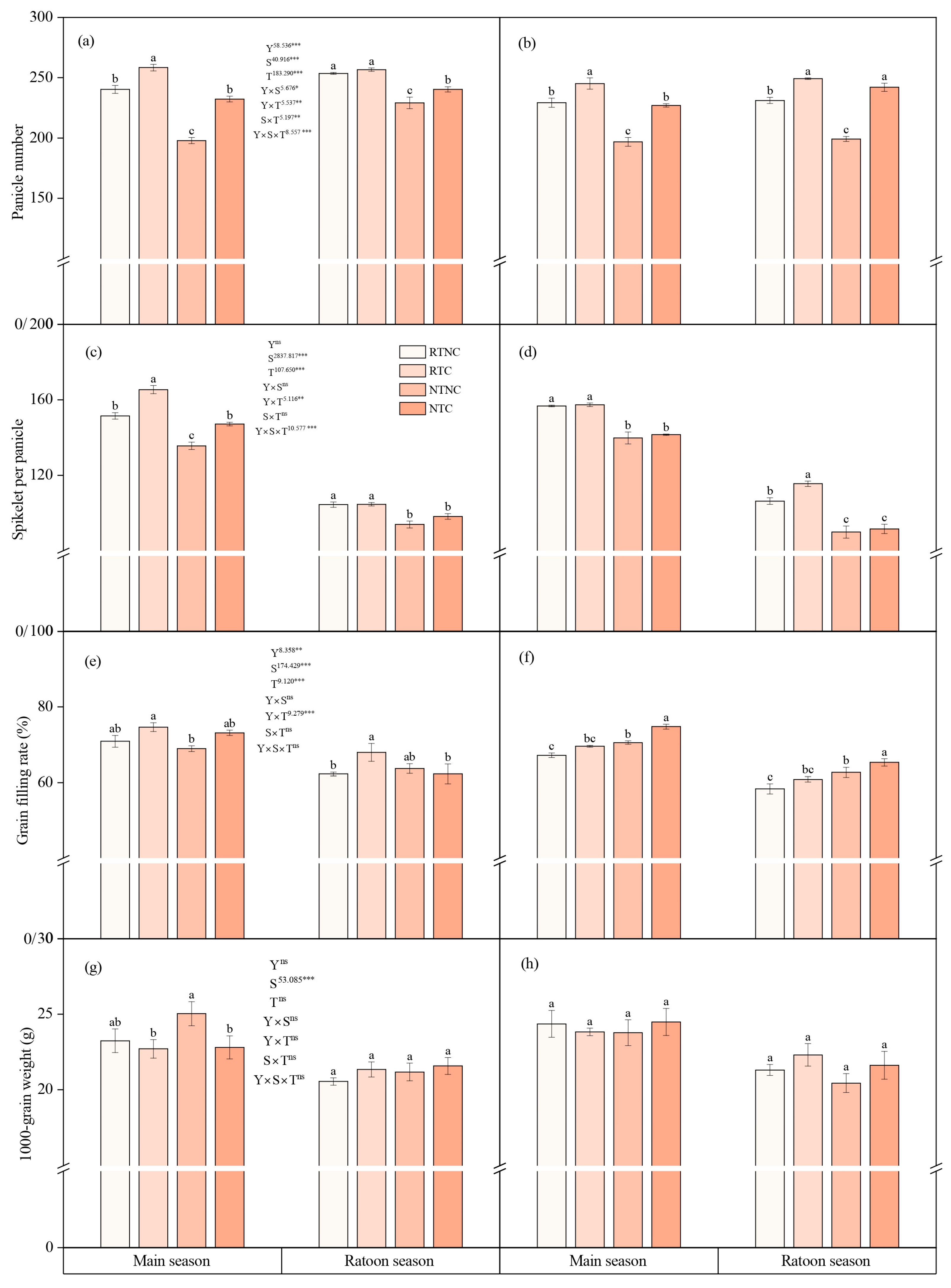
3.1. Yield and Yield Components
3.2. Grain Quality
3.3. MDA Content and Activities of SOD, CAT, and POD
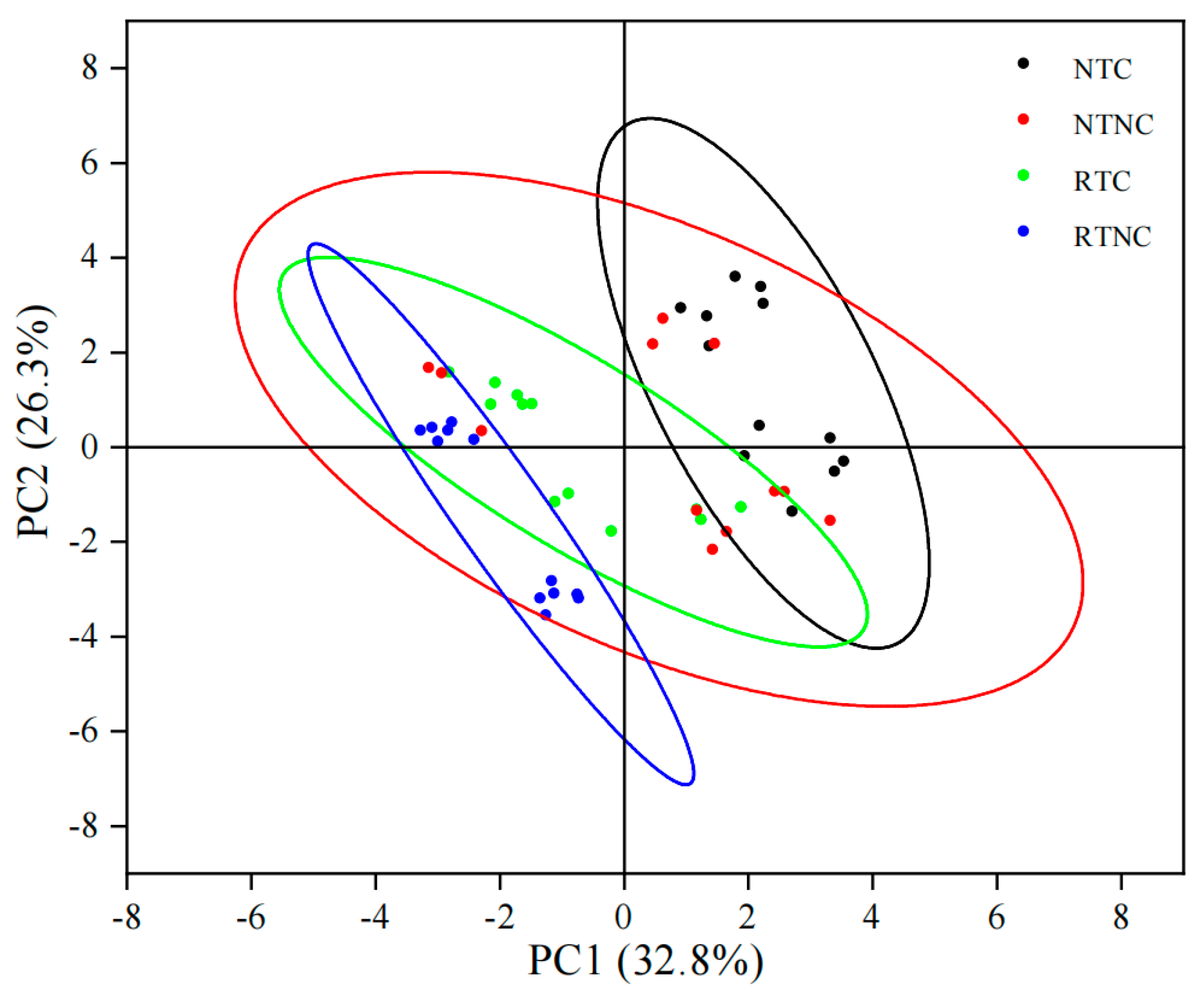
3.4. Principal Component Analysis
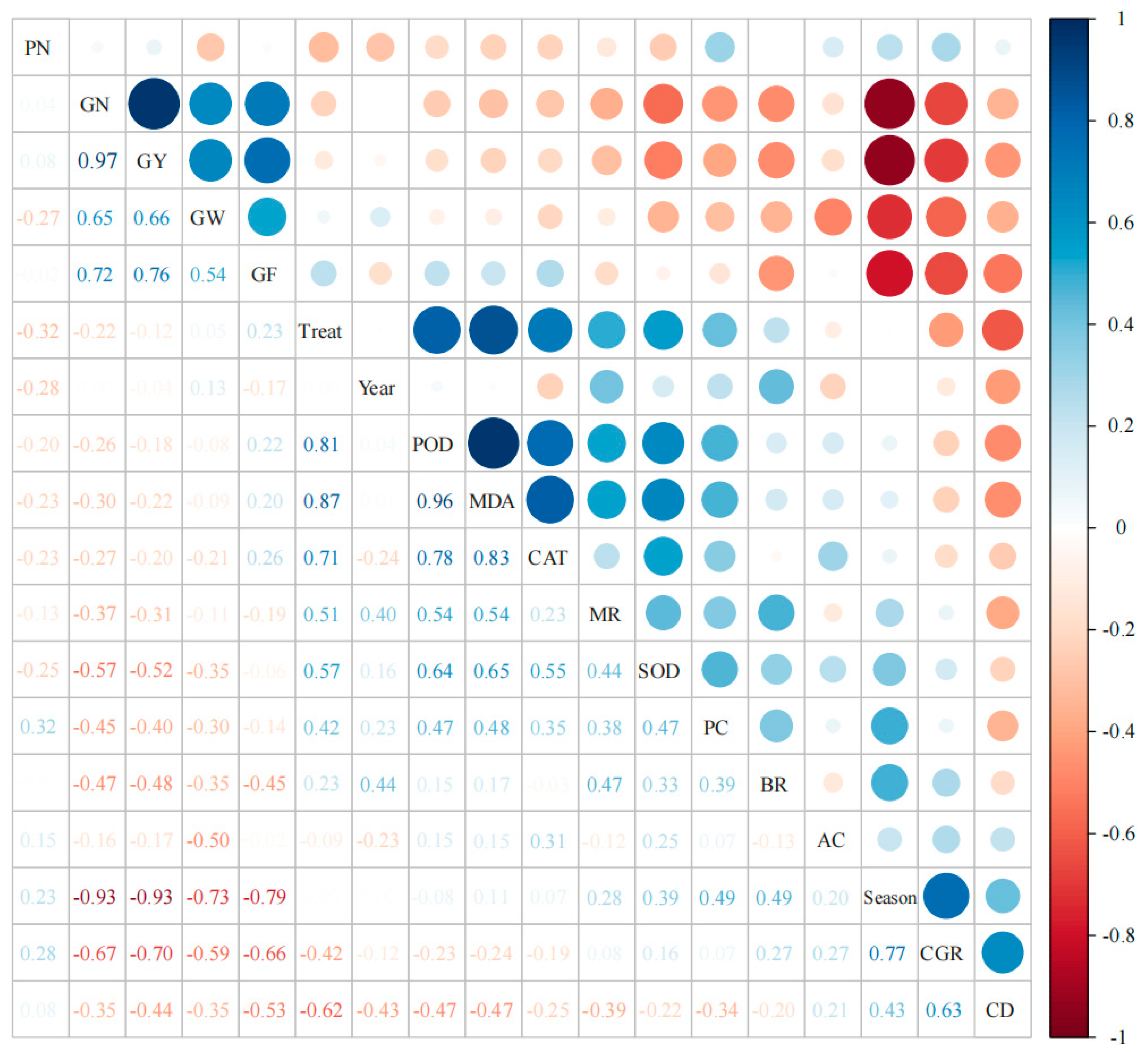
3.5. Correlation Analyses
4. Discussion
4.1. Grian Yield and Yield Components
4.2. Grain Quality and Antioxidant Enzyme Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van, H.P.; Chau, H.T.; Phi, N.T. In Vitro digestibility and in vivo glucose response of native and physically modified rice starches varying amylose contents. Food Chem. 2016, 191, 74–80. [Google Scholar]
- Lin, W.-x. Developmental status and problems of rice ratooning. J. Integr. Agric. 2019, 18, 246–247. [Google Scholar] [CrossRef]
- Lin, W.X.; Chen, H.F.; Zhang, Z.X.; Xu, Q.H.; Tu, N.M.; Fang, C.X.; Ren, W.J. Research and prospect on physio-ecological properties of ratoon rice yield formation its key cultivation technology. Chin. J. Eco-Agric. 2015, 23, 392–401, (In Chinese with English abstract). [Google Scholar]
- Deng, Q.J.; Cao, Z.G.; Li, C.F. Effects of different ratooning cultivation modes on greenhouse gas emissions and grain yields in paddy fields. J. Agro-Environ. Sci. 2019, 38, 1373–1380, (In Chinese with English abstract). [Google Scholar]
- Wu, Q.; Wang, Y.Z.; Chen, T.T.; Zheng, J.L.; Sun, Y.D.; Chi, D.C. Soil nitrogen regulation using clinoptilolite for grain filling and grain quality improvements in rice. Soil Tillage Res. 2020, 199, 104547. [Google Scholar] [CrossRef]
- Lemaire, G.; Franzluebbers, A.; de Faccio Carvalho, P.C.; Dedieu, B. Integrated crop–livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Liu, L.C.; Tong, H.N.; Xiao, Y.H.; Che, R.H.; Xu, F.; Hu, B.; Liang, C.Z.; Chu, J.F.; Li, J.Y.; Chu, C.C. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, B.; Yao, Y.Y.; Guo, Z.F.; Jia, H.Y.; Kong, L.R.; Zhang, A.M.; Ma, W.J.; Ni, Z.F.; Xu, S.B. Wheat genomic study for genetic improvement of traits in China. Sci. China Life Sci. 2022, 65, 1718–1775. [Google Scholar] [CrossRef]
- Begum, M.K.; Hasan, K.M.; Hossain, S.M.A.; Hossain, M.A. Effect of culm cutting height and nitrogenous fertilizer on the yield of ratoon of late boro rice. Pak. J. Agron. 2002, 1, 136–138. [Google Scholar]
- Harrell, D.L.; Bond, J.A.; Blanche, S. Evaluation of main-crop stubble height on ratoon rice growth and development. Field Crops Res. 2009, 114, 396–403. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Xu, F.X.; Zhang, L.; Zhou, X.B.; Guo, X.Y.; Zhu, Y.C.; Liu, M.; Xiong, H.; Jiang, P. The ratoon rice system with high yield and high efficiency in China: Progress, trend of theory and technology. Field Crops Res. 2021, 272, 108282. [Google Scholar] [CrossRef]
- Kim, J.; Shon, J.; Lee, C.K.; Yang, W.; Yoon, Y.; Yang, W.H.; Kim, Y.G.; Lee, B.W. Relationship between grain filling duration and leaf senescence of temperate rice under high temperature. Field Crops Res. 2011, 122, 207–213. [Google Scholar] [CrossRef]
- Chen, Z.K.; Li, P.; Xiao, J.C.; Jiang, Y.; Cai, M.L.; Wang, J.P.; Li, C.F.; Zhan, M.; Cao, C.G. Dry cultivation with ratoon system impacts rice quality using rice flour physicochemical traits, fatty and amino acids contents. Food Res. Int. 2021, 150, 110764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, Q.Y.; Li, L.L.; Xu, H.Q.; Zheng, H.B.; Wang, J.L.; Hua, Y.J.; Ren, L.J.; Tang, J.W. Ratoon rice with direct seeding improves soil carbon sequestration in rice fields and increases grain quality. J. Environ. Manag. 2022, 317, 115374. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Zeng, Y.H.; Zhao, X.F.; Fan, C.G.; Pan, X.H.; Shi, Q.H. Effects of tillage methods on yield of double cropping rice with machine-transplanted and soil physical-chemical properties. J. Hunan Agric. Univ. 2017, 43, 581–585. [Google Scholar]
- Xu, S.Q.; Zhang, M.Y.; Sun, G.F.; Shang, W.G.; Chen, F.; Zhang, H.L. Assessment of tillage effects on soil quality for double-rice paddy with tilth index. Sci. Agric. Sin. 2011, 44, 3999–4006. [Google Scholar]
- Quan, M.H.; Hu, A.S.; Ou, L.J.; Hu, D.Y.; Li, B.H. Effects of tillage mode on characteristics of photosynthesis and root growth of rice. Hybrid Rice 2012, 27, 71–75. [Google Scholar]
- Chen, D.G.; Zhou, X.Q.; Li, L.J.; Liu, C.G.; Zhang, X.; Chen, Y.D. Relationship between root morphological characteristics and yield components of major commercial Indica rice in South China. Acta Agron. Sin. 2013, 39, 1899–1908. [Google Scholar] [CrossRef]
- Yadav, K.; Jagadevan, S. Influence of process parameters on synthesis of biochar by pyrolysis of biomass: An alternative source of energy. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2019. [Google Scholar]
- Yang, Y.; Zuo, P.; Qu, S. Adjusting hydrophily and aromaticity strategy for pitch-based hierarchical porous carbon and its application in flexible supercapacitor. Fuel 2022, 311, 122514. [Google Scholar] [CrossRef]
- Kochanek, J.; Soo, R.M.; Martinez, C.; Dakuidreketi, A.; Mudge, A.M. Biochar for intensification of plant-related industries to meet productivity, sustainability and economic goals: A review. Resour. Conserv. Recycl. 2022, 179, 106109. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adeyemo, A.J.; Awodun, M.A. Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma 2019, 336, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, C.; Kong, X.S.; Hou, D.P.; Gu, J.F.; Liu, L.J.; Wang, Z.Q.; Yang, J.C. Progressive integrative crop managements increase grain yield, nitrogen use efficiency and irrigation water productivity in rice. Field Crops Res. 2018, 215, 1–11. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with alow possibility to amplify eukaryotic rRNA genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, S.; Kumar, R.; Das, A.; Kumari, R.; Choudhary, C.; Sharma, R. Effect of long-term integrated nutrient management on crop yield, nutrition and soil fertility under rice-wheat system. J. Appl. Nat. Sci. 2017, 9, 1801–1807. [Google Scholar] [CrossRef]
- Yang, C.; Dou, S.; Guo, D.; Zhao, H. The Application of Biochar Enhances Soil Organic Carbon and Rice Yields. Agronomy 2024, 14, 455. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.N.; Wu, Q.X. Impact of Tillage and Straw Management on Soil Properties and Rice Yield in a Rice-Ratoon Rice System. Agronomy 2023, 13, 1762. [Google Scholar] [CrossRef]
- Cao, X.C.; Yuan, L.; Liu, X.X.; Zhu, L.F.; Zhu, C.Q.; Kong, Y.L.; Wu, L.L.; Tian, C.; Lu, R.H.; Zhang, J.H. Benefits of controlled-release/stable fertilizers plus biochar for rice grain yield and nitrogen utilization under alternating wet and dry irrigation. Eur. J. Agron. 2021, 129, 126338. [Google Scholar] [CrossRef]
- Liu, B.T.; Li, H.L.; Li, H.B.; Zhang, A.P.; Rengel, Z. Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. GCB Bioenergy 2021, 13, 257–268. [Google Scholar] [CrossRef]
- Sashidhar, P.; Kochar, M.; Singh, B.; Gupta, M.; Cahill, D.; Adholeya, A.; Dubey, M. Biochar for delivery of agri-inputs: Current status and future perspectives. Sci. Total Environ. 2020, 703, 134892. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Hu, T.; Mahmoud, A.; Li, J.; Zhu, R.; Jiao, X.; Jing, P. A quantitative review of the effects of biochar application on rice yield and nitrogen use efficiency in paddy fields: A meta-analysis. Sci. Total Environ. 2022, 830, 154792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Jiao, X.Y.; Li, H.D.; Hu, T.S.; Jiang, H.Z.; Mahmoud, A. Effects of biochar on water quality and rice productivity under straw returning condition in a rice-wheat rotation region. Sci. Total Environ. 2022, 819, 152063. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, F.; Ren, W.J. Shading tolerance in rice is related to better light harvesting and use efficiency and grain filling rate during grain filling period. Field Crops Res. 2015, 180, 54–62. [Google Scholar] [CrossRef]
- Si, L.L.; Xie, Y.N.; Ma, Q.X.; Wu, L.H. The short-term effects of rice straw biochar, nitrogen and phosphorus fertilizer on rice yield and soil properties in a cold waterlogged paddy field. Sustainability 2018, 10, 537. [Google Scholar] [CrossRef]
- Roy, P.; Ijiri, T.; Okadome, H.; Nei, D.; Orikasa, T.; Nakamura, N.; Shiina, T. Effect of processing conditions on overall energy consumption and quality of rice (Oryza sativa L.). J. Food Eng. 2008, 89, 343–348. [Google Scholar] [CrossRef]
- Deng, F.; Li, Q.P.; Chen, H.; Zeng, Y.L.; Li, B.; Zhong, X.Y.; Wang, L.; Ren, W.J. Relationship between chalkiness and the structural and thermal properties of rice starch after shading during grain-filling stage. Carbohydr. Polym. 2021, 252, 117212. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mao, B.G.; Zhang, C.Q.; Shao, Y.; Wu, T.H.; Hu, L.M.; Hu, Y.Y.; Tang, L.; Li, Y.K.; Tang, W.B. Influence of physicochemical properties and starch fine structure on the eating quality of hybrid rice with similar apparent amylose content. Food Chem. 2021, 353, 129461. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.Y.; Luo, H.W.; Wu, F.D.; He, L.X.; Lai, R.F.; Tang, X.R. Organic cultivation induced regulation in yield formation, grain quality attributes, and volatile organic compounds of fragrant rice. Food Chem. 2023, 405, 134845. [Google Scholar] [CrossRef]
- Lin, F.F.; Rensing, C.; Pang, Z.Q.; Zou, J.N.; Lin, S.; Letuma, P.L.; Zhang, Z.X.; Lin, W.X. Metabolomic analysis reveals differential metabolites and pathways involved in grain chalkiness improvement under rice ratooning. Field Crops Res. 2022, 283, 108521. [Google Scholar] [CrossRef]
- Huang, J.W.; Pan, Y.P.; Chen, H.F.; Zhang, Z.X.; Fang, C.X.; Shao, C.H.; Amjad, H.; Lin, W.W.; Lin, W.X. Physiochemical mechanisms involved in the improvement of grain-filling, rice quality mediated by related enzyme activities in the ratoon cultivation system. Field Crops Res. 2020, 258, 107962. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.B.; Kirui, W.K.; Lei, M.; Lu, Q.M.; Bah, H.; Dong, R.J. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, C.; He, L.H.; Liao, S.; Li, Q.P.; Li, B.; Zhu, S.L.; Gao, Y.T.; Tao, Y.F.; Zhou, W. Delayed sowing date improves the quality of mechanically transplanted rice by optimizing temperature conditions during growth season. Field Crops Res. 2022, 281, 108493. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.C.; Wu, Y.Z.; Yi, W.T.; Gu, Q.C.; Tang, X.R. Integrative Effect of Reduced Tillage and Shading Enhanced Yield and Grain Quality of Fragrant Rice. Agronomy 2023, 13, 2010. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Gan, Y.T.; Niu, Y.N.; Zhang, R.Z.; Li, L.L.; Cai, L.Q.; Xie, J.H. Soil quality indicators and crop yield under long-term tillage systems. Exp. Agric. 2017, 53, 497–511. [Google Scholar] [CrossRef]
- French, E.; Iyer-Pascuzzi, A.S. A role for the gibberellin pathway in biochar-mediated growth promotion. Sci. Rep. 2018, 8, 5389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant steroidal hormone epibrassinolide regulate–Heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]



| Year | Season | Treatment | Brown Rice Rate | Milled Rice Rate | Chalkiness Degree | Chalk Grain Rate | Amylose Content | Protein Content |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |||
| 2020 | Main season | RTNC | 71.33 a | 63.79 b | 5.84 a | 20.83 a | 18.69 a | 8.00 bc |
| RTC | 73.63 a | 65.02 b | 5.22 b | 20.27 ab | 18.76 a | 9.24 a | ||
| NTNC | 73.03 a | 66.13 ab | 5.26 b | 19.58 b | 16.55 a | 7.58 c | ||
| NTC | 76.66 a | 70.53 a | 4.61 c | 16.60 c | 18.56 a | 8.65 ab | ||
| Mean | 73.66 C | 66.37 B | 5.23 A | 19.32 A | 18.14 B | 8.37 B | ||
| Ratoon season | RTNC | 76.07 ab | 68.28 c | 4.91 a | 16.31 a | 18.63 a | 8.70 b | |
| RTC | 73.68 b | 68.79 bc | 4.04 b | 14.39 ab | 20.49 a | 10.08 a | ||
| NTNC | 79.40 a | 70.72 b | 4.72 a | 14.59 ab | 17.97 a | 8.55 b | ||
| NTC | 76.53 ab | 72.99 a | 3.14 c | 14.04 b | 17.95 a | 9.68 a | ||
| Mean | 76.41 B | 70.19 A | 4.20 B | 14.83 B | 18.76 A | 9.25 A | ||
| 2021 | Main season | RTNC | 74.00 b | 70.28 ab | 5.27 a | 20.39 a | 17.86 a | 7.42 c |
| RTC | 78.11 a | 70.99 ab | 4.28 b | 16.34 c | 16.86 a | 8.64 b | ||
| NTNC | 75.44 ab | 69.24 b | 4.18 b | 18.49 b | 18.28 a | 8.70 b | ||
| NTC | 76.66 ab | 73.49 a | 3.09 c | 17.35 bc | 17.00 a | 10.23 a | ||
| Mean | 76.05 B | 71.00 A | 4.20 B | 18.14 A | 17.50 B | 8.74 B | ||
| Ratoon season | RTNC | 80.75 a | 69.30 a | 4.57 a | 17.07 a | 17.66 a | 8.73 c | |
| RTC | 79.86 a | 69.16 a | 3.28 b | 15.11 b | 17.06 a | 10.30 a | ||
| NTNC | 79.02 a | 71.62 a | 4.37 a | 13.11 c | 19.52 a | 9.51 b | ||
| NTC | 81.56 a | 73.09 a | 2.36 c | 14.02 bc | 18.04 a | 10.42 a | ||
| Mean | 80.30 A | 70.79 A | 3.64 C | 14.83 B | 18.07 B | 9.74 A | ||
| ANOVA | Year (Y) | 17.6 *** | 16.8 *** | 289.5 *** | 5.9 * | ns | 18.8 *** | |
| Season (S) | 22 *** | 8.1 ** | 289.1 *** | 256.9 *** | ns | 88.8 *** | ||
| Treatment (T) | ns | 10.4 *** | 280.4 *** | 30 *** | ns | 55.6 *** | ||
| Y × S | ns | 10 ** | 25.1 *** | 5.8 * | ns | ns | ||
| Y × T | ns | ns | 9.6 *** | 4.2 * | 5.5 ** | 14.8 *** | ||
| S × T | ns | ns | 21.8 *** | 3.8 * | ns | ns | ||
| Y × S × T | ns | ns | ns | 6.4 ** | ns | 3.6 * |
| Loadings | PC1 | PC2 | PC3 |
|---|---|---|---|
| Panicle number | −0.06101 | −0.15576 | 0.07535 |
| Spikelet per panicle | −0.29941 | 0.35113 | 0.05058 |
| Grain filling rate | −0.07008 | 0.4391 | 0.21072 |
| 1000-grain weight | −0.20296 | 0.36358 | −0.21581 |
| Brown rice rate | 0.21939 | −0.17473 | −0.46284 |
| Milled rice rate | 0.30808 | 0.02451 | −0.36398 |
| Chalkiness degree | −0.15315 | −0.38056 | 0.25936 |
| Chalk grain rate | 0.05584 | −0.46237 | 0.04858 |
| Amylose content | 0.11225 | −0.12632 | 0.57625 |
| Protein content | 0.31542 | −0.03356 | −0.11718 |
| SOD | 0.38651 | −0.01062 | 0.06277 |
| POD | 0.39245 | 0.21577 | 0.09373 |
| CAT | 0.33571 | 0.17148 | 0.34234 |
| MDA | 0.40337 | 0.21105 | 0.09347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Zhang, W.; Liu, Q.; Duan, X.; Yao, Y.; Wang, Y.; Li, J.; Yao, X. Biochar Application in Combination with No Tillage Enhanced Yield and Grain Quality of Ratoon Rice. Agriculture 2024, 14, 1407. https://doi.org/10.3390/agriculture14081407
Du B, Zhang W, Liu Q, Duan X, Yao Y, Wang Y, Li J, Yao X. Biochar Application in Combination with No Tillage Enhanced Yield and Grain Quality of Ratoon Rice. Agriculture. 2024; 14(8):1407. https://doi.org/10.3390/agriculture14081407
Chicago/Turabian StyleDu, Bin, Wujun Zhang, Qiangming Liu, Xiujian Duan, Yanjie Yao, Yu Wang, Jingyong Li, and Xiong Yao. 2024. "Biochar Application in Combination with No Tillage Enhanced Yield and Grain Quality of Ratoon Rice" Agriculture 14, no. 8: 1407. https://doi.org/10.3390/agriculture14081407
APA StyleDu, B., Zhang, W., Liu, Q., Duan, X., Yao, Y., Wang, Y., Li, J., & Yao, X. (2024). Biochar Application in Combination with No Tillage Enhanced Yield and Grain Quality of Ratoon Rice. Agriculture, 14(8), 1407. https://doi.org/10.3390/agriculture14081407






