Free Gossypol Removal and Nutritional Value Enhancement of Cottonseed Meal via Solid-State Fermentation with Rhodotorula mucilaginosa TG529
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of the Gossypol-Biodegrading Strains
2.3. Identification of the Strains
2.4. Determination of Growth Curves
2.5. Optimization of Solid-State Fermentation Conditions
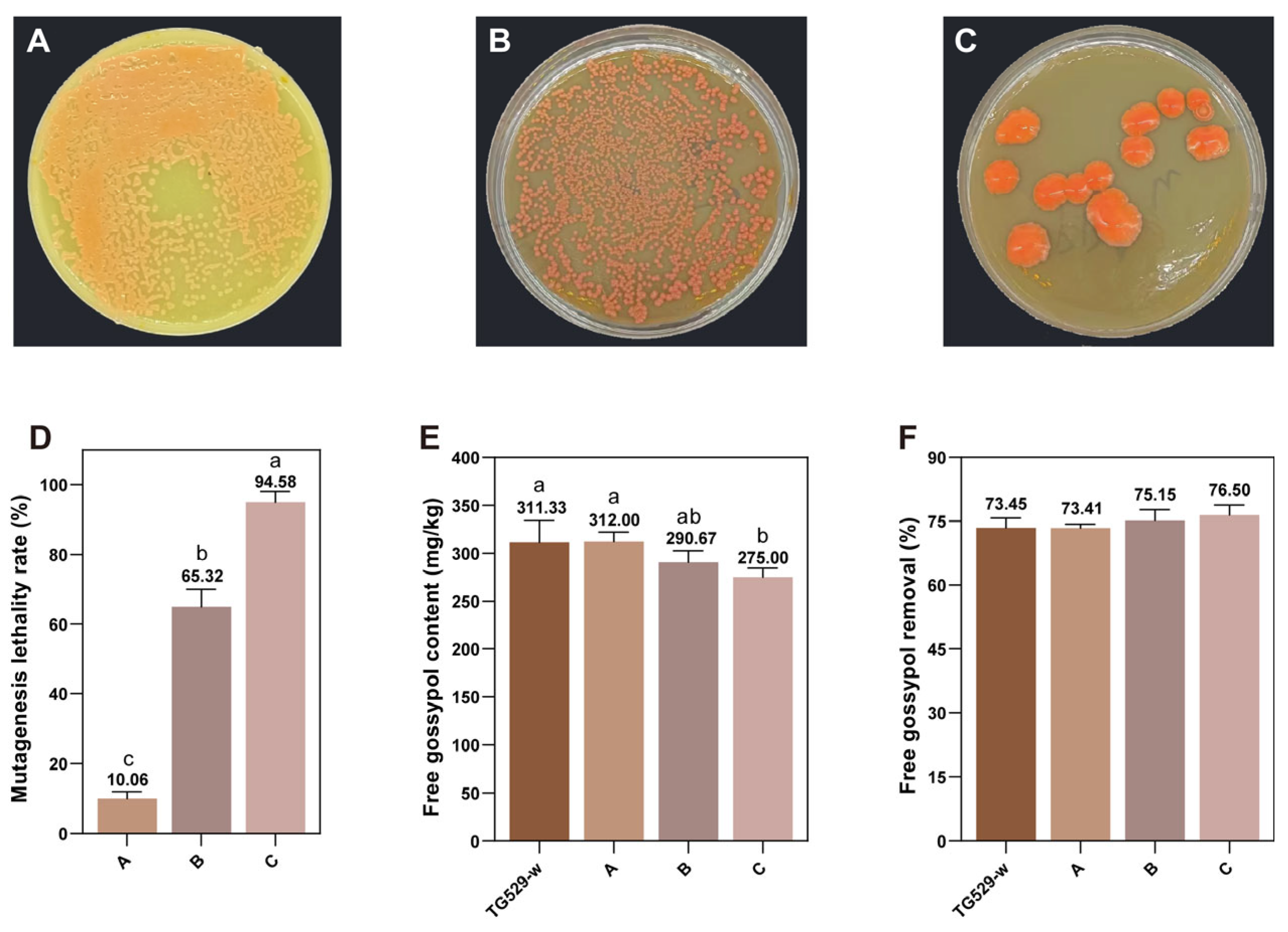
2.6. ARTP Mutation Breeding of Strains
2.7. Chemical Composition of the FCSM
2.8. Statistical Analysis
3. Results
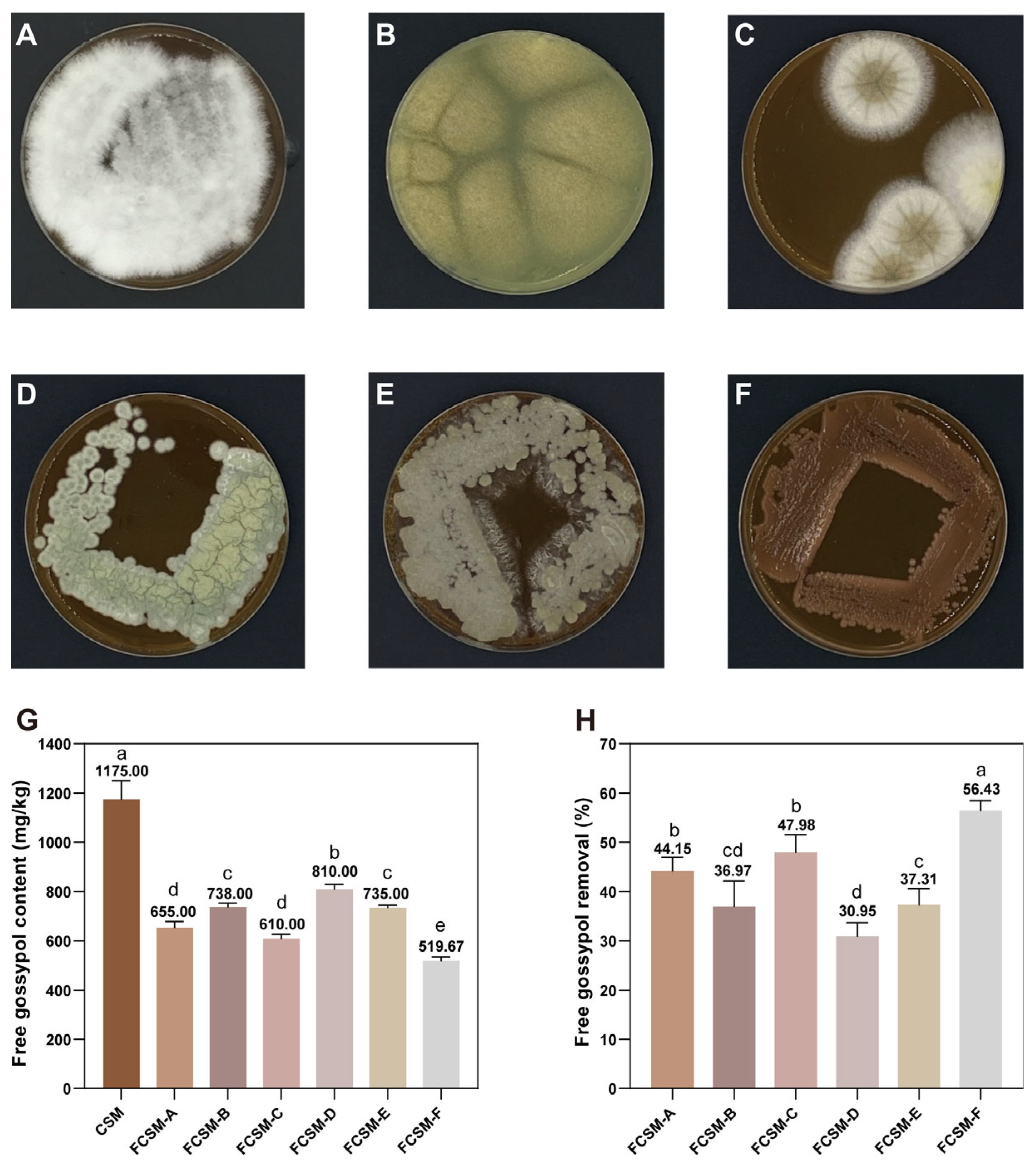
3.1. Strain Screening and Identification
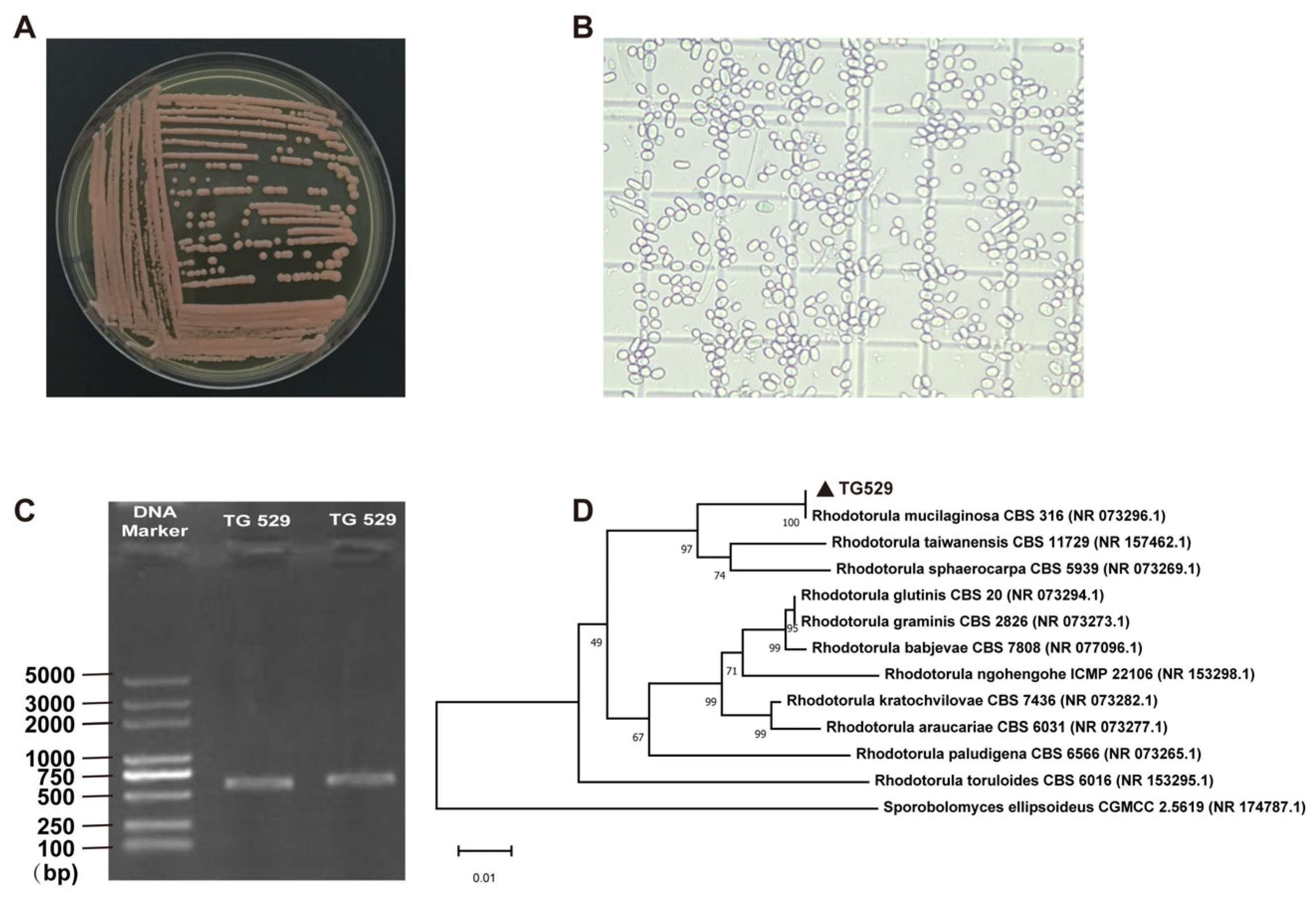
3.2. Identification of the FG Biodegrading Strain
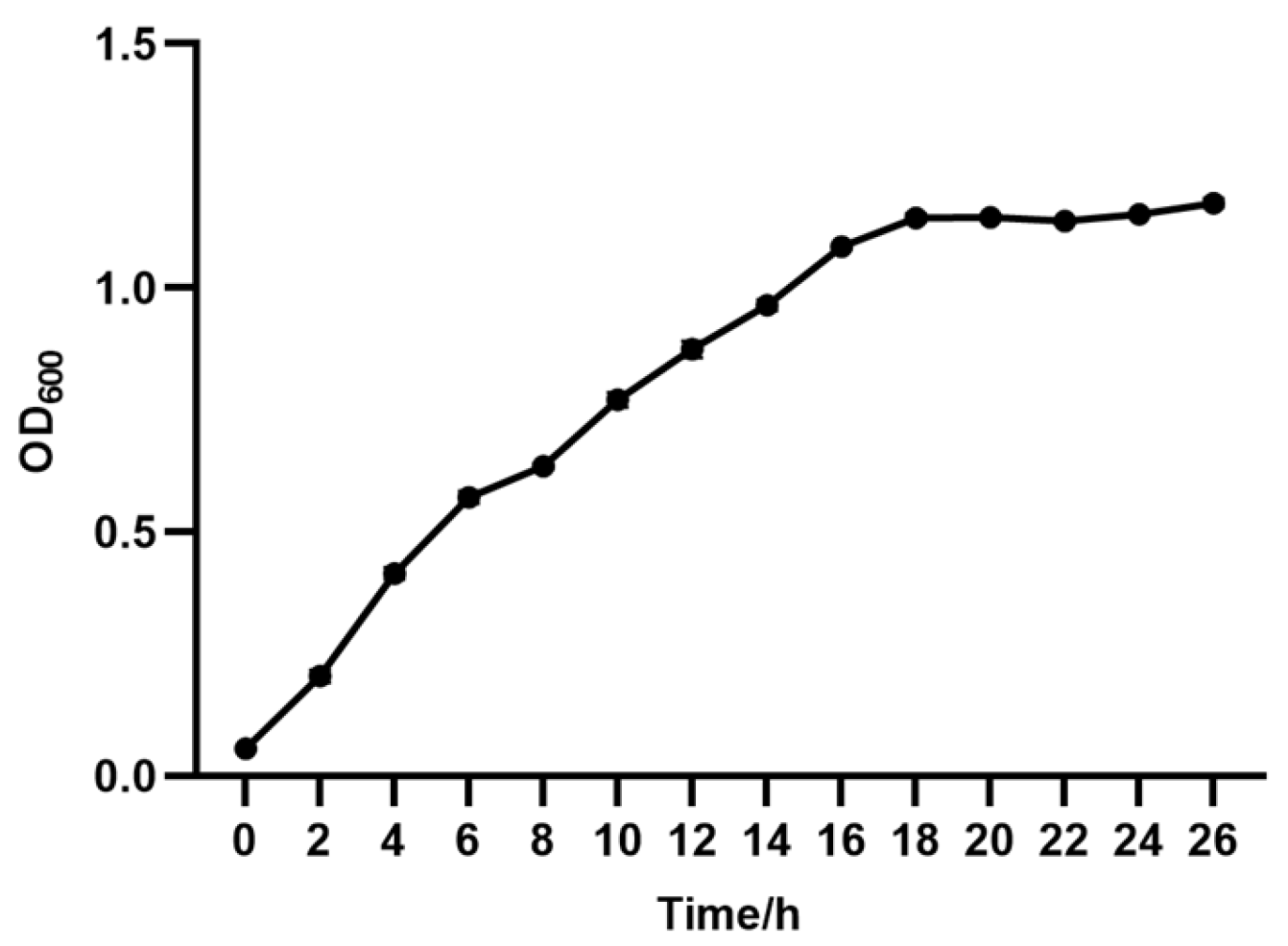
3.3. Growth Study of R. mucilaginosa TG529
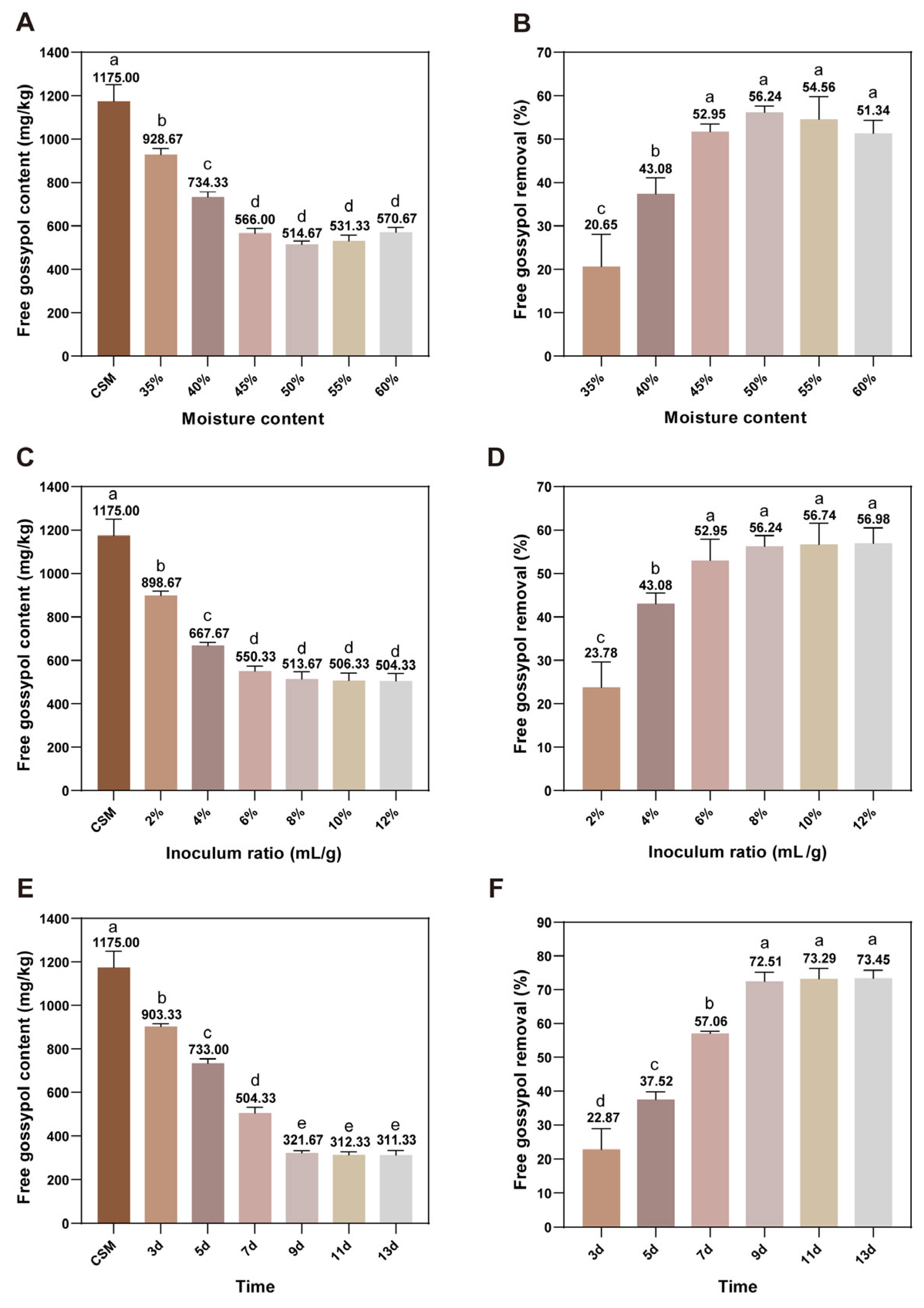
3.4. Optimization of Solid-State Fermentation Condition
3.5. Nutritional Value of R. mucilaginosa-Fermented Cottonseed Meal
3.6. ARTP Mutagenesis in R. mucilaginosa TG 529
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD/FAO: Pairs France, 2023. [Google Scholar]
- Betchem, G.; Monto, A.R.; Lu, F.; Billong, L.F.; Ma, H.L. Prospects and application of solid-state fermentation in animal feed production—A review. Ann. Anim. Sci. 2024, 96, 1338–1349. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, X.; Dai, G.; Tong, B.; Wang, H.; Oenema, O.; van Zanten, H.H.E.; Gerber, P.; Hou, Y. Low-opportunity-cost feed can reduce land-use-related environmental impacts by about one-third in China. Nat. Food 2023, 4, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, D.; Wang, Z.; Li, Y. Structural Evolution of Global Soybean Trade Network and the Implications to China. Foods 2023, 12, 1550–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Hou, L.; He, Z.; Cao, S.; Wen, X.; Liu, S.; Li, Y.; Chen, S.; Zheng, H.; Deng, D.; et al. Effect of Miscellaneous Meals Replacing Soybean Meal in Feed on Growth Performance, Serum Biochemical Parameters, and Microbiota Composition of 25–50 kg Growing Pigs. Animals 2024, 14, 1354–1370. [Google Scholar] [CrossRef]
- Ma, D.L.; Ma, X.K.; Liu, L.; Zhang, S. Chemical composition, energy, and amino acid digestibility in 7 cottonseed co-products fed to growing pigs. Anim. Sci. J. 2018, 96, 1338–1349. [Google Scholar] [CrossRef]
- Tan, C.F.; Kwan, S.H.; Lee, C.S.; Soh, Y.N.A.; Ho, Y.S.; Bi, X. Cottonseed Meal Protein Isolate as a New Source of Alternative Proteins: A Proteomics Perspective. Int. J. Mol. Sci. 2022, 23, 10105–10124. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, N.; Prakash, S.; Sharma, N.; Radha; Sharma, K.; Chandran, D.; Raman, P.; Panesar, P.S. Cottonseed Meal: Eliminating Gossypol for Securing Another Source of Protein. In Oilseed Meal as a Sustainable Contributor to Plant-Based Protein; Springer International Publishing: Cham, Switzerland, 2024; pp. 145–167. [Google Scholar]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. The use of cottonseed meal as a protein source for poultry: An updated review. World Poult. Sci. J. 2016, 72, 473–484. [Google Scholar] [CrossRef]
- Gadelha, I.C.; Fonseca, N.B.; Oloris, S.C.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 2014, 231635–231646. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zou, X.; Wang, Y.; Jiang, X.; Sun, M.; Xu, S.; Lin, Y.; Hua, L.; Li, J.; Feng, B.; et al. Nutritional values of cottonseed meal from different sources fed to gestating and non-pregnant sows. J. Anim. Sci. 2023, 101, 118. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, W.; Liu, H.; Shen, L.; Zhu, M.; Zhou, T.; Zhang, L.; Xiao, D.; Chen, L. Rumen Microbiota Transplantation Alleviates Gossypol Diet-Induced Reproductive, Liver, and Intestinal Damage in Male Mice. Animals 2024, 14, 2206–2218. [Google Scholar] [CrossRef]
- Kadam, D.M.; Kumar, M.; Kasara, A. Application of high energy electromagnetic radiations in elimination of anti-nutritional factors from oilseeds. LWT Food Sci. Technol. 2021, 151, 112085–112094. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, H.; Zhang, X.; Chen, X.; Liu, Y.; Tang, Y.; Zhang, W.; Wang, Z.; Zhao, L.; Guo, Y. Effective Degradation of Free Gossypol in Defatted Cottonseed Meal by Bacterial Laccases: Performance and Toxicity Analysis. Foods 2024, 13, 566–580. [Google Scholar] [CrossRef]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Xu, Z.-R.; Zhao, S.-H.; Sun, J.-Y.; Yang, X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed Sci. Tech. 2007, 135, 176–186. [Google Scholar] [CrossRef]
- Suntara, C.; Cherdthong, A.; Uriyapongson, S.; Wanapat, M.; Chanjula, P. Comparison Effects of Ruminal Crabtree-Negative Yeasts and Crabtree-Positive Yeasts for Improving Ensiled Rice Straw Quality and Ruminal Digestion Using In Vitro Gas Production. J. Fungi 2020, 6, 109–129. [Google Scholar] [CrossRef]
- Tang, J.W.; Sun, H.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of Replacement of Soybean Meal by Fermented Cottonseed Meal on Growth Performance, Serum Biochemical Parameters and Immune Function of Yellow-feathered Broilers. Asian Australas. J. Anim. Sci. 2012, 25, 393–400. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Effect of lactic acid bacteria and yeast supplementation on anti-nutritional factors and chemical composition of fermented total mixed ration containing cottonseed meal or rapeseed meal. Anim. Biosci. 2022, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Zhu, M.; Wang, Y.; Zhou, T.; Wan, F.; Zhang, Y.; Chen, L. Isolation of Bacillus velezensis from Silage and Its Effect on Aerobic Stability and In Vitro Methane Production of Whole-Plant Corn Silage. Agriculture 2024, 14, 830–844. [Google Scholar] [CrossRef]
- ISO 6866-1985; Animal Feeding Stuffs-Determination of Free and Total Gossypol. Internal Organization for Standardization: Geneva, Switzerland, 1985.
- Satankar, V.; Jhodkar, D.; Singh, M.; Kumar, M.; Mageshwaran, V.; Palanisamy, S.; Ayrilmis, N.; Khan, T. Two-step process for gossypol reduction and protein enhancement in cottonseed kernel powder. Biomass Conv. Bioref. 2024. [Google Scholar] [CrossRef]
- GB/T 38580-2020; Technical Specification for Mutagenesis Breeding of Microorganisms. State Administration for Market Regulation. Standardization Administration of the People’s Repub.: Beijing, China, 2020.
- Berbert; Queiroz, D.M.; Melo, E.C. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Rockville, MA, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Henneberg, W.; Stohmann, F. Stohmann Über das Erhaltungsfutter volljährigen Rindviehs. J. Landwirtsch. 1859, 3, 485–551. [Google Scholar]
- QB/T2653-2004; Soy Peptides Powder. National Development and Reform Commission: Beijing, China, 2004.
- Liu, J.; Song, W.J.; Zhang, N.Y.; Tan, J.; Krumm, C.S.; Sun, L.H.; Qi, D.S. Biodetoxification of aflatoxin B1 in cottonseed meal by fermentation of Cellulosimicrobium funkei in duckling diet. Poult. Sci. 2017, 96, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Bharagava, R.N. Environmental Contaminants: Ecological Implications and Management; Springer: Berlin/Heidelberg, Germany, 2019; Volume 14. [Google Scholar]
- Barnes, N.M.; Khodse, V.B.; Lotlikar, N.P.; Meena, R.M.; Damare, S.R. Bioremediation potential of hydrocarbon-utilizing fungi from select marine niches of India. 3 Biotech 2018, 8, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Xu, Z.R.; Zhao, S.H.; Jiang, J.F.; Wang, Y.B.; Yan, X.H. Optimization of process parameters for reduction of gossypol levels in cottonseed meal by Candida tropicalis ZD-3 during solid substrate fermentation. Toxicon 2006, 48, 221–236. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xu, Z.R.; Sun, J.Y.; Yang, X. Effect of selected fungi on the reduction of gossypol levels and nutritional value during solid substrate fermentation of cottonseed meal. J. Zhejiang Univ. Sci. B 2006, 7, 690–695. [Google Scholar] [CrossRef]
- Neto, C.B.S.; Gomes, T.G.; Filho, E.X.F.; Fontes, W.; Ricart, C.A.O.; de Almeida, J.R.M.; de Siqueira, F.G.; Miller, R.N.G. An Enzymatic and Proteomic Analysis of Panus lecomtei during Biodegradation of Gossypol in Cottonseed. J. Fungi 2024, 10, 321–341. [Google Scholar] [CrossRef]
- Mageshwaran, V.; Satankar, V.; Paul, S. Solid-state fermentation for gossypol detoxification and nutritive enrichment of cottonseed cake: A scale-up of batch fermentation process. BioResources 2023, 19, 1107–1118. [Google Scholar] [CrossRef]
- Lv, L.; Xiong, F.; Liu, Y.; Pei, S.; He, S.; Li, S.; Yang, H. The rumen-derived Lact. mucosae LLK-XR1 exhibited greater free gossypol degradation capacity during solid-state fermentation of cottonseed meal and probiotic potential. BMC Microbiol. 2024, 24, 15–29. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Polerecky, L.; Dombrowski, N.; Kienhuis, M.V.M.; Posthuma, I.; Gerritse, J.; Boekhout, T.; Niemann, H. Polyethylene degradation and assimilation by the marine yeast Rhodotorula mucilaginosa. ISME Commun. 2023, 3, 68–76. [Google Scholar] [CrossRef]
- Angeles de Paz, G.; Martínez-Gutierrez, H.; Ramírez-Granillo, A.; López-Villegas, E.O.; Medina-Canales, M.G.; Rodríguez-Tovar, A.V. Rhodotorula mucilaginosa YR29 is able to accumulate Pb2+ in vacuoles: A yeast with bioremediation potential. World J. Microb. Biot. 2023, 39, 238–254. [Google Scholar] [CrossRef]
- Wang, W.-K.; Li, W.-J.; Wu, Q.-C.; Wang, Y.-L.; Li, S.-L.; Yang, H.-J. Isolation and Identification of a Rumen Lactobacillus Bacteria and Its Degradation Potential of Gossypol in Cottonseed Meal during Solid-State Fermentation. Microorganisms 2021, 9, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Bi, Y.; Sun, S. Free fatty acids increase gossypol losses in soybean oil during heating. Eur. J. Lipid Sci. Tech. 2016, 118, 584–591. [Google Scholar] [CrossRef]
- Yu, F.; Mcnabb, W.C.; Barry, T.N.; Moughan, P.J. Effect of Heat Treatment Upon the Chemical Composition of Cottonseed Meal and Upon the Reactivity of Cottonseed Condensed Tannins. J. Sci. Food Agric. 1996, 72, 263–272. [Google Scholar] [CrossRef]
- Soares Neto, C.B.; Conceição, A.A.; Gomes, T.G.; de Aquino Ribeiro, J.A.; Campanha, R.B.; Barroso, P.A.V.; Machado, A.E.V.; Mendonça, S.; De Siqueira, F.G.; Miller, R.N.G. A Comparison of Physical, Chemical, Biological and Combined Treatments for Detoxification of Free Gossypol in Crushed Whole Cottonseed. Waste Biomass Valoriz. 2020, 12, 3965–3975. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L. Presence of free gossypol in whole cottonseed. EFSA J. 2017, 15, e04850–e04865. [Google Scholar]
- Duodu, C.P.; Adjei-Boateng, D.; Edziyie, R.E.; Agbo, N.W.; Owusu-Boateng, G.; Larsen, B.K.; Skov, P.V. Processing techniques of selected oilseed by-products of potential use in animal feed: Effects on proximate nutrient composition, amino acid profile and antinutrients. Anim. Nutr. 2018, 4, 442–451. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Su, L.W.; Cheng, Y.H.; Hsiao, F.S.; Han, J.C.; Yu, Y.H. Optimization of Mixed Solid-state Fermentation of Soybean Meal by Lactobacillus Species and Clostridium butyricum. Pol. J. Microbiol. 2018, 67, 297–305. [Google Scholar] [CrossRef]
- Handa, C.L.; de Lima, F.S.; Guelfi, M.F.G.; Fernandes, M.D.S.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef]
- Weng, X.Y.; Sun, J.Y. Biodegradation of free gossypol by a new strain of Candida tropicalis under solid state fermentation: Effects of fermentation parameters. Process Biochem. 2006, 41, 1663–1668. [Google Scholar] [CrossRef]
- Olukosi, O.A.; Walker, R.L.; Houdijk, J.G.M. Evaluation of the nutritive value of legume alternatives to soybean meal for broiler chickens. Poult. Sci. 2019, 98, 5778–5788. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Moharam, M.E.; El-Gamal, N.N.; Khalil, B.E. Enhancement of novel Endo-polygalacturonase expression in Rhodotorula mucilaginosa PY18: Insights from mutagenesis and molecular docking. Microb. Cell Factories 2023, 22, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42–52. [Google Scholar] [CrossRef]
- Rehemujiang, H.; Yusuf, H.A.; Ma, T.; Diao, Q.; Kong, L.; Kang, L.; Tu, Y. Evaluating Fermentation Quality, Aerobic Stability, and Rumen-Degradation (In Situ) Characteristics of Various Protein-Based Total Mixed Rations. Animals 2023, 13, 2730–2745. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Langousi, I.; Kougia, E.; Saxami, G.; Markou, G.; Haroutounian, S.A.; Arapoglou, D. Solid-State Fermentation Initiated by Pleurotus ostreatus of a Cottonseed Cake and Lathyrus clymenum Pericarp Mixture: Impact on Nutritional Profile and Gossypol Content. Appl. Sci. 2024, 14, 5066–5078. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, M.; Zannini, E.; Arendt, E. Impact of Saccharomyces cerevisiae metabolites produced during fermentation on bread quality parameters: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1152–1164. [Google Scholar] [CrossRef]
- Xu, H.; Dai, C.; Tang, Y.; Xu, X.; Umego, E.C.; He, R.; Ma, H. The selective breeding and mutagenesis mechanism of high-yielding surfactin Bacillus subtilis strains with atmospheric and room temperature plasma. J. Sci. Food Agric. 2022, 102, 1851–1861. [Google Scholar] [CrossRef]
- Zhang, A.D.; Ma, Y.D.; Deng, Y.; Zhou, Z.W.; Cao, Y.; Yang, B.; Bai, J.; Sun, Q. Enhancing Protease and Amylase Activities in Bacillus licheniformis XS-4 for Traditional Soy Sauce Fermentation Using ARTP Mutagenesis. Foods 2023, 12, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.C.; Lu, M.J.; Cui, X.Y.; Wang, X.Q.; Jiang, M.Y.; Wang, Y.S.; Wang, Z.Q.; Yu, X.X.; Tang, S.S.; Chen, G.; et al. Enhanced laccase production by mutagenized Myrothecium verrucaria using corn stover as a carbon source and its potential in the degradation of 2-chlorophen. Bioproc. Biosyst. Eng. 2022, 45, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
| Items | CSM | FCSM | FCSM-ARTP |
|---|---|---|---|
| Gross energy (MJ/kg) | 19.34 ± 0.05 | 19.30 ± 0.03 | 19.33 ± 0.02 |
| Crude protein content (%) | 46.04 ± 0.08 b | 51.23 ± 0.02 a | 51.67 ± 0.32 |
| Acid-soluble protein contents (%) | 4.58 ± 0.04 b | 8.49 ± 0.29 Ba | 10.47 ± 0.23 Aa |
| Dry matter (%) | 95.03 ± 0.22 | 95.01 ± 0.31 | 95.92 ± 0.09 |
| Ether extract (%) | 1.15 ± 0.05 | 1.24 ± 0.05 | 1.26 ± 0.02 |
| Crude fiber (%) | 16.45 ± 0.08 a | 14.30 ± 0.29 b | 14.20 ± 0.15 |
| NDF (%) | 34.55 ± 0.42 a | 29.92 ± 1.21 Ab | 25.07 ± 2.32 Ba |
| ADF (%) | 21.02 ± 0.23 a | 20.38 ± 0.29 b | 21.27 ± 0.81 |
| Items | CSM | FCSM | FCSM-ARTP |
|---|---|---|---|
| Lysine | 1.55 ± 0.01 b | 2.13 ± 0.02 a | 2.10 ± 0.10 |
| Methionine | 0.22 ± 0.02 b | 0.57 ± 0.04 a | 0.59 ± 0.08 |
| Threonine | 1.05 ± 0.05 b | 1.44 ± 0.12 a | 1.56 ± 0.09 |
| Tryptophan | 0.29 ± 0.02 b | 0.64 ± 0.05 a | 0.63 ± 0.07 |
| Arginine | 3.57 ± 0.04 b | 5.32 ± 0.12 a | 5.35 ± 0.12 |
| Histidine | 0.89 ± 0.06 b | 1.29 ± 0.03 a | 1.30 ± 0.08 |
| Isoleucine | 1.02 ± 0.03 b | 1.64 ± 0.07 a | 1.65 ± 0.03 |
| Leucine | 2.02 ± 0.09 b | 2.54 ± 0.12 a | 2.50 ± 0.14 |
| Phenylalanine | 1.96 ± 0.02 b | 2.50 ± 0.05 aB | 2.80 ± 0.09 A |
| Valine | 1.50 ± 0.05 b | 2.42 ± 0.10 a | 2.33 ± 0.07 |
| Alanine | 1.33 ± 0.02 b | 1.94 ± 0.03 a | 1.93 ± 0.13 |
| Aspartic acid | 3.10 ± 0.03 b | 4.48 ± 0.13 a | 4.49 ± 0.19 |
| Cystine | 0.34 ± 0.04 b | 0.69 ± 0.02 a | 0.64 ± 0.11 |
| Glutamic acid | 6.52 ± 0.30 b | 10.57 ± 0.32 a | 10.50 ± 0.15 |
| Glycine | 1.36 ± 0.01 b | 1.81 ± 0.07 a | 1.76 ± 0.21 |
| Proline | 1.39 ± 0.03 b | 2.02 ± 0.07 a | 2.10 ± 0.20 |
| Serine | 1.23 ± 0.03 b | 1.75 ± 0.05 a | 1.67 ± 0.15 |
| Tyrosine | 0.89 ± 0.03 b | 1.22 ± 0.13 a | 1.24 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liu, H.; Liu, D.; Zhou, M.; Jiang, Q.; Ma, X.; Wang, J.; Tan, B.; Zhang, C. Free Gossypol Removal and Nutritional Value Enhancement of Cottonseed Meal via Solid-State Fermentation with Rhodotorula mucilaginosa TG529. Agriculture 2024, 14, 1463. https://doi.org/10.3390/agriculture14091463
Liu B, Liu H, Liu D, Zhou M, Jiang Q, Ma X, Wang J, Tan B, Zhang C. Free Gossypol Removal and Nutritional Value Enhancement of Cottonseed Meal via Solid-State Fermentation with Rhodotorula mucilaginosa TG529. Agriculture. 2024; 14(9):1463. https://doi.org/10.3390/agriculture14091463
Chicago/Turabian StyleLiu, Bifan, Huanyu Liu, Daohe Liu, Miao Zhou, Qian Jiang, Xiaokang Ma, Jing Wang, Bi’e Tan, and Chen Zhang. 2024. "Free Gossypol Removal and Nutritional Value Enhancement of Cottonseed Meal via Solid-State Fermentation with Rhodotorula mucilaginosa TG529" Agriculture 14, no. 9: 1463. https://doi.org/10.3390/agriculture14091463







