First Report: Senna multijuga Subsp. multijuga (Fabales: Fabaceae) as an Attractant and Bioinsecticide for Oryctes rhinoceros (Coleoptera: Scarabaeidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of “Hujan Emas” (Golden Rain) Plants
2.1.1. Morphological Characteristics
2.1.2. Molecular Identification
- DNA Extraction
- DNA Amplification
- Sequencing and Analysis of Results
2.2. The Potential of the “Hujan Emas” (Golden Rain) Plant as an Attractant for O. rhinoceros Beetles
2.3. Laboratory Testing of the Plant Extract’s Ability as a Bioinsecticide against O. rhinoceros
2.3.1. Preparation of the Golden Rain Plant Extract
2.3.2. Experimental Design
2.3.3. Preparation of Test Insects
2.3.4. Application of Plant Extracts
2.3.5. Observations and Data Collection
2.3.6. Data Analysis
3. Results
3.1. Senna multijuga Has the Identity of the “Hujan Emas” (Golden Rain) Plant
3.1.1. Morphological Observations Indicate That the Golden Rain Plant Is Senna multijuga
3.1.2. Molecular Identification Confirmed That the Golden Rain Plant Is Senna multijuga
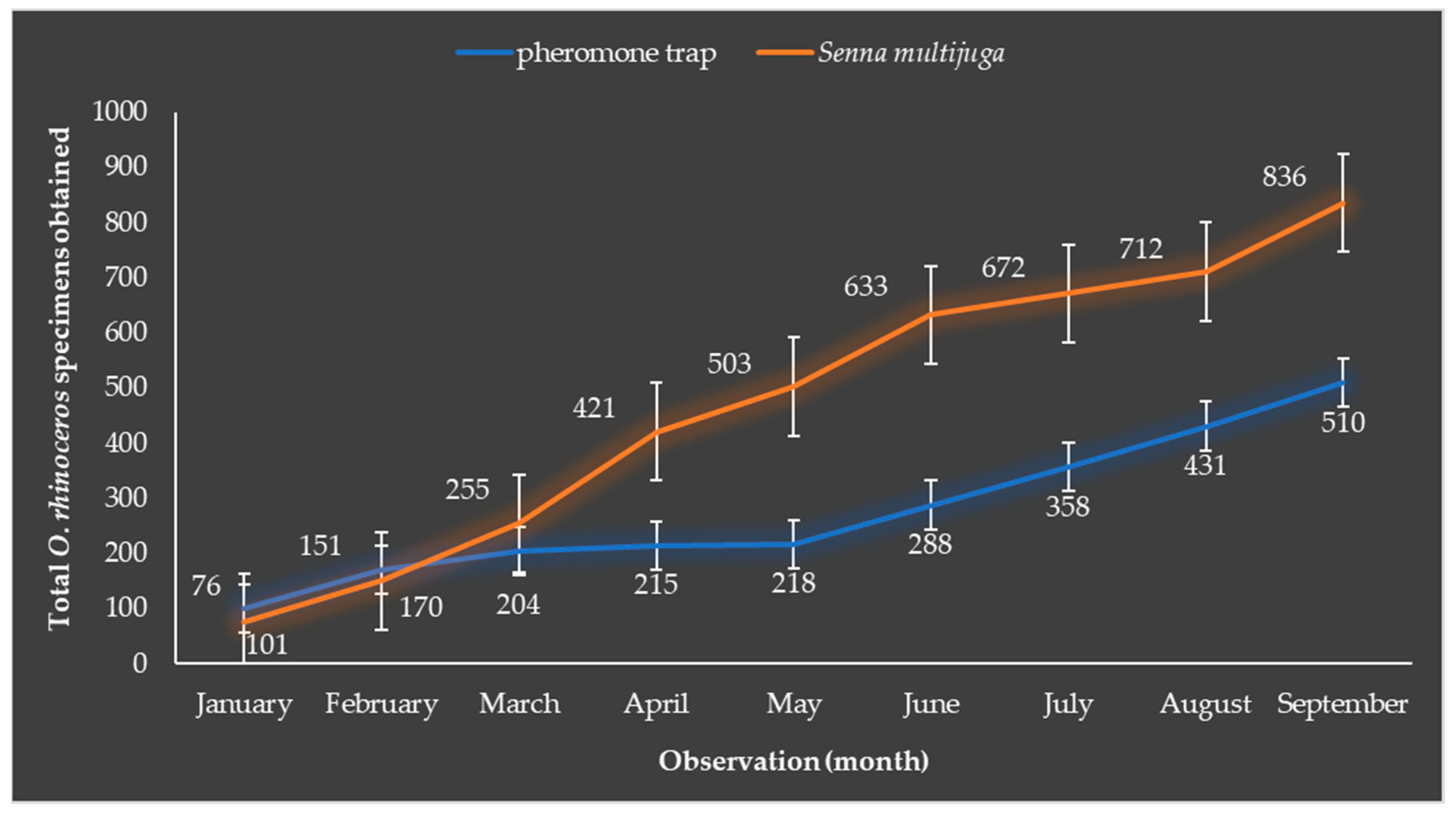
3.2. Senna multijuga Has the Ability to Attract Adult O. rhinoceros
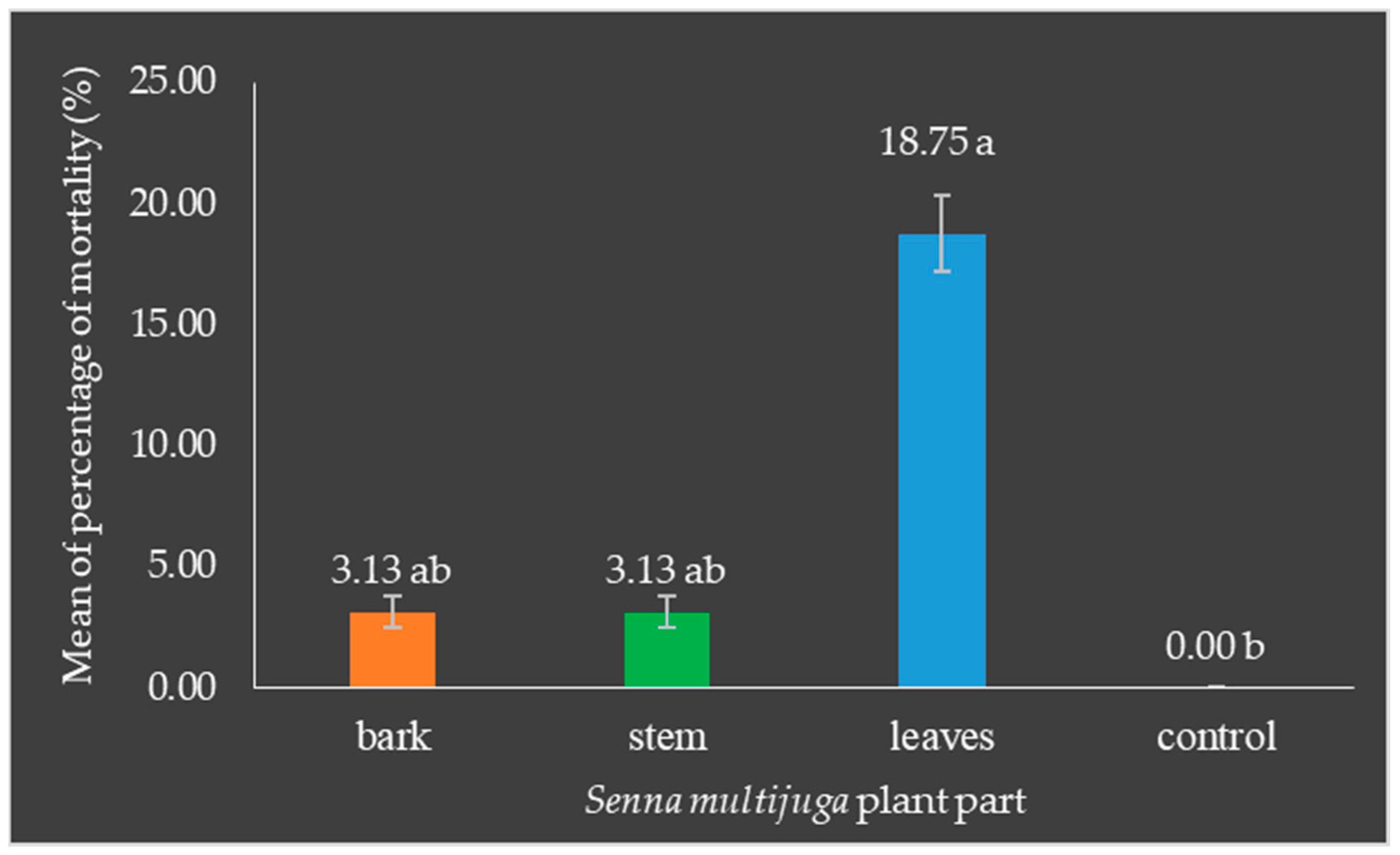
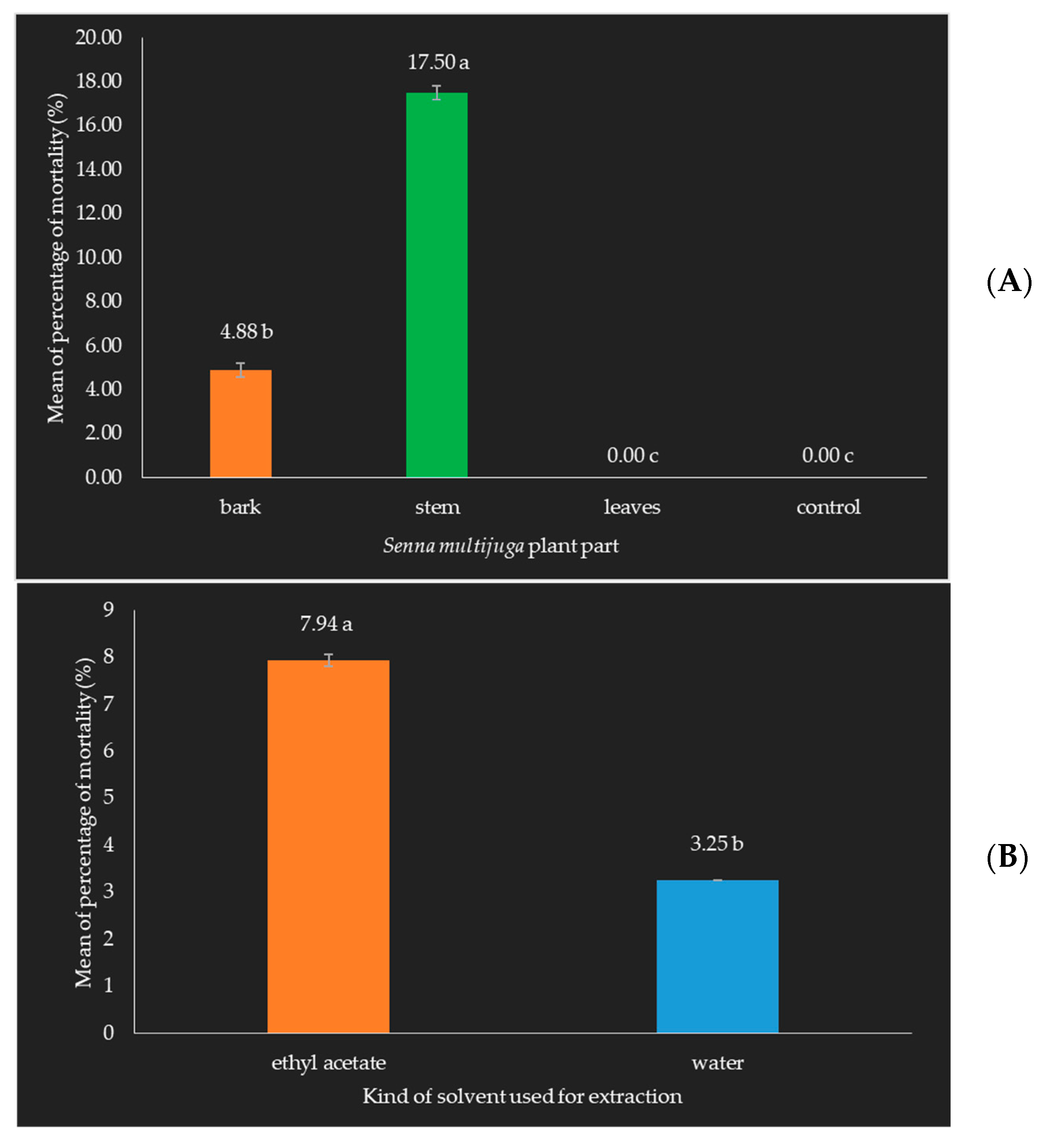
3.3. Senna multijuga Has Potential as a Bioinsecticide for O. rhinoceros
- Mortality of Oryctes rhinoceros larvae
- Mortality of Oryctes rhinoceros beetle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedford, G.O. Biology, ecology, and control of palm rhinoceros beetles. Annu. Rev. Entomol. 1980, 25, 309–339. [Google Scholar] [CrossRef]
- Hochberg, M.E.; Waage, J.K. A model for the biological control of Oryctes rhinoceros (Coleopteran: Scarabaeidae) by means of pathogens. J. Appl. Ecol. 1991, 28, 514–531. [Google Scholar] [CrossRef]
- Kalidas, P. Pest problems of oil palm and management strategies for sustainability. Agrotechnology 2012, SS11, 1. [Google Scholar] [CrossRef]
- Lever, R. Pests of the Coconut Palm; Food and Agriculture Organization: Rome, Italy, 1969; pp. 125–133. [Google Scholar]
- Manjeri, G.; Muhamad, R.; Tan, S.G. Oryctes rhinoceros beetles, an oil palm pest in Malaysia. Annu. Res. Rev. 2014, 4, 3429–3439. [Google Scholar] [CrossRef]
- Kalshoven, L.G.E. The Pests of Crops in Indonesia; Ichtiar Baru-Van Hoeve: Jakarta, Indonesia, 1981; pp. 463–468. [Google Scholar]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Kelchner, S.A. Group II introns as phylogenetic tools: Structure, function, and evolutionary constraints. Am. J. Bot. 2002, 89, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, B.; Endress, P.K.; Queiroz, L.P.; Conti, E. Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: Patterns in the evolution of floral symmetry and extrafloral nectaries. Am. J. Bot. 2006, 93, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Suharjo, R.; Swibawa, G.; Prasetyo, J.; Fitriana, Y.; Danaatmadja, Y.; Budiawan, A.; Roberts, S.; Noorhajati, N.; Amad, M.; Thines, M. Peronosclerospora australiensis is a synonym of P. maydis, which is widespread on Sumatra, and distinct from the most prevalent Java maize downy mildew pathogen. Mycol. Prog. 2020, 19, 1309–1315. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 4, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Maimunah, D.; Irwan, S.N.R.; Indradewa, D. Pertumbuhan widelia (Wedelia trilobata (L) Hitchc) pada tingkat naungan berbeda di jalur hijau Kota Yogyakarta [Widelia (Wedelia trilobata (L) Hitchc) growth at different shading levels in the roadside greenery of Yogyakarta City]. J. Ilmu Pertan. Indones. 2020, 25, 547–555. [Google Scholar] [CrossRef]
- Salinas-Sánchez, D.O.; Flores-Franco, G.; Avilés-Montes, D.; Valladares-Cisneros, M.G.; Arias-Ataide, D.M.; Mendoza-Catalán, M.A.; Sotelo-Leyva, C. Bioactivity of a linoleic acid–rich fraction of Ricinus communis L. (Euphorbiaceae) leaves against the yellow sugarcane aphid, Sipha flava (Hemiptera: Aphididae). J. Food Prot. 2021, 84, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Fitriana, Y.; Purnomo; Hariri, A.M. Uji efikasi ekstrak gulma siam terhadap mortalitas hama pencucuk buah kakao (Helopeltis spp.) di laboratorium) [Efficacy test of siam weed extract on the mortality of cocoa mirid bugs (Helopeltis spp.) in the laboratory]. J. HPT. Tropika. 2012, 12, 85–91. [Google Scholar] [CrossRef]
- R Core Team. R: A Languane and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 January 2024).
- Irwin, H.S.; Barneby, R.C. The American Cassiinae: A Synoptical Revision of Leguminosae Tribe Cassieae Subtribe Cassiinae in the New World; New York Botanical Garden: New York, NY, USA, 1982; pp. 1–918. [Google Scholar]
- Datiles, M.J.; Acevedo-Rodrígue, P. Senna multijuga (November Shower); CABI Compendium: Oxford, UK, 2014. [Google Scholar] [CrossRef]
- Ribeiro-costa, C.S.; Reynaud, D.T. Bruchids from Senna multijuga (Rich) I. & B (Caesalpinaceae) in Brazil with description of two new species. Coleopt. Bull. 1998, 52, 245–252. [Google Scholar]
- Local Land Services. North Coast Regional Strategic Weed Management Plan 2023–2027; North Coast Local Land Services: New South Wales, Australia, 2022; pp. 1–98. [Google Scholar]
- Serrano, M.A.; Pivatto, M.; Francisco, W.; Danuello, A.; Regasini, L.O.; Lopes, E.M.; Lopes, M.N.; Young, M.C.; Bolzani, V.S. Acetylcholinesterase inhibitory pyridine alkaloids of the leaves of Senna multijuga. J. Nat. Prod. 2010, 73, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Francisco, W.; Pivatto, M.; Danuello, A.; Regasini, L.O.; Baccini, L.R.; Young, M.C.; Lopes, N.P.; Lopes, J.L.; Bolzani, V.S. Pyridine alkaloids from Senna multijuga as acetylcholinesterase inhibitors. J. Nat. Prod. 2012, 75, 408–413. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Adelowoc, F.E.; Oluyoria, A.P. The genus Senna (Fabaceae): A review on its traditional uses, botany, phytochemistry, pharmacology and toxicology. S. Afr. J. Bot. 2021, 138, 1–32. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Tutuncu, S.; Aydar, E.F.; Topkaya, C.; Mertdinc, Z.; Ozcelik, B.; Aital, M.; et al. A review of recent studies on the antioxidant and antiinfectious properties of senna plants. Oxidative Med. Cell. Longev. 2022, 6025900. [Google Scholar] [CrossRef]
- Zibaee, E.; Javadi, B.; Sobhani, Z.; Akaberi, M.; Farhadi, F.; Amiri, M.S.; Baharara, H.; Sahebkarf, A.; Emami, S.A. Cassia species: A review of traditional uses, phytochemistry and pharmacology. Pharmacol. Res. Mod. Chin. Med. 2023, 9, 100325. [Google Scholar] [CrossRef]
- Hazuki, I.N.; Shukor, M.Y. Acetylcholinesterase as an in vitro assay for insecticides: A mini review. J. Environ. Microbiol. Toxicol. 2018, 6, 7–12. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, Y.; Raghavendra, A.; Bakthavatsalam, N. Acetylcholinesterase inhibition by biofumigant (Coumaran) from leaves of Lantana camara in stored grain and household insect pests. BioMed Res. Int. 2014, 187019. [Google Scholar] [CrossRef]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Persp. 1990, 87, 245–254. [Google Scholar] [CrossRef]
- Bedford, G.O. Advances in the control of rhinoceros beetle, Oryctes rhinoceros in oil palm. J. Oil Palm Res. 2014, 26, 183–194. [Google Scholar]
- Bedford, G.O. Biology and management of palm dynastid beetles: Recent advances. Annu. Rev. Entomol. 2013, 58, 353–372. [Google Scholar] [CrossRef]
- Paudel, S.; Jackson, T.A.; Mansfield, S.; Ero, M.; Moore, A.; Marshall, S.D.G. Use of pheromones for monitoring and control strategies of coconut rhinoceros beetle (Oryctes rhinoceros): A review. Crop Prot. 2023, 17, 106400. [Google Scholar] [CrossRef]
- Munawaroh, L.; Wirman, S.P.; Fitrya, N.; Syahputra, R.F.; Gesriantuti, N. Pendekatan baru pegendalian kumbang tanduk secara otomatis pada tanaman kelapa sawit yang ditanam ulang: Perangkap feromon kulit nanas [A novel approach for automated rhinoceros beetle control in oil palm replanting: Pineapple peel-derived trap]. J. Ilm. Pertan. 2013, 20, 200–208. [Google Scholar] [CrossRef]
- Alfiler, A.R.R. Increased attraction of Oryctes rhinoceros aggregation pheromone, ethyl 4-methyloctanoate, with coconut wood. CORD 1999, 15, 34. [Google Scholar] [CrossRef]
- Huber, M.; Roder, T.; Irmisch, S.; Riedel, A.; Gablenz, S.; Fricke, J.; Rahfeld, P.; Reichelt, M.; Paetz, C.; Liechti, N.; et al. A beta-glucosidase of an insect herbivore determines both toxicity and deterrence of a dandelion defense metabolite. Elife 2021, 10, e68642. [Google Scholar] [CrossRef]
- Oakeshott, J.G.; Horne, I.; Sutherland, T.D.; Russell, R.J. The genomics of insecticide resistance. Genome Biol. 2003, 4, 202. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H. The molecular genetics of insecticide resistance. Genetics 2013, 194, 807–815. [Google Scholar] [CrossRef]
- Ranson, H.; Claudianos, C.; Ortelli, F.; Abgrall, C.; Hemingway, J.; Sharakhova, M.V.; Unger, M.F.; Collins, F.H.; Feyereisen, R. Evolution of supergene families associated with insecticide resistance. Science 2002, 298, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-C.; Chien, T.-Y.; Hu, C.-C.; Chen, M.-J.M.; Wu, W.-J.; Feng, H.-T.; Haymer, D.S.; Chen, C.-Y. Discovery of genes related to insecticide resistance in bactrocera dorsalis by functional genomic analysis of a de novo assembled transcriptome. PLoS ONE 2012, 7, e40950. [Google Scholar] [CrossRef] [PubMed]
- Blanton, A.G.; Peterson, B.F. Symbiont-mediated insecticide detoxification as an emerging problem in insect pests. Front. Microbiol. 2020, 11, 547108. [Google Scholar] [CrossRef] [PubMed]
- Rupawate, P.S.; Roylawar, P.; Khandagale, K.; Gawande, S.; Ade, A.B.; Jaiswal, D.K.; Borgave, S. Role of gut symbionts of insect pests: A novel target for insect-pest control. Front. Microbiol. 2023, 14, 1146390. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Chen, M.; Yue, L.; Xing, K.; Li, T.; Kang, K.; Liang, Z.; Yuan, L.; Zhang, W. A distinct strain of Arsenophonus symbiont decreases insecticide resistance in its insect host. PLoS Genet. 2018, 14, e1007725. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011, 5, 446–460. [Google Scholar] [CrossRef]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Boush, G.M.; Matsumura, F. Insecticidal degradation by Pseudomonas melophthora, the bacterial symbiote of the apple maggot. J. Econ. Entomol. 1967, 60, 918–920. [Google Scholar] [CrossRef]
- Shen, S.K.; Dowd, P.F. Detoxification spectrum of the cigarette beetle symbiont Symbiotaphrina kochii in culture. Entomol. Exp. Appl. 1991, 60, 51–59. [Google Scholar] [CrossRef]
- Berticat, C.; Rousset, F.; Raymond, M.; Berthomieu, A.; Weill, M. High Wolbachia density in insecticide-resistant mosquitoes. Proc. R. Soc. London Ser. B 2002, 269, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect microbial symbionts: Ecology, interactions, and biological significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 11–20. [Google Scholar] [CrossRef] [PubMed]






| Primer | Sequence (5′–3′) | Length of the PCR Product (bp) |
|---|---|---|
| matKsenF | TAAGGYTCTTTCTTTATGAG | 749 |
| matKsenR | ATACAAACTCTTTTTTGTTG | |
| rps16senF | GCCGTGGATTCTTACATCCA | 825 |
| rps16senR | ACGTATAAGAAGTTTTCTCC | |
| rpl16senF | CTCCTCGCGAATGAAASGAT | 846 |
| rpl16senR | GATATATTGGCGATATTATT |
| Gene | Identity | Total Score | Query Coverage | Percentage Identity | Acc. No |
|---|---|---|---|---|---|
| MaturaseK (MatK) | Senna multijuga | 1341 | 100% | 99.86% | KX538469 |
| chloroplast rps16 (rps16) | Senna multijuga var. multijuga | 1517 | 100% | 99.76% | AM086962 |
| chloroplast rpl16 (rpl16) | Senna multijuga var. multijuga | 1568 | 100% | 99.77% | AM08676 |
| Source | DF | Anova SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Block | 3 | 468.750000 | 156.250000 | 0.84 | 0.4872 |
| Plant part | 3 | 1718.750000 | 572.916667 | 3.08 | 0.0496 |
| Solvent | 1 | 78.125000 | 78.125000 | 0.42 | 0.5240 |
| Plant part × Solvent | 3 | 78.125000 | 26.041667 | 0.14 | 0.9349 |
| Source | DF | Anova SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Block | 3 | 58.593750 | 19.531250 | 2.33 | 0.1037 |
| Plant part | 3 | 1638.843750 | 546.281250 | 66.12 | <0001 |
| Solvent | 1 | 175.781250 | 175.781250 | 20.96 | 0.0002 |
| Plant part × Solvent | 3 | 176.343750 | 58.781250 | 7.01 | 0.0019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wandri, R.; Alam, S.; Ayundra, S.D.; Apriansa, A.; Asmono, D.; Subeki, S.; Fitriana, Y.; Hasibuan, R.; Suharjo, R. First Report: Senna multijuga Subsp. multijuga (Fabales: Fabaceae) as an Attractant and Bioinsecticide for Oryctes rhinoceros (Coleoptera: Scarabaeidae). Agriculture 2024, 14, 1477. https://doi.org/10.3390/agriculture14091477
Wandri R, Alam S, Ayundra SD, Apriansa A, Asmono D, Subeki S, Fitriana Y, Hasibuan R, Suharjo R. First Report: Senna multijuga Subsp. multijuga (Fabales: Fabaceae) as an Attractant and Bioinsecticide for Oryctes rhinoceros (Coleoptera: Scarabaeidae). Agriculture. 2024; 14(9):1477. https://doi.org/10.3390/agriculture14091477
Chicago/Turabian StyleWandri, Ruli, Samsu Alam, Shervinia Dwi Ayundra, Azharudin Apriansa, Dwi Asmono, Subeki Subeki, Yuyun Fitriana, Rosma Hasibuan, and Radix Suharjo. 2024. "First Report: Senna multijuga Subsp. multijuga (Fabales: Fabaceae) as an Attractant and Bioinsecticide for Oryctes rhinoceros (Coleoptera: Scarabaeidae)" Agriculture 14, no. 9: 1477. https://doi.org/10.3390/agriculture14091477





