Improving the Storage Quality of Ready-to-Eat Clementine Fruits Using Lemon By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Antioxidant Compounds from Lemon Pomace (LP)
2.2. Clementine Fruit Sample Preparation
2.3. Chemical Analyses of LPE and Clementine Fruits
2.4. Physical Analyses of Clementine Fruits
2.5. Microbiological Analyses of Clementine Fruits
2.6. Sensory Analyses of Clementine Fruits
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of LPE
3.2. Chemical, Physical, Microbiological, and Sensory Analyses Results of Clementine Fruits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzoni, L.; Ariza Fernández, M.T.; Capocasa, F. Potential health benefits of fruits and vegetables. Appl. Sci. 2021, 11, 8951. [Google Scholar] [CrossRef]
- Fabroni, S.; Romeo, F.V.; Rapisarda, P. Nutritional composition of clementine (Citrus × clementina) cultivars. In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2016; pp. 149–172. [Google Scholar]
- Temgire, S.; Borah, A.; Kumthekar, S.; Idate, A. Recent trends in ready-to-eat/cook food products. Pharma Innov. J. 2021, 10, 211–217. [Google Scholar] [CrossRef]
- Zahr, S.; Zahr, R.; El Hajj, R.; Khalil, M. Phytochemistry and biological activities of Citrus sinensis and Citrus limon: An update. J. Herb. Med. 2023, 41, 100737. [Google Scholar] [CrossRef]
- Adetunji, J.A.; Fasae, K.D.; Awe, A.I.; Paimo, O.K.; Adegoke, A.M.; Akintunde, J.K.; Sekhoacha, M.P. The protective roles of citrus flavonoids, naringenin, and naringin on endothelial cell dysfunction in diseases. Heliyon 2023, 9, e17166. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Mula, H.M.; López, J.P.; Serrano, M.; Pretel, M.T. A New Ready-to-Eat Product Based on Enzymatically Peeled ‘Hernandina’ Clementine Segments and Citrus Syrup. Foods 2023, 12, 3977. [Google Scholar] [CrossRef] [PubMed]
- Makahleh, A.; Saad, B.; Bari, M.F. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; pp. 51–78. [Google Scholar]
- Ghatak, P.D.; Sen, C.K. 20 Antioxidant Additives in Food Preservation and Human Health. In Food Toxicology; CRC Press: Boca Raton, FL, USA, 2016; p. 377. [Google Scholar]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Thompson, A.K.; Prange, R.K.; Bancroft, R.; Puttongsiri, T. Controlled Atmosphere Storage of Fruit and Vegetables; CABI: Wallingford, UK, 2018. [Google Scholar]
- Rao, C.G. Engineering for Storage of Fruits and Vegetables: Cold Storage, Controlled Atmosphere Storage, Modified Atmosphere Storage; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Lohita, B.; Srijaya, M. Novel Technologies for Shelf-Life Extension of Food Products as a Competitive Advantage: A Review. In Food Production, Diversity, and Safety Under Climate Change; Springer: Berlin/Heidelberg, Germany, 2024; Volume 2, pp. 285–306. [Google Scholar]
- Giacondino, C.; De Bruno, A.; Puntorieri, D.; Pizzimenti, M.; Piscopo, A. Impact of Antioxidant-Enriched Edible Gel Coatings and Bio-Based Packaging on Cherry Tomato Preservation. Gels 2024, 10, 549. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Hasan, K.; Islam, R.; Hasan, M.; Sarker, S.H.; Biswas, M.H. Effect of alginate edible coatings enriched with black cumin extract for improving postharvest quality characteristics of guava (Psidium guajava L.) fruit. Food Bioprocess Technol. 2022, 15, 2050–2064. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S. Alginate/carboxymethyl cellulose/starch-based active coating with grapefruit seed extract to extend the shelf life of green chilli. Ind. Crops Prod. 2023, 199, 116752. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Y.; Sun, L.; Xia, L.; Jia, H.; Li, Q.; Pan, J. Sodium alginate coating with plant extract affected microbial communities, biogenic amine formation and quality properties of abalone (Haliotis discus hannai Ino) during chill storage. LWT 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Shankar, S.; Danneels, F.; Lacroix, M. Coating with alginate containing a mixture of essential oils and citrus extract in combination with ozonation or gamma irradiation increased the shelf life of Merluccius sp. fillets. Food Packag. Shelf Life 2019, 22, 100434. [Google Scholar] [CrossRef]
- Han, J.H. Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus by-products: Valuable source of bioactive compounds for food applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Nardella, S.; Conte, A.; Del Nobile, M.A. State-of-Art on the recycling of by-products from fruits and vegetables of Mediterranean countries to prolong food shelf life. Foods 2022, 11, 665. [Google Scholar] [CrossRef]
- Gattuso, A.; Piscopo, A.; Santacaterina, S.; Imeneo, E.; De Bruno, A.; Poiana, M. Fortification of vegetable fat with natural antioxidants recovered by bergamot pomace for use as an ingredient for the production of biscuits. Sustain. Food Technol. 2023, 1, 951–961. [Google Scholar] [CrossRef]
- Gattuso, A.; Piscopo, A.; Romeo, R.; De Bruno, A.; Poiana, M. Recovery of bioactive compounds from Calabrian bergamot citrus waste: Selection of best green extraction. Agriculture 2023, 13, 1095. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Al-Farga, A. Prolonged the shelf life of different foods using the citrus by-products as antimicrobials: A review article. Ann. Agric. Crop Sci. 2019, 4, 1039. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Rastegar, S.; Hassanzadeh Khankahdani, H.; Rahimzadeh, M. Effectiveness of alginate coating on antioxidant enzymes and biochemical changes during storage of mango fruit. J. Food Biochem. 2019, 43, e12990. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT 2021, 152, 112346. [Google Scholar] [CrossRef]
- Li, X.Y.; Du, X.L.; Liu, Y.; Tong, L.J.; Wang, Q.; Li, J.L. Rhubarb extract incorporated into an alginate-based edible coating for peach preservation. Sci. Hortic. 2019, 257, 108685. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-Coatings for Preservation of Fresh Fruits and Vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer Science: New York, NY, USA, 2009; Volume 9. [Google Scholar]
- Castro-Yobal, M.A.; Contreras-Oliva, A.; Saucedo-Rivalcoba, V.; Rivera-Armenta, J.L.; Hernández-Ramírez, G.; Salinas-Ruiz, J.; Herrera-Corredor, A. Evaluation of physicochemical properties of film-based alginate for food packing applications. e-Polymers 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Boninsegna, M.A.; De Bruno, A.; Piscopo, A. Quality Evaluation of Ready-to-Eat Coated Clementine (Citrus x clementina) Fruits. Coatings 2023, 13, 1562. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; Official Methods: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Legua, P.; Forner, J.B.; Hernandez, F.C.A.; Forner-Giner, M.A. Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Sci. Hortic. 2014, 174, 60–64. [Google Scholar] [CrossRef]
- Jurić, S.; Bureš, M.S.; Vlahoviček-Kahlina, K.; Stracenski, K.S.; Fruk, G.; Jalšenjak, N.; Bandić, L.M. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem. X 2023, 17, 100575. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; De Bruno, A.; Piscopo, A. Green-sustainable extraction techniques for the recovery of antioxidant compounds from “citrus Limon” by-products. J. Environ. Sci. Health Part B 2022, 57, 220–232. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 2019, 43, e14211. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Glicerina, V.; Siroli, L.; Betoret, E.; Canali, G.; Dalla Rosa, M.; Lanciotti, R.; Romani, S. Characterization and evaluation of the influence of an alginate, cocoa and a bilayer alginate–cocoa coating on the quality of fresh-cut oranges during storage. J. Sci. Food Agr. 2022, 102, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Weight loss. In Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1994. [Google Scholar]
- Dobson, C.C.; Mottawea, W.; Rodrigue, A.; Pereira, B.L.B.; Hammami, R.; Power, K.A.; Bordenave, N. Impact of molecular interactions with phenolic compounds on food polysaccharides functionality. Adv. Food Nutr. Res. 2019, 90, 135–181. [Google Scholar]
- Tietel, Z.; Plotto, A.; Fallik, E.; Lewinsohn, E.; Porat, R. Taste and aroma of fresh and stored mandarins. J. Sci. Food Agric. 2011, 91, 14–23. [Google Scholar] [CrossRef]
- Ureña, M.; Carullo, D.; Phùng, T.T.T.; Fournier, P.; Farris, S.; Lagorce, A.; Karbowiak, T. Effect of polymer structure on the functional properties of alginate for film or coating applications. Food Hydrocoll. 2024, 149, 109557. [Google Scholar] [CrossRef]
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Cross-linked alginate edible coatings incorporated with hexyl acetate: Film characteristics and its application on fresh-cut rose apple. Food Biosci. 2023, 52, 102410. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; De Claville Christiansen, J. Mechanical properties of alginate hydrogels cross-linked with multivalent cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Andriamanantoanina, H.; Rinaudo, M. Relationship between the molecular structure of alginates and their gelation in acidic conditions. Polym. Int. 2010, 59, 1531–1541. [Google Scholar] [CrossRef]
- Messaoud, G.B.; Sánchez-González, L.; Probst, L.; Jeandel, C.; Arab-Tehrany, E.; Desobry, S. Physico-chemical properties of alginate/shellac aqueous-core capsules: Influence of membrane architecture on riboflavin release. Carbohydr. Polym. 2016, 144, 428–437. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Fanciullino, A.L.; Bert, L.; Urban, L. Effect of fruit load on maturity and carotenoid content of clementine (Citrus clementina Hort. ex Tan.) fruits. J. Sci. Food Agric. 2012, 92, 2076–2083. [Google Scholar] [CrossRef]
- Hijaz, F.; Gmitter, F.G., Jr.; Bai, J.; Baldwin, E.; Biotteau, A.; Leclair, C.; McCollum, T.G.; Plotto, A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Gupta, A.K.; Pathak, U.; Tongbram, T.; Medhi, M.; Terdwongworakul, A.; Magwaza, L.S.; Mditshwa, A.; Chen, T.; Mishra, P. Emerging approaches to determine maturity of citrus fruit. Crit. Rev. Food Sci. Nutr. 2022, 62, 5245–5266. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Ghidelli, C.; Pérez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; De, J.; Berrios, J.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2010, 10, 165–174. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Ehteshami, S.; Dastjerdi, A.M.; Ramezanian, A.; Etemadipoor, R.; Abdollahi, F.; Salari, M.; Shamili, M. Effects of edible alginate coating enriched with organic acids on quality of mango fruit during storage. J. Food Meas. Charact. 2022, 16, 400–409. [Google Scholar] [CrossRef]
- Khan, S.A.; Beekwilder, J.; Schaart, J.G.; Mumm, R.; Soriano, J.M.; Jacobsen, E.; Schouten, H.J. Differences in acidity of apples are probably mainly caused by a malic acid transporter gene on LG16. Tree Genet. Genomes 2013, 9, 475–487. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, S.; Xu, R.; Tu, S.; Tu, K. Interactions among chilling tolerance, sucrose degradation and organic acid metabolism in UV-C-irradiated peach fruit during postharvest cold storage. Acta Physiol. Plant. 2019, 41, 79. [Google Scholar] [CrossRef]
- Tang, N.; Deng, W.; Hu, N.; Chen, N.; Li, Z. Metabolite and transcriptomic analysis reveals metabolic and regulatory features associated with Powell orange pulp deterioration during room temperature and cold storage. Postharvest Biol. Technol. 2016, 112, 75–86. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum postharvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Cebadera Miranda, M.E.; Dias, M.I.; Barros, L.; Fernández-Ruiz, V.; Cámara, R.M.; Del Pino, Á.; Santos Buelga, C.; Ferreira, I.C.F.R.; Morales, P.; Cámara, M. Characterization of Extra Early Spanish Clementine Varieties (Citrus clementina Hort ex Tan) as a Relevant Source of Bioactive Compounds with Antioxidant Activity. Foods 2020, 9, 642. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y. Effect of different postharvest temperatures on the accumulation of sugars, organic acids, and amino acids in the juice sacs of Satsuma mandarin (Citrus unshiu Marc.) fruit. J. Agric. Food Chem. 2012, 60, 9900–9909. [Google Scholar] [CrossRef]
- Rey, F.; Zacarías, L.; Rodrigo, M.J. Carotenoids, vitamin c, and antioxidant capacity in the peel of mandarin fruit in relation to the susceptibility to chilling injury during postharvest cold storage. Antioxidants 2020, 9, 1296. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar] [PubMed]
- Simonne, A.H.; Ritenour, M.A.; Terry, L.A. Citrus (Orange, Lemon, Mandarin, Grapefruit, Lime and Other citrus fruits). In Health-Promoting Properties of Fruits and Vegetables; CAB International: Oxfordshire, UK, 2011; pp. 90–117. [Google Scholar]
- Milella, L.; Caruso, M.; Galgano, F.; Favati, F.; Padula, M.C.; Martelli, G. Role of the cultivar in choosing Clementine fruits with a high level of health-promoting compounds. J. Agric. Food Chem. 2011, 59, 5293–5298. [Google Scholar] [CrossRef]
- Khan, M.K.; Huma, Z.E.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Choi, M.Y.; Chai, C.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Effects of storage period and heat treatment on phenolic compound composition in dried Citrus peels (Chenpi) and discrimination of Chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J. Pharm. Biomed. Anal. 2011, 54, 638–645. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Palafox-Carlos, H.; Yahia, E.M.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. Maintaining antioxidant potential of fresh fruits and vegetables after harvest. Crit. Rev. Food Sci. Nutr. 2015, 55, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Garg, M.; Prajapati, P.; Chopra, R. Chemistry of Citrus Fruits. In Citrus Fruits and Juice: Processing and Quality Profiling; Springer Nature: Singapore, 2024; pp. 45–68. [Google Scholar]
- Lafuente, M.T.; Ballester, A.R.; Calejero, J.; González-Candelas, L. Effect of high-temperature-conditioning treatments on quality, flavonoid composition and vitamin C of cold stored ‘Fortune’ mandarins. Food Chem. 2011, 128, 1080–1086. [Google Scholar] [CrossRef]
- De Ancos, B.; Cilla, A.; Barberá, R.; Sánchez-Moreno, C.; Cano, M.P. Influence of orange cultivar and mandarin postharvest storage on polyphenols, ascorbic acid and antioxidant activity during gastrointestinal digestion. Food Chem. 2017, 225, 114–124. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays—A practical approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Sobrino-López, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Shelf-life extension of fresh-cut “Fuji” apples at different ripeness stages using natural substances. Postharvest Biol. Technol. 2007, 45, 265–275. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, C.; Prakash, D.; Garg, A.P. Comparative study of antimicrobial effects of lemon oil and peel extract against food-spoilage microbes. JNHFS 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Najafi Marghmaleki, S.; Mortazavi, S.M.H.; Saei, H.; Mostaan, A. The effect of alginate-based edible coating enriched with citric acid and ascorbic acid on texture, appearance and eating quality of apple fresh-cut. Int. J. Fruit Sci. 2021, 21, 40–51. [Google Scholar] [CrossRef]
- Reyes-Avalos, M.C.; Femenia, A.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Aguilar-González, C.N.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Improvement of the quality and the shelf life of figs (Ficus carica) using an alginate–chitosan edible film. Food Bioprocess Technol. 2016, 9, 2114–2124. [Google Scholar] [CrossRef]
- Huang, C.; Hou, J.; Huang, M.; Hu, M.; Deng, L.; Zeng, K.; Yao, S. A comprehensive review of segment drying (vesicle granulation and collapse) in citrus fruit: Current state and future directions. Sci. Hortic. 2023, 309, 111683. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, Q.; Niu, B.; Liu, R.; Chen, H.; Xiao, S.; Wu, W. The dual function of calcium ion in fruit edible coating: Regulating polymer internal crosslinking state and improving fruit postharvest quality. Food Chem. 2024, 5, 138952. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
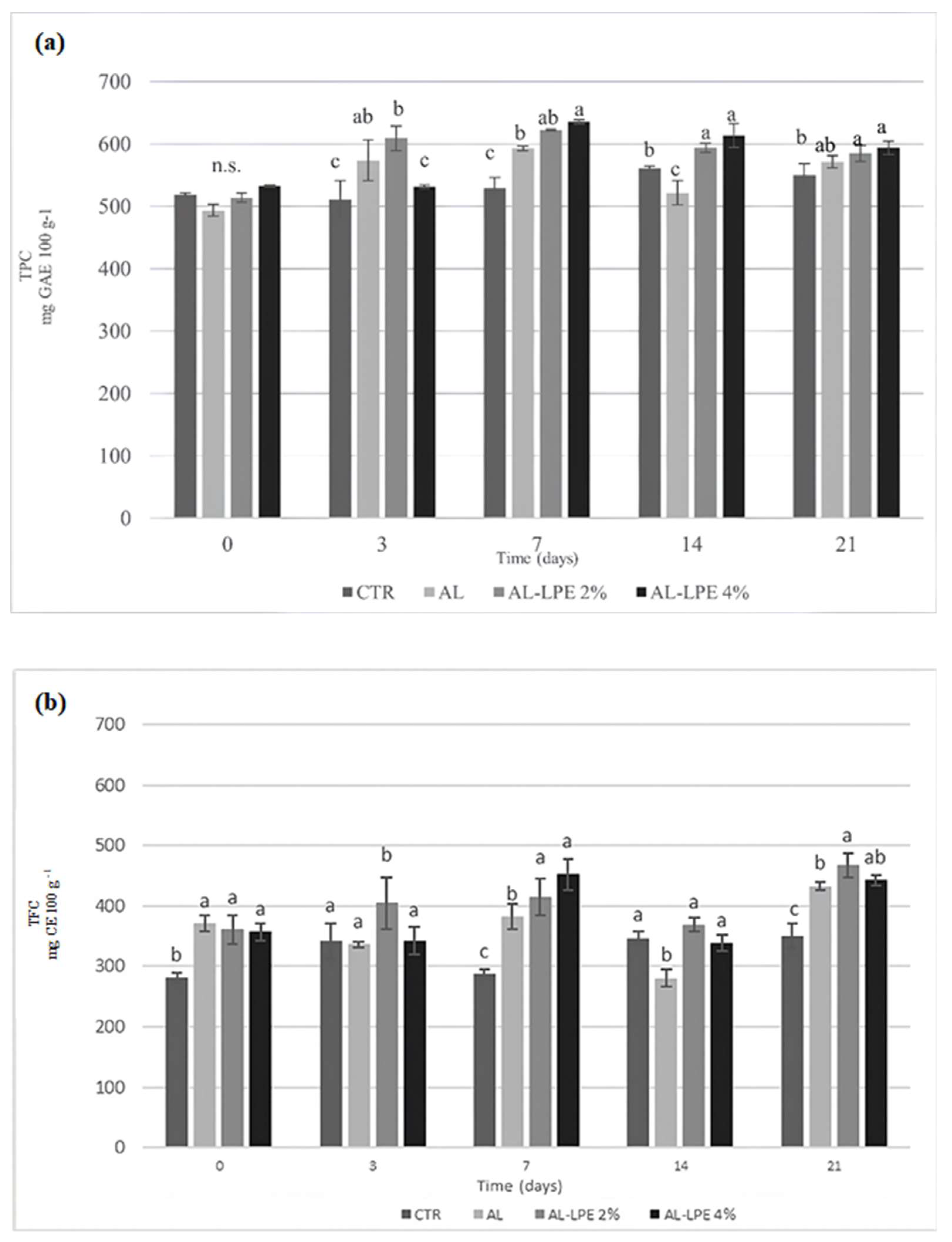
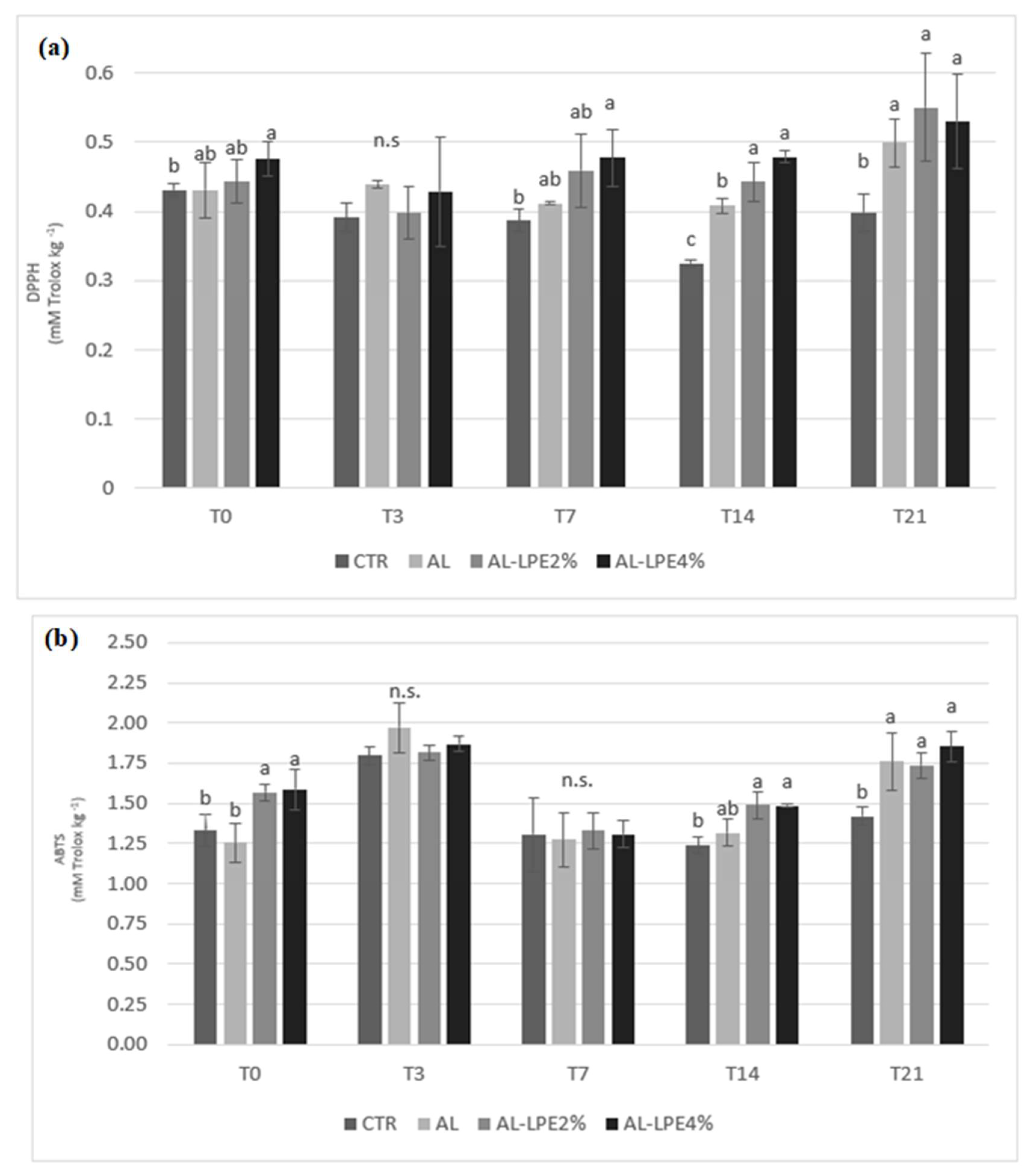
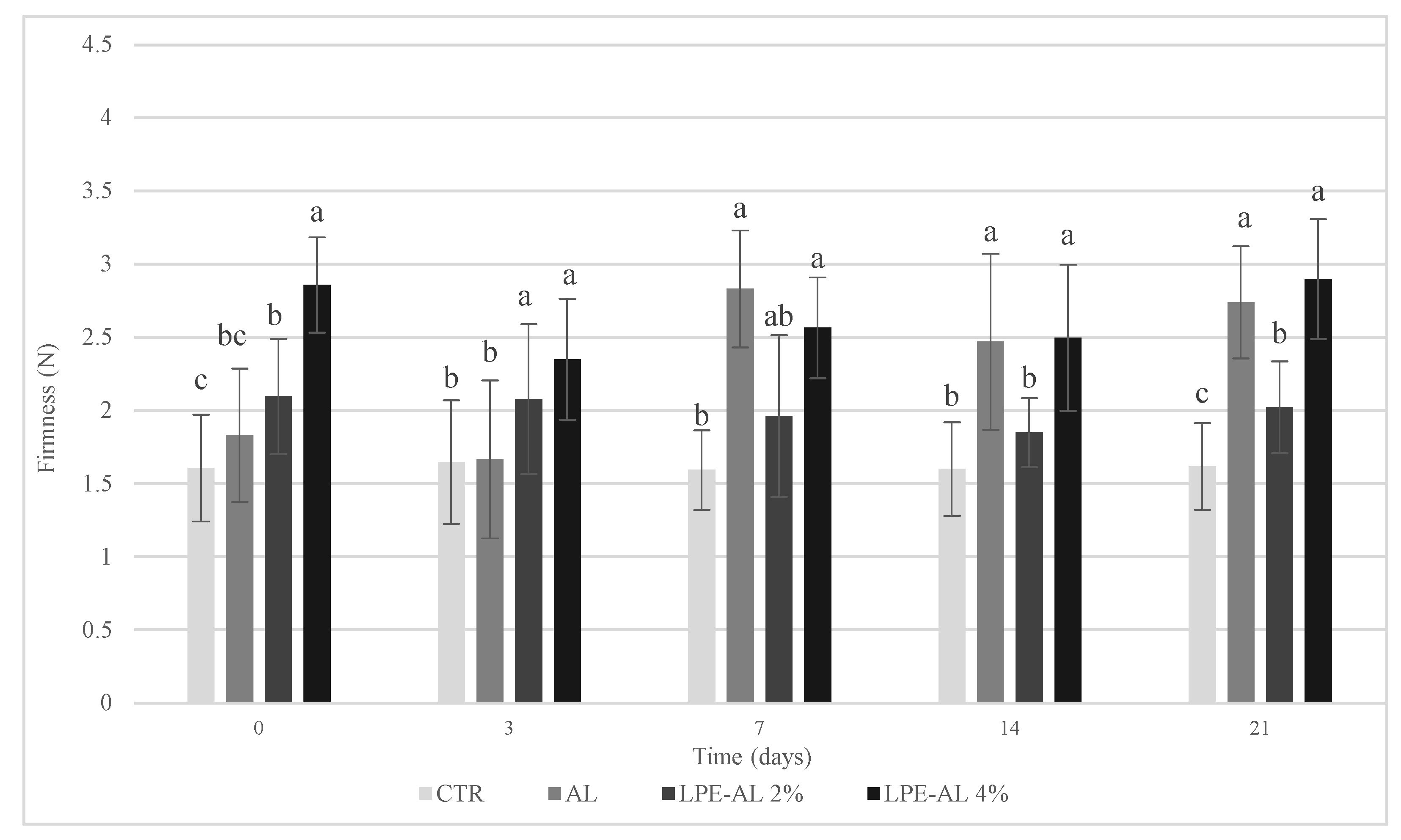
| Time (min) | Eluent A (%) | Eluent B (%) | Flow (mL/min) |
|---|---|---|---|
| Initial | 95.00 | 5.00 | 0.40 |
| 3.00 | 95.00 | 5.00 | 0.40 |
| 17.00 | 60.00 | 40.00 | 0.40 |
| 17.50 | 0.00 | 100.00 | 0.40 |
| 20.00 | 95.00 | 5.00 | 0.40 |
| 21.00 | 95.00 | 5.00 | 0.40 |
| Parameter | Results |
|---|---|
| pH | 3.95 ± 0.02 |
| TPC (mg GAE g−1 d.w.) | 12.67 ± 0.17 |
| TFC (mg CE g−1 d.w.) | 2.10 ± 0.11 |
| Hesperidin (mg g−1 d.w.) | 3.88 ± 0.35 |
| Eriocitrin (mg g−1 d.w.) | 1.51 ± 0.39 |
| Narirutin (mg g−1 d.w.) | 0.03 ± 0.00 |
| Naringin (mg g−1 d.w.) | 0.01 ± 0.05 |
| Neoeriocitrin (mg g−1 d.w.) | 0.01 ± 0.04 |
| DPPH (μM TE g−1 d.w.) | 22.97 ± 0.53 |
| ABTS (μM TE g−1 d.w.) | 18.90 ± 0.29 |
| Parameter | Sample | Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | Sig. | ||
| TSS (°Bx) | CTRL | 12.10 ± 0.05 AB | 12.15 ± 0.17 AB | 10.25 ± 0.72 bC | 12.18 ± 0.10 A | 11.47 ± 0.38 B | ** |
| AL | 11.88 ± 0.13 | 12.20 ± 0.23 | 12.57 ± 0.78 a | 12.12 ± 0.07 | 11.85 ± 0.06 | n.s. | |
| AL-LPE 2% | 12.13 ± 0.61 | 12.90 ± 0.69 | 12.08 ± 0.55 a | 12.02 ± 0.00 | 11.80 ± 0.92 | n.s. | |
| AL-LPE 4% | 11.75 ± 0.40 | 12.30 ± 0.69 | 11.37 ± 0.43 a | 11.60 ± 0.00 | 11.20 ± 0.58 | n.s. | |
| Sig. | n.s. | n.s. | ** | n.s. | n.s. | ||
| TTA (%) | CTRL | 0.59 ± 0.03 bcAB | 0.64 ± 0.08 abA | 0.53 ± 0.04 bAB | 0.49 ± 0.0 bB | 0.51 ± 0.02 cB | ** |
| AL | 0.51 ± 0.03 bB | 0.55 ± 0.02 cAB | 0.58 ± 0.05 abA | 0.48 ± 0.00 bB | 0.54 ± 0.01 abAB | * | |
| AL-LPE 2% | 0.63 ± 0.04 ab | 0.65 ± 0.03 a | 0.62 ± 0.04 a | 0.65 ± 0.03 a | 0.63 ± 0.01 a | n.s. | |
| AL-LPE 4% | 0.68 ± 0.08 aA | 0.61 ± 0.02 bB | 0.64 ± 0.04 abAB | 0.69 ± 0.03 aA | 0.61 ± 0.02 bB | * | |
| Sign. | ** | * | ** | ** | * | ||
| pH | CTRL | 3.84 ± 0.01 aB | 3.85 ± 0.01 aB | 3.91 ± 0.01 bAB | 3.93 ± 0.01 aAB | 4.07 ± 0.22 aA | * |
| AL | 3.75 ± 0.01 abB | 3.77 ± 0.02 abB | 3.99 ± 0.04 aA | 3.78 ± 0.02 bB | 3.80 ± 0.02 cB | ** | |
| AL-LPE 2% | 3.59 ± 0.07 cC | 3.68 ± 0.02 abBC | 3.83 ± 0.01 cA | 3.81 ± 0.03 bAB | 3.69 ± 0.13 bcC | ** | |
| AL-LPE 4% | 3.67 ± 0.08 bc | 3.64 ± 0.15 b | 3.74 ± 0.03 d | 3.77 ± 0.04 b | 3.71 ± 0.13 bc | n.s. | |
| Sign. | ** | * | ** | ** | * | ||
| Moisture (g/100 g) | CTRL | 85.29 ± 0.55 AB | 88.46 ± 2.00 A | 87.60 ± 1.90 AB | 85.59 ± 1.85 AB | 83.20 ± 1.16 Bb | * |
| AL | 85.38 ± 2.00 | 85.56 ± 0.30 | 86.74 ± 1.97 | 85.80 ± 1.35 | 86.77 ± 1.23 a | n.s. | |
| AL-LPE 2% | 85.69 ± 1.61 | 86.63 ± 1.83 | 85.57 ± 2.21 | 85.17 ± 1.49 | 86.58 ± 0.89 a | n.s. | |
| AL-LPE 4% | 85.76 ± 1.41 | 86.89 ± 1.53 | 86.04 ± 1.32 | 86.42 ± 0.50 | 86.15 ± 0.79 a | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | * | ||
| Organic Acids (mg 100 g−1) | Sample | Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | Sig. | ||
| Citric acid | CTRL | 473.77 ± 19.74 | 455.9 ± 43.93 | 501.37 ± 2.88 | 444 ± 3.43 | 425.6 ± 66.4 | n.s. |
| AL | 500.52 ± 2.07 | 423.52 ± 21.23 | 499.74 ± 53.59 | 463.21 ± 7.20 | 464.46 ± 2.75 | n.s. | |
| AL-LPE 2% | 516.75 ± 18.34 | 477.42 ± 43.89 | 540.55 ± 66.43 | 482.49 ± 56.63 | 483.04 ± 67.26 | n.s. | |
| AL-LPE 4% | 489.48 ± 2.82 | 426.6 ± 21.78 | 422.11 ± 8.95 | 465.44 ± 0.00 | 439.97 ± 23.93 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Malic acid | CTRL | 86.2 ± 4.28 a | 82.57 ± 6.28 a | 71.24 ± 5.40 ab | 63.88 ± 14.29 ab | 68.13 ± 11.13 | n.s. |
| AL | 62.45 ± 5.43 b | 59.37 ± 4.90 b | 55.38 ± 9.19 b | 48.31 ± 0.03 b | 62.55 ± 6.71 | n.s. | |
| AL-LPE 2% | 57.99 ± 11.6 Bb | 84.84 ± 16.66 Aa | 76.52 ± 0.31 ABa | 71.26 ± 0.15 ABab | 76.07 ± 13.11 AB | * | |
| AL-LPE 4% | 59.43 ± 5.64 bB | 87.74 ± 13.37 Aa | 52.21 ± 4.34 Bb | 75.8 ± 0.26 Aba | 52.63 ± 23.29 B | * | |
| Sign. | ** | * | * | ** | n.s. | ||
| Ascorbic acid | CTRL | 152.99 + 3.19 aA | 104.62 + 13.14 bB | 94.56 + 1.85 bB | 97.19 + 1.65 bB | 90.51 + 0.99 cB | ** |
| AL | 124.49 + 18.75 b | 112.18 + 35.52 b | 169.82 + 14.13 a | 170.78 ± 3.11 a | 124.28 ± 1.21 bc | n.s. | |
| AL-LPE 2% | 205.69 + 36.80 ab | 175.41 + 4.95 a | 176.35 + 5.72 a | 177.99 ± 2.52 a | 202.96 ± 12.10 a | n.s. | |
| AL-LPE 4% | 172.31 + 7.61 ab | 180.92 + 11.50 a | 175.31 + 6.37 a | 180.61 ± 1.98 a | 184.37 ± 8.26 ab | n.s. | |
| Sign. | ** | * | ** | ** | ** | ||
| Sample | Time (Days) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | Sign. | ||
| Narirutin | CTRL | 170.01 ± 3.37 | 190.44 ± 17.82 | 170.62 ± 9.56 | 165.35 ± 3.01 b | 168.80 ± 3.53 | n.s. |
| AL | 175.32 ± 6.74 CB | 207.38 ± 6.02 A | 175.04 ± 0.42 B | 152.61 ± 0.66 cC | 173.02 ± 4.91 BC | ** | |
| AL-LPE 2% | 187.50 ± 2.36 A | 213.05 ± 6.46 B | 181.76 ± 4.60 AB | 192.56 ± 2.01 aAB | 188.78 ± 8.24 AB | * | |
| AL-LPE 4% | 191.65 ± 10.11 | 201.86 ± 0.09 | 183.02 ± 13.55 | 167.81 ± 0.76 b | 178.98 ± 2.12 | n.s. | |
| Sig. | n.s. | n.s. | n.s. | ** | n.s. | ||
| Hesperidin | CTRL | 96.39 ± 13.34 cC | 124.56 ± 12.21 A | 96.24 ± 12.83 cC | 102.23 ± 12.83 cCB | 116.90 ± 13.65 AB | * |
| AL | 123.61 ± 6.08 bBC | 128.44 ± 4.95 A | 125.03 ± 18.47 bA | 104.75 ± 18.74 bcC | 115.57 ± 6.08 B | ** | |
| AL-LPE 2% | 125.54 ± 13.68 bA | 138.62 ± 12.65 B | 126.49 ± 6.20 bC | 141.77 ± 6.52 aAB | 117.35 ± 12.52 C | ** | |
| AL-LPE 4% | 142.78 ± 17.21 aA | 129.11 ± 7.62 AB | 142.92 ± 8.91 aA | 115.22 ± 8.91 bBC | 110.90 ± 21.21 C | ** | |
| Sign. | ** | n.s. | ** | ** | n.s. | ||
| Eriocitrin | CTRL | 0.39 ± 0.01 cA | 0.40 ± 0.00 bA | 0.22 ± 0.01 cB | 0.07 ± 0.00 cC | 0.08 ± 0.00 bC | ** |
| AL | 0.38 ± 0.00 dA | 0.37 ± 0.01 bA | 0.36 ± 0.00 abA | 0.06 ± 0.00 cB | 0.08 ± 0.00 bB | ** | |
| AL-LPE 2% | 0.99 ± 0.01 bA | 0.76 ± 0.02 abB | 0.46 ± 0.00 abC | 0.35 ± 0.00 bD | 0.49 ± 0.00 aC | ** | |
| AL-LPE 4% | 1.06 ± 0.01 aAB | 1.19 ± 0.02 aA | 0.65 ± 0.01 aAB | 0.55 ± 0.00 aAB | 0.51 ± 0.00 aB | * | |
| Sign. | ** | * | * | ** | ** | ||
| Parameter | Sample | Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | Sig. | ||
| L* | CTRL | 50.19 ± 1.69 | 50.72 ± 1.42 | 53.25 ± 1.22 | 52.86 ± 2.65 | 51.71 ± 1.67 | n.s. |
| AL | 54.80 ± 1.33 | 52.62 ± 1.69 | 53.84 ± 1.16 | 52.57 ± 1.54 | 50.92 ± 2.10 | n.s. | |
| AL-LPE 2% | 49.67 ± 5.70 | 53.15 ± 1.80 | 52.31 ± 1.83 | 51.99 ± 2.10 | 51.58 ± 6.11 | n.s. | |
| AL-LPE 4% | 51.90 ± 5.86 | 50.54 ± 2.39 | 50.98 ± 2.48 | 50.70 ± 3.19 | 50.40 ± 2.09 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| a* | CTRL | 8.30 ± 1.21 | 8.41 ± 1.27 | 8.74 ± 1.01 | 8.85 ± 2.14 | 8.67 ± 1.32 | n.s. |
| AL | 8.18 ± 1.30 | 9.27 ± 2.01 | 9.42 ± 2.38 | 8.60 ± 1.96 | 9.46 ± 1.56 | n.s. | |
| AL-LPE 2% | 8.80 ± 3.06 | 8.12 ± 1.50 | 8.91 ± 2.29 | 8.39 ± 1.47 | 8.79 ± 2.29 | n.s. | |
| AL-LPE 4% | 8.31 ± 2.95 | 8.88 ± 2.10 | 8.54 ± 1.90 | 7.84 ± 1.89 | 8.19 ± 1.21 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| b* | CTRL | 19.48 ± 1.07 | 20.90 ± 1.54 | 19.78 ± 1.43 | 21.71 ± 1.97 | 20.21 ± 1.82 | n.s. |
| AL | 20.27 ± 1.50 | 20.48 ± 1.41 | 24.23 ± 2.40 | 20.60 ± 2.63 | 21.39 ± 1.58 | n.s. | |
| AL-LPE 2% | 19.39 ± 2.16 | 19.66 ± 2.07 | 21.43 ± 1.53 | 20.71 ± 2.08 | 21.47 ± 2.96 | n.s. | |
| AL-LPE 4% | 20.83 ± 1.74 | 21.9 ± 1.45 | 21.07 ± 2.30 | 18.67 ± 2.75 | 19.21 ± 2.81 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Weight loss (g 100 g−1) | CTRL | 0.00 ± 0.00 D | 0.03 ± 0.01 C | 0.02 ± 0.01 Cb | 0.07 ± 0.02 B | 0.10 ± 0.01 Aa | ** |
| AL | 0.00 ± 0.00 C | 0.05 ± 0.02 B | 0.05 ± 0.01 Ba | 0.07 ± 0.01 AB | 0.10 ± 0.01 Aa | ** | |
| AL-LPE 2% | 0.00 ± 0.00 D | 0.04 ± 0.02 BC | 0.03 ± 0.01 ABCab | 0.06 ± 0.02 AB | 0.08 ± 0.00 Aab | * | |
| AL-LPE 4% | 0.00 ± 0.00 B | 0.05 ± 0.03 A | 0.05 ± 0.01 Aa | 0.07 ± 0.02 A | 0.05 ± 0.02 Ab | * | |
| Sign. | n.s. | n.s. | * | n.s. | * | ||
| O2 (%) | CTRL | 21.00 ± 0.00 A | 14.52 ± 1.11 Bb | 14.30 ± 1.90 B | 7.10 ± 0.99 Cb | 5.6 ± 0.71 Cc | ** |
| AL | 21.00 ± 0.00 A | 16.40 ± 0.43 Ba | 13.30 ± 0.63 C | 8.25 ± 0.57 Dab | 8.30 ± 0.68 Db | ** | |
| AL-LPE 2% | 21.00 ± 0.00 A | 17.40 ± 0.38 Ba | 14.70 ± 1.86 C | 9.40 ± 0.14 Cab | 10.60 ± 0.21 Ca | ** | |
| AL-LPE 4% | 21.00 ± 0.00 A | 17.70 ± 0.75 Ba | 14.92 ± 1.06 C | 12.50 ± 2.12 Da | 8.80 ± 0.64 Eb | ** | |
| Sign. | n.s. | ** | n.s. | ** | ** | ||
| CO2 (%) | CTRL | 0.02 ± 0.00 D | 9.40 ± 1.37 C | 10.00 ± 2.30 BC | 13.00 ± 1.13 ABb | 19.90 ± 0.14 Aa | ** |
| AL | 0.02 ± 0.00 D | 9.20 ± 0.43 C | 9.80 ± 0.81 C | 14.90 ± 0.10 Ba | 18.25 ± 0.51 Aab | ** | |
| AL-LPE 2% | 0.02 ± 0.00 C | 7.75 ± 1.82 B | 8.90 ± 1.69 B | 16.80 ± 0.92 Aa | 17.30 ± 0.78 Ab | ** | |
| AL-LPE 4% | 0.02 ± 0.00 D | 7.70 ± 0.79 C | 10.30 ± 1.22 C | 13.50 ± 2.25 Bab | 17.50 ± 1.82 Aab | ** | |
| Sign. | n.s. | n.s. | n.s. | * | * | ||
| CBT (Log10 CFU g−1) | CTRL | 0.00 ± 0.00 D | 0.00 ± 0.00 D | 1.45 ± 0.26 abC | 3.00 ± 0.04 aB | 5.11 ± 0.08 aA | ** |
| AL | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 1.89 ± 0.35 aB | 1.50 ± 0.28 bB | 2.75 ± 0.25 bA | ** | |
| AL-LPE 2% | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 1.10 ± 0.17 bB | 1.09 ± 0.12 bcB | 2.45 ± 0.58 bA | ** | |
| AL-LPE 4% | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.96 ± 0.24 bB | 1.00 ± 0.00 cB | 2.32 ± 0.51 bA | ** | |
| Sign. | n.s. | n.s. | * | ** | ** | ||
| Mold (Log10 CFU g−1) | CTRL | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 1.77 ± 0.10 aB | 2.15 ± 0.63 aA | ** |
| AL | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 1.03 ± 0.10 aB | 1.94 ± 0.34 aA | ** | |
| AL-LPE 2% | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 bB | 1.66 ± 0.00 aA | ** | |
| AL-LPE 4% | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | n.s. | |
| Sign. | n.s. | n.s. | n.s. | ** | * | ||
| Days | CTR | AL | AL-LPE2% | AL-LPE4% | Sign. | |
|---|---|---|---|---|---|---|
| Color | 0 | 7.00 ± 0.50 b | 8.67 ± 0.47 a | 8.33 ± 0.47 a | 8.50 ± 0.49 a | ** |
| 21 | 6.83 ± 0.86 b | 8.00 ± 0.82 a | 7.5 ± 0.96 ab | 8.00 ± 0.63 a | * | |
| Visual Appearance | 0 | 7.83 ± 0.76 | 8.5 ± 0.50 | 8.5 ± 0.76 | 8.33 ± 0.49 | n.s. |
| 21 | 8.17 ± 0.69 | 8.17 ± 0.37 | 8.33 ± 0.94 | 7.67 ± 1.02 | n.s. | |
| Fruity | 0 | 7.17 ± 1.34 | 6.33 ± 0.47 | 6.83 ± 0.69 | 7.00 ± 0.63 | n.s. |
| 21 | 6.33 ± 0.47 ab | 6.00 ± 0.57 b | 7.00 ± 0.58 a | 6.50 ± 0.49 ab | * | |
| Citrusy | 0 | 6.50 ± 0.76 | 6.67 ± 0.75 | 6.83 ± 0.69 | 6.83 ± 0.80 | n.s. |
| 21 | 5.83 ± 0.68 | 6.50 ± 0.05 | 6.83 ± 0.68 | 6.67 ± 0.80 | n.s. | |
| Sweetness | 0 | 6.83 ± 0.75 | 5.93 ± 1.05 | 6.33 ± 0.55 | 6.33 ± 0.62 | n.s. |
| 21 | 4.33 ± 0.70 | 5.10 ± 0.55 | 5.00 ± 0.66 | 5.00 ± 0.75 | n.s. | |
| Acidity | 0 | 3.00 ± 0.89 | 3.05 ± 0.57 | 2.33 ± 0.74 | 2.66 ± 0.48 | n.s. |
| 21 | 5.67 ± 0.95 a | 4.33 ± 0.75 a | 2.66 ± 0.55 b | 2.83 ± 0.55 b | ** | |
| Aftertaste | 0 | 7.00 ± 1.45 | 8.16 ± 0.85 | 8.00 ± 1.10 | 7.33 ± 0.95 | n.s. |
| 21 | 6.33 ± 0.51 b | 8.00 ± 0.65 a | 8.33 ± 0.45 a | 7.66 ± 1.15 a | * | |
| Crunchiness | 0 | 6.66 ± 0.47 b | 8.16 ± 0.68 a | 8.17 ± 0.72 a | 7.70 ± 1.01 ab | * |
| 21 | 4.5 ± 0.89 b | 7.00 ± 0.67 ab | 8.00 ± 0.48 a | 7.33 ± 0.45 a | ** | |
| Firmness | 0 | 6.83 ± 1.07 b | 8.00 ± 0.58 a | 8.33 ± 0.47 a | 8.17 ± 0.63 a | ** |
| 21 | 6.17 ± 0.75 b | 7.00 ± 0.95 ab | 8.17 ± 0.37 a | 8.00 ± 0.75 a | ** | |
| Overall acceptability | 0 | 6.60 ± 0.80 | 6.40 ± 0.49 | 6.80 ± 0.75 | 6.80 ± 0.40 | n.s. |
| 21 | 5.33 ± 0.82 b | 6.17 ± 0.41 ab | 6.83 ± 0.41 a | 7.00 ± 0.89 a | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boninsegna, M.A.; De Bruno, A.; Piscopo, A. Improving the Storage Quality of Ready-to-Eat Clementine Fruits Using Lemon By-Products. Agriculture 2024, 14, 1488. https://doi.org/10.3390/agriculture14091488
Boninsegna MA, De Bruno A, Piscopo A. Improving the Storage Quality of Ready-to-Eat Clementine Fruits Using Lemon By-Products. Agriculture. 2024; 14(9):1488. https://doi.org/10.3390/agriculture14091488
Chicago/Turabian StyleBoninsegna, Miriam Arianna, Alessandra De Bruno, and Amalia Piscopo. 2024. "Improving the Storage Quality of Ready-to-Eat Clementine Fruits Using Lemon By-Products" Agriculture 14, no. 9: 1488. https://doi.org/10.3390/agriculture14091488
APA StyleBoninsegna, M. A., De Bruno, A., & Piscopo, A. (2024). Improving the Storage Quality of Ready-to-Eat Clementine Fruits Using Lemon By-Products. Agriculture, 14(9), 1488. https://doi.org/10.3390/agriculture14091488








