Long-Term Manuring Enhanced Compositional Stability of Glomalin-Related Soil Proteins through Arbuscular Mycorrhizal Fungi Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling Analysis
2.3. GRSP Determination
2.4. Structural Characterizations of GRSP
2.5. Statistical Analysis
3. Results
3.1. Differences between C and N Content and Proportion in GRSP under Fertilization Regimes
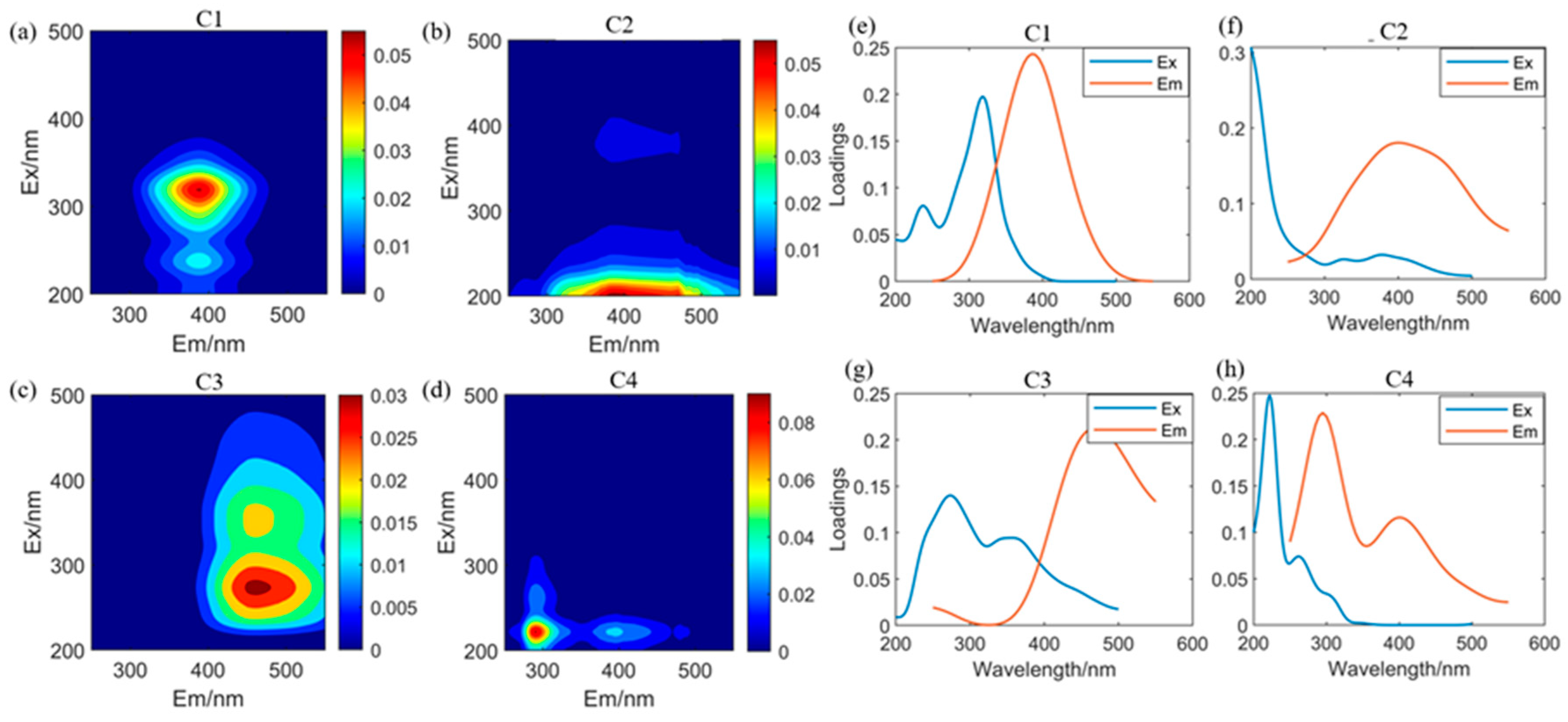
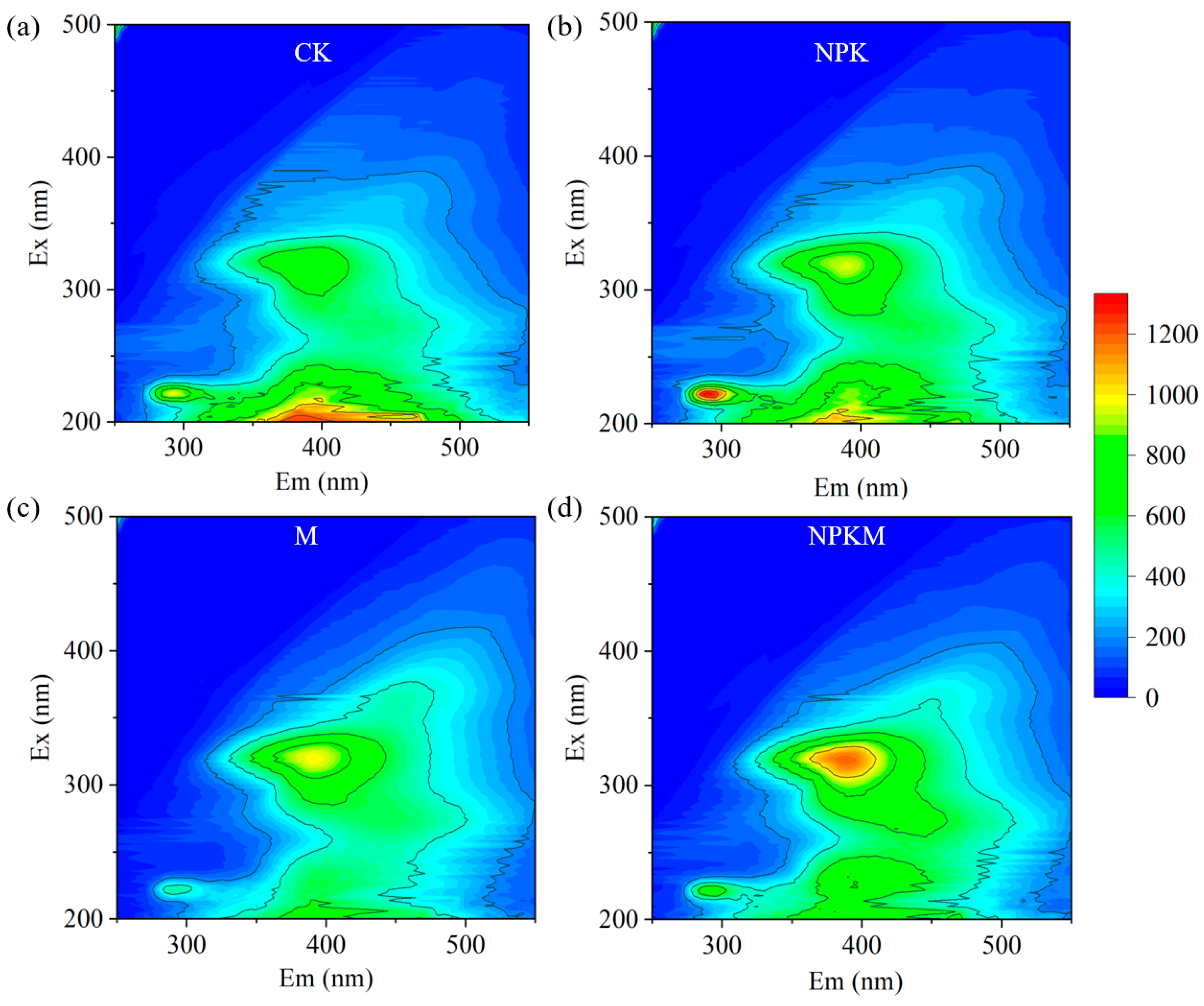
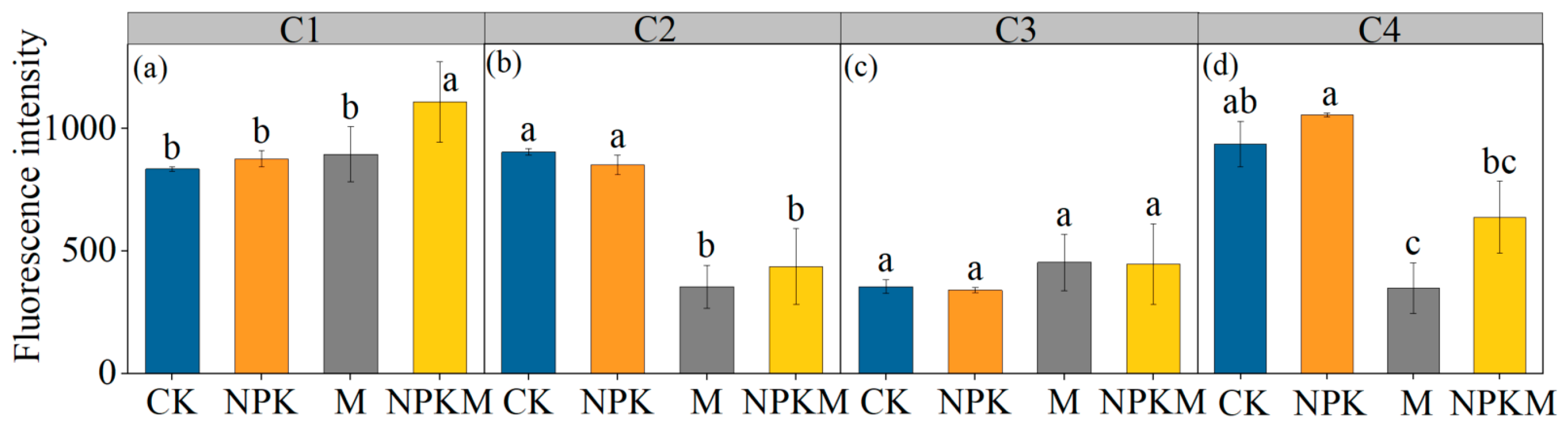
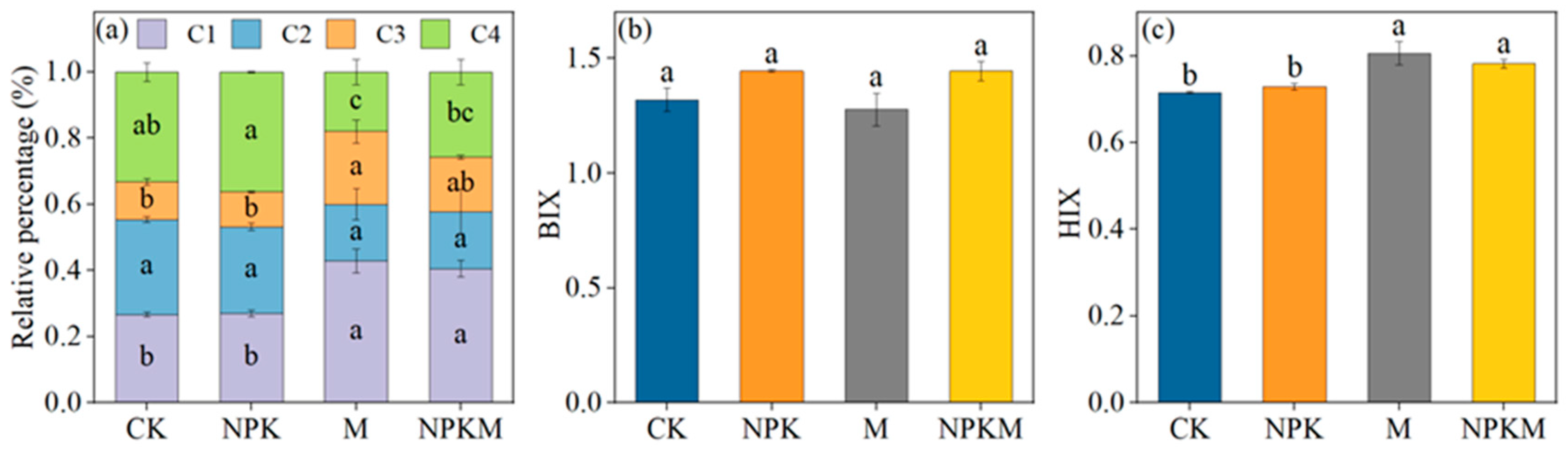
3.2. Fluorescence Characteristics Analysis of GRSP
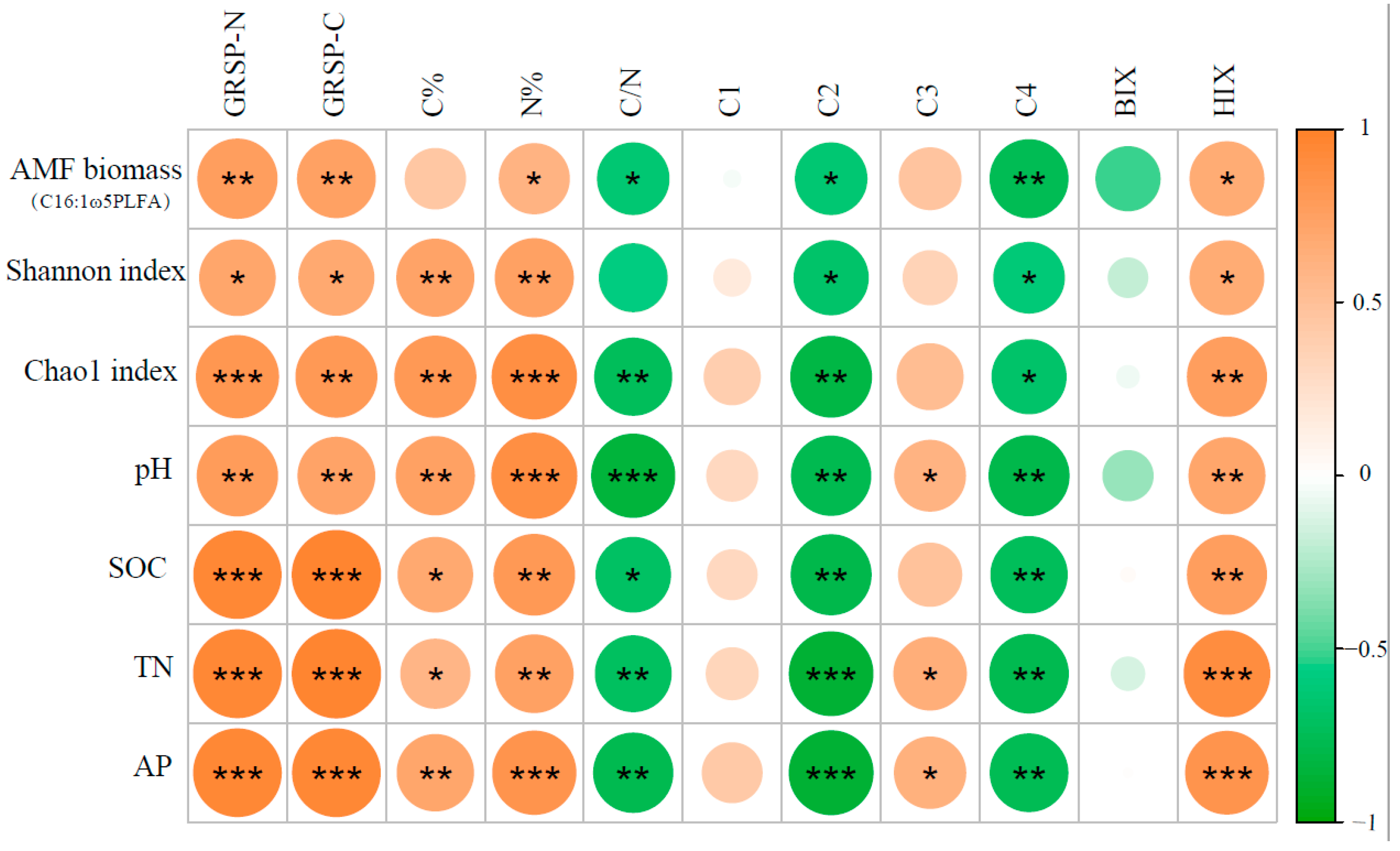
3.3. The Relationship between Structural Characterizations of GRSP and the Properties of Edaphic and AMF
4. Discussion
4.1. Effects of Fertilization Regimes on C and N Contents in GRSP
4.2. Effects of Fertilization Regimes on Fluorescence Components in GRSP
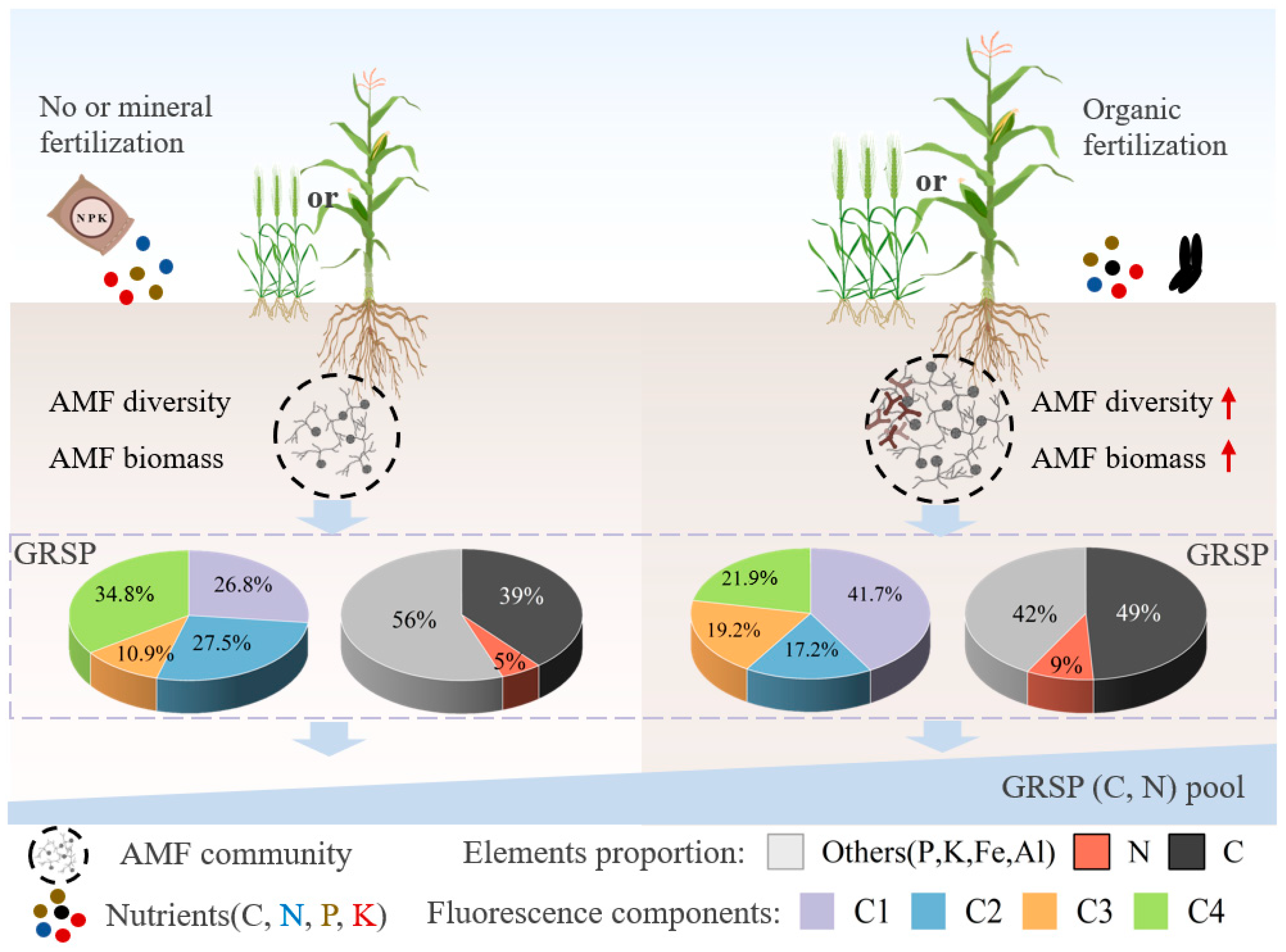
4.3. Implications and Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, S.F.; Upadhyaya, A. Extraction of an Abundant and Unusual Protein from Soil and Comparison with Hyphal Protein of Arbuscular Mycorrhizal Fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Gillespie, A.W.; Farrell, R.E.; Walley, F.L.; Ross, A.R.S.; Leinweber, P.; Eckhardt, K.-U.; Regier, T.Z.; Blyth, R.I.R. Glomalin-Related Soil Protein Contains Non-Mycorrhizal-Related Heat-Stable Proteins, Lipids and Humic Materials. Soil Biol. Biochem. 2011, 43, 766–777. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Zhong, S.; Yin, G.; Gao, Y.; He, X. Recalcitrant Carbon Components in Glomalin-Related Soil Protein Facilitate Soil Organic Carbon Preservation in Tropical Forests. Sci. Rep. 2017, 7, 2391. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Manna, M.C.; Kurganova, I.; Kuzyakov, Y. Glycoproteins of Arbuscular Mycorrhiza for Soil Carbon Sequestration: Review of Mechanisms and Controls. Sci. Total Environ. 2022, 806, 150571. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Wright, S.F.; Clark, D.A.; Ruess, R.W. Soil Stocks of Glomalin Produced by Arbuscular Mycorrhizal Fungi across a Tropical Rain Forest Landscape. J. Ecol. 2004, 92, 278–287. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The Role of Glomalin in Mitigation of Multiple Soil Degradation Problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, J.; Wu, J.; Yang, D.; Mei, K.; Li, H.; Lu, H.; Yan, C. Spatial Heterogeneity in Chemical Composition and Stability of Glomalin-Related Soil Protein in the Coastal Wetlands. Sci. Total Environ. 2022, 835, 155351. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, J.; Zeng, H.; Xiao, X.; Liu, M.; Hong, H.; Lu, H.; Yan, C. Variation of Glomalin-Metal Binding Capacity in 1 m Soil Profiles from Mangrove Forests to Mudflat and Affected Factor Analysis. Sci. Total Environ. 2023, 863, 160890. [Google Scholar] [CrossRef] [PubMed]
- Holátko, J.; Brtnický, M.; Kučerík, J.; Kotianová, M.; Elbl, J.; Kintl, A.; Kynický, J.; Benada, O.; Datta, R.; Jansa, J. Glomalin–Truths, Myths, and the Future of This Elusive Soil Glycoprotein. Soil Biol. Biochem. 2021, 153, 108116. [Google Scholar] [CrossRef]
- Lin, H.; Xia, X.; Bi, S.; Jiang, X.; Wang, H.; Zhai, Y.; Wen, W. Quantifying Bioavailability of Pyrene Associated with Dissolved Organic Matter of Various Molecular Weights to Daphnia Magna. Environ. Sci. Technol. 2018, 52, 644–653. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Chen, J.; Hong, H.; Lu, H.; Liu, J.; Dong, Y.; Yan, C. Glomalin-Related Soil Protein Deposition and Carbon Sequestration in the Old Yellow River Delta. Sci. Total Environ. 2018, 625, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Wu, Z.; Lin, C.; Zhou, S. Terrestrially Derived Glomalin-Related Soil Protein Quality as a Potential Ecological Indicator in a Peri-Urban Watershed. Environ. Monit. Assess. 2017, 189, 315. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, Q.; Huang, Y.; Cai, Z.; Li, D.; Wu, L.; Meersmans, J.; Colinet, G.; Zhang, W. Long-Term Manuring Facilitates Glomalin-Related Soil Proteins Accumulation by Chemical Composition Shifts and Macro-Aggregation Formation. Soil Tillage Res. 2024, 235, 105904. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Wang, W.; Zhong, Z.; Pei, Z.; Ren, J.; Wang, H.; Zu, Y. Spatial Variations in Concentration, Compositions of Glomalin Related Soil Protein in Poplar Plantations in Northeastern China, and Possible Relations with Soil Physicochemical Properties. Sci. World J. 2014, 2014, 160403. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, W.; Wang, Q.; Wu, Y.; Wang, H.; Pei, Z. Glomalin Amount and Compositional Variation, and Their Associations with Soil Properties in Farmland, Northeastern China. J. Plant Nutr. Soil Sci. 2017, 180, 563–575. [Google Scholar] [CrossRef]
- Wang, J.G.; Lu, J.F. Analyses on the Changes of Soil Fertility of North China Plain and the Affecting Factors. J. Eco. Rural Environ. 1998, 14, 12–16. (In Chinese) [Google Scholar]
- Tao, L.; Li, F.; Liu, C.; Feng, X.; Gu, L.; Wang, B.; Wen, S.; Xu, M. Mitigation of Soil Acidification through Changes in Soil Mineralogy Due to Long-Term Fertilization in Southern China. Catena 2019, 174, 227–234. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, M.; Wang, B.; Wang, X. Soil Organic Carbon, Total Nitrogen and Grain Yields under Long-Term Fertilizations in the Upland Red Soil of Southern China. Nutr. Cycl. Agroecosystems 2009, 84, 59–69. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Cargill, R.I.M.; Van Nuland, M.E.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal Mycelium as a Global Carbon Pool. Curr. Biol. 2023, 33, R560–R573. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular Mycorrhizal Fungi Conducting the Hyphosphere Bacterial Orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Application of microbial biofilms in biocatalysis and biodegradation. In Enzymes for Pollutant Degradation; Springer Nature: Singapore, 2022; pp. 93–118. [Google Scholar]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of beneficial microorganisms in soil remediation and quality improvement of medicinal plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A.; Buyer, J.S. Comparison of N-Linked Oligosaccharides of Glomalin from Arbuscular Mycorrhizal Fungi and Soils by Capillary Electrophoresis. Soil Biol. Biochem. 1998, 30, 1853–1857. [Google Scholar] [CrossRef]
- Gao, J.; Liang, C.; Shen, G.; Lv, J.; Wu, H. Spectral Characteristics of Dissolved Organic Matter in Various Agricultural Soils throughout China. Chemosphere 2017, 176, 108–116. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with Fluorescence Spectroscopy the Sources of Dissolved Organic Matter in Soils Subjected to Drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of Fluorescent Dissolved Organic Matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Williams, A.; Manoharan, L.; Rosenstock, N.P.; Olsson, P.A.; Hedlund, K. Long-term Agricultural Fertilization Alters Arbuscular Mycorrhizal Fungal Community Composition and Barley (H Ordeum Vulgare) Mycorrhizal Carbon and Phosphorus Exchange. New Phytol. 2017, 213, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, Y.; Li, Q.; Xiao, W.; Song, X. Arbuscular Mycorrhizal Fungal Colonization and Soil PH Induced by Nitrogen and Phosphorus Additions Affects Leaf C: N: P Stoichiometry in Chinese Fir (Cunninghamia Lanceolata) Forests. Plant Soil 2021, 461, 421–440. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Schindler, F.V.; Mercer, E.J.; Rice, J.A. Chemical Characteristics of Glomalin-Related Soil Protein (GRSP) Extracted from Soils of Varying Organic Matter Content. Soil Biol. Biochem. 2007, 39, 320–329. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, H.; Chen, J.; Hong, H.; Liu, J.; Li, J.; Yan, C. Spatial Distribution of Glomalin-Related Soil Protein and Its Relationship with Sediment Carbon Sequestration across a Mangrove Forest. Sci. Total Environ. 2018, 613, 548–556. [Google Scholar] [CrossRef]
- Koorem, K.; Gazol, A.; Oepik, M.; Moora, M.; Saks, Ü.; Uibopuu, A.; Sober, V.; Zobel, M. Soil Nutrient Content Influences the Abundance of Soil Microbes but Not Plant Biomass at the Small-Scale. PLoS ONE 2014, 9, e91998. [Google Scholar] [CrossRef] [PubMed]
- Masebo, N.; Birhane, E.; Takele, S.; Belay, Z.; Lucena, J.J.; Anjulo, A. Richness of Arbuscular Mycorrhizal Fungi Under Different Agroforestry Practices in the Drylands of Southern Ethiopia. BMC Plant Biology 2023, 23, 634. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, L.; Zuo, X.; Ye, X.; Wang, R.; Huang, Z.; Liu, G.; Cornelissen, J.H.C. Plant Diversity Has Stronger Linkage with Soil Fungal Diversity than with Bacterial Diversity across Grasslands of Northern China. Glob. Ecol. Biogeogr. 2022, 31, 886–900. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Wang, H.; Nie, S.; Liang, Z. Effects of Soil Salinity on the Content, Composition, and Ion Binding Capacity of Glomalin-Related Soil Protein (GRSP). Sci. Total Environ. 2017, 581, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lin, L.; Hong, H.; Li, H.; Liu, S.; Tang, S.; Lu, H.; Liu, J.; Yan, C. Enhanced Cr (VI) Stabilization by Terrestrial-Derived Soil Protein: Photoelectrochemical Properties and Reduction Mechanisms. J. Hazard. Mater. 2024, 465, 133153. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; He, X.; Zhang, W.; Song, K.; Han, S. Role and Variation of the Amount and Composition of Glomalin in Soil Properties in Farmland and Adjacent Plantations with Reference to a Primary Forest in North-Eastern China. PLoS ONE 2015, 10, e0139623. [Google Scholar] [CrossRef]
- Van Geel, M.; Verbruggen, E.; De Beenhouwer, M.; Van Rennes, G.; Lievens, B.; Honnay, O. High Soil Phosphorus Levels Overrule the Potential Benefits of Organic Farming on Arbuscular Mycorrhizal Diversity in Northern Vineyards. Agric. Ecosyst. Environ. 2017, 248, 144–152. [Google Scholar] [CrossRef]
- Gosling, P.; Mead, A.; Proctor, M.; Hammond, J.P.; Bending, G.D. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol. 2013, 198, 546–556. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large Contribution of Arbuscular Mycorrhizal Fungi to Soil Carbon Pools in Tropical Forest Soils. Plant Soil 2001, 233, 167–177. [Google Scholar] [CrossRef]


| Treatments | Fertilization (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Maize | Wheat | |||||||
| N | P2O5 | K2O | Fresh Manure | N | P2O5 | K2O | Fresh Manure | |
| CK | - | - | - | - | - | - | - | - |
| NPK | 210 | 84 | 84 | 90 | 36 | 36 | - | |
| M | - | - | - | 42,000 | - | - | - | 18,000 |
| NPKM | 63 | 84 | 84 | 29,400 | 27 | 36 | 36 | 12,600 |
| Component | Excitation Max (nm) | Emission Max (nm) | Likely Structure |
|---|---|---|---|
| C1 | 320 | 390 | UVA humic acid-like |
| C2 | 200 | 385 | Fulvic acid-like |
| C3 | 274 | 460 | UVA humic acid-like |
| C4 | 222 | 290 | Protein-like substance (tyrosine) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Cai, Z.; De Clerck, C.; Meersmans, J.; Colinet, G.; Zhang, W. Long-Term Manuring Enhanced Compositional Stability of Glomalin-Related Soil Proteins through Arbuscular Mycorrhizal Fungi Regulation. Agriculture 2024, 14, 1510. https://doi.org/10.3390/agriculture14091510
Yang H, Cai Z, De Clerck C, Meersmans J, Colinet G, Zhang W. Long-Term Manuring Enhanced Compositional Stability of Glomalin-Related Soil Proteins through Arbuscular Mycorrhizal Fungi Regulation. Agriculture. 2024; 14(9):1510. https://doi.org/10.3390/agriculture14091510
Chicago/Turabian StyleYang, Hongbo, Zejiang Cai, Caroline De Clerck, Jeroen Meersmans, Gilles Colinet, and Wenju Zhang. 2024. "Long-Term Manuring Enhanced Compositional Stability of Glomalin-Related Soil Proteins through Arbuscular Mycorrhizal Fungi Regulation" Agriculture 14, no. 9: 1510. https://doi.org/10.3390/agriculture14091510







