Genetic Diversity and Zoonotic Potential of Shiga Toxin-Producing E. coli (STEC) in Cattle and Buffaloes from Islamabad, Pakistan
Abstract
:1. Introduction
2. Materials and Methods
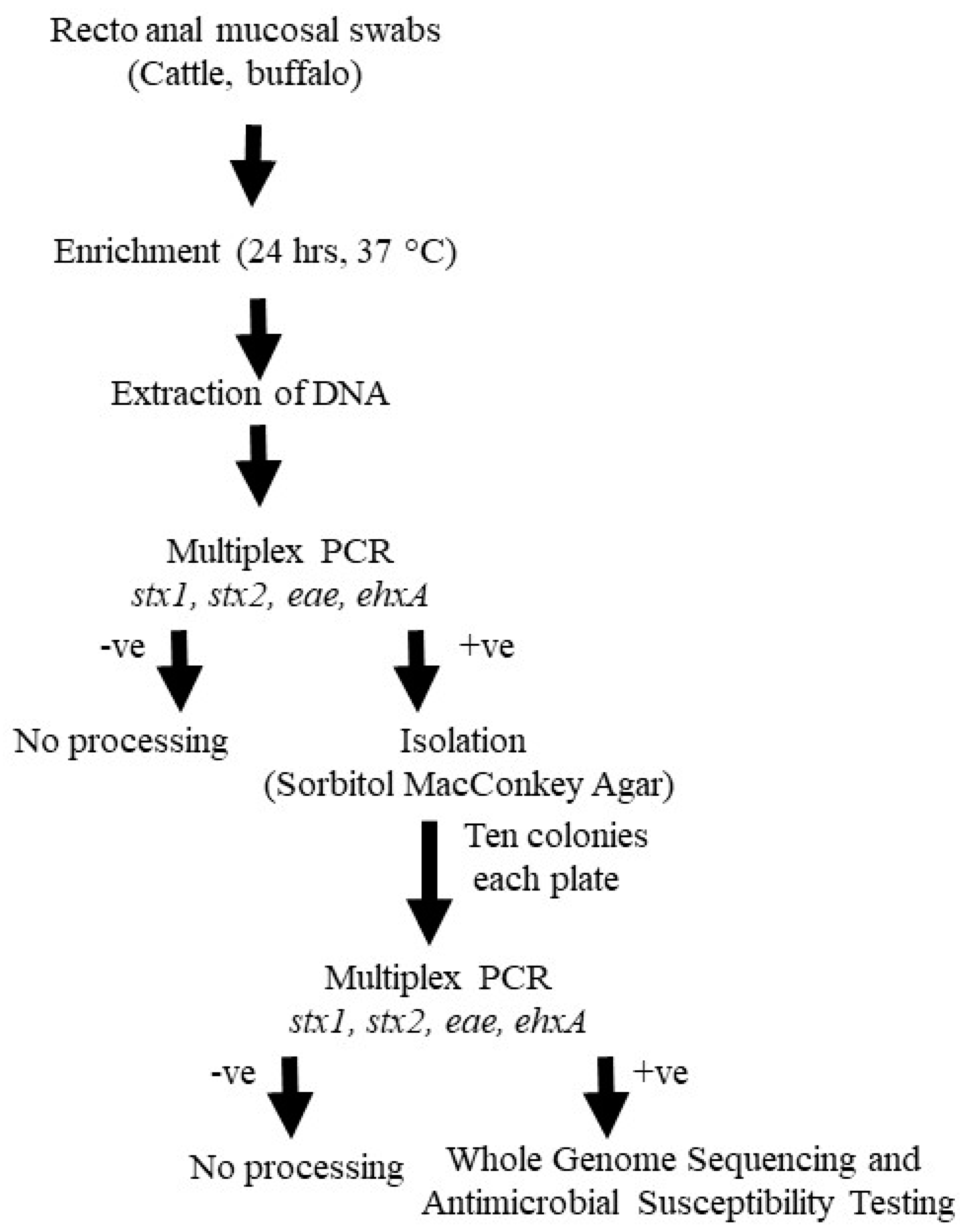
2.1. Study Population and Sample Collection
2.2. Isolation and Identification of STEC
2.3. Antimicrobial Susceptibility Testing of STEC
2.4. Whole Genome Sequencing and Sequence Analysis
3. Results
3.1. Identification of E. coli Serotypes and Virulence Gene Profiling
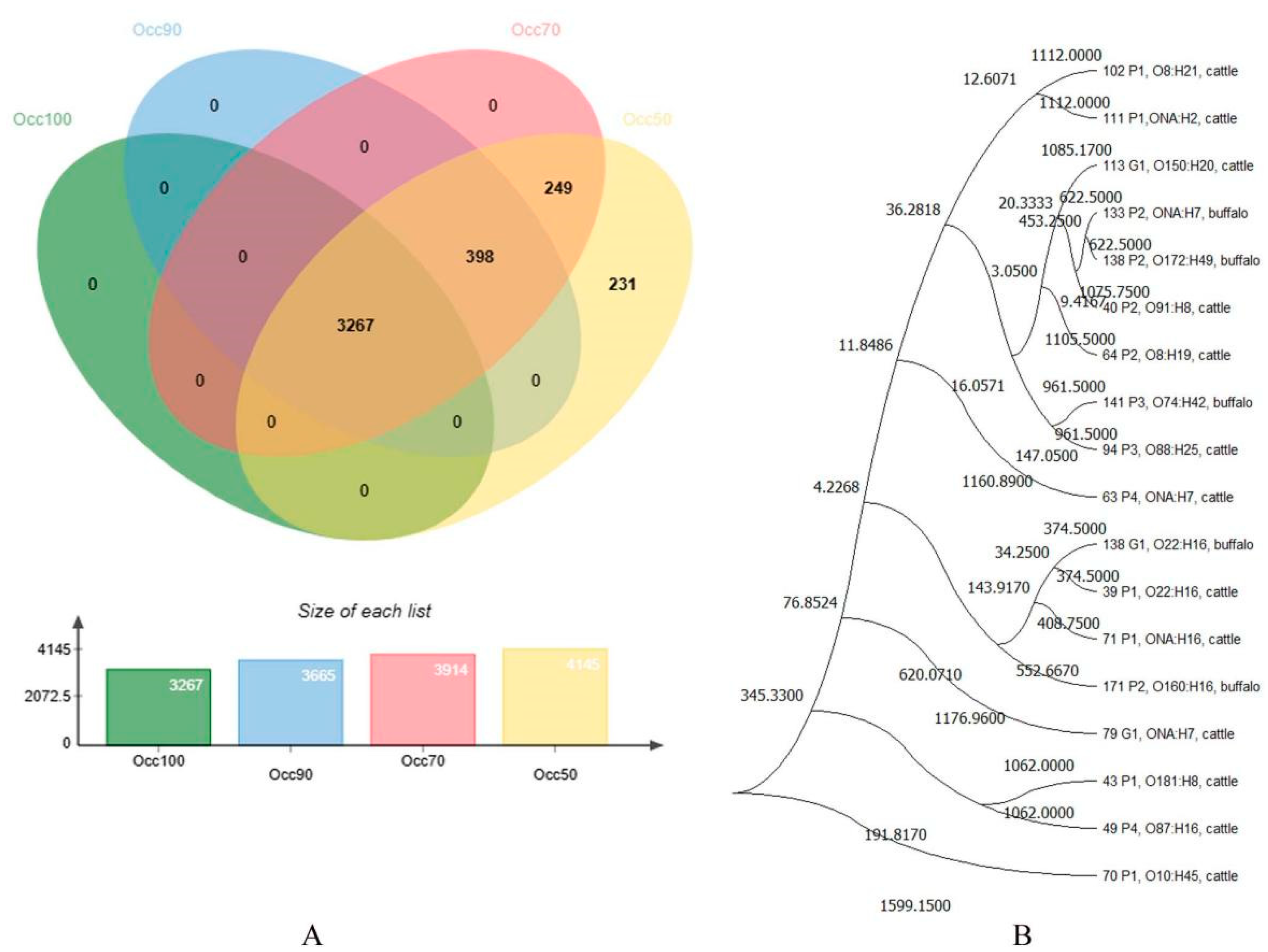
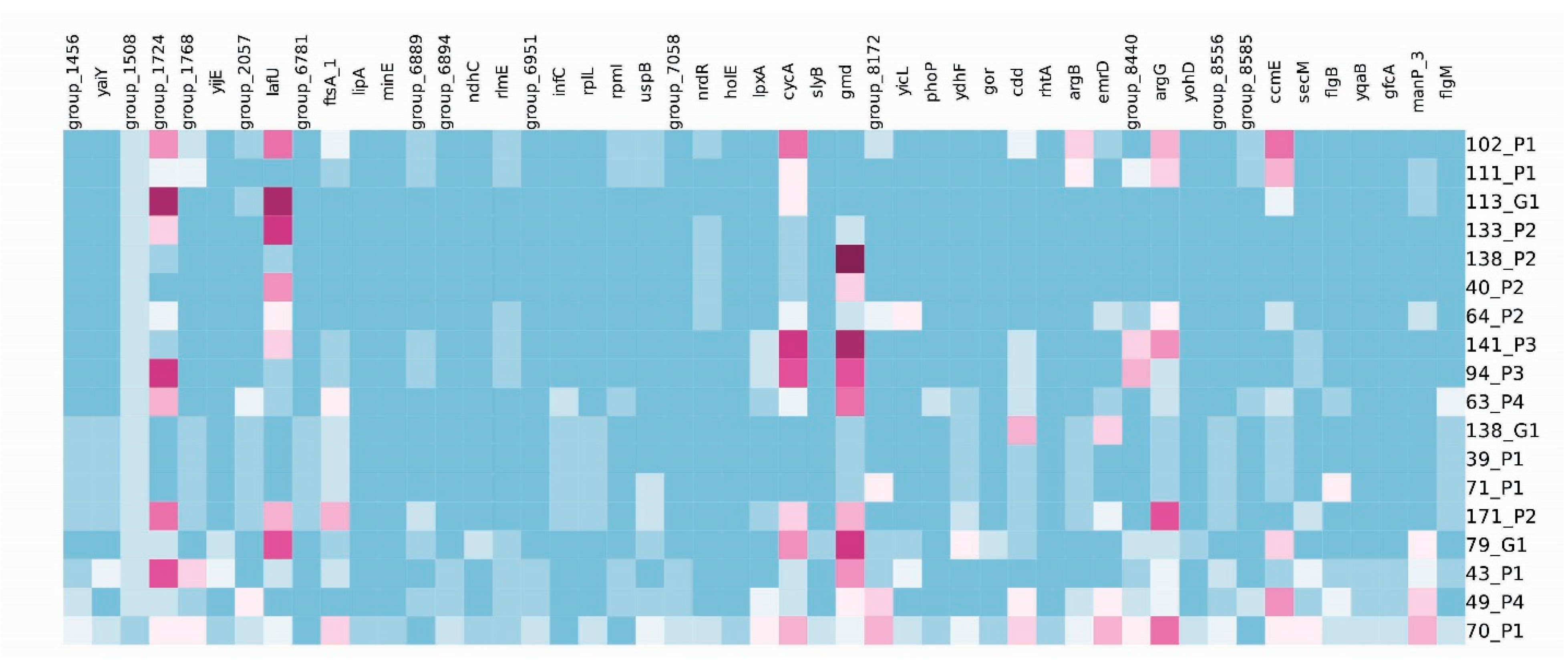
3.2. cgMLST Analyses
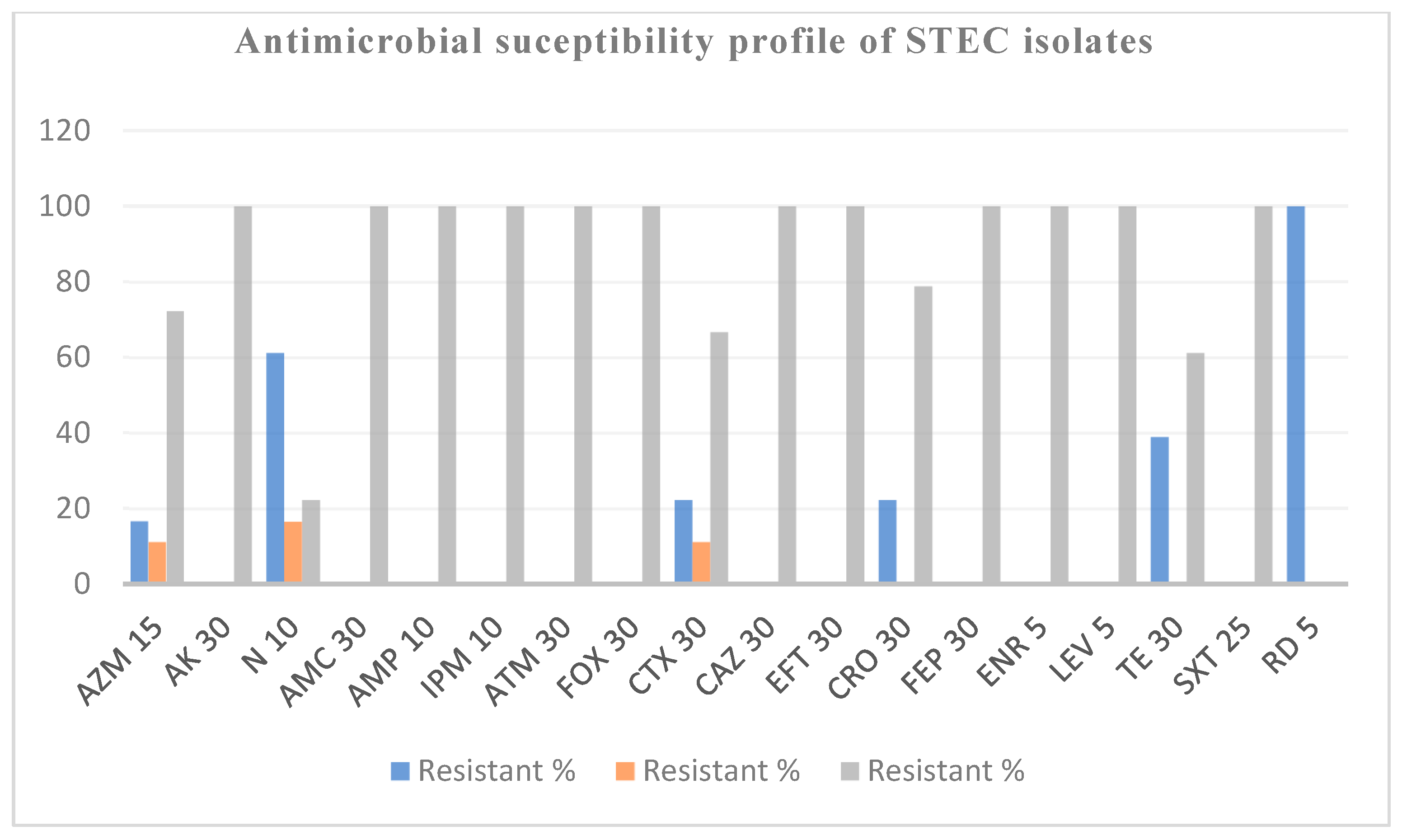
3.3. Phenotypic and Genotypic Characterization of STEC Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bettelheim, K.A. The non-O157 Shiga-toxigenic (verocytotoxigenic) Escherichia coli; under-rated pathogens. Crit. Rev. Microbiol. 2007, 33, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.M. Shiga toxin-producing Escherichia coli (STEC). Clin. Lab. Med. 2010, 30, 21–45. [Google Scholar] [CrossRef]
- Mcpherson, M.; Kirk, M.D.; Raupach, J.; Combs, B.; Butler, J.R.G. Economic Costs of Shiga Toxin-Producing Escherichia coli Infection in Australia. Foodborne Pathog. Dis. 2011, 8, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, A.; Yahata, Y.; Arima, Y.; Takahashi, T.; Saitoh, T.; Kanou, K.; Kawabata, K.; Sunagawa, T.; Matsui, T.; Oishi, K. Enterohemorrhagic Escherichia coli outbreaks related to childcare facilities in Japan, 2010–2013. BMC Infect. Dis. 2015, 15, 539. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Hebert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; et al. Hemorrhagic Colitis Associated with a Rare Escherichia coli Serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Naseer, U.; Løbersli, I.; Hindrum, M.; Bruvik, T.; Brandal, L.T. Virulence factors of Shiga toxin-producing Escherichia coli and the risk of developing haemolytic uraemic syndrome in Norway, 1992–2013. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1613–1620. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol. Spectr. 2014, 2, 10–1128. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Shiga toxin-producing Escherichia coli. In Advances in Applied Microbiology; Academic Press Inc.: Cambridge, MA, USA, 2014; pp. 145–197. [Google Scholar]
- González-Escalona, N.; Kase, J.A. Virulence gene profiles and phylogeny of Shiga toxin-positive Escherichia coli strains isolated from FDA regulated foods during 2010–2017. PLoS ONE 2019, 14, e0214620. [Google Scholar] [CrossRef]
- Fan, R.; Shao, K.; Yang, X.; Bai, X.; Fu, S.; Sun, H.; Xu, Y.; Wang, H.; Li, Q.; Hu, B.; et al. High prevalence of non-O157 Shiga toxin-producing Escherichia coli in beef cattle was detected by combining four selective agars. BMC Microbiol. 2019, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, M.S.; Tzipori, S.; McKee, M.L.; O’Brien, A.D.; Alroy, J.; Kaper, J.B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 1993, 92, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.R.; Brooks, H.J.L.; O’Brien, R. Prevalence of Shiga toxin-producing and enteropathogenic Escherichia coli marker genes in diarrhoeic stools in a New Zealand catchment area. J. Clin. Pathol. 2017, 70, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, M.; Friedrich, A.W.; Grundmann, H.; de Boer, R.F.; Croughs, P.D.; Islam, M.A.; Kluytmans-van den Bergh, M.F.; Kooistra-Smid, A.M.; Rossen, J.W. Molecular characterization and phylogeny of Shiga toxin–producing Escherichia coli isolates obtained from two Dutch regions using whole genome sequencing. Clin. Microbiol. Infect. 2016, 22, 642.e1–642.e9. [Google Scholar] [CrossRef]
- Savarino, S.J.; Fasano, A.; Watson, J.; Martin, B.M.; Levine, M.M.; Guandalini, S.; Guerry, P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc. Natl. Acad. Sci. USA 1993, 90, 3093–3097. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, J.W.; Hovde, C.J. A brief overview of Escherichia coli O157 and its plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, B.; Bai, X.; Yang, X.; Cao, L.; Liu, Q.; Sun, H.; Li, J.; Zhang, J.; Jin, D.; et al. Antimicrobial resistance of non-o157 shiga toxin-producing Escherichia coli isolated from humans and domestic animals. Antibiotics 2021, 10, 74. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hashem, M.E.A.; Alfifi, K.J.; Al-Otaibi, A.S.; Alatawy, M.; Eltarabili, R.M.; Abd El-Ghany, W.A.; Hetta, H.F.; Hamouda, A.M.; Elewa, A.A.; et al. Sequence Analysis, Antibiogram Profile, Virulence and Antibiotic Resistance Genes of XDR and MDR Gallibacterium anatis Isolated from Layer Chickens in Egypt. Infect. Drug Resist. 2022, 15, 4321–4334. [Google Scholar] [CrossRef]
- Algammal, A.M.; El-Kholy, A.W.; Riad, E.M.; Mohamed, H.E.; Elhaig, M.M.; Al Yousef, S.A.; Hozzein, W.N.; Ghobashy, M.O. Genes encoding the virulence and the antimicrobial resistance in enterotoxigenic, and shiga-toxigenic E. coli isolated from diarrheic calves. Toxins 2020, 12, 383. [Google Scholar] [CrossRef]
- Mahanti, A.; Samanta, I.; Bandopaddhay, S.; Joardar, S.N.; Dutta, T.K.; Batabyal, S.; Sar, T.K.; Isore, D.P. Isolation, molecular characterization and antibiotic resistance of Shiga Toxin-Producing Escherichia coli (STEC) from buffalo in India. Lett. Appl. Microbiol. 2013, 56, 291–298. [Google Scholar] [CrossRef]
- Conrad, C.; Stanford, K.; Narvaez-Bravo, C.; Neumann, N.; Munns, K.; Tymensen, L.; Jokinen, C.; McAllister, T.A. Zoonotic Fecal Pathogens and Antimicrobial Resistance in Canadian Petting Zoos. Microorganisms 2018, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Fathi Sharafa, E.; Shabanaa, I.I. Prevalence and molecular characterization of Shiga toxin-producing Escherichia coli isolates from human and sheep in Al-Madinah Al-Munawarah. Infectio 2017, 21, 81–87. [Google Scholar] [CrossRef]
- Jacob, M.E.; Foster, D.M.; Rogers, A.T.; Balcomb, C.C.; Shi, X.; Nagaraja, T.G. Evidence of Non-O157 Shiga toxin-producing Escherichia coli in the feces of meat goats at a U.S. slaughter plant. J. Food Prot. 2013, 76, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Bumunang, E.W.; McAllister, T.A.; Zaheer, R.; Ortega Polo, R.; Stanford, K.; King, R.; Niu, Y.D.; Ateba, C.N. Characterization of Non-O157 Escherichia coli from Cattle Faecal Samples in the North-West Province of South Africa. Microorganisms 2019, 7, 272. [Google Scholar] [CrossRef]
- Samad, A.; Abbas, F.; Ahmad, Z.; Tanveer, Z.; Ahmad, I.; Patching, S.G.; Nawaz, N.; Asmat, M.T.; Raziq, A.; Asadullah; et al. Multiplex polymerase chain reaction detection of Shiga toxin genes and antibiotic sensitivity of Escherichia coli O157 isolated from beef meat in Quetta, Pakistan. J. Food Saf. 2018, 38, e12540. [Google Scholar] [CrossRef]
- Erickson, M.C.; Doyle, M.P. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 2007, 70, 2426–2449. [Google Scholar] [CrossRef] [PubMed]
- Irshad, H.; Cookson, A.L.; Hotter, G.; Besser, T.E.; On, S.L.W.; French, N.P. Epidemiology of Shiga toxin-producing Escherichia coli O157 in very young calves in the North Island of New Zealand. N. Z. Vet. J. 2012, 60, 21–26. [Google Scholar] [CrossRef]
- Sharma, V.K.; Dean-Nystrom, E.A. Detection of enterohemorrhagic Escherichia coli O157 by using a multiplex real-time PCR assay for genes encoding intimin and Shiga toxins. Vet. Microbiol. 2003, 93, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Paton, A.W.; Paton, J.C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR Assays for stx1, stx2, eaeA, Enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clinic. Microbiol. 1998, 36, 598–602. [Google Scholar] [CrossRef]
- Mahe, A. Effect of Citric Acid at Different pH on the Survival of Escherichia coli. Bayero J. Pure Appl. Sci. 2021, 14, 79–84. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M 100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- WHO. Integrated Surveillance of Antimicrobial Resistance—Guidance from WHO Advisory Group; WHO: Geneva, Switzerland, 2013; pp. 1–100. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Siddique, A.; Azim, S.; Ali, A.; Andleeb, S.; Ahsan, A.; Imran, M.; Rahman, A. Antimicrobial resistance profiling of biofilm forming non typhoidal salmonella enterica isolates from poultry and its associated food products from Pakistan. Antibiotics 2021, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Ibrahim, R.A.; Alfifi, K.J.; Ghabban, H.; Alghamdi, S.; Kabrah, A.; Khafagy, A.R.; Abou-Elela, G.M.; Abu-Elala, N.M.; Donadu, M.G. A First Report of Molecular Typing, Virulence Traits, and Phenotypic and Genotypic Resistance Patterns of Newly Emerging XDR and MDR Aeromonas veronii in Mugil seheli. Pathogens 2022, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; Krueger, F.; Segonds-Pichon, A.; Biggins, L.; Krueger, C., 3rd; Wingett, S.; Babraham Bioinformatics; FastQC. A Quality Control Tool for High Throughput Sequence Data (Online). 2019. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 22 January 2022).
- Krueger, F. Trim Galore: A wrapper tool around Cutadapt and FastQC to consistently apply quality and adapter trimming to FastQ files, with some extra functionality for MspI-digested RRBS-type (Reduced Representation Bisufite-Seq) libraries. 2012. Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 22 January 2022).
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Zhou, Z.; Sergeant, M.J.; Achtman, M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018, 14, e1007261. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lin, J.W.; Chen, C.C. Cano-wgMLST_BacCompare: A bacterial genome analysis platform for epidemiological investigation and comparative genomic analysis. Front. Microbiol. 2019, 10, 1687. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, J.L.; O’Toole, D.; Creekmore, T.; Peckham, E.; Killion, H.; Vance, M.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F. Mycoplasma bovis Infections in Free-Ranging Pronghorn, Wyoming, USA. Emerg. Infect. Dis. 2020, 26, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.L.; Pouseele, H.; Chen, J.C.; Strockbine, N.A.; Carleton, H.A. Implementation of whole genome sequencing (WGS) for identification and characterization of Shiga toxin-producing Escherichia coli (STEC) in the United States. Front. Microbiol. 2016, 7, 766. [Google Scholar] [CrossRef] [PubMed]
- Bettelheim, K.A.; Kuzevski, A.; Gilbert, R.A.; Krause, D.O.; McSweeney, C.S. The diversity of Escherichia coli serotypes and biotypes in cattle faeces. J. Appl. Microbiol. 2005, 98, 699–709. [Google Scholar] [CrossRef]
- Karmali, M.A. Infection by Verocytotoxin-Producing Escherichia coli. Clin. Microbiol. Rev. 1989, 2, 15–38. [Google Scholar] [CrossRef]
- Pikrard, D.; Stevens, D.; Moriau, L.; Lior, H.; Lauwers, S. Isolation and virulence factors of vero cyto toxin-producing Escherichia coli in human stool samples. Clin. Microbiol. Infect. 1997, 3, 531–540. [Google Scholar] [CrossRef]
- Schmidt, H.; Karch, H. Enterohemolytic Phenotypes and Genotypes of Shiga Toxin-Producing Escherichia coli O111 Strains from Patients with Diarrhea and Hemolytic-Uremic Syndrome. J. Clin. Microbiol. 1996, 34, 2364–2367. [Google Scholar] [CrossRef]
- Scotland, S.M.; Willshaw, G.A.; Smith, H.R.; Rowe, B. Properties of strains of Escherichia coli belonging to serogroup O 157 with special reference to production of Vero cytotoxins VT1 and VT2. Epidemiol. Infect. 1987, 99, 613–624. [Google Scholar] [CrossRef]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between Virulence Factors of Shiga Toxin-Producing Escherichia coli and Disease in Humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar] [CrossRef]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic Profile of Shiga-Toxin–Producing Escherichia coli O104 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Brandal, L.T.; Tunsjø, H.S.; Ranheim, T.E.; Løbersli, I.; Lange, H.; Wester, A.L. Shiga Toxin 2a in Escherichia albertii. J. Clin. Microbiol. 2015, 53, 1454–1455. [Google Scholar] [CrossRef]
- Carter, M.Q.; Pham, A.; Huynh, S.; He, X. Complete genome sequence of a shiga toxin-producing Enterobacter cloacae clinical isolate. Genome Announc. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Skinner, C.; Patfield, S.; He, X. Phage-mediated Shiga toxin (Stx) horizontal gene transfer and expression in non-Shiga toxigenic Enterobacter and Escherichia coli strains. Pathog. Dis. 2016, 74, ftw037. [Google Scholar] [CrossRef] [PubMed]
- Pradel, N.; Bertin, Y.; Martin, C.; Livrelli, V. Molecular analysis of Shiga toxin-producing Escherichia coli strains isolated from hemolytic-uremic syndrome patients and dairy samples in France. Appl. Environ. Microbiol. 2008, 74, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Irshad, H.; Binyamin, I.; Ahsan, A.; Riaz, A.; Shahzad, M.A.; Qayyum, M.; Kanchanakhan, N.; Siriwong, W.; Pumpaibool, T.; Hussain, M.; et al. Occurrence and molecular characterization of shiga toxin-producing Escherichia coli isolates recovered from cattle and goat meat obtained from retail meat shops in Rawalpindi and Islamabad, Pakistan. Pak. Vet. J. 2020, 40, 295–300. [Google Scholar]
- Shahzad, A.; Ullah, F.; Irshad, H.; Ahmed, S.; Shakeela, Q.; Mian, A.H. Molecular detection of Shiga toxin-producing Escherichia coli (STEC) O157 in sheep, goats, cows and buffaloes. Mol. Biol. Rep. 2021, 48, 6113–6121. [Google Scholar] [CrossRef]
- Toma, C.; Espinosa, E.M.; Song, T.; Miliwebsky, E.; Chinen, I.; Iyoda, S.; Iwanaga, M.; Rivas, M. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 2004, 42, 4937–4946. [Google Scholar] [CrossRef]
- Cordonnier, C.; Etienne-Mesmin, L.; Thévenot, J.; Rougeron, A.; Rénier, S.; Chassaing, B.; Darfeuille-Michaud, A.; Barnich, N.; Blanquet-Diot, S.; Livrelli, V. Enterohemorrhagic Escherichia coli pathogenesis: Role of long polar fimbriae in Peyer’s patches interactions. Sci. Rep. 2017, 7, 44655. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, J.; Ambikan, A.; Jernberg, C.; Ehricht, R.; Scheutz, F.; Xiong, Y.; Matussek, A. Molecular Characterization and Comparative Genomics of Clinical Hybrid Shiga Toxin-Producing and Enterotoxigenic Escherichia coli (STEC/ETEC) Strains in Sweden. Sci. Rep. 2019, 9, 5619. [Google Scholar] [CrossRef]
- Baschera, M.; Cernela, N.; Stevens, M.J.A.; Liljander, A.; Jores, J.; Corman, V.M.; Nüesch-Inderbinen, M.; Stephan, R. Shiga toxin-producing Escherichia coli (STEC) isolated from fecal samples of African dromedary camels. One Health 2019, 7, 100087. [Google Scholar] [CrossRef]
- Colello, R.; Krüger, A.; Velez, M.V.; Del Canto, F.; Etcheverría, A.I.; Vidal, R.; Padola, N.L. Identification and detection of iha subtypes in LEE-negative Shiga toxin-producing Escherichia coli (STEC) strains isolated from humans, cattle and food. Heliyon 2019, 5, e03015. [Google Scholar] [CrossRef] [PubMed]
- Mundy, R.; Jenkins, C.; Yu, J.; Smith, H.; Frankel, G. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J. Med. Microbiol. 2004, 53, 1145–1149. [Google Scholar] [CrossRef]
- Beutin, L.; Prada, J.; Zimmermann, S.; Stephan, R.; Ørskov, I.; Ørskov, F. Enterohemolysin, a new type of hemolysin produced by some strains of enteropathogenic E. coli (EPEC). Zentralbl. Bakteriol. Mikrobiol. Hyg. 1988, 267, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Beutin, L.; Karch, H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157 strain EDL 933. Infect. Immun. 1995, 63, 1055–1061. [Google Scholar] [CrossRef]
- Karch, H.; Janetzki-Mittmann, C.; Aleksic, S.; Datz, M. Isolation of enterohemorrhagic Escherichia coli O157 strains from patients with hemolytic-uremic syndrome by using immunomagnetic separation, DNA-based methods, and direct culture. J. Clin. Microbiol. 1996, 34, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk-Zurek, M.; Chmielarczyk, A.; Gosiewski, T.; Tomusiak, A.; Adamski, P.; Zwolinska-Wcislo, M.; Mach, T.; Heczko, P.B.; Strus, M. Possible role of Escherichia coli in propagation and perpetuation of chronic inflammation in ulcerative colitis. BMC Gastroenterol. 2013, 13, 61. [Google Scholar] [CrossRef]
- Feng, Y.; Mannion, A.; Madden, C.M.; Swennes, A.G.; Townes, C.; Byrd, C.; Marini, R.P.; Fox, J.G. Cytotoxic Escherichia coli strains encoding colibactin and cytotoxic necrotizing factor (CNF) colonize laboratory macaques. Gut Pathog. 2017, 9, 71. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Phan, M.D.; Peters, K.M.; Walker, M.J.; Schembri, M.A.; McEwan, A.G. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6818–6823. [Google Scholar] [CrossRef]
- Zhang, W.L.; Köhler, B.; Oswald, E.; Beutin, L.; Karch, H.; Morabito, S.; Caprioli, A.; Suerbaum, S.; Schmidt, H. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 2002, 40, 4486–4492. [Google Scholar] [CrossRef]
- Fitzhenry, R.J.; Pickard, D.J.; Hartland, E.L.; Reece, S.; Dougan, G.; Phillips, A.D. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157. Gut. 2002, 50, 180–185. [Google Scholar] [CrossRef]
- Phillips, A.D.; Frankel, G. Intimin-Mediated Tissue Specificity in Enteropathogenic Escherichia coli Interaction with Human Intestinal Organ Cultures. J. Infect. Dis. 2000, 181, 1496–1506. Available online: https://academic.oup.com/jid/article/181/4/1496/865392 (accessed on 22 January 2022). [CrossRef] [PubMed]
- Beutin, L.; Martin, A. Outbreak of shiga toxin-producing Escherichia coli (STEC) O104 infection in Germany causes a paradigm shift regarding human pathogenicity of STEC strains. J. Food Prot. 2012, 75, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, F.; Palma, F.; Trevisani, M.; Parisi, A.; Lucchi, A.; De Cesare, A.; Manfreda, G. Whole genome sequencing based typing and characterisation of shiga-toxin producing Escherichia coli strains belonging to O157 and O26 serotypes and isolated in dairy farms. Ital. J. Food Saf. 2018, 7, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.S.; Biggs, P.J.; Wilkinson, D.A.; Cookson, A.L.; Midwinter, A.C.; Bloomfield, S.J.; Hranac, C.R.; Rogers, L.E.; Marshall, J.C.; Benschop, J. Use of genomics to investigate historical importation of shiga toxin-producing Escherichia coli serogroup O26 and nontoxigenic variants into New Zealand. Emerg. Infect. Dis. 2019, 25, 489–500. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Moussa, I.; Abalkhail, A.; Ibrahem, M.; Hamada, M.; Sindi, W.; Alzaben, F.; Almuzaini, A.M. Pseudomonas species prevalence, protein analysis, and antibiotic resistance: An evolving public health challenge. AMB Express 2022, 12, 53. [Google Scholar] [CrossRef]
- Zhang, Q.; An, X.; Liu, H.; Wang, S.; Xiao, T.; Liu, H. Uncovering the Resistance Mechanism of Mycobacterium tuberculosis to Rifampicin Due to RNA Polymerase H451D/Y/R Mutations from Computational Perspective. Front. Chem. 2019, 7, 819. [Google Scholar] [CrossRef]
- Shafiq, M.; Zeng, M.; Permana, B.; Bilal, H.; Huang, J.; Yao, F.; Algammal, A.M.; Li, X.; Yuan, Y.; Jiao, X. Coexistence of blaNDM–5 and tet(X4) in international high-risk Escherichia coli clone ST648 of human origin in China. Front. Microbiol. 2022, 13, 1031688. [Google Scholar] [CrossRef]
| Species of Animal | |||
|---|---|---|---|
| Cattle | Buffalo | ||
| Virulence Genes | No. of Positive Samples | Virulence Genes | No. of Positive Samples |
| stx1, stx2, ehxA | 18 | stx1, stx2, ehxA | 29 |
| stx1 | 17 | stx1, ehxA | 20 |
| stx1, ehxA | 15 | stx1 | 7 |
| stx1, stx2 | 5 | stx1, stx2 | 3 |
| ehxA | 3 | ehxA | 0 |
| stx2 | 1 | stx2 | 0 |
| Total | 59/104 (56.7%) | Total | 59/96 (61.4%) |
| Isolate ID | Serotype | Specie | Shiga Toxin Type | Virulence Genes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stx1 | Stx2 | espI | Iha | ireA | iss | IpfA | espP | ehxA | gad | epeA | mcmA | mch | ast | celB | eilA | katP | capU | |||
| E. coli 39P1 | O22:H16 | Cattle | Stx1a | Stx2a | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | |
| E. coli 40P2 | O91:H8 | Cattle | Stx1a | Stx2c | − | + | − | + | + | + | + | + | − | − | − | − | − | − | − | − |
| E. coli 43P1 | O181:H8 | Cattle | Stx1a | Stx2c | − | + | − | + | + | + | + | + | + | − | − | − | − | − | − | − |
| E. coli 49P4 | O87:H16 | Cattle | Stx1a | Stx2c | − | + | − | + | + | − | + | + | + | − | − | − | − | − | − | − |
| E. coli 63P4 | ONA:H7 | Cattle | Stx1a | Stx2c | − | + | − | + | + | + | + | + | − | + | + | + | + | − | − | − |
| E. coli 64P2 | O8:H19 | Cattle | - | - | − | − | − | + | + | − | − | + | − | − | − | − | − | − | − | − |
| E. coli 70P1 | O10:H45 | Cattle | - | - | − | − | − | + | − | − | − | + | − | − | − | − | − | + | − | − |
| E. coli 71P1 | ONA:H16 | Cattle | Stx1a | Stx2c | − | + | − | + | + | − | − | + | − | − | − | − | − | − | − | − |
| E. coli 79G1 | ONA:H7 | Cattle | Stx1a | Stx2c | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − |
| E. coli 94P3 | O88:H25 | Cattle | Stx1a | Stx2a | − | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − |
| E. coli 102P1 | O8:H21 | Cattle | - | Stx2a | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | − |
| E. coli 111P1 | ONA:H2 | Cattle | - | - | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − |
| E. coli 113G1 | O150:H20 | Cattle | - | - | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | + |
| E. coli 133P2 | ONA:H7 | Buffalo | - | - | − | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − |
| E. coli 138P2 | O172:H49 | Buffalo | Stx1a | Stx2b | − | + | − | + | + | − | + | + | − | − | − | − | − | − | − | − |
| E. coli 138G1 | O22:H16 | Buffalo | Stx1a | - | − | − | − | + | + | − | − | + | − | − | + | − | + | − | − | − |
| E. coli 141P3 | O74:H42 | Buffalo | Stx1a | - | − | + | − | + | + | − | + | + | − | − | − | − | + | − | − | − |
| E. coli 171P2 | O160:H16 | Cattle | Stx1a | Stx2a | + | + | − | + | + | − | + | + | − | − | − | − | − | − | − | − |
| Total | 12 | 11 | 3 | 12 | 1 | 15 | 17 | 4 | 8 | 16 | 2 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | ||
| Isolate ID | Serotype | Specie | GenBank Accession Number | No. of Contigs | Coverage | Total no. of Genes |
|---|---|---|---|---|---|---|
| 39P1 | O22:H16 | Cattle | DAERGV000000000.1 | 402 | 108× | 5392 |
| 40P2 | O91:H8 | Cattle | DAERHR000000000.1 | 180 | 152× | 4959 |
| 43P1 | O181:H8 | Cattle | DAERHL000000000.1 | 112 | 136× | 4834 |
| 49P4 | O87:H16 | Cattle | DAERGQ000000000.1 | 123 | 127× | 4792 |
| 63P4 | ONA:H7 | Cattle | DAERHQ000000000.1 | 142 | 129× | 4861 |
| 64P2 | O8:H19 | Cattle | DAERGG000000000.1 | 86 | 90× | 4509 |
| 70P1 | O10:H45 | Cattle | DAERGZ000000000.1 | 174 | 138× | 4762 |
| 71P1 | ONA:H16 | Cattle | DAERHF000000000.1 | 211 | 142× | 5122 |
| 79G1 | ONA:H7 | Cattle | DAERHH000000000.1 | 161 | 106× | 4939 |
| 94P3 | O88:H25 | Cattle | DAERGJ000000000.1 | 135 | 135× | 4982 |
| 102P1 | O8:H21 | Cattle | DAERHO000000000.1 | 246 | 163× | 5055 |
| 111P1 | ONA:H2 | Cattle | DAERHG000000000.1 | 126 | 131× | 4481 |
| 113G1 | O150:H20 | Cattle | DAERHA000000000.1 | 92 | 160× | 4598 |
| 133P2 | ONA:H7 | Buffalo | DAERHQ000000000.1 | 142 | 129× | 4861 |
| 138P2 | O172:H49 | Buffalo | DAERHS000000000.1 | 132 | 254 | 4749 |
| 138G1 | O22:H16 | Buffalo | DAERGN000000000.1 | 189 | 117 | 4963 |
| 141_P3 | O74:H42 | Buffalo | DAERGW000000000.1 | 158 | 126× | 5136 |
| 171_P2 | O160:H16 | Cattle | DAERGS000000000.1 | 218 | 105× | 4972 |
| Isolate ID | Serotype | Phenotypic Resistance Profile | MAR Index | Resistance Genes |
|---|---|---|---|---|
| 39P1 | O22:H16 | RIF | 0.06 | rpoB |
| 40P2 | O91:H8 | NEO, AZM, TCY, RIF | 0.25 | ramA, evgA, adeL, emrY, acrAB-TolC, mexR, tet (M), tetA, tetB, rpoB |
| 43P1 | O181:H8 | NEO, TCY, RIF, CTX | 0.25 | ramA, evgA, adeL, emrY, acrAB-TolC, mexR, tetA, tetB, rpoB |
| 49P4 | O87:H16 | RIF | 0.06 | rpoB |
| 63P4 | ONA:H7 | RIF | 0.06 | rpoB |
| 64P2 | O8:H19 | RIF | 0.12 | rpoB |
| 70P1 | O10:H45 | TCY, RIF, NEO | 0.12 | ramA, evgA, adeL, emrY, acrAB-TolC, mexR, tetA, tetB, rpoB |
| 71P1 | ONA:H16 | TCY, RIF | 0.12 | ramA, evgA adeL, emrY, acrAB-TolC, tetA, tet (M), tetB, rpoB |
| 79G1 | ONA:H7 | NEO, RIF | 0.12 | rpoB |
| 94P3 | O88:H25 | NEO, RIF, CRO | 0.18 | rpoB |
| 102P1 | O8:H21 | NEO, RIF | 0.12 | rpoB |
| 111P1 | ONA:H2 | RIF | 0.06 | rpoB |
| 113G1 | O150:H20 | NEO, RIF, CRO | 0.18 | rpoB |
| 133P2 | ONA:H7 | NEO, TCY, RIF, CTX, CRO | 0.31 | ramA, evgA, adeL, emrY, acrAB-TolC, mexR, tetA, tetB, rpoB |
| 138P2 | O172:H49 | NEO, AZM, TCY, RIF, CRO | 0.31 | ramA, evgA adeL, emrY, acrAB-TolC, tetA, tet (M), tetB, rpoB |
| 138G1 | O22:H16 | AZM, RIF, CTX | 0.18 | ramA, evgA, adeL, emrY, acrAB-TolC, mexR, rpoB |
| 141P3 | O74:H42 | RIF | 0.06 | rpoB |
| 171P2 | O160:H16 | NEO, RIF, CRO | 0.18 | rpoB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irshad, H.; Ahsan, A.; Yousaf, A.; Kanchanakhan, N.; Pumpaibool, T.; Siriwong, W.; Prombutara, P.; Ahmed, I.; Basharat, Z.; Nawaz, M.; et al. Genetic Diversity and Zoonotic Potential of Shiga Toxin-Producing E. coli (STEC) in Cattle and Buffaloes from Islamabad, Pakistan. Agriculture 2024, 14, 1537. https://doi.org/10.3390/agriculture14091537
Irshad H, Ahsan A, Yousaf A, Kanchanakhan N, Pumpaibool T, Siriwong W, Prombutara P, Ahmed I, Basharat Z, Nawaz M, et al. Genetic Diversity and Zoonotic Potential of Shiga Toxin-Producing E. coli (STEC) in Cattle and Buffaloes from Islamabad, Pakistan. Agriculture. 2024; 14(9):1537. https://doi.org/10.3390/agriculture14091537
Chicago/Turabian StyleIrshad, Hamid, Aitezaz Ahsan, Arfan Yousaf, Naowarat Kanchanakhan, Tepanata Pumpaibool, Wattasit Siriwong, Pinidphon Prombutara, Ibrar Ahmed, Zarrin Basharat, Mudussar Nawaz, and et al. 2024. "Genetic Diversity and Zoonotic Potential of Shiga Toxin-Producing E. coli (STEC) in Cattle and Buffaloes from Islamabad, Pakistan" Agriculture 14, no. 9: 1537. https://doi.org/10.3390/agriculture14091537









