Dietary Effects of Black-Oat-Rich Polyphenols on Production Traits, Metabolic Profile, Antioxidative Status, and Carcass Quality of Fattening Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design and Body Weight Analysis
2.2. Analyses of Feed Mixture and Hay
2.3. Blood Sampling and Analysis
2.4. Analyses of Carcass Measures and Meat Quality
2.5. Extraction of Antioxidants from Lamb Muscle, Liver, and Kidney Samples to Assess Antioxidative Status
2.5.1. Free-Radical-Scavenging Activity of 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.5.2. Thiobarbituric-Acid-Reactive Substances (TBARS)
2.6. Statistical Analysis
3. Results
3.1. Production Traits
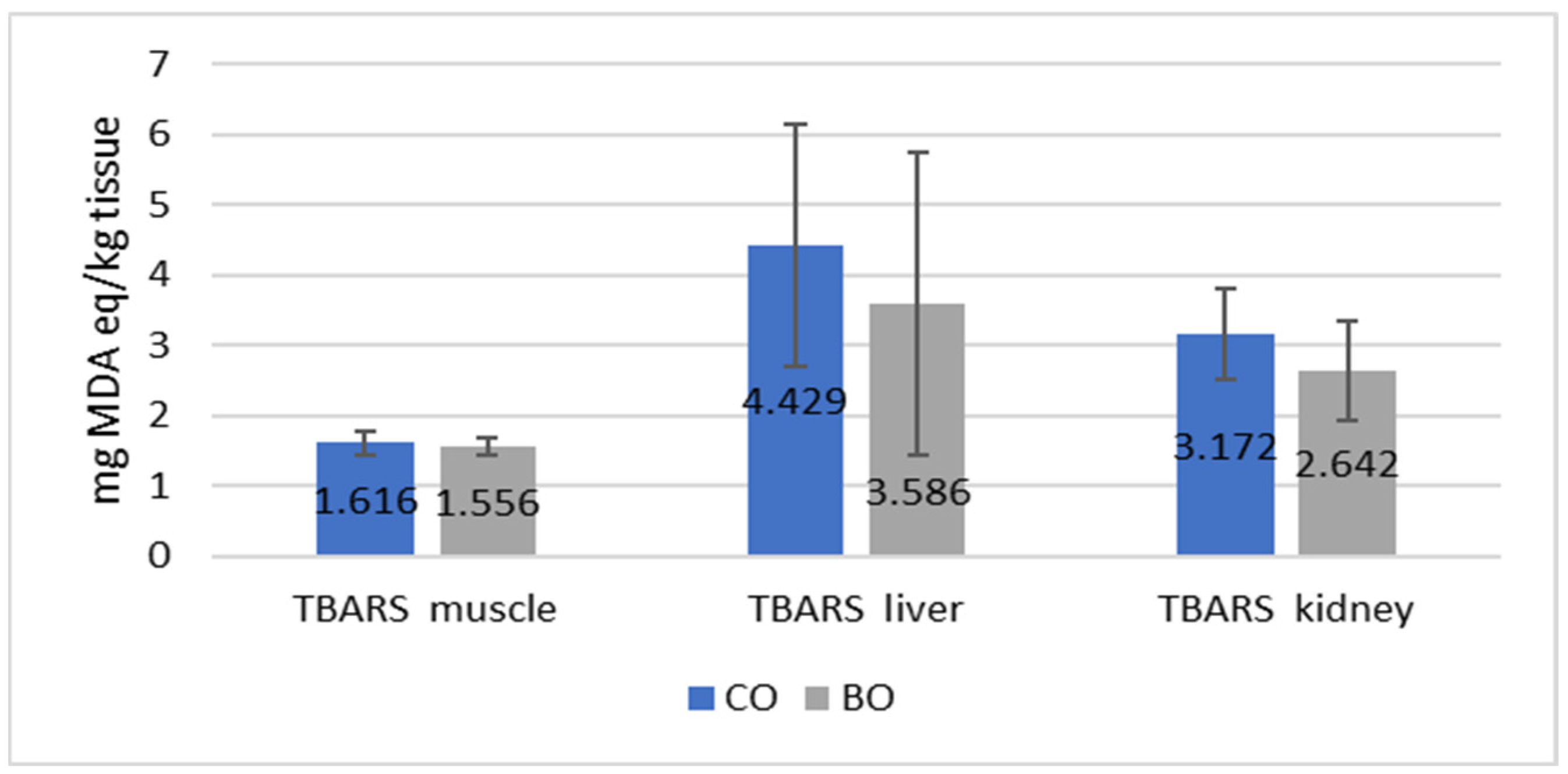
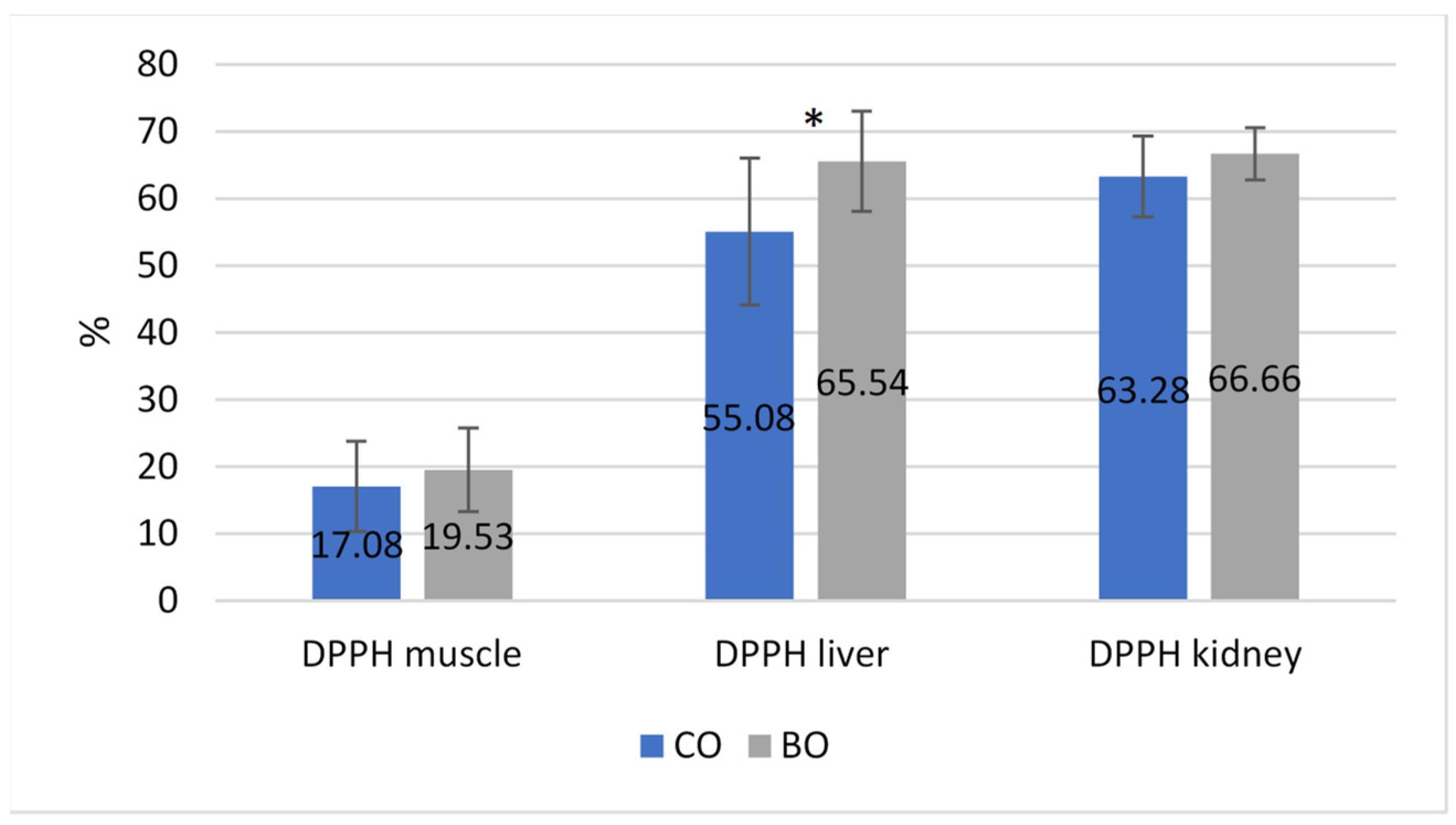
3.2. Blood Parameters and Antioxidative Status
4. Discussion
4.1. Production Traits
4.2. Blood Parameters and Antioxidative Status
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lammer, P. Small Grains for Livestock. A Meta-Analysis; University of Wisconsin-Platteville: Platteville, WI, USA, 2017. [Google Scholar]
- Klose, C.; Schehl, B.D.; Arendt, E.K. Fundamental study on protein changes taking place during malting of oats. J. Cereal Sci. 2009, 49, 83–91. [Google Scholar] [CrossRef]
- Čech, M.; Ivanišová, E.; Hozlár, P.; Tokár, M.; Zagula, G.; Gumul, D.; Kačániová, M.; Sterczyńska, M.; Haščík, P. Nutritional composition, biological activity and technological properties of new Slovakian black oat varieties. Microbiol. Biotech. Food Sci. 2021, 11, e4238. [Google Scholar] [CrossRef]
- Xie, F.; Lei, Y.; Han, X.; Zhao, Y.; Zhang, S. Antioxidant ability of polyphenols from black rice, buckwheat and oats: In vitro and in vivo. Czech J. Food Sci. 2020, 38, 242–247. [Google Scholar] [CrossRef]
- Hussain, T.; Wang, J.; Murtaza, G.; Metwally, E.; Yang, H.; Kalhoro, M.S.; Kalhoro, D.H.; Rahu, B.A.; Tan, B.; Sahito, R.G.A.; et al. The role of polyphenols in regulation of heat shock proteins and gut microbiota in weaning stress. Oxid. Med. Cell. Longev. 2021, 6, 6676444. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Čech, M.; Hozlár, P.; Zaguła, G.; Gumul, D.; Grygorieva, O.; Makowska, A.; Kowalczewski, P.Ł. Nutritional, antioxidant and sensory characteristics of bread enriched with whole meal flour from Slovakian black aat varieties. Appl. Sci. 2023, 13, 4485. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Yan, J.; Peng, Y.; Weng, W.; Yao, X.; Gao, A.; Cheng, J.; Ruan, J.; Xu, B. Bioactive components and health functions of oat. Food Rev. Int. 2022, 39, 4545–4564. [Google Scholar] [CrossRef]
- Singh, R.; De, S.; Belkheir, A. Avena sativa (oat) a potential nutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Teshager, M.A.; Assegie, A.A.; Amare, E. Proximate, mineral and anti-nutrient compositions of oat grains (Avena sativa) cultivated in Ethiopia: Implications for nutrition and mineral bioavailability. Heliyon 2021, 7, e07722. [Google Scholar] [CrossRef]
- Menon, R.; Gonzalez, T.; Ferruzzi, M.; Jackson, E.; Winderl, D.; Watson, J. Oats—from farm to fork. Adv. Food Nutr. Res. 2016, 77, 1–55. [Google Scholar] [PubMed]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Stover, M.G.; Watson, R.R. Polyphenols in foods and dietary supplements: Role in veterinary medicine and animal health. In Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2014; Chapter 1; pp. 3–7. [Google Scholar]
- Abdel-Aal, E.S.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple and red cereal grains. J. Agric. Food Che. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic composition and antioxidant activity of colored oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Fontaneli, R.S.; dos Santos, H.P.; Fontaneli, R.S.; Oliveira, J.T.; Lehmen, R.I.; Dreon, G. Winter forage grasses. In Forrageiras Para Integração Lavoura-Pecuária-Floresta NA Região Sul-Brasileira; Fontaneli, R.S., dos Santos, H.P., Fontaneli, R.S., Eds.; EMBRAPA: Brasilia, Brazi, 2012. [Google Scholar]
- Derenevicz Faisca, L.; Peres, M.T.P.; Fernandes, S.R.; Bonnet, O.J.F.; Batista, R.; Deiss, L.; Monteiro, A.G. A new insight about the selection and intake of forage by ewes and lambs in different production systems on pasture. Small Rumin. Res. 2023, 221, 106949. [Google Scholar] [CrossRef]
- Vega-García, J.I.; López-González, F.; Estrada-Flores, J.G.; Flores-Calvete, G.; Prospero-Bernal, F.; Arriaga-Jordán, C.M. Black oat (Avena strigosa Schreb.) grazing or silage for small-scale dairy systems in the highlands of central Mexico. Part I. Crop and dairy cow performance. Chil. J. Agric. Res. 2020, 80, 515–525. [Google Scholar] [CrossRef]
- Vonz, D.; Menezes, L.F.G.; Paris, W.; Kuss, F.; Silveira, M.F.; Venturini, T.; Stanqueviski, F.; Boito, B. Performance of steers fed on pasture receiving different seeding rates of vetch in an integrated crop-livestock system. Span. J. Agric. Res. 2021, 19, e06SC01. [Google Scholar] [CrossRef]
- Silva, T.B.P.; Del Valle, T.A.; Ghizzi, L.G.; Silva, G.G.; Gheller, L.S.; Marques, J.A.; Dias, M.S.S.; Nunes, A.T.; Grigoletto, N.T.S.; Takiya, C.S.; et al. Partial replacement of corn silage with whole-plant soybean and black oat silages for dairy cows. J. Dairy Sci. 2021, 104, 9842–9852. [Google Scholar] [CrossRef]
- Bernardes, G.M.C.; Carvalho, S.; Pires, C.C.; Motta, J.H.; Teixeira, W.S.; Borges, L.I.; Fleig, M.; Pilecco, V.M.; Farinha, E.T.; Venturini, R.S. Consumption, performance and economic analysis of the feeding of lambs finished in feedlot as the use of high-grain diets. Arq. Bras. Med. Vet. Zootec. 2015, 67, 1684–1692. [Google Scholar] [CrossRef]
- Bernardes, G.M.C.; Carvalho, S.; Venturini, R.S.; Teixeira, W.S.; Motta, J.H.; Borges, L.I.; Rosa, J.S.; Pesamosca, A.C.; Cocco, A.; Mello, V.L.; et al. Carcass characteristics and tissue composition of the meat of feedlot lambs fed high-grain diets. Semin. Ciências Agrárias 2018, 39, 2637–2646. [Google Scholar]
- Karakuş, F. Weaning stress in lambs. J. Int. Sci. Pub. Agric. Food 2014, 2, 165–170. [Google Scholar]
- Lynch, E.; McGee, M.; Earley, B. Weaning management of beef calves with implications 322 for animal health and welfare. J. Appl. Anim. Res. 2019, 47, 167–175. [Google Scholar] [CrossRef]
- Freitas-de-Melo, A.; Orihuela, A.; Hötzel, M.J.; Ungerfeld, R. What do we know and need to know about weaning in sheep? An overview of weaning practises, stress and welfare. Front. Anim. Sci. 2022, 3, 823188. [Google Scholar]
- Freitas-de-Melo, A.; Ungerfeld, R. Destete artificial en ovinos: Respuesta de estrés y bienestar animal. Rev. Mex. Cienc. Pecu. 2016, 7, 361–375. [Google Scholar] [CrossRef]
- Damián, J.P.; Hötzel, M.J.; Banchero, G.; Ungerfeld, R. Behavioural response of grazing lambs to changes associated with feeding and separation from their mothers at weaning. Res. Vet. Sci. 2013, 95, 913–918. [Google Scholar] [CrossRef]
- Freitas-de-Melo, A.; Ungerfeld, R.; Pérez-Clariget, R. Behavioral pattern in Texel × Corriedale terminal crossbreeding: Maternal behavior score at birth, lambs’ feeding behaviors, and behavioral responses of lambs to abrupt weaning. J. Vet. Behav. 2019, 30, 9–15. [Google Scholar] [CrossRef]
- Schichowski, C.; Moors, E.; Gauly, M. Effects of weaning lambs in two stages or by abrupt separation on their behavior and growth rate. J. Anim. Sci. 2008, 86, 220–225. [Google Scholar] [CrossRef]
- Selaive-Villarroel, A.B.; Maciel, M.B.; de Oliveira, N.M. Effects of weaning age and weight on lamb growth rate of Morada Nova breed raised in a tropical extensive production system. Ciência Rural. Santa Maria 2008, 38, 784–788. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, F.; Wang, J.; Wu, P.; Qiu, X.; He, X.; Xiao, Y.; Gan, S. Effect of tea polyphenols on intestinal barrier and immune function in weaned lambs. Front. Vet. Sci. 2024, 11, 1361507. [Google Scholar] [CrossRef]
- Russel, A. Body condition scoring of sheep. In Sheep and Goat Practice; Boden, E., Ed.; Bailliere Tindall: Philadelphia, PA, USA, 1991; p. 3. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academy Press: Washington, DC, USA, 2007; p. 293. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2006. [Google Scholar]
- Antunovic, Z.; Novoselec, J.; Klir Šalavardic, Ž.; Steiner, Z.; Šperanda, M.; Jakobek Barron, L.; Ronta, M.; Pavic, V. Influence of red corn rich in anthocyanins on productive traits, blood metabolic profile, and antioxidative status of fattening lambs. Animals 2022, 12, 612. [Google Scholar] [CrossRef]
- INRAE-CIRAD-AFZ. Feed tables composition and nutritive values of feeds for cattle, sheep, goats, pigs, poultry, rabbits, horses and salmonids. Available online: https://www.feedtables.com (accessed on 10 July 2024).
- Jakobek, L.; Matić, P.; Ištuk, J.; Barron, A.R. Study of interactions between individual phenolics of aronia with barley β-glucan. Pol. J. Food Nutr. Sci. 2021, 71, 187–196. [Google Scholar] [CrossRef]
- Antunović, Z.; Domaćinović, M.; Šperanda, M.; Liker, B.; Mioč, B.; Šerić, V.; Šperanda, T. Effect of roasted cereals and soybean in feed mixtures on fattening and slaughter traits as well as blood composition in fattening lambs. Arch. Tierz. 2009, 52, 512–526. [Google Scholar] [CrossRef]
- CIE (Commission Internationale de l’Eclairage). Colorimetry, Official Recommendations of the International Commission on Illumination; Publication CIE No. 15 (E-1.3.1); Bureau Central dela CIE: Paris, France, 1976. [Google Scholar]
- Sierra, I. Contributions to the study of the Belgian White_Landrace cross: Productive characters, carcass quality and meat quality. Rev. Inst. Econom. Prod. Ebro. 1973, 16, 43–48. [Google Scholar]
- Qwele, K.; Hugo, A.; Oyedemi, S.O.; Moyo, B.; Masika, P.J.; Muchenje, V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 2013, 93, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dai, R.; Zhu, J.; Li, X. Optimizing color and lipid stability of beef patties with a mixture design incorporating with tea catechins, carnosine, and α-tocopherol. J. Food Eng. 2010, 98, 170–177. [Google Scholar] [CrossRef]
- TIBCO. Statistica, version 13.3.0; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Lepherd, M.L.; Canfield, P.J.; Hunt, G.B.; Bosward, K.L. Haematological, biochemical and selected acute phase protein reference intervals for weaned female Merino lambs. Aust. Vet. J. 2009, 87, 5–11. [Google Scholar] [CrossRef]
- Latimer, K.S.; Maheffey, E.A.; Prasse, K.W. Duncan and Prasse’s Veterinary Labaratory Medicine: Clinical Pathology, 4th ed. In Plumb’s Veterinary Drug Handbook, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2003; pp. 1241–1249. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2008; p. 963. [Google Scholar]
- Antunović, Z.; Novoselec, J.; Šperanda, M.; Domaćinović, M.; Đidara, M. Monitoring nutritional status of lambs in organic breeding. Krmiva 2010, 52, 27–34. [Google Scholar]
- Antunović, Z.; Klir Šalavardić, Ž.; Steiner, Z.; Đidara, M.; Drenjančević, M.; Ronta, M.; Pavić, V.; Jakobek Barron, L.; Novoselec, J. Meat quality, metabolic profile and antioxidant status of lambs fed on seedless grape pomace (Vitis vinifera L.). Ann. Anim. Sci. 2023, 23, 809–818. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z.; Yang, Y.; Wang, Y. Functional properties of polyphenols in grains and effects of physicochemical processing on polyphenols. Hindawi J. Food Qual. 2019, 2019, 2793973. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, J.; Luo, Q.; Wang, X.; Wang, T.; Zhou, D.; Xie, L.; Ban, C.; Lu, Q. Effects of purple corn Anthocyanin on growth performance, meat quality, muscle antioxidant status, and fatty acid profiles in goats. Foods 2022, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Hernández-García, P.A. Growth performance, carcass characteristics, and blood metabolites of lambs supplemented with a polyherbal mixture. Animals 2021, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, R.; Barone, C.M.A.; Claps, S.; Varrivvhio, E.; Rufrano, D.; Caroprese, M.; Albenzi, M.; De Palo, P.; Neglia, G. Effect of dietary supplementation with polyphenols on meat quality in Saanen goat kids. BMC Vet. Res. 2018, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Muela, E.; Alonso, V.; Campo, M.M.; Sañudo, C.; Beltrán, J.A. Antioxidant diet supplementation and lamb quality throughout preservation time. Meat Sci. 2014, 98, 289–295. [Google Scholar] [CrossRef]
- Kholif, A.E.; Hassan, A.A.; El Ashry, G.M.; Bakr, M.H.; El-Zaiat, H.M.; Olafadehan, O.A.; Matloup, O.H. Phytogenic feed additives mixture enhances the lactational performance, feed utilization and ruminal fermentation of Friesian cows. Anim. Biotechnol. 2020, 32, 708–718. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Rad. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- Antunović, Z.; Novoselec, J.; Klir, Ž. Hematological parameters in ewes during lactation in organic farming. Poljoprivreda 2017, 23, 46–52. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, X.; Liu, Y.; Wang, H.; Luo, J.; Luo, Y.; An, P. Effects of dietary polyphenol supplementation on iron status and erythropoiesis: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 780–793. [Google Scholar]
- Youdim, K.A.; Shukitt-Hale, B.; MacKinnon, S.; Kalt, W.; Joseph, J.A. Polyphenolics enhance red blood cell resistance to oxidative stress: In vitro and in vivo. Biochim. Biophys. Acta 2000, 1523, 117–122. [Google Scholar] [CrossRef]
- Antunović, Z.; Mioč, B.; Klir Šalavardić, Ž.; Širić, I.; Držaić, V.; Đidara, M.; Novoselec, J. The effect of lactation stage on the hematological and serum-related biochemical parameters of the Travnik pramenka ewe. Poljoprivreda 2021, 27, 56–62. [Google Scholar] [CrossRef]
- Balcells, J.; Aris, A.; Serrano, A.; Seradj, A.R.; Crespo, J.; Devant, M. Effects of an extract of plant flavonoids (Bioflavex) on rumen fermentation and performance in heifers fed high-concentrate diets. J. Anim. Sci. 2012, 90, 4975–4984. [Google Scholar] [CrossRef]
- Mbuh, J.V.; Mbwaye, J. Serological changes in goats experimentally infected with Fasciola gigantica in Buea sub-division of S.W.P. Cameroon. Vet. Parasitol. 2005, 131, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Skoufos, J.; Giannakopoulos, C.; Wiemann, M.; Gortzi, O.; Lalas, S.; Kyriazakis, I. Effects of essential oils on milk production, milk composition, and rumen microbiota in Chios dairy ewes. J. Dairy Sci. 2011, 94, 5569–5577. [Google Scholar] [CrossRef]
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Silanikove, N.; Tiomokin, D. Toxicity induced by poultry litter consumption: Effect on parameters reflecting liver function in beef cows. Anim. Prod. 1992, 54, 203–209. [Google Scholar] [CrossRef]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; McAllister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.; Wang, S.; Wang, Y. Effect of purple prairie clover (Dalea purpurea Vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.-P.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Distribution of procyanidins and their metabolites in rat plasma and tissues after an acute intake of hazelnut extract. Food Funct. 2011, 2, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Zeng, Q.S.; Deng, Y.Y.; Zhang, M.W.; Wei, Z.C.; Tang, X.J. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2012, 136, 11691176. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 70, 123–129. [Google Scholar] [CrossRef]
- Callcott, E.T.; Santhakumar, A.B.; Luo, J.; Blanchard, C.L. Terapeutic potential of rice-derived polyphenols on obesity-related oxidative stress and inflammation. J. Appl. Biomed. 2018, 16, 255–262. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Wild lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res. Int. 2018, 107, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.; Saavedra, N.; Salazar, L.; Abdalla, D. Modulation of immune function by polyphenols: Possible contribution of epigenetic factors: Possible con-tribution of epigenetic factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef]
- Bañón, S.; Méndez, L.; Almela, E. Effects of dietary rosemary extract on lamb spoilage under retail display conditions. Meat Sci. 2012, 90, 579–583. [Google Scholar] [CrossRef]
- Sañudo, C.; Santolaria, M.P.; Maria, G.; Osorio, M.; Sierra, I. Influence of carcass weight on instrumental and sensory lamb meat quality in intensive production systems. Meat Sci. 1996, 42, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Dalle, Z.O. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest. Prod. Sci. 2002, 75, 11–32. [Google Scholar] [CrossRef]
| Ingredient (%) | Group | Meadow Hay | Black Oat | |
|---|---|---|---|---|
| CO | BO | |||
| Ingredients composition | ||||
| Corn | 40.00 | 40.00 | ||
| Oat | 15.00 * | - | ||
| Black oat | - | 15.00 | ||
| Barley | 21.30 | 21.30 | ||
| Soybean meal | 8.00 | 8.00 | ||
| Soybean toasted | 12.00 | 12.00 | ||
| Limestone | 0.30 | 0.30 | ||
| Cattle salt | 0.40 | 0.40 | ||
| Mineral premix ** | 3.00 | 3.00 | ||
| Chemical composition (g/kg DM) | ||||
| Dry matter | 950.00 | 945.00 | 946.70 | 950.20 |
| Crude proteins | 155.00 | 152.90 | 102.70 | 73.60 |
| Ether extract | 48.30 | 48.60 | 7.00 | 48.70 |
| Crude fibre | 22.30 | 36.90 | 353.70 | 101.30 |
| Ash | 66.90 | 60.40 | 51.50 | 28.80 |
| NEM, MJ/kg | 8.00 | 7.00 | 4.50 | - |
| Polyphenols (total; mg gallic acid equivalents (GAE)/kg) | 1631.15 | 2234.63 | 4730.30 | 1538.46 |
| Traits | Measuring Time (Days) | Groups (Mean ± sd) | SEM | p-Values | |
|---|---|---|---|---|---|
| CO | BO | ||||
| Body mass (kg) | 1 | 23.71 ± 1.58 | 24.75 ± 1.34 | 0.34 | 0.13 |
| 30 | 33.71 ± 2.65 | 34.00 ± 2.27 | 0.54 | 0.80 | |
| Daily gain (kg) | 1–30 | 0.333 ± 0.05 | 0.309 ± 0.05 | 0.01 | 0.29 |
| Feed conversion (DM/g gain) | 1–30 | 2.86 | 2.88 | ||
| Feed consumption (g/days) | 1–30 | 0.95 | 0.89 | ||
| BCS (point) | 1 | 3.53 ± 0.27 | 3.66 ± 0.23 | 0.06 | 0.26 |
| 30 | 3.88 ± 0.23 | 4.00 ± 0.21 | 0.05 | 0.06 | |
| Indicators | Groups (Mean ± sd) | SEM | p-Value | |
|---|---|---|---|---|
| CO | BO | |||
| Pre-slaughter mass (kg) | 33.71 ± 2.65 | 34.00 ± 2.27 | 0.54 | 0.80 |
| Carcass mass (kg) | 19.06 ± 1.33 | 18.47 ± 1.03 | 0.27 | 0.28 |
| Entrails mass * (kg) | 1.50 ± 0.30 | 1.45 ± 0.26 | 0.06 | 0.71 |
| Skin and legs mass (kg) | 4.52 ± 0.40 | 4.64 ± 0.59 | 0.11 | 0.60 |
| Forestomach and intestines mass (kg) | 7.78 ± 0.87 | 7.09 ± 0.59 | 0.18 | 0.06 |
| Carcass dressing (%) | 56.73 ± 4.54 | 54.37 ± 1.50 | 0.78 | 0.14 |
| Measures | Groups (Mean ± sd) | SEM | p-Value | |
|---|---|---|---|---|
| CO | BO | |||
| Carcass length 1 (cm) | 73.90 ± 3.03 | 74.40 ± 3.06 | 0.67 | 0.72 |
| Carcass length 2 (cm) | 51.95 ± 2.59 | 53.40 ± 3.45 | 0.68 | 0.30 |
| Carcass length 3 (cm) | 29.00 ± 1.53 | 29.90 ± 1.70 | 0.37 | 0.23 |
| Carcass circumference (cm) | 65.85 ± 1.84 | 66.90 ± 1.58 | 0.44 | 0.06 |
| Hind legs length (cm) | 30.60 ± 4.01 | 30.15 ± 2.54 | 0.73 | 0.21 |
| Ham circumference (cm) | 52.60 ± 1.90 | 54.05 ±1.89 | 0.44 | 0.11 |
| Indicators | Groups (Mean ± sd) | SEM | p-Value | |
|---|---|---|---|---|
| CO | BO | |||
| pH1 | 6.51 ± 0.15 | 6.37 ± 0.10 | 0.03 | 0.03 |
| pH2 | 5.69 ± 0.05 | 5.66 ± 0.05 | 0.12 | 0.24 |
| WHC (%) | 25.50 ± 5.07 | 28.23 ± 3.88 | 1.03 | 0.19 |
| Color | ||||
| L* | 44.32 ± 1.24 | 43.00 ± 1.82 | 0.37 | 0.08 |
| a* | 19.03 ± 1.41 | 19.30 ± 0.80 | 0.25 | 0.14 |
| b* | 2.47 ± 0.26 | 2.42 ± 0.27 | 0.06 | 0.68 |
| Hue angle | 7.44 ± 0.90 | 7.17 ± 0.88 | 0.20 | 0.51 |
| Chroma | 19.19 ± 1.40 | 19.45 ± 0.79 | 0.25 | 0.61 |
| Indicators | Measuring Time (Day) | Groups (Mean ± sd) | SEM | p-Values | Ref. Values [46] | |
|---|---|---|---|---|---|---|
| CO | BO | |||||
| WBC × 109 L | 1 | 10.51 ± 2.74 | 10.39 ± 1.85 | 0.51 | 0.91 | 5.10–15.90 |
| 30 | 11.31 ± 3.02 | 12.12 ± 3.60 | 0.73 | 0.59 | ||
| RBC × 1012 L | 1 | 9.74 ± 1.19 | 10.50 ± 0.88 | 0.24 | 0.14 | 9.20–13.00 |
| 30 | 9.17 ± 3.02 | 10.45 ± 0.63 | 0.27 | 0.01 | ||
| HGB (g/L) | 1 | 117.70 ± 9.90 | 120.70 ± 2.62 | 2.02 | 0.47 | 105.00–137.00 |
| 30 | 121.80 ± 10.41 | 129.00 ± 9.84 | 2.35 | 0.13 | ||
| HCT (L/L) | 1 | 0.44 ± 0.11 | 0.48 ± 0.12 | 0.03 | 0.41 | 0.28–0.47 |
| 30 | 0.34 ± 0.06 | 0.40 ± 0.03 | 0.01 | 0.003 | ||
| MCV (fL) | 1 | 46.09 ± 18.50 | 46.50 ± 15.24 | 0.69 | 0.96 | 28–41 |
| 30 | 36.46 ± 0.06 | 38.09 ± 1.29 | 0.35 | 0.02 | ||
| MCH (pg) | 1 | 12.22 ± 1.86 | 11.57 ± 0.62 | 0.31 | 0.31 | 10–13 |
| 30 | 13.61 ± 3.02 | 12.41 ± 1.54 | 0.54 | 0.28 | ||
| MCHC (g/L) | 1 | 279.00 ± 41.70 | 261.30 ± 43.92 | 9.54 | 0.37 | 332–392 |
| 30 | 357.90 ± 101.63 | 327.80 ± 50.85 | 18.34 | 0.20 | ||
| PLT × 109 L | 1 | 680.80 ± 68.87 | 634.60 ± 155.73 | 26.74 | 0.40 | 426.00– |
| 30 | 610.53 ± 47.09 | 591.80 ± 129.74 | 21.35 | 0.67 | 1142.00 | |
| Differential blood smears (%) | ||||||
| EOS | 1 | 4.50 ± 3.60 | 5.40 ± 8.46 | 1.42 | 0.76 | 1–8 [47] |
| 30 | 2.60 ± 1.65 | 1.00 ± 0.67 | 0.33 | 0.01 | ||
| SEG | 1 | 33.30 ± 7.82 | 29.20 ± 5.79 | 1.70 | 0.07 | 10–50 [47] |
| 30 | 26.10 ± 6.74 | 24.40 ± 11.02 | 1.99 | 0.68 | ||
| BAC | 1 | 0.20 ± 0.42 | 0.40 ± 1.71 | 0.37 | 0.06 | 0 [47] |
| 30 | 0 | 0.10 ± 0.32 | 0.05 | 0.33 | ||
| LYMPH | 1 | 56.60 ± 9.24 | 64.50 ± 11.96 | 2.50 | 0.16 | 50–75 [47] |
| 30 | 69.50 ± 8.44 | 73.60 ± 10.78 | 2.16 | 0.36 | ||
| MONO | 1 | 4.80 ± 6.63 | 0.90 ± 1.20 | 1.13 | 0.08 | 0–4 [47] |
| 30 | 1.70 ± 2.00 | 0.70 ± 0.95 | 0.36 | 0.17 | ||
| BAS | 1 | 0.59 ± 0.70 | 0.60 ± 1.08 | 0.20 | 0.99 | 0–1 [47] |
| 30 | 0.10 ± 0.32 | 0.20 ± 0.42 | 0.08 | 0.56 | ||
| Indicators | Measuring Time (Day) | Groups (Mean ± sd) | SEM | p-Values | Ref. Values [46] | |
|---|---|---|---|---|---|---|
| CO | BO | |||||
| Ca (mmol/L) | 1 | 2.77 ± 0.16 | 2.89 ± 0.23 | 0.05 | 0.20 | 2.42–2.92 |
| 30 | 2.85 ± 0.21 | 2.68 ± 0.28 | 0.08 | 0.12 | ||
| P-inorganic (mmol/L) | 1 | 3.04 ± 0.60 | 3.31 ± 0.52 | 0.13 | 0.29 | 1.88–3.34 |
| 30 | 3.05 ± 0.35 | 2.99 ± 0.38 | 0.10 | 0.08 | ||
| Mg (mmol/L) | 1 | 1.55 ± 0.19 | 1.61 ± 0.22 | 0.05 | 0.52 | 0.91–1.31 |
| 30 | 1.52 ± 0.18 | 1.42 ± 0.14 | 0.04 | 0.17 | ||
| Glucose (mmol/L) | 1 | 6.62 ± 0.52 | 6.39 ± 0.58 | 0.12 | 0.37 | 2.70–4.80 |
| 30 | 6.57 ± 0.34 | 6.06 ± 0.67 | 0.13 | 0.046 | ||
| Urea (mmol/L) | 1 | 6.55 ± 1.07 | 6.80 ± 1.83 | 0.33 | 0.72 | 5.00–9.10 |
| 30 | 9.76 ± 1.51 | 9.67 ± 0.97 | 0.28 | 0.89 | ||
| Total | 1 | 60.80 ± 4.01 | 59.19 ± 4.32 | 0.93 | 0.40 | 51.00–64.00 |
| proteins (g/L) | 30 | 58.50 ± 3.19 | 65.64 ± 4.30 | 1.16 | 0.007 | |
| ALB (g/L) | 1 | 30.87 ± 2.47 | 28.97 ± 2.01 | 0.54 | 0.08 | 30.00–37.00 |
| 30 | 28.64 ± 4.57 | 32.32 ± 1.69 | 0.86 | 0.03 | ||
| GLOB (g/L) | 1 | 29.93 ± 3.63 | 30.22 ± 3.64 | 0.79 | 0.86 | 19.00–30.00 |
| 30 | 29.82 ± 3.62 | 33.33 ± 3.33 | 0.86 | 0.04 | ||
| CHOL (mmol/L) | 1 | 1.42 ± 0.46 | 1.63 ± 0.76 | 0.14 | 0.46 | 1.35–1.97 [48] |
| 30 | 1.43 ± 0.22 | 1.29 ± 0.30 | 0.06 | 0.26 | ||
| HDL (mmol/L) | 1 | 0.73 ± 0.26 | 0.87 ± 0.19 | 0.05 | 0.19 | 0.68–0.97 [49] |
| 30 | 0.83 ± 0.09 | 0.77 ± 0.17 | 0.03 | 0.09 | ||
| LDL (mmol/L) | 1 | 0.42 ± 0.26 | 0.64 ± 0.49 | 0.09 | 0.22 | 0.10–0.50 [49] |
| 30 | 0.45 ± 0.14 | 0.39 ± 0.17 | 0.03 | 0.80 | ||
| TRIG (mmol/L) | 1 | 0.43 ± 0.10 | 0.40 ± 0.07 | 0.02 | 0.48 | |
| 30 | 0.33 ± 0.14 | 0.29 ± 0.04 | 0.02 | 0.78 | 0.00–2.00 [48] | |
| NEFA (mmol/L) | 1 | 0.35 ± 0.47 | 0.17 ± 0.18 | 0.08 | 0.27 | <0.4 |
| 30 | 0.27 ± 0.22 | 0.26 ± 0.14 | 0.04 | 0.88 | ||
| BHB (mmol/L) | 1 | 0.29 ± 0.12 | 0.28 ± 0.13 | 0.03 | 0.87 | 0.2–0.7 |
| 30 | 0.30 ± 0.10 | 0.38 ± 0.12 | 0.03 | 0.11 | ||
| Indicators | Measuring Time (Day) | Groups (Mean ± sd) | SEM | p-Values | Ref. Values [46] | |
|---|---|---|---|---|---|---|
| CO | BO | |||||
| AST (U/L) | 1 | 107.88 ± 8.84 | 116.03 ± 11.98 | 2.48 | 0.10 | 83.00–140.00 |
| 30 | 130.55 ± 16.11 | 119.27 ± 5.76 | 2.93 | 0.052 | ||
| ALT (U/L) | 1 | 13.43 ± 3.24 | 15.29 ± 5.24 | 0.97 | 0.35 | 6.00–20.00 [48] |
| 30 | 16.06 ± 2.53 | 12.55 ± 0.69 | 0.57 | 0.0006 | ||
| ALP (U/L) | 1 | 441.97 ± 114.50 | 459.56 ± 98.01 | 23.28 | 0.72 | 300–500 [50] |
| 30 | 590.11 ± 59.36 | 508.66 ± 64.23 | 16.39 | 0.009 | ||
| GGT (U/L) | 1 | 81.31 ± 10.09 | 68.58 ± 19.44 | 3.67 | 0.08 | 56.00–110.00 |
| 30 | 72.49 ± 9.50 | 70.10 ± 10.30 | 2.14 | 0.59 | ||
| GPx (U/L) | 1 | 524.02 ± 37.77 | 524.73 ± 38.98 | 8.35 | 0.97 | >600 [50] |
| 30 | 510.27 ± 36.74 | 676.73 ±109.68 | 25.85 | 0.0005 | ||
| SOD (U/mL) | 1 | 0.39 ± 0.10 | 0.43 ± 0.11 | 0.04 | 0.366 | 0.39–0.67 [50] |
| 30 | 0.54 ± 0.08 | 0.63 ± 0.10 | 0.03 | 0.047 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunović, Z.; Klir Šalavardić, Ž.; Mioč, B.; Steiner, Z.; Đidara, M.; Sičaja, V.; Pavić, V.; Mihajlović, L.; Jakobek, L.; Novoselec, J. Dietary Effects of Black-Oat-Rich Polyphenols on Production Traits, Metabolic Profile, Antioxidative Status, and Carcass Quality of Fattening Lambs. Agriculture 2024, 14, 1550. https://doi.org/10.3390/agriculture14091550
Antunović Z, Klir Šalavardić Ž, Mioč B, Steiner Z, Đidara M, Sičaja V, Pavić V, Mihajlović L, Jakobek L, Novoselec J. Dietary Effects of Black-Oat-Rich Polyphenols on Production Traits, Metabolic Profile, Antioxidative Status, and Carcass Quality of Fattening Lambs. Agriculture. 2024; 14(9):1550. https://doi.org/10.3390/agriculture14091550
Chicago/Turabian StyleAntunović, Zvonko, Željka Klir Šalavardić, Boro Mioč, Zvonimir Steiner, Mislav Đidara, Vinko Sičaja, Valentina Pavić, Lovro Mihajlović, Lidija Jakobek, and Josip Novoselec. 2024. "Dietary Effects of Black-Oat-Rich Polyphenols on Production Traits, Metabolic Profile, Antioxidative Status, and Carcass Quality of Fattening Lambs" Agriculture 14, no. 9: 1550. https://doi.org/10.3390/agriculture14091550






