Biological and Physiological Changes in Spodoptera frugiperda Larvae Induced by Non-Consumptive Effects of the Predator Harmonia axyridis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Experimental Setup
2.3. Non-Consumptive Effects of Predator Presence on the Biology of S. frugiperda
2.4. Antioxidant Enzyme Activities of S. frugiperda
2.5. Determination of Nutrients in Larvae after Stress
2.6. Data Analysis
3. Results
3.1. Non-Consumptive Effects of H. axyridis on the Biology of S. frugiperda
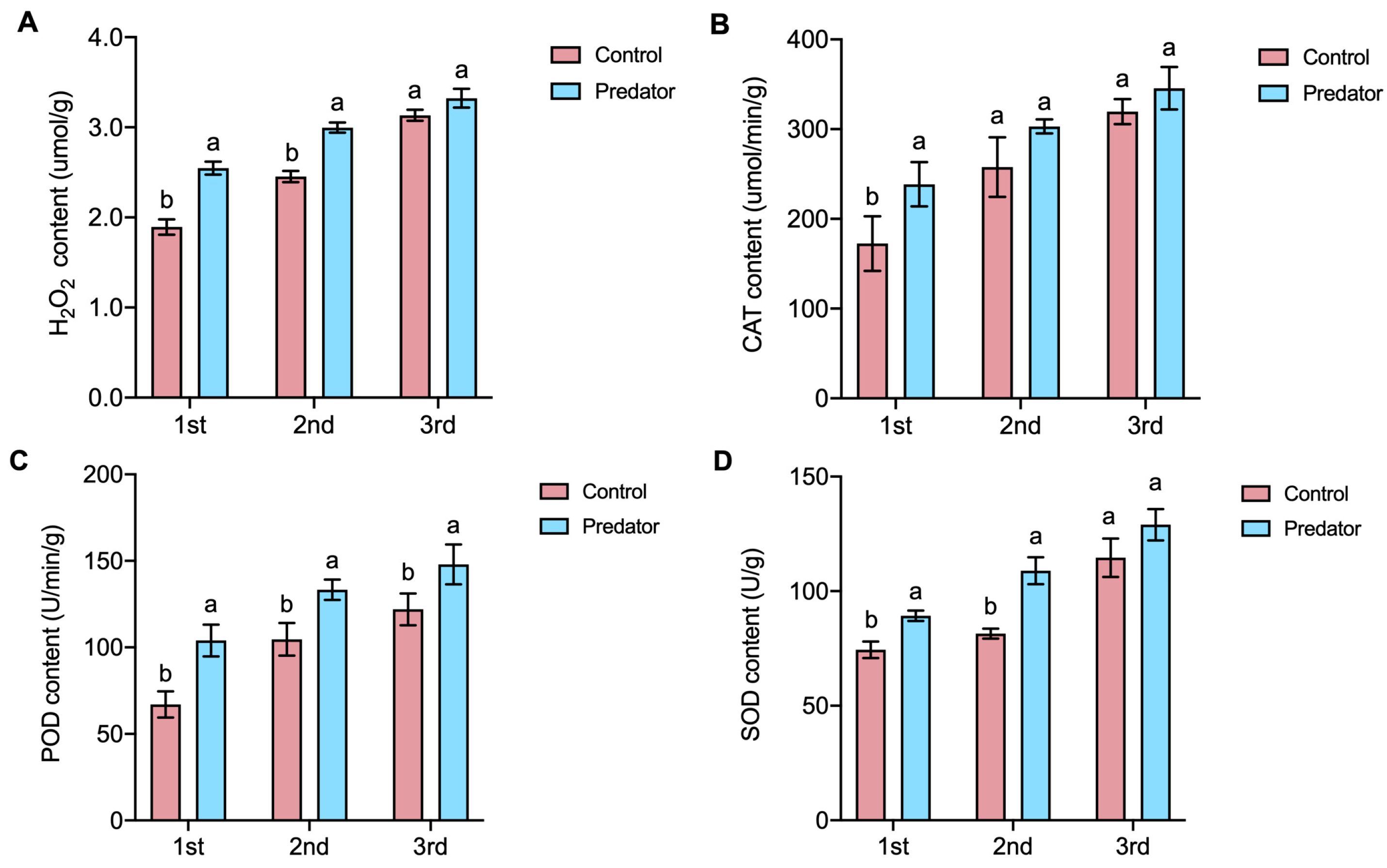
3.2. Non-Consumptive Effects of S. frugiperda on Antioxidant Enzyme Activities
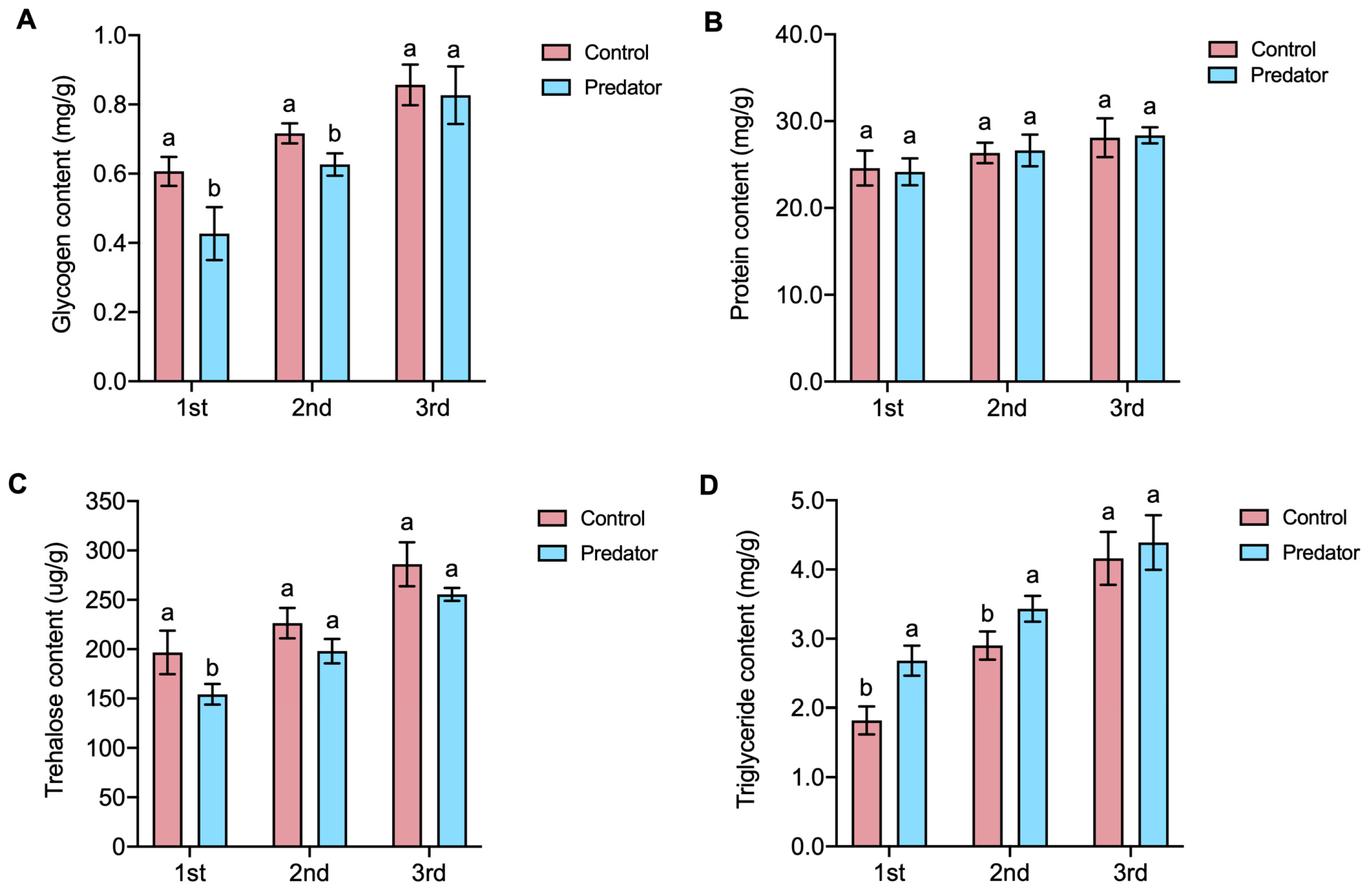
3.3. Non-Consumptive Effects on Nutrients in S. frugiperda Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barratt, B.I.P.; Ferguson, C.M.; Goldson, S.L.; Phillips, C.M.; Hannah, D.J. Predicting the risk from biological control agent introductions: A New Zealand approach. In Nontarget Effects of Biological Control; Springer: Boston, MA, USA, 2000; pp. 59–75. [Google Scholar] [CrossRef]
- Bouvet, J.P.R.; Urbaneja, A.; Pérez-Hedo, M.; Monzó, C. Contribution of predation to the biological control of a key herbivorous pest in citrus agroecosystems. J. Anim. Ecol. 2019, 88, 915–926. [Google Scholar] [CrossRef]
- Frank, D.A. Evidence for top predator control of a grazing ecosystem. Oikos 2008, 117, 1718–1724. [Google Scholar] [CrossRef]
- Wang, L.; Atlihan, R.; Chai, R.; Dong, Y.; Luo, C.; Hu, Z. Assessment of non-consumptive predation risk of Coccinella septempunctata (Coleoptera: Coccinellidae) on the population growth of Sitobion miscanthi (Hemiptera: Aphididae). Insects 2022, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.F.; Michaud, J.P.; Li, Z.; Wu, P.X.; Chu, Y.N.; Zhang, Q.W.; Liu, X.X. Chronic, predator-induced stress alters development and reproductive performance of the cotton bollworm, Helicoverpa armigera. BioControl 2015, 60, 827–837. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Francis, F.; Cheng, D.F.; Sun, J.R.; Chen, J.L. Orco mediates olfactory behaviors and winged morph differentiation induced by alarm pheromone in the grain aphid, Sitobion avenae. Insect Biochem. Mol. Biol. 2015, 64, 16–24. [Google Scholar] [CrossRef]
- Pauwels, K.; Stoks, R.; De Meester, L. Coping with predator stress: Interclonal differences in induction of heat-shock proteins in the water flea Daphnia magna. J. Evol. Biol. 2005, 18, 867–872. [Google Scholar] [CrossRef]
- Rypstra, A.L.; Buddle, C.M. Spider silk reduces insect herbivory. Biol. Lett. 2013, 9, 20120948. [Google Scholar] [CrossRef]
- Sendoya, S.F.; Freitas, A.V.L.; Oliveira, P.S. Egg-laying butterflies distinguish predaceous ants by sight. Am. Nat. 2009, 174, 134–140. [Google Scholar] [CrossRef]
- Wasserberg, G.; White, L.; Bullard, A.; King, J.; Maxwell, R. Oviposition site selection in Aedes albopictus (Diptera: Culicidae): Are the effects of predation risk and food level independent? J. Med. Entomol. 2013, 50, 1159–1164. [Google Scholar] [CrossRef]
- Lagrue, C.; Besson, A.A.; Lecerf, A. Interspecific differences in antipredator strategies determine the strength of non-consumptive predator effects on stream detritivores. Oikos 2015, 124, 1589–1596. [Google Scholar] [CrossRef]
- Wilson, M.R.; Leather, S.R. The effect of past natural enemy activity on host-plant preference of two aphid species. Entomol. Exp. Appl. 2012, 144, 216–222. [Google Scholar] [CrossRef]
- Sloggett, J.J.; Weisser, W.W. Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos 2002, 98, 323–333. [Google Scholar] [CrossRef]
- Kersch-Becker, M.F.; Thaler, J.S. Plant resistance reduces the strength of consumptive and non-consumptive effects of predators on aphids. J. Anim. Ecol. 2015, 84, 1222–1232. [Google Scholar] [CrossRef]
- Stoks, R.; McPeek, M.A. Antipredator behavior and physiology determine Lestes species turnover along the pond-permanence gradient. Ecology 2003, 84, 3327–3338. [Google Scholar] [CrossRef]
- Hawlena, D.; Schmitz, O.J. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc. Natl. Acad. Sci. USA 2010, 107, 15503–15507. [Google Scholar] [CrossRef] [PubMed]
- Venkanna, Y.; Suroshe, S.S.; Dahuja, A. Non-consumptive effects of the zigzag ladybird beetle, Cheilomenes sexmaculata on its prey, the cotton aphid, Aphis gossypii Glover. Biocontrol Sci. Technol. 2021, 31, 1204–1219. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Fan, S.; Chen, C.; Zhao, Q.; Wei, J.; Zhang, H. Identifying Potentially climatic suitability areas for Arma custos (Hemiptera: Pentatomidae) in China under climate change. Insects 2020, 11, 674. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Wang, M.; Liu, C.; Mao, J.; Chen, H.; Zhang, L. Predation of Hippodamia variegata and Harmonia axyridis to young larvae of Spodoptera frugiperda. Chin. J. Biol. Control 2019, 35, 709–714. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Xiang, M.; Wang, M.; Mao, J.; Chen, H.; Zhang, L. Predation capacity of Chrysopa pallens larvae to young larvae of Spodoptera frugiperda. Chin. J. Biol. Control 2020, 36, 513–519. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, M.; Chen, H.; Wang, Y.; Zhang, H.; Chen, F.; Zhao, X.; Zhang, L. Predatory capacity and behavior of Picromerus lewisi Scott against Spodoptera frugiperda higher instar larve. Chin. J. Biol. Control 2019, 35, 698–703. [Google Scholar] [CrossRef]
- Koch, R.L. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 2003, 3, 32. [Google Scholar] [CrossRef]
- Dutra, C.C.; Koch, R.L.; Burkness, E.C.; Meissle, M.; Romeis, J.; Hutchison, W.D.; Fernandes, M.G. Harmonia axyridis (Coleoptera: Coccinellidae) exhibits no preference between bt and non-bt maize fed Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2012, 7, e44867. [Google Scholar] [CrossRef] [PubMed]
- Di, N.; Zhang, K.; Xu, Q.; Zhang, F.; Harwood, J.D.; Wang, S.; Desneux, N. Predatory ability of Harmonia axyridis (Coleoptera: Coccinellidae) and Orius sauteri (Hemiptera: Anthocoridae) for suppression of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2021, 12, 12. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Peacor, S.D.; Hawlena, D.; Thaker, M. Non-consumptive predator effects on prey population size: A dearth of evidence. J. Anim. Ecol. 2020, 89, 1302–1316. [Google Scholar] [CrossRef]
- Santos, L.M.; Redaelli, L.R.; Diefenbach, L.M.; Efrom, C.F. Larval and pupal stage of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in sweet and field corn genotypes. Braz. J. Biol. 2003, 63, 627–633. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D. Antioxidant enzymes in Spodoptera littoralis (Boisduval): Are they enhanced to protect gut tissues during oxidative stress? J. Insect Physiol. 2006, 52, 11–20. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Vetter, J.L.; Steinberg, M.P.; Nelson, A.I. Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 1958, 6, 39–41. [Google Scholar] [CrossRef]
- Brodin, T.; Mikolajewski, D.J.; Johansson, F. Behavioural and life history effects of predator diet cues during ontogeny in damselfly larvae. Oecologia 2006, 148, 162–169. [Google Scholar] [CrossRef]
- Stoks, R. Food stress and predator-induced stress shape developmental performance in a damselfly. Oecologia 2001, 127, 222–229. [Google Scholar] [CrossRef]
- Walzer, A.; Schausberger, P. Non-consumptive effects of predatory mites on thrips and its host plant. Oikos 2009, 118, 934–940. [Google Scholar] [CrossRef]
- Mondor, E.B.; Roitberg, B.D. Pea aphid, Acyrthosiphon pisum, cornicle ontogeny as an adaptation to differential predation risk. Can. J. Zool. 2002, 80, 2131–2136. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Zhu, Z.P.; Peng, J.; Chen, X.Y.; Lu, Z.T.; Pan, H.P.; Qiu, B.L. Non-consumptive effects of Encarsia formosa on the reproduction and metabolism of the whitefly Bemisia tabaci. BioControl 2021, 66, 639–648. [Google Scholar] [CrossRef]
- Thaler, J.S.; Contreras, H.; Davidowitz, G. Effects of predation risk and plant resistance on Manduca sexta Caterpillar feeding behaviour and physiology. Ecol. Entomol. 2014, 39, 210–216. [Google Scholar] [CrossRef]
- Slos, S.; De Block, M.; Stoks, R. Autotomy reduces immune function and antioxidant defence. Biol. Lett. 2009, 5, 90–92. [Google Scholar] [CrossRef]
- Slos, S.; De Meester, L.; Stoks, R. Food level and sex shape predator-induced physiological stress: Immune defence and antioxidant defence. Oecologia 2009, 161, 461–467. [Google Scholar] [CrossRef]
- Slos, S.; Meester, L.D.; Stoks, R. Behavioural activity levels and expression of stress proteins under predation risk in two damselfly species. Ecol. Entomol. 2009, 34, 297–303. [Google Scholar] [CrossRef]
- Slos, S.; Stoks, R. Predation risk induces stress proteins and reduces antioxidant defense. Funct. Ecol. 2008, 22, 637–642. [Google Scholar] [CrossRef]
- Kaplan, I.; McArt, S.H.; Thaler, J.S. Plant defenses and predation risk differentially shape patterns of consumption, growth, and digestive efficiency in a guild of leaf- chewing insects. PLoS ONE 2014, 9, e93714. [Google Scholar] [CrossRef] [PubMed]
- Van Dievel, M.; Janssens, L.; Stoks, R. Short- and long-term behavioural, physiological and stoichiometric responses to predation risk indicate chronic stress and compensatory mechanisms. Oecologia 2016, 181, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M. Anti-predator defence and the complexity-stability relationship of food webs. Proc. Roy Soc. B-Biol. Sci. 2007, 274, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Weissburg, M.; Smee, D.L.; Ferner, M.C. The sensory ecology of nonconsumptive predator effects. Am. Nat. 2014, 184, 141–157. [Google Scholar] [CrossRef]
- Magal, C.; Dangles, O.; Caparroy, P.; Casas, J. Hair canopy of cricket sensory system tuned to predator signals. J. Theor. Biol. 2006, 241, 459–466. [Google Scholar] [CrossRef]
- Takahashi, L.K. Olfactory systems and neural circuits that modulate predator odor fear. Front. Behav. Neurosci. 2014, 8, 72. [Google Scholar] [CrossRef]
- Madhwal, S.; Shin, M.; Kapoor, A.; Goyal, M.; Joshi, M.K.; Ur Rehman, P.M.; Gor, K.; Shim, J.; Mukherjee, T. Metabolic control of cellular immune-competency by odors in Drosophila. eLife 2020, 9, e60376. [Google Scholar] [CrossRef]
- Pang, L.; Liu, Z.; Chen, J.; Dong, Z.; Zhou, S.; Zhang, Q.; Lu, Y.; Sheng, Y.; Chen, X.; Huang, J. Search performance and octopamine neuronal signaling mediate parasitoid induced changes in Drosophila oviposition behavior. Nat. Commun. 2022, 13, 4476. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Wang, Z.; Lin, T.; Feng, J.; Jiang, T. Effects of predation risks of bats on the growth, development, reproduction, and hormone levels of Spodoptera litura. Front. Ecol. Evol. 2023, 11, 1126253. [Google Scholar] [CrossRef]
- Janssens, L.; Stoks, R. Chronic predation risk reduces escape speed by increasing oxidative damage: A deadly cost of an adaptive antipredator response. PLoS ONE 2014, 9, e101273. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Thaler, J.S.; Ugine, T.A.; Inouye, B.D.; Underwood, N. Predator densities alter the influence of non-consumptive effects on the population dynamics of an agricultural pest. Ecol. Entomol. 2024, 49, 306–318. [Google Scholar] [CrossRef]
- Wen, J.; Ueno, T. Application of predator-associated cues to control small brown planthoppers: Non-consumptive effects of predators suppress the pest population. BioControl 2021, 66, 813–824. [Google Scholar] [CrossRef]
- Aflitto, N.C.; Thaler, J.S. Predator pheromone elicits a temporally dependent non-consumptive effect in prey. Ecol. Entomol. 2020, 45, 1190–1199. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Kong, W.; Ran, X.; Lv, X.; Ma, C.; Yan, H. Biological and Physiological Changes in Spodoptera frugiperda Larvae Induced by Non-Consumptive Effects of the Predator Harmonia axyridis. Agriculture 2024, 14, 1566. https://doi.org/10.3390/agriculture14091566
Fan Z, Kong W, Ran X, Lv X, Ma C, Yan H. Biological and Physiological Changes in Spodoptera frugiperda Larvae Induced by Non-Consumptive Effects of the Predator Harmonia axyridis. Agriculture. 2024; 14(9):1566. https://doi.org/10.3390/agriculture14091566
Chicago/Turabian StyleFan, Zeyun, Weizhen Kong, Xiaotong Ran, Xiaolu Lv, Chongjian Ma, and He Yan. 2024. "Biological and Physiological Changes in Spodoptera frugiperda Larvae Induced by Non-Consumptive Effects of the Predator Harmonia axyridis" Agriculture 14, no. 9: 1566. https://doi.org/10.3390/agriculture14091566






