Abstract
Nocturnal root and meristem temperature (RMT) can have a strong effect on rice growth and yield. However, underlying mechanisms are not well understood. To investigate the effects of night-time RMT on photosynthesis biomass allocation and activities of antioxidant enzymes, we designed a hydroponic system that maintained the following daily patterns of day/night temperature: 18/28 °C (HNT) or 28/18 °C (LNT). Rice plants cv. IR64 were grown in the greenhouse and subjected to either HNT or LNT. HNT stimulated growth and tillering but did not affect biomass allocation. HNT plants increased total biomass by 16 and 35%, depending on time of exposure. HNT increased rates of photosynthesis (Pn) compared to LNT plants in leaves of different ages. Overnight carbohydrate remobilisation was larger in HNT than in LNT plants, particularly at 16 days after treatment (dat), when Pn and relative growth rates were highest. Leaf soluble protein concentrations and specific leaf area were not affected by RMT, indicating higher photosynthetic nitrogen use efficiency in HNT plants. Super Oxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase activities did not respond to RMT, indicating no change in the production of reactive oxygen species in LNT plants despite lower photosynthesis rates. HNT increased sink demand by stimulating tillering, the increased sink demand upregulated the source activity through a larger leaf area per plant and a higher Pn throughout the canopy. The hydroponic system described here was able to control the temperature of the nutrient solution effectively, the installation of a second pump directly circulating the nutrient solution from and back to the reservoir through the cooling system allowed reaching the target temperature within 1 h. This system opens new opportunities to characterise plant responses to RMT alone or in combination with other environmental drivers.
1. Introduction
Global mean air temperature is predicted to increase between 1.0 and 3.7 °C above the current level by the end of this century [1], and an impact on yield of the major crop species is expected [2,3,4] as temperature affects many metabolic and physiological processes that determine crop growth and yield. Minimum temperatures are increasing at a faster rate than maximum temperatures [5,6] and are reported to have a larger effect than maximum temperatures on yields of crops such as maize, soybean, or wheat [7]. Several authors reported strong negative effects of increases in night-time temperature on yield, aboveground biomass, and yield components in rice [8,9]. However, some other reports have shown that high night temperature (HNT) can stimulate biomass production, and such a stimulating effect may depend on the development stage [7,10,11].
Most studies addressing the effects of either minimal or maximal temperatures on crop growth have focused on air temperature, and much less attention has been paid to substrate temperature. Rice is a staple food for over two thirds of the world’s population [12], and most of it is grown in flooded conditions in which the meristems are either surrounded by or immersed in ponded water from seedling establishment to the panicle initiation stage. Furthermore, root functioning and metabolism are affected by substrate temperature, which consequently will influence the water and nutrient uptake necessary to support aboveground plant growth [13]. In this respect, Shimono and Ishii (2012) [14] found that water temperature can affect seed set almost independently of air temperature. In the field, leaf area development and yield components were highly affected by soil night temperature [15], highlighting the importance of meristem and root thermal environment on rice growth and seed production. Kanno et al. (2009) found that a high night root temperature stimulated CO2 uptake as well as leaf area development and tillering, together with biomass allocation and carbohydrate concentrations in leaf blades and sheaths [11]. Thus, night temperature of the root zone in rice should be of paramount importance, particularly during the vegetative phase, firstly because during this period a large fraction of the carbon and nitrogen are fixed and assimilated for further mobilisation during grain formation; and secondly because during this period the canopy is still open and large day–night temperature fluctuations can occur. Studying root- and meristem-zone temperature (RMT) can be challenging when using solid substrates due to their heterogeneous nature. In soil, differences in texture and bulk density can make it difficult to ensure a uniform temperature distribution throughout the rhizosphere and gradients can develop, masking the actual response of the plant to RMT. On the other hand, the experimental approach to study the impact of either air or RMT, has focused on increasing or decreasing both night and day temperatures, or narrowing the difference between day and night, with the concomitant effect of changing the daily average temperature. The effects of mean day temperature on leaf emergence and development in rice depend on a specific daily average temperature being attained by means of warmer days than nights or vice versa [16].
Here, we describe a hydroponic set up as a useful and reliable tool to study the effects of RMT on rice plants during the vegetative stage. To overcome the confounding effect of changing daily mean temperature, the inversion of the daily patterns of RMT was used as an experimental approach to directly elucidate the effects of night-time RMT without altering the average temperature.
The objectives of this study were, firstly, to present a hydroponic set up able to accurately control the temperature of the nutrient solution and uniformly distribute it to a relatively large number of plants and, secondly, to determine the effects of RMT regimes on gas exchange and assimilate partitioning and relate this to leaf area production, dry matter accumulation, and activities of key enzymes associated with oxidative stress in rice.
We hypothesised that the effects of water temperature on growth and biomass production should be determined by shifted balances between carbon acquisition by photosynthesis and biomass and assimilate partitioning within the plant. We expect that the contribution of lower leaves within the canopy to the carbon balance of the plant will help explain the differences in leaf area development and biomass production under varying RMT regimes.
2. Materials and Methods
The hydroponic system used in this study is shown in Figure 1. The nutrient solution flowed from a 60 L tank to the pots/boxes containing the plants by means of a 350 W pump (Eheim GmbH, Deizisau, Germany, 10 L min−1 capacity) connected to two 4-outlet manifolds linked to each other via a T connector including a flow controlling valve. To each outlet a 1 cm i.d. Teflon tube was tightly connected and immersed into either 1 L capacity square plastic pots or plastic boxes in which PVC cylinders were inserted (see below). The flow-controlling valve was adjusted to provide a flow rate of 0.5 L min−1. Each pot/box had an overflow outlet located 1 cm below the border connected to an “L” connector and Teflon tube that drained back to the 60 L tank. A second pump (150 W) was connected to the temperature control systems (Teco, TR/TC 10, Croydon, UK). This pump took nutrient solution from the 60 L tank and passed it through the temperature control system and back to the 60 L tank. Temperature sensors connected to TinyTag data loggers (Chichester, UK) were inserted into randomly selected pots/boxes to monitor temperature in the nutrient solution and adjust the settings of the temperature controlling system. Temperatures were targeted at 28 °C (day) and 18 °C (night), this regime was denoted as low night temperature (LNTu and 18 °C (day) and 28 °C (night), denoted as high night temperature HNT. The typical day–night water temperature profile is shown in Figure 2.
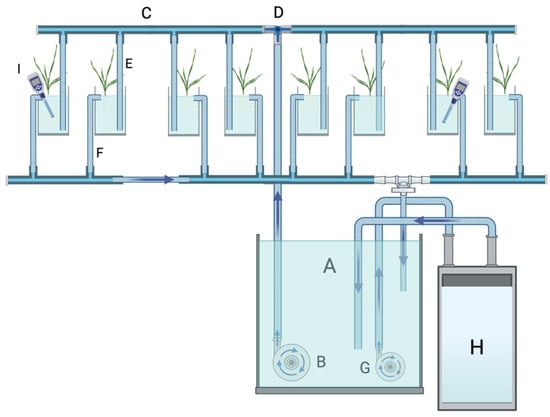
Figure 1.
Simplified diagram of the hydroponic system designed for this study. Nutrient solution contained in a 60L tank (A) is pumped (B) to a 4-outlet manifold (C) via a “T” connector (D) with a flow-controlling valve. Outlets of the manifold were plugged into Teflon flexible tubes (E) that delivered nutrient solution to 1 L pots containing plants. Each pot was provided with an overflow drainage system consisting of a flexible Teflon tube (F) inserted in a small hole drilled at 1 cm below the pot upper border that drained back to A. A second pump (G) circulated the nutrient solution from A to a temperature control system (H) and back to A. Thermometers (I) were inserted in randomly selected pots to monitor the temperature. The system depicted in the figure is the one used in experiment 2. For experiment 1, plastic boxes 36.5 cm long, 26.5 cm wide, and 11.5 cm deep were used, in which PVC cylinders 9 cm long and 3.5 cm in internal diameter were inserted.
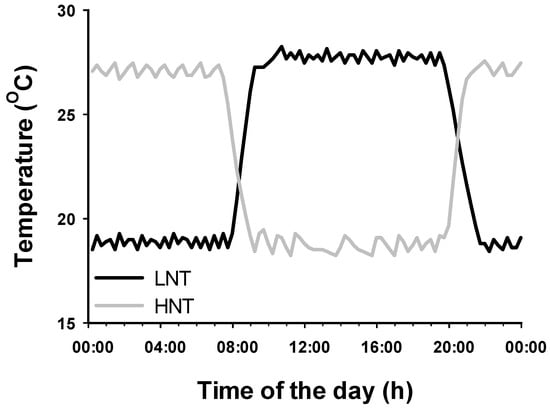
Figure 2.
Day and night temperature profile of the nutrient solution in which rice plants cv. IR64 were grown for different lengths of time. HNT: night temperature targeted at 28 °C and day temperature at 18 °C; LNT: target night temperature at 18 °C and day temperature at 28 °C.
Rice seeds cv. IR64 were germinated in plastic boxes on wet filter paper. Seven days after germination, which corresponded to developmental stage V2 [17], seedlings were transferred to the hydroponic circulating system described above with half strength Yoshida nutrient solution [18] under the LNT regime. One week later, a full-strength nutrient solution was used. The nutrient solution was replaced every week to prevent nutrient depletion due to plant uptake. Photosynthetic photon flux density was on average 1250 μmol m−2 s−1 at the top of the canopy and was provided by metal halide lamps with a photoperiod of 12 h. Air temperature and vapour pressure deficit (VPD) were maintained at 30.16/27.7 °C and 1.17/0.9 KPa day/night, respectively. All experiments were carried out in a greenhouse at the University of Hohenheim Stuttgart, Germany.
For experiment 1, plants in growth stage V5 [17] were transplanted to PVC cylinders 9.0 cm long and 3.5 cm in diameter, placed in plastic boxes 36.5 cm long, 26.5 cm wide, and 11.5 cm deep, and subjected to either LNT or HNT regimes for 7 days, after which plants were measured and sampled. For experiment 2, plants at the same growth stage as experiment 1 were grown in 1 L pots, were subjected to either HNT or LNT for 30 days, and samples/measurements were taken at 16 and 28 days of treatment.
Plants from the LNT and HNT treatments were harvested, separated into organs, and dried in an oven at 60 °C until a constant weight was achieved. The area of leaf blades was measured with a leaf area meter LiCor, Li3000C (Bad Homburg, Germany). For biochemical analysis, leaf sections were taken 6 h into the photoperiod and at the end of the dark period, immediately frozen in liquid N2, and stored at −80 °C until analysis. In both experiments the three uppermost leaves were selected and numbered as leaf 1 (L1), leaf 2 (L2), and leaf 3 (L3), with L1 being the oldest and L3 being the youngest leaf (experiment 1). In experiment 2, sampled leaves were L3, L4, and L5. Growth parameters were calculated as in [19].
Light-saturated rates of CO2 assimilation were measured on plants from experiment 2 at 16 and 28 days after transplant (dat) with an IRGA Walz GFS 3000, and measurements were taken between 0800 and 1100 h. Conditions in the leaf cuvette were as follows: CO2 molar fraction was 400 μmol mol−1, temperature was 30 °C, relative humidity was 65%, and photosynthetic photon flux density was 2000 μmol m−2 s−1 and was provided by LEDs attached to the leaf cuvette. L3, L4, and L5 were used for measurements. After a steady rate of photosynthesis was attained, readings were recorded, the lamp was switched off, and the rate of respiration (Rd) was measured once a stable reading was attained, typically within 3–5 min.
Leaf sections of known areas were immersed in 0.5 mL of 70% (v/v) ethanol at 80 °C for 30 min. to extract soluble carbohydrates. Soluble carbohydrates were determined by the production of NADPH from the conversion of sucrose and fructose to glucose-6-P and 2,6-phosphogluconate as described by [20]. Reactions were carried out using a microplate reader (Tecan, Infinite M2000Pro, Männedorf, Switzerland).
All enzymes were extracted with a homogenizing system FastPrep 24 (MP biochemicals, Eching, Germany) using ceramic balls. A ratio of 100–200 mg FW or 1 cm2 of leaf blade to 1 mL of extraction buffer was typically used, and the extraction was performed during 60 s. Extracts were centrifuged at 3 °C and 10,000 g for 5 min., and the supernatant was decanted and then used for enzyme assays. All enzymatic assays were carried out in a microplate reader (Tecan, Infinite M200Pro. Männedorf, Switzerland).
Super Oxide Dismutase (SOD) was extracted with 10 mM sodium phosphate buffer pH 7.8 containing 0.1 mM EDTA, and its activity was determined by the method described by [21] by the inhibition of the photoreduction of NBT. The method was adapted for small volumes [22] and the absorbance at 560 nm was recorded.
Ascorbate Peroxidase (APX) and Glutathione Reductase (GR) were extracted with 50 mM potassium phosphate buffer, pH 7.0 containing 1% (w/v) PVP, 0.2 mM EDTA, and 5 mM Ascorbate. Determination of APX activity was conducted according to [23], by tracking the reduction in Ascorbate at 290 nm, and activity of GR was determined as in [24] following the oxidation of NADPH at 340 nm.
Aliquots of the leaf extracts used for antioxidant enzyme determination were taken for soluble protein analysis using the Bradford reagent [25].
Data were analysed using a t-test for independent samples and by a two- or one-way ANOVA with RMT regime, time of exposure to treatments, or leaf age as main effects, using R (v.4.2.0). For remobilised carbon, a randomisation of the quotient (CH2OPM-CH2OAM)/(CH2OPM) was performed, the median of the randomized quotient was calculated, and the Mann-Witney U-test was used.
3. Results
3.1. Biomass, Growth Parameters, and Photosynthesis
Plants grown at HNT showed a larger total and organ biomass than LNT plants (Figure 3). But differences were statistically significant after 16 days of exposure to HNT (p < 0.05). The exception was root biomass, which was not affected by HNT at 16 dat, resulting in a transient increase in the root/shoot ratio of LNT plants (Table 1). The increased biomass observed in HNT plants was associated with a larger number of tillers and a larger leaf area (Table 1), which were both significantly increased by HNT. Similar to the total and organ biomass, the number of tillers and the leaf area per plant were not statistically affected by the root–meristem night temperature 7 dat. The relative growth rate (RGR) was higher in HNT than in LNT plants at 16 and 28 days of treatment, whereas the leaf area ratio (LAR) was higher in the HNT only at 16 dat (Table 2). Specific leaf area (SLA) was not affected by the root and meristem temperature regime.
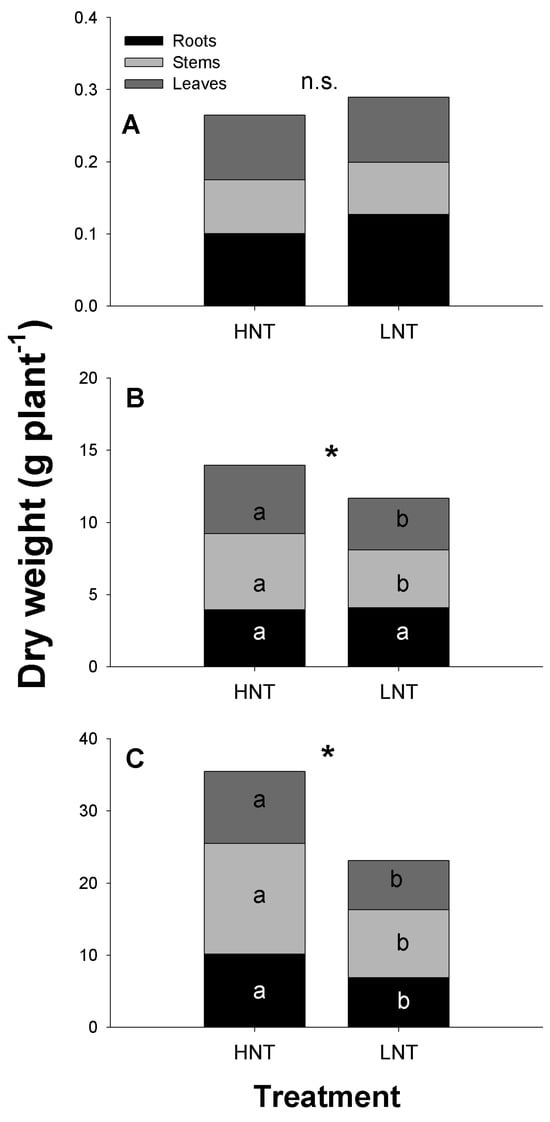
Figure 3.
Total and organ dry weight of rice plants cv. IR64 subjected to high (HNT) or low (LNT) night root and meristem temperature for different lengths of time. (A): experiment 1, 7 days after treatment (dat); (B): experiment 2, 16 dat; and (C): 28 dat. Data were analysed by a two-way ANOVA with time after treatment, and root and meristem night-time temperature as main factors. When factors were statistically significant, the post-hoc Tukey test was performed. * Indicates significant differences in total and organ plant biomass due to root and meristem night-time temperature at p < 0.05, and n.s. indicates not significant at p < 0.05. Plant organs of different treatments labelled with different letters are statistically significant at p < 0.05.

Table 1.
Leaf area per plant, tiller number, and root to shoot ratio of rice plants cv. IR64 grown at different night root and meristem temperatures (RMTs) and sampled on different days after treatment (DAT). HNT: high night RMT; LNT: low night RMT. A two-way ANOVA was used with RMT and time of exposure to treatment (Time) as main factors.

Table 2.
Relative growth rate (RGR), leaf area ratio (LAR), and specific leaf area (SLA) of rice plants cv. IR64 grown at different night root and meristem temperatures (RMTs) and sampled on different days after treatment (DAT). HNT: high night RMT; LNT: low night RMT. A two-way ANOVA was used with RMT and time of exposure to treatment (Time) as main factors.
The light-saturated rate of photosynthesis was stimulated by HNT, particularly in the youngest fully expanded leaf (L5 at 16 dat, and L4 and L5 at 28 dat) (Figure 4). When leaves of different ages within the same treatment were compared, no differences were detected in HNT plants, whereas in LNT plants, L3 (oldest leaf) was significantly lower than L4 and L5 at both sampling dates (Figure 4A,B; p < 0.05). Rd was not significantly affected by the root–meristem temperature regime, although HNT plants tended to have lower Rd than LNT plants (Figure 4C).
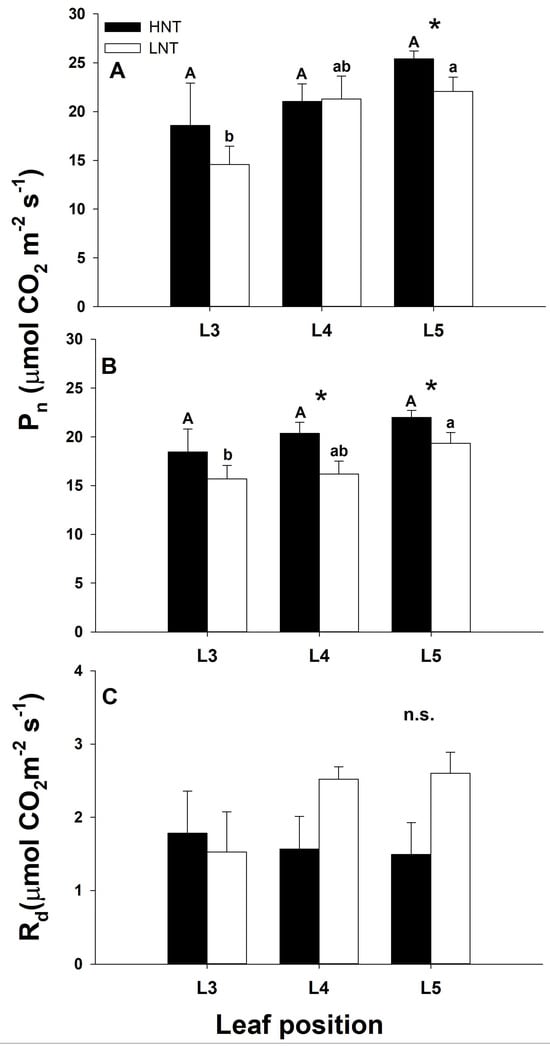
Figure 4.
Rates of photosynthesis measured at a photosynthetic photon flux density of 2000 μmol m−2 s−1, and 400 μmol mol−1 CO2 in leaves of different ages from rice plants cv. IR64 subjected to high (HNT) or low (LNT) root and meristem night-time temperature (RMT) during the night for different lengths of time in experiment 2. (A): 16 days after treatment (dat); (B): 28 dat; (C): day respiration rates in leaves of different ages subjected to the treatments for 28 dat. Data were analysed using a two-way ANOVA with leaf age and RMT as main factors. When factors were statistically significant, the post-hoc Tukey test was performed. * Indicates significant differences at p < 0.05 between leaves of the same age from different RMT. Leaves of different ages within the same treatment and the same letter are not statistically significant at p < 0.05. n.s. indicates not statistically significant at p < 0.05. Uppercase letters: HNT; lowercase letters: LNT.
3.2. Carbohydrate Concentration and Soluble Proteins
Minimum soluble carbohydrate concentrations (taken at the end of the night period) were not affected by the RMT regime nor by leaf age (Figure 5A,D,G); only L3 of plants grown under the LNT 28 dat (Figure 5G) had higher soluble carbohydrate concentrations than the corresponding leaf from HNT plants. In the middle of the photoperiod, HNT increased soluble carbohydrates at 7 dat in L1 and at 16 dat in L4 (Figure 5B,E). In plants exposed to LNT for 7 days, there was a clear gradient of carbohydrate concentration, with older leaves showing less soluble carbohydrates than younger leaves (Figure 5B), whereas in HNT plants, all leaves had similar carbohydrate concentrations. In successive sampling dates, little differences were detected due to either night RMT or to leaf age. Calculating the fraction of accumulated carbohydrates mobilised during the night (Figure 5C,F,I) showed that leaves from the HNT treatment exported a larger fraction of accumulated carbon (58%, 7 dat; 31%, 16 dat; and 39%, 28 dat) compared to 54%, 15%, and 32% in LNT plants, 7, 16, and 28 dat, respectively. The oldest leaf of HNT plants (L1, 7 dat or L3, 16 and 28 dat) consistently exported a larger fraction of accumulated carbon than the corresponding leaf of LNT plants.
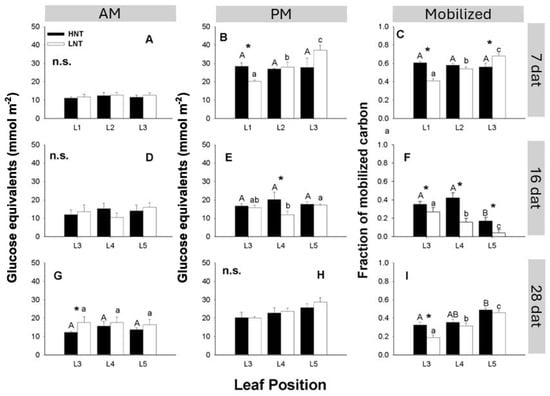
Figure 5.
Soluble non-structural carbohydrates as glucose equivalents in leaves of different ages from rice plants cv. IR64 subjected to high (HNT) or low (LNT) root and meristem night-time temperature (RMT) for different lengths of time. Upper panel: experiment 1, 7 dat; middle panel: experiment 2, 16 dat; lower panel: experiment 2, 28 dat. (A,D,G), samples taken at 0800 h (beginning of the photoperiod). (B,E,H), samples taken at 1400 h (six hours into the photoperiod). (C,F,I) carbohydrates difference between PM and AM as a fraction of maximum accumulated carbohydrates. Data were analysed using a two-way ANOVA with leaf age and RMT as main factors. When factors were statistically significant, the post-hoc Tukey test was performed. * Indicates significant differences at p < 0.05, between leaves of the same age from different RMT treatments. n.s. indicates not statistically significant at p < 0.05. Leaves of different age within the same treatment and the same letter are not statistically significant at p < 0.05. Uppercase letters: HNT; lowercase letters: LNT.
Night RMT had no effect on leaf protein concentrations regardless of the length of exposure (Figure 6), whereas leaf age affected leaf protein concentrations (Figure 6B,C) with younger leaves (L5 and L4) having a higher protein concentration than older leaves. In young plants (exposed for 7 days to treatment), no gradient due to leaf age was observed.
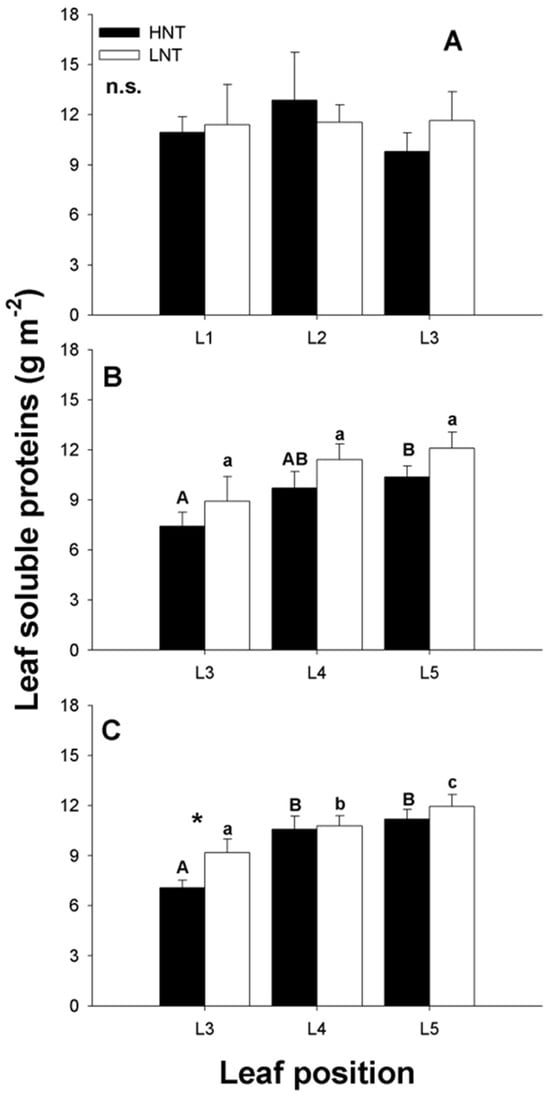
Figure 6.
Soluble protein concentration in leaves of different ages from rice plants cv. IR64 subjected to high (HNT) or low (LNT) root and meristem temperature (RMT) during the night for different lengths of time. (A): Experiment 1, 7 days after transplant (dat); (B): experiment 2, 16 dat; and (C): experiment 2, 28 dat. Data were analysed using a two-way ANOVA with leaf age and RMT as main factors. When factors were statistically significant, the post-hoc Tukey test was performed. * Indicates significant differences at p < 0.05, between leaves of the same age from different RMT treatments. n.s. indicates not statistically significant at p < 0.05. Leaves of different age within the same treatment and the same letter are not statistically significant at p < 0.05. Uppercase letters: HNT; lowercase letters: LNT.
3.3. Antioxidative Stress Proteins
To investigate if lower rates of photosynthesis in the LNT plants could be associated with the generation of oxidative stress, we measured the activities of APX, GR, and SOD (Figure 7). In general SOD activity was higher in older plants (16 dat) than in plants exposed to the treatments for 7 days (Figure 7A,D). In plants exposed to HNT for 7 days, no significant effect was detected, whereas in plants exposed for 16 days the increase in SOD caused by high night RMT was detected in L4 and L5. APX was not affected by RMT, and activities showed similar values irrespective of plant age (Figure 7B,E), but in plants exposed to treatments for 7 days, APX activity was lower in younger than in older leaves. A similar trend was observed for GR activity (Figure 7C,F). Night RMT had no effect on GR, but in older plants the activity was lower, as in plants exposed for 7 days to treatments.
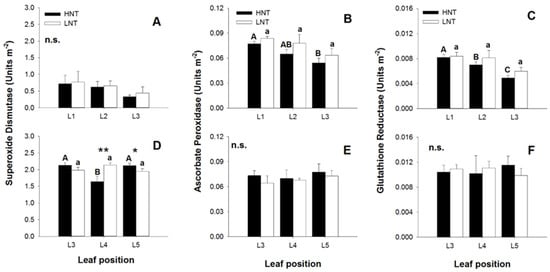
Figure 7.
Activities of Superoxide Dismutase (A,D), Ascorbate Peroxidase (B,E) and Glutathione Reductase (C,F) determined in leaves of different ages from rice plants cv. IR64 subjected to high (HNT) or low (LNT) root and meristem temperature (RMT) during the night for different lengths of time. One unit is defined as 1 μmol min−1. Upper panel: experiment 1, 7 days after treatment (dat); lower panel: experiment 2, 16 dat. Data were analysed using a two-way ANOVA with leaf age and RMT as main factors. When factors were statistically significant, the post-hoc Tukey test was performed. ** Indicates significant differences at p < 0.01, between leaves of the same age from different RMT treatments. * Indicates significant differences at p < 0.05, between leaves of the same age from different RMT treatments. n.s. indicates not statistically significant at p < 0.05. Leaves of different ages within the same treatment and the same letter are not statistically significant at p < 0.05. Uppercase letters: HNT; lowercase letters: LNT.
4. Discussion
Here, we presented an effective hydroponic system that allows the control of nutrient solution temperature. The installation of a second pump (G in Figure 1) that directly circulated the nutrient solution from and back to the reservoir through the cooling system allowed a fast and accurate control of nutrient solution temperature. Within 1 h the temperature reached the targeted value and remained stable with a variation coefficient of 0.25% at 28.0 °C and 0.20% at 18.0 °C.
Several studies trying to elucidate the effects of night RMT have set different combinations of day and night temperatures with a resultant change in daily mean temperature. Our experimental approach of inverting the daily temperature regime overcomes this problem, as in both HNT and LNT treatments, daily average temperature remained the same at 23 °C. This is not trivial, as it is well known that daily average RMT has an important effect on leaf appearance and leaf area development in rice [15,16]. Thus, with this setup, the actual impact of night-time RMT can be addressed and clearly differentiated from that of daily average temperature.
High night RMT stimulated growth during the vegetative stage, and stimulation was dependent on duration of treatment exposure. Plants exposed for 16 days to HNT increased biomass by 16% and by 35% when exposed for 28 days compared to LNT plants. The time-dependent response of rice growth to RMT has been reported elsewhere [10,11,26]. Kanno et al. [11] found an 18% and 23% stimulation of biomass production in rice plants exposed to high night-time root temperature for 42 and 63 days, respectively. The higher biomass under high night RMT reported here was the result of a faster RGR, mainly within the period of 7–16 dat and to a lesser extent within the period of 16–28 dat. This was possibly due to a nearly doubling of leaf area in HNT compared to LNT plants not only in absolute terms but also as a fraction of total plant biomass (LAR), which provided a larger photosynthetic surface that increased the potential for carbon gain. Total leaf area per plant is the result of the number of leaves per tiller, the number of tillers per plant, and the area of individual leaves. Kanno et al. [11] reported that high root temperature increased plant leaf area by stimulating the expansion rate of individual leaves and tiller number and this was achieved by the production of thinner leaves as evidenced by a higher SLA. However, we did not observe changes in SLA as influenced by root and meristem night-time temperature, which could be associated with thinner and/or larger individual leaves. In our experimental system, both roots and meristems were exposed to the substrate temperature regime. A higher night-time temperature at the meristem level could have increased metabolism with the concomitant increase in cell growth, thus stimulating the production of tillers. Similar results were reported by Yin and Kropff [16] and by Shimono et al., [27], who found that root and meristem temperature can influence tillering with little effect on the number of leaves on the main stem. Moreover, a close correlation between relative leaf growth rate and tillering rate has been reported [28,29].
HNT increased photosynthetic rates in the youngest fully expanded leaf but also in the two leaves below, and their photosynthetic activity remained for longer compared to LNT plants, which together with the larger leaf area found in HNT plants would have provided a larger CO2 assimilation capacity on a per plant basis. Nevertheless, the question remains on how high night RMT stimulated photosynthesis? Rates of CO2 fixation must be coordinated with those of assimilate metabolism and export in order to maintain a balance between assimilate production and efficient utilization by sinks [30,31]. In our study, stimulation of tillering by HNT could have increased sink demand and induced a faster translocation of soluble carbohydrates to growing points, as observed in HNT leaves that exported on average 10% more carbohydrates than LNT leaves, and this could have prevented any feedback inhibition of photosynthesis through carbohydrate build up, maintaining high photosynthetic rates for longer. Lafitte and Travis, [30] found that in rice, varieties with a large sink demand showed higher photosynthesis and had lower carbohydrate accumulation in source leaves. This response would allow the re-establishment of a balance between source activity and sink demand as has been reported elsewhere [11,31,32]. Although respiratory metabolism is an important process accounting for carbohydrate depletion overnight, in our study respiration was little affected by night RMT as Rd was not significantly different between HNT and LNT plants, so any differences in over-night sugar depletion due to root and meristem night temperature cannot be accounted for by respiration.
An increased sink demand has also been reported to delay the loss of photosynthetic activity related to senescence as reported in other systems in which the source:sink ratio was decreased by partial defoliation or by imposing new carbon sinks [33,34]. It is likely that the increased sink demand observed in HNT plants could have a similar effect of keeping older leaves more active as more carbon is required to sustain the stimulated growth.
Accelerated development, and thus early senescence, has also been associated with high water temperature in rice [35]. However, in our experiments, leaf proteins (as a proxy for leaf senescence) were not affected by root–meristem night temperature and rates of photosynthesis were higher in HNT plants. These results rule out the accelerated senescence hypothesis and rather suggest an increased photosynthetic N use efficiency caused by HNT, particularly in older leaves as has been reported for rice varieties with a high sink demand [31].
The occurrence of oxidative stress is frequent under high light intensities as those used in this study [36,37], but its occurrence can be minimized in conditions that promote photosynthesis as a larger fraction of excitation energy is efficiently funneled through linear electron transport. Thus, we hypothesized that HNT-grown plants used excitation energy and reductant power more efficiently than LNT plants, decreasing the possibilities of oxidative stress. This altered balance should be reflected in activities of key antioxidant enzymes such as SOD, APX, and GR. However, we only detected significant differences in activities of SOD caused by the RMT regime, which suggests an enhanced production of superoxide radical under LNT. Superoxide is one of the major ROS causing photo-oxidative-induced cell death [38]. Thus, it is not surprising that SOD, the enzyme metabolizing superoxide to H2O2, was the enzyme that responded to RMT. Increased SOD activity will increase H2O2 production with an expected stimulation of APX activity to convert H2O2 to H2O. However, this expected increase in APX activity was not observed. A possible explanation to this is the dual role of H2O2 as a ROS and as a signaling molecule of the cell redox state, activating dissipation processes and safely quenching any excess of excitation energy due to lower photosynthetic rates compared to HNT leaves.
In summary, high night RMT stimulated growth and biomass production by inducing tillering, which represented a larger sink for carbohydrates, that accounted for the larger over-night depletion of the carbohydrates accumulated during the light hours. To match the increased demand for photo-assimilates, the source activity was upregulated by means of a larger leaf area and higher photosynthetic rate maintained for longer in older leaves; it is likely that upregulation of source activity was signalled by a faster carbohydrate translocation overnight. Despite being grown under high light intensity, no signs of oxidative stress were detected based on the activities of the main antioxidant enzymes. Finally, the potential of hydroponics as an experimental tool to study crop physiological processes is wide. Combining RMT with other environmental factors can provide useful insights in the response of traits with potential for breeding climate change resilient crops. Vu et al. [38] for example, implemented a similar system to that described here to study N uptake under different evaporative demands [39].
Author Contributions
Conceptualization: A.J.P., S.S. and F.A.; methodology: A.J.P., S.S. and J.A.; writing—original draft preparation: A.J.P.; writing—review and editing: A.J.P. and F.A.; funding acquisition: F.A. and A.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Baden-Württemberg Stiftung; Ministerium für Wissenschaft, Forschung und Kunst Baden-Württemberg, DAAD grant No. 57378441, and DFG project AS 246/8-1.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- IPCC. Working group I contribution to the fifth assessment report of the intergovernmental panel on climate change 2013: The physical science basis. In Summary for Policy Makers; IPCC: Geneva, Switzerland, 2014; pp. 1–1552. [Google Scholar]
- Brown, R.A.; Rosenberg, N.J. Sensitivity of crop yield and water use to change in a range of climatic factors and CO2 concentrations: A simulation study applying EPIC to the central USA. Agric. For. Meteorol. 1997, 83, 171. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Craufurd, P.Q.; Ellis, R.H.; Porter, J.R.; Vara Prasad, P.V. Temperature variability and the yield of annual crops. Agric. Ecosyst. Environ. 2000, 82, 159. [Google Scholar] [CrossRef]
- Easterling, W.; Apps, M. Assessing the consequences of climate change for food and forest resources: A view from the IPCC. Clim. Chang. 2005, 70, 165. [Google Scholar] [CrossRef]
- Alward, R.D. Grassland Vegetation Changes and Nocturnal Global Warming. Science 1999, 283, 229. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Tubiello, F.N. Effects of changes in minimum and maximum temperature on wheat yields in the central US A simulation study. Agric. For. Meteorol. 1996, 80, 215. [Google Scholar] [CrossRef]
- Peters, D.B.; Pendleton, J.W.; Hageman, R.H.; Brown, C.M. Effect of night air temperature on grain yield of corn, wheat, and soybeans. Agron. J. 1971, 63, 809. [Google Scholar] [CrossRef]
- Ziska, L.H.; Manalo, P.A. Increasing Night Temperature Can Reduce Seed Set and Potential Yield of Tropical Rice. Aust. J. Plant Physiol. 1996, 23, 791–794. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Kush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef]
- Yoshida, S. Effects of temperature on growth of the rice plant (Oryza sativa L.) in a controlled environment. Soil Sci. Plant Nutr. 1973, 19, 299. [Google Scholar] [CrossRef]
- Kanno, K.; Mae, T.; Makino, A. High night temperature stimulates photosynthesis, biomass production and growth during the vegetative stage of rice plants. Soil Sci. Plant Nutr. 2009, 55, 124. [Google Scholar] [CrossRef]
- Fairhurst, A.; Dobermann, D.A. Rice in the Global Food Supply. Better Crop. Int. 2002, 16, 1. [Google Scholar]
- Murai-Hatano, M.; Kuwagata, T.; Sakurai, J.; Nonami, H.; Ahamed, A.; Nagasuga, K.; Matsunami, T.; Fukushi, K.; Maeshima, M.; Okada, M. Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant Cell Physiol. 2008, 49, 1294. [Google Scholar] [CrossRef] [PubMed]
- Shimono, H.; Ishii, A. Poor grain growth in rice under high temperatures affected by water temperature during vegetative stage. J. Agric. Meteorol. 2012, 68, 205. [Google Scholar] [CrossRef][Green Version]
- Stuerz, S.; Sow, A.; Muller, B.; Manneh, B.; Asch, F. Leaf area development in response to meristem temperature and irrigation system in lowland rice. Field Crop. Res. 2014, 163, 74. [Google Scholar] [CrossRef]
- Yin, X.; Kropff, M.J. The Effect of Temperature on Leaf Appearance in Rice. Ann. Bot. 1996, 77, 215. [Google Scholar] [CrossRef][Green Version]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objective and adaptive system for expressing rice development. Crop Sci. 2000, 40, 436. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; IRRI: Los Baños, Philippines, 1976; pp. 1–83. [Google Scholar]
- Hunt, R. Growth Analysis, Individual plants. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Sidney, Australia, 2017; Volume 2, pp. 421–429. [Google Scholar] [CrossRef]
- Sttit, M.; Lilley, R.M.; Gerhardt, R.; Heldt, H.W. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989, 174, 518. [Google Scholar] [CrossRef]
- Gianopolitis, C.N.; Ries, S.K. Superoxide dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Hartmann, J.; Asch, F. Extraction, Storage Duration, and Storage Temperature Affect the Activity of Ascorbate Peroxidase, Glutathione Reductase, and Superoxide Dismutase in Rice Tissue. Biology 2019, 8, 70. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Kuwagata, T.; Ishikawa-Sakurai, J.; Hayashi, H.; Nagasuga, K.; Fukushi, K.; Ahamed, A.; Takasugi, K.; Katsuhara, M.; Murai-Hatano, M. Influence of low air humidity and low root temperature on water uptake, growth and aquaporin expression in rice plants. Plant Cell Physiol. 2012, 53, 1418. [Google Scholar] [CrossRef]
- Shimono, H.; Okada, M.; Kanda, E.; Arakawa, I. Low temperature-induced sterility in rice: Evidence for the effects of temperature before panicle initiation. Field Crop. Res. 2007, 101, 221. [Google Scholar] [CrossRef]
- Shimono, H.; Hasegawa, T.; Iwama, K. Response of growth and grain yield in paddy rice to cool water at different growth stages. Field Crop. Res. 2002, 73, 67. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Travis, R.L. Photosynthesis assimilate partitioning in closely related lines of rice exhibiting different sink:source relationships. Crop Sci. 1984, 24, 447. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source-sink perspective on growth rate. J. Exp. Bot. 2015, 67, 31. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate Export from the Leaf: A Highly Regulated Process and Target to Enhance Photosynthesis and Productivity. Plant Physiol. 2011, 155, 64. [Google Scholar] [CrossRef]
- Hodgkinson, K.C. Influence of Partial Defoliation on Photosynthesis, Photorespiration and Transpiration by Lucerne Leaves of Different Ages. Funct. Plant Biol. 1974, 1, 561. [Google Scholar] [CrossRef]
- Kaschuk, G.; Hungria, M.; Leffelaar, P.A.; Giller, K.E.; Kuyper, T.W. Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol. 2009, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Kuroda, E.; Shimono, H. Effect of high water temperature during vegetative growth on rice growth and yield under a cool climate. Field Crop. Res. 2011, 121, 88. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxygen processing in photosynthesis: Regulation and signaling. New Phytol. 2000, 146, 359. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Mueller, M.J. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008, 148, 960. [Google Scholar] [CrossRef]
- Vu, H.D.; Stuerz, S.; Asch, F. Leaf gas exchange of lowland rice in response to nitrogen source and vapor pressure deficit. J. Plant Nutr. Soil Sci. 2021, 184, 448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).