Evaluating the Efficacy of Selected Plant Growth-Promoting Microorganisms in Optimizing Plant Growth and Soil Health in Diverse Soil Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Used Microorganisms
2.2. Experimental Design
2.2.1. Experiment for the PGP Strain Test
2.2.2. Pot Experiment with Mixed Microbial Inoculations and Alginite-Amended Soils
2.3. Determination of the Soils’ Physico-Chemical Parameters
2.4. Examination of the Soils’ Biological Parameters
2.4.1. Examination of Microbial Abundance in the Soils
2.4.2. Methodology for Determining Soil Enzyme Activities
2.5. Measurement of Organic Matter Decomposition
2.6. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, A.N.; Meena, B.R.; Pandey, K.K.; Singh, J. Microbial Bioagents in Agriculture: Current Status and Prospects. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 331–368. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Dudas, A.; Kotroczó, Z.; Videki, E.; Wass-Matics, H.; Kocsis, T.; Szalai, Z.; Végvári, G.; Biró, B. Fruit Quality of Tomato Affected by Single and Combined Bioeffectors in Organically System. Pak. J. Agric. Sci. 2017, 54, 847–856. [Google Scholar] [CrossRef]
- Dudás, A.; Szalai, Z.; Vidéki, E.; Wass-Matics, H.; Kocsis, T.; Végvári, G.; Kotroczó, Z.; Biró, B. Sporeforming Bacillus Bioeffectors for Healthier Fruit Quality of Tomato in Pots and Field. Appl. Ecol. Environ. Res. 2017, 15, 1399–1418. [Google Scholar] [CrossRef]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Béni, Á.; Lajtha, K.; Kozma, J.; Fekete, I. Application of a Stir Bar Sorptive Extraction Sample Preparation Method with HPLC for Soil Fungal Biomass Determination in Soils from a Detrital Manipulation Study. J. Microbiol. Methods 2017, 136, 1–5. [Google Scholar] [CrossRef]
- Fekete, I.; Lajtha, K.; Kotroczó, Z.; Várbíró, G.; Varga, C.; Tóth, J.A.; Demeter, I.; Veperdi, G.; Berki, I. Long-Term Effects of Climate Change on Carbon Storage and Tree Species Composition in a Dry Deciduous Forest. Glob. Chang. Biol. 2017, 23, 3154–3168. [Google Scholar] [CrossRef]
- Liddle, K.; McGonigle, T.; Koiter, A. Microbe Biomass in Relation to Organic Carbon and Clay in Soil. Soil Syst. 2020, 4, 41. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial Indole-3-Acetic Acid: A Key Regulator for Plant Growth, Plant-Microbe Interactions, and Agricultural Adaptive Resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef]
- Lopes, M.J.d.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Khan, W.; Zhu, Y.; Khan, A.; Zhao, L.; Yang, Y.-M.; Wang, N.; Hao, M.; Ma, Y.; Nepal, J.; Ullah, F.; et al. Above-and below-Ground Feedback Loop of Maize Is Jointly Enhanced by Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungi in Drier Soil. Sci. Total Environ. 2024, 917, 170417. [Google Scholar] [CrossRef]
- Nagrale, D.T.; Chaurasia, A.; Kumar, S.; Gawande, S.P.; Hiremani, N.S.; Shankar, R.; Gokte-Narkhedkar, N.; Renu; Prasad, Y.G. PGPR: The Treasure of Multifarious Beneficial Microorganisms for Nutrient Mobilization, Pest Biocontrol and Plant Growth Promotion in Field Crops. World J. Microbiol. Biotechnol. 2023, 39, 100. [Google Scholar] [CrossRef]
- David, B.V.; Chandrasehar, G.; Selvam, P.N. Pseudomonas fluorescens: A Plant-Growth-Promoting Rhizobacterium (PGPR) With Potential Role in Biocontrol of Pests of Crops. In Crop Improvement Through Microbial Biotechnology; Prasad, R., Gill, S.S., Tuteja, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–243. [Google Scholar] [CrossRef]
- Orhan, E.; Esitken, A.; Ercisli, S.; Turan, M.; Sahin, F. Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Yield, Growth and Nutrient Contents in Organically Growing Raspberry. Sci. Hortic. 2006, 111, 38–43. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Do Inoculated Microbial Consortia Perform Better than Single Strains in Living Soil? A Meta-Analysis. Appl. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Gomes, E.A.; de Sousa, S.M.; de Paula Lana, U.G.; Coelho, A.M.; Marriel, I.E.; de Oliveira-Paiva, C.A. Co-Inoculation with Tropical Strains of Azospirillum and Bacillus Is More Efficient than Single Inoculation for Improving Plant Growth and Nutrient Uptake in Maize. Arch. Microbiol. 2022, 204, 143. [Google Scholar] [CrossRef] [PubMed]
- Shoebitz, M.; Ribaudo, C.M.; Pardo, M.A.; Cantore, M.L.; Ciampi, L.; Curá, J.A. Plant Growth Promoting Properties of a Strain of Enterobacter ludwigii Isolated from Lolium perenne Rhizosphere. Soil Biol. Biochem. 2009, 41, 1768–1774. [Google Scholar] [CrossRef]
- Dolkar, D.; Dolkar, P.; Angmo, S.; Chaurasia, O.P.; Stobdan, T. Stress Tolerance and Plant Growth Promotion Potential of Enterobacter ludwigii PS1 Isolated from Seabuckthorn Rhizosphere. Biocatal. Agric. Biotechnol. 2018, 14, 438–443. [Google Scholar] [CrossRef]
- Singh, R.; Mishra, S.; Jha, P.; Raghuvanshi, S.; Jha, P. Effect of Inoculation of Zinc-Resistant Bacterium Enterobacter ludwigii CDP-14 on Growth, Biochemical Parameters and Zinc Uptake in Wheat (Triticum aestivum L.) Plant. Ecol. Eng. 2018, 116, 163–173. [Google Scholar] [CrossRef]
- Do, T.T.T.; Dang, T.N.T.; Minh, T.H. Characterization of Phosphate and Potassium Solubilization, and Antifungal Activity of Bacteria Isolated from Rhizosphere of Allium Ascalonicum (L.) Grown in Ninh Hai District, Ninh Thuan Province, Vietnam. World J. Adv. Res. Rev. 2023, 17, 018–027. [Google Scholar] [CrossRef]
- Xiao-ying, G.; Chun-e, H.; Tao, L.; Zhu, O. Effect of Bacillus subtilis and Pseudomonas fluorescens on Growth of Greenhouse Tomato and Rhizosphere Microbial Community. J. Northeast. Agric. Univ. 2015, 22, 32–42. [Google Scholar] [CrossRef]
- Ahmad, M.; Adil, Z.; Hussain, A.; Mumtaz, M.; Nafees, M.; Ahmad, I.; Jamil, M. Potential of Phosphate Solubilizing Bacillus Strains for Improving Growth and Nutrient Uptake in Mungbean and Maize Crops. Pak. J. Agric. Sci. 2019, 56, 283–289. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, S.-C.; Cho, Y.-Y.; Oh, H.-J.; Ko, Y.H. Isolation of Cellulolytic Bacillus subtilis Strains from Agricultural Environments. ISRN Microbiol. 2012, 2012, 650563. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, E.; Ramirez-Bahena, M.H.; Peix, A.; Tejedor, C.; Mulas, R.; González-Andrés, F.; Martínez-Molina, E.; Velázquez, E. Analysis of Cultivable Endophytic Bacteria in Roots of Maize in a Soil from León Province in Mainland Spain. In Biological Nitrogen Fixation and Beneficial Plant-Microbe Interaction; González-Andrés, F., James, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 45–53. [Google Scholar] [CrossRef]
- Brady, C.; Cleenwerck, I.; Venter, S.; Coutinho, T.; De Vos, P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. orachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst. Appl. Microbiol. 2013, 36, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhou, Q.; Lin, L.; Hu, C.; Shen, P.; Yang, L.; An, Q.; Xie, G.; Li, Y. Enterobacter sacchari sp. nov., a Nitrogen-Fixing Bacterium Associated with Sugar Cane (Saccharum officinarum L.). Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 7, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Z.; Hu, C.; Zhang, X.; Chang, S.; Yang, L.; Li, Y.; An, Q. Plant Growth-Promoting Nitrogen-Fixing Enterobacteria Are in Association with Sugarcane Plants Growing in Guangxi, China. Microbes Environ. 2012, 27, 391–398. [Google Scholar] [CrossRef]
- Sherpa, M.T.; Sharma, L.; Bag, N.; Das, S. Isolation, Characterization, and Evaluation of Native Rhizobacterial Consortia Developed From the Rhizosphere of Rice Grown in Organic State Sikkim, India, and Their Effect on Plant Growth. Front. Microbiol. 2021, 12, 713660. [Google Scholar] [CrossRef]
- Pau-Roblot, C.; Lequart-Pillon, M.; Apanga, L.; Pilard, S.; Courtois, J.; Pawlicki-Jullian, N. Structural Features and Bioremediation Activity of an Exopolysaccharide Produced by a Strain of Enterobacter Ludwigii Isolated in the Chernobyl Exclusion Zone. Carbohydr. Polym. 2013, 93, 154–162. [Google Scholar] [CrossRef]
- Gao, H.; Lu, C.; Wang, H.; Wang, L.; Yang, Y.; Jiang, T.; Li, S.; Xu, D.; Wu, L. Production Exopolysaccharide from Kosakonia Cowanii LT-1 through Solid-State Fermentation and Its Application as a Plant Growth Promoter. Int. J. Biol. Macromol. 2020, 150, 955–964. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.; Gregory, A.S.; White, R.P.; Watts, C.W. Soil Organic Carbon, Extracellular Polymeric Substances (EPS), and Soil Structural Stability as Affected by Previous and Current Land-Use. Geoderma 2020, 363, 114143. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; Shabana, Y.M.; Ismail, A.E.; Rashad, Y.M. Trichoderma harzianum: A Biocontrol Agent against Bipolaris oryzae. Mycopathologia 2007, 164, 81–89. [Google Scholar] [CrossRef]
- Haddadin, M.S.Y.; Haddadin, J.; Arabiyat, O.I.; Hattar, B. Biological Conversion of Olive Pomace into Compost by Using Trichoderma harzianum and Phanerochaete chrysosporium. Bioresour. Technol. 2009, 100, 4773–4782. [Google Scholar] [CrossRef] [PubMed]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Borah, P.; Gogoi, N.; Asad, S.A.; Rabha, A.J.; Farooq, M. An Insight into Plant Growth-Promoting Rhizobacteria-Mediated Mitigation of Stresses in Plant. J. Plant Growth Regul. 2023, 42, 3229–3256. [Google Scholar] [CrossRef]
- Bashan, Y.; Kamnev, A.; de-Bashan, L. Tricalcium Phosphate Is Inappropriate as a Universal Selection Factor for Isolating and Testing Phosphate-Solubilizing Bacteria That Enhance Plant Growth: A Proposal for an Alternative Procedure. Biol. Fertil. Soils 2013, 49, 465–469. [Google Scholar] [CrossRef]
- Bradáčová, K.; Sittinger, M.; Tietz, K.; Neuhäuser, B.; Kandeler, E.; Berger, N.; Ludewig, U.; Neumann, G. Maize Inoculation with Microbial Consortia: Contrasting Effects on Rhizosphere Activities, Nutrient Acquisition and Early Growth in Different Soils. Microorganisms 2019, 7, 329. [Google Scholar] [CrossRef]
- Kincses, S.; Filep, T.; Kátai, J. Effect of organic, artificial, and bacterial fertilizers on the nutrient content of soils extractable with 0.01 M CaCl2 solution. Talajvédelem 2008, 423–430. (In Hungarian) [Google Scholar]
- Ayala-Zepeda, M.; Rojas-Padilla, J.; Díaz-Rodríguez, A.M.; Chávez-Luzanía, R.A.; Parra Cota, F.I.; Villalobos, S.d.l.S. Chapter 12—Performance Evaluation of Bacterial Inoculants in the Field. In New Insights, Trends, and Challenges in the Development and Applications of Microbial Inoculants in Agriculture; Villalobos, S.d.l.S., Ed.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2024; pp. 117–127. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, H.; Macdonald, C.A.; Singh, B.K. Microbial Inoculants with Higher Capacity to Colonize Soils Improved Wheat Drought Tolerance. Microb. Biotechnol. 2023, 16, 2131–2144. [Google Scholar] [CrossRef]
- Iqbal, R.; Valipour, M.; Ali, B.; Zulfiqar, U.; Aziz, U.; Zaheer, M.S.; Sarfraz, A.; Javed, M.A.; Afridi, M.S.; Ercisli, S.; et al. Maximizing Wheat Yield through Soil Quality Enhancement: A Combined Approach with Azospirillum Brasilense and Bentonite. Plant Stress 2024, 11, 100321. [Google Scholar] [CrossRef]
- Ntanos, E.; Kekelis, P.; Assimakopoulou, A.; Gasparatos, D.; Denaxa, N.-K.; Tsafouros, A.; Roussos, P.A. Amelioration Effects against Salinity Stress in Strawberry by Bentonite–Zeolite Mixture, Glycine Betaine, and Bacillus Amyloliquefaciens in Terms of Plant Growth, Nutrient Content, Soil Properties, Yield, and Fruit Quality Characteristics. Appl. Sci. 2021, 11, 8796. [Google Scholar] [CrossRef]
- Solti, G. Soil-Improving, and Ecological Nutrient-Management; Mezőgazda Press: Budapest, Hungary, 2000. (In Hungarian) [Google Scholar]
- Kátai, J.; Magdolna, T.; Sándor, Z.; Zsuposné, Á. Effect of Bentonite and Zeolite on Some Characteristics of Acidic Sandy Soil and on the Biomass of a Test Plant. Agrokémia Talajt. 2010, 59, 165–174. [Google Scholar] [CrossRef][Green Version]
- Ragályi, P.; Kádár, I.; Csathó, P.; Murányi, A.; Radimszky, L.; Gajdó, A. Effect of Gérce Alginit on the Fertility of an Acidic Sandy Soil During Five Years; Magyar Tudományos Akadémia Agrártudományi Kutatóközpont, Alginit Kft: Martonvásár, Hungary; Budapest, Hungary, 2019. (In Hungarian) [Google Scholar]
- Kardos, L.; Juhasz, A.; Palko, G.; Olah, J.; Barkács, K.; Záray, G. Enzyme Activity Analyses of Anaerobic Fermented Sewage Sludges. Appl. Ecol. Environ. Res. 2011, 9, 333–339. [Google Scholar] [CrossRef]
- Kovács, R.; Imre, C.; Puspán, I.; Rizó, B.; Imri, Á.; Pék, N.; Kárpáti, É.; Árvay, G.; Romsics, C.; Kutasi, J. Selection and creation of a strain collection of stress-tolerant bacteria adapted to degraded soils with adverse pH and salt conditions. Talajvédelem 2017, 85–96. (In Hungarian) [Google Scholar]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Iosa, I.; Agrimonti, C.; Marmiroli, N. Real-Time PCR (qtPCR) to Discover the Fate of Plant Growth-Promoting Rhizobacteria (PGPR) in Agricultural Soils. Microorganisms 2024, 12, 1002. [Google Scholar] [CrossRef]
- Gupta, G.; Jha, P. Screening of Potential PGPR Candidates as Future Biofertilizers-A Strategic Approach from Lab to Field. Res. J. Biotechnol. 2015, 10, 48–62. [Google Scholar]
- Zhang, X.; Wang, Y.; Guo, J.; Yu, Y.; Li, Y.; Liu, C. Comparing Two Functions for Optical Density and Cell Numbers in Bacterial Exponential Growth Phase. J. Pure Appl. Microbiol. 2015, 9, 299–305. [Google Scholar]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W. Untersuchungen Über Die Chemische Bodenanalyse Als Grundlage Für Die Beurteilung Des Nährstoffzustandes Der Böden II. K. Lantbrukshögskolans Ann. Chem. Extraktionsmethoden Zur Phosphor-Und Kaliumbestimmung 1960, 26, 199–215. [Google Scholar]
- Tyurin, I.V. Analytic Procedure for a Comperative Study of Soil Humus. Tr. PochvInst Dokuchayeva 1951, 38, 5–21. [Google Scholar]
- MSZ-08-0206/2-1978; Evaluation of Some Chemical Properties of the Soil. Laboratory Tests. (pH Value), Phenolphtalein Alkalinity Expressed in Soda, Total Water-Soluble Salt Content, Hydrolytic (y1 Value) and Exchangeable Acidity (y2 Value). Hungarian Standard Association: Budapest, Hungary, 1978. (In Hungarian)
- Buzás, I. A talaj Arany-féle kötöttségi számának (KA) meghatározása kézi keveréssel. In Talaj—és Agrokémiai Vizsgálati Módszerkönyv 1; Buzás, I., Ed.; Inda: Budapest, Hungary, 1993; p. 48. [Google Scholar]
- Olsen, P.E.; Sanda, E.S.; Keyser, H.H. The Enumeration and Identification of Rhizobial Bacteria in Legume Inoculant Quality Control Procedures; NifTAL Center: Paia, HI, USA, 1996; p. 96. [Google Scholar]
- Reichart, O. Some Remarks on the Bias of the MPN Method. Int. J. Food Microbiol. 1991, 13, 131–141. [Google Scholar] [CrossRef]
- Cochran, W.G. Estimation of Bacterial Densities by Means of the “Most Probable Number”. Biometrics 1950, 6, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Veres, Z.; Kotroczó, Z.; Magyaros, K.; Tóth, J.A.; Tóthmérész, B. Dehydrogenase Activity in a Litter Manipulation Experiment in Temperate Forest Soil. Acta Silv. Lignaria Hung. 2013, 9, 25–33. [Google Scholar] [CrossRef][Green Version]
- Thalmann, A. Dehydrogenase activity. In 1995 Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press Ltd.: New York, NY, USA, 1968; pp. 228–230. [Google Scholar]
- Villányi, I.; Füzy, A.; Angerer, I.; Biró, B. Total Catabolic Enzyme Activity of Microbial Communities. Fluorescein Diacetate Analysis (FDA). In Understanding and Modelling Plant-Soil Interactions in the Rhizosphere Environment. Handbook of Methods Used in Rhizosphere Research; Jones, D.L., Ed.; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2006; pp. 441–442. [Google Scholar]
- Unger, H. The Cellulose Test as a Method for Determining the Cellulose-Decomposing Activity of Soil in Field Experiments. Z. Pflanzenernahr. Dung. Bodenkd. 1960, 91, 44–52. [Google Scholar] [CrossRef]
- Kaymak, H.C. Potential of PGPR in Agricultural Innovations. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2010; Volume 18, pp. 45–79. [Google Scholar] [CrossRef]
- Minuț, M.; Diaconu, M.; Roșca, M.; Cozma, P.; Bulgariu, L.; Gavrilescu, M. Screening of Azotobacter, Bacillus and Pseudomonas Species as Plant Growth-Promoting Bacteria. Processes 2023, 11, 80. [Google Scholar] [CrossRef]
- Zaballa, J.I.; Golluscio, R.; Ribaudo, C.M. Effect of the Phosphorus-Solubilizing Bacterium Enterobacter Ludwigii on Barley Growth Promotion. Am. Sci. Res. J. Eng. Technol. Sci. 2020, 63, 144–157. [Google Scholar]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant Growth-Promoting Rhizobacteria (PGPR) Improve the Growth and Nutrient Use Efficiency in Maize (Zea mays L.) under Water Deficit Conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Manjunath, M.; Prasanna, R.; Sharma, P.; Nain, L.; Singh, R. Developing PGPR Consortia Using Novel Genera Providencia and Alcaligenes along with Cyanobacteria for Wheat. Arch. Agron. Soil Sci. 2011, 57, 873–887. [Google Scholar] [CrossRef]
- Telesiński, A.; Krzyśko-Łupicka, T.; Cybulska, K.; Pawłowska, B.; Biczak, R.; Śnieg, M.; Wróbel, J. Comparison of Oxidoreductive Enzyme Activities in Three Coal Tar Creosote-Contaminated Soils. Soil Res. 2019, 57, 814–824. [Google Scholar] [CrossRef]
- Abou-Zeid, M. Improvement of Soybean Growth by Co-Inoculation with Rhizobium and Plant Growth-Promoting Rhizobacteria. Egypt. J. Appl. Sci. 2011, 26, 445–449. [Google Scholar]
- Bandyopadhyay, S.; Maiti, S.K. Different Soil Factors Influencing Dehydrogenase Activity in Mine Degraded Lands—State-of-Art Review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Kutateladze, L.Y.; Zakariashvili, N.G.; Jobava, M.D.; Burduli, T.A.; Sadunishvili, T.A. Microscopic Fungi Spread in Different Types of Soils in Western Georgia. Ann. Agrar. Sci. 2016, 14, 227–232. [Google Scholar] [CrossRef]
- Gazdag, O.; Takács, T.; Ködöböcz, L.; Krett, G.; Szili-Kovács, T. Alphaproteobacteria Communities Depend More on Soil Types than Land Managements. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 147–154. [Google Scholar] [CrossRef]
- Javed, S.; Javaid, A.; Hanif, U.; Bahadur, S.; Sultana, S.; Shuaib, M.; Ali, S. Effect of Necrotrophic Fungus and PGPR on the Comparative Histochemistry of Vigna Radiata by Using Multiple Microscopic Techniques. Microsc. Res. Tech. 2021, 84, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.D.; Elliott, E.T.; Paustian, K. Bacterial and Fungal Abundance and Biomass in Conventional and No-Tillage Agroecosystems along Two Climatic Gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Mallon, C.A.; Poly, F.; Le Roux, X.; Marring, I.; van Elsas, J.D.; Salles, J.F. Resource Pulses Can Alleviate the Biodiversity–Invasion Relationship in Soil Microbial Communities. Ecology 2015, 96, 915–926. [Google Scholar] [CrossRef]
- Mawarda, P.C.; Mallon, C.A.; Le Roux, X.; van Elsas, J.D.; Salles, J.F. Interactions between Bacterial Inoculants and Native Soil Bacterial Community: The Case of Spore-Forming Bacillus spp. FEMS Microbiol. Ecol. 2022, 98, fiac127. [Google Scholar] [CrossRef]
- Chmolowska, D.; Hamda, N.; Laskowski, R. Cellulose Decomposed Faster in Fallow Soil than in Meadow Soil Due to a Shorter Lag Time. J. Soils Sediments 2017, 17, 299–305. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zhao, B.; Zhang, J. Relationship between the Chemical Structure of Straw and Composition of Main Microbial Groups during the Decomposition of Wheat and Maize Straws as Affected by Soil Texture. Biol. Fertil. Soils 2020, 56, 11–24. [Google Scholar] [CrossRef]
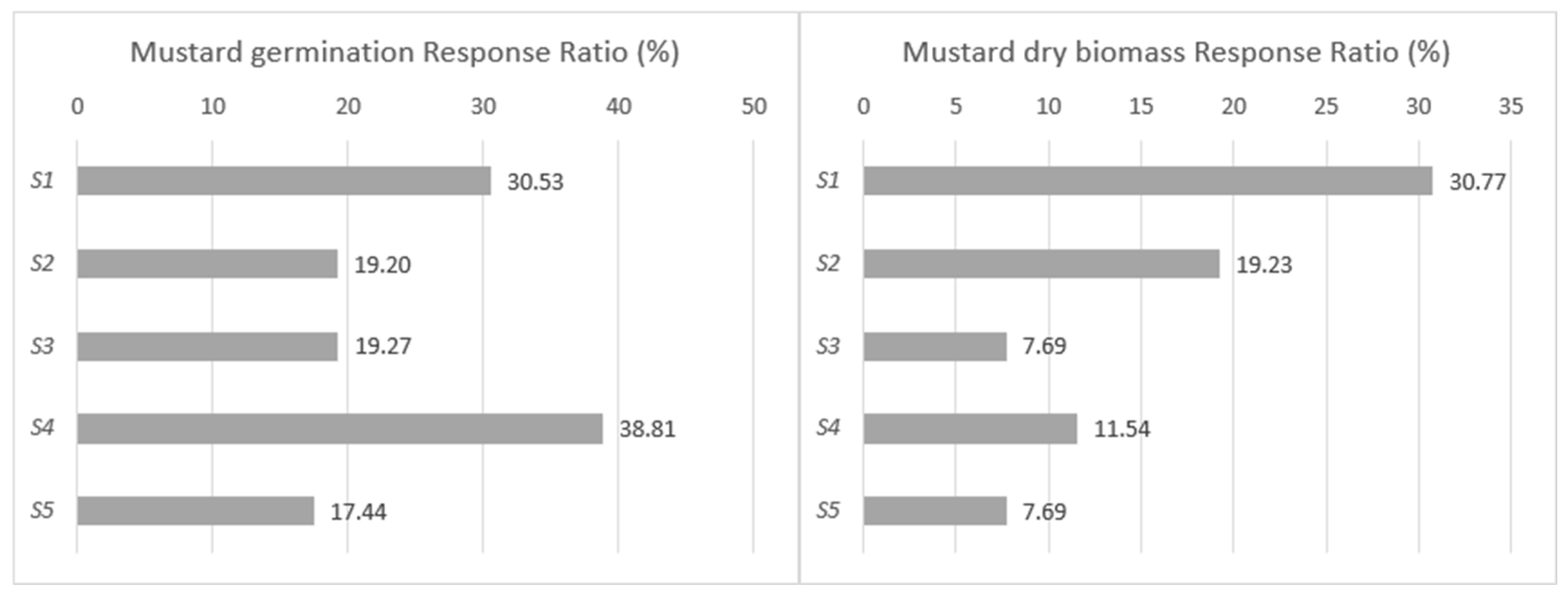
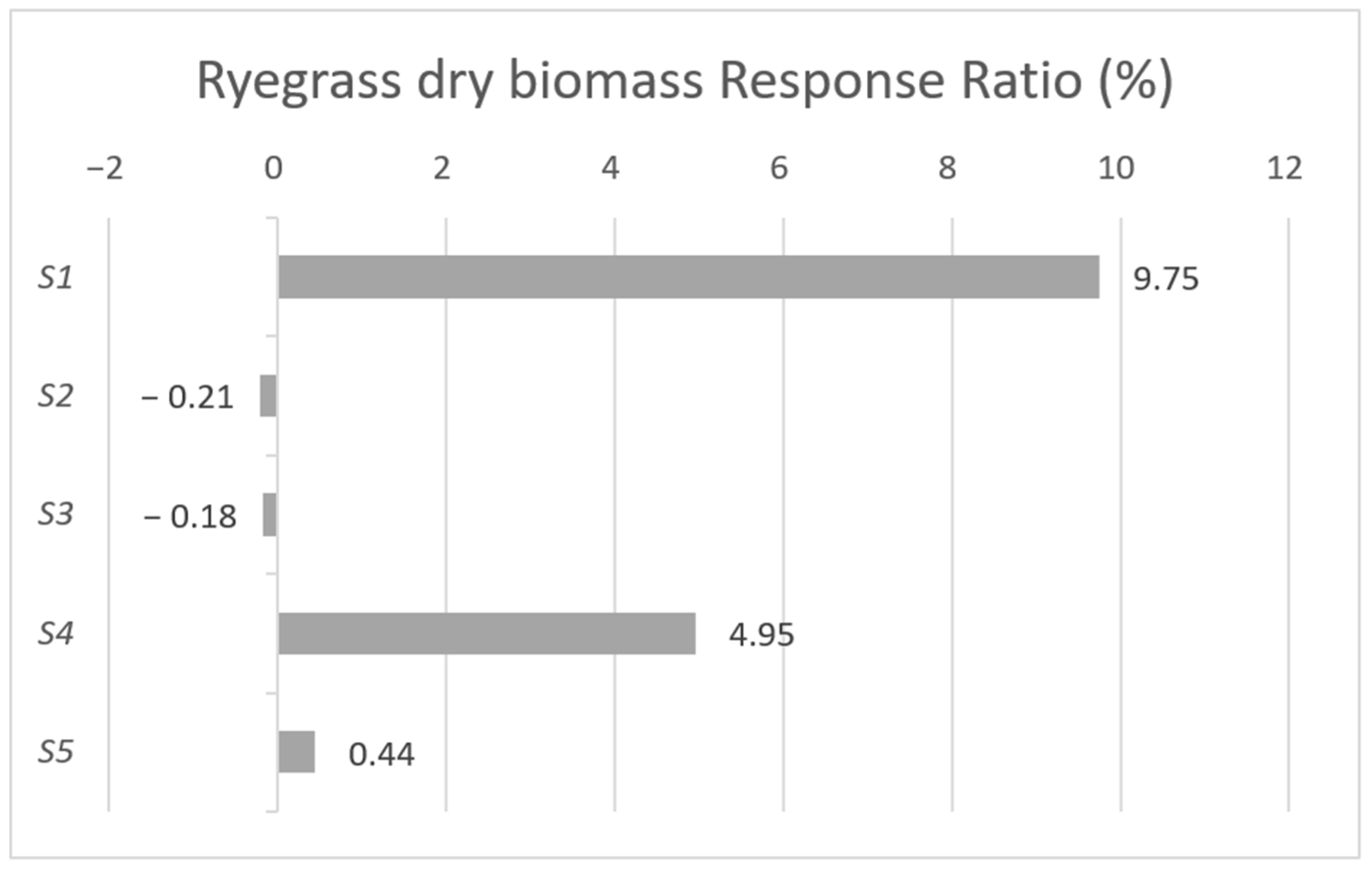


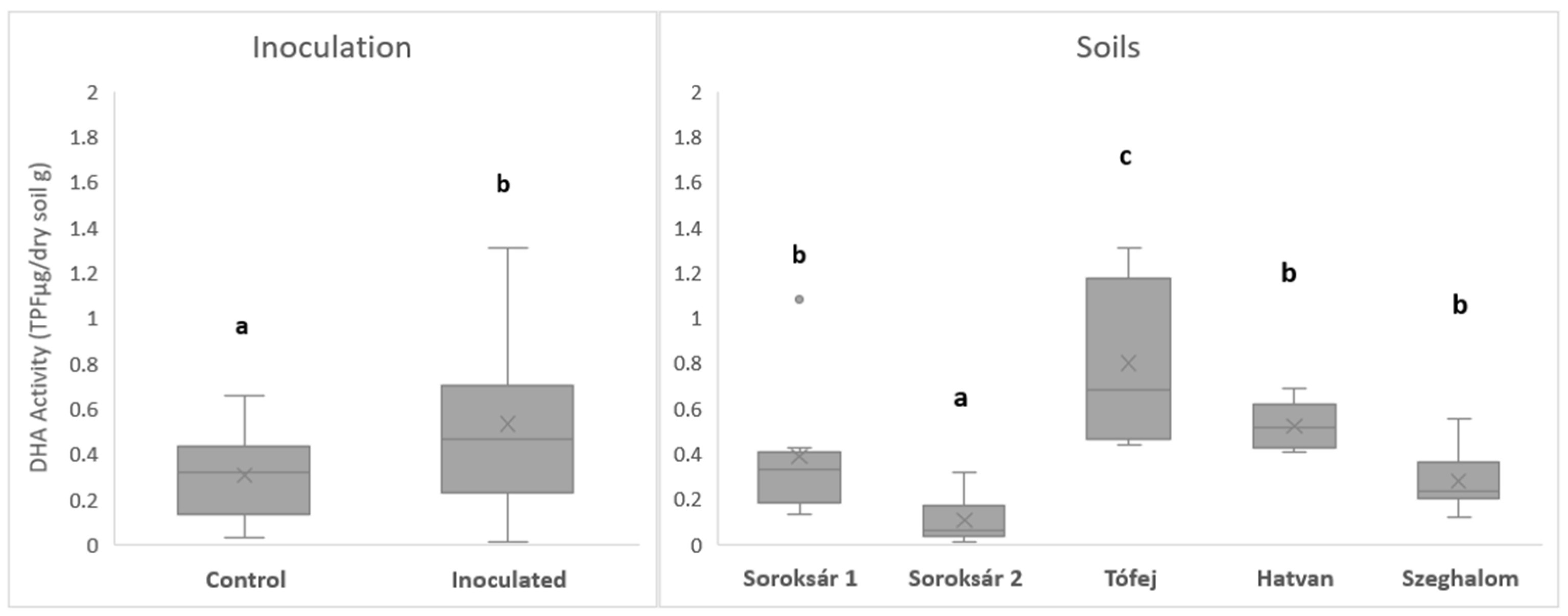
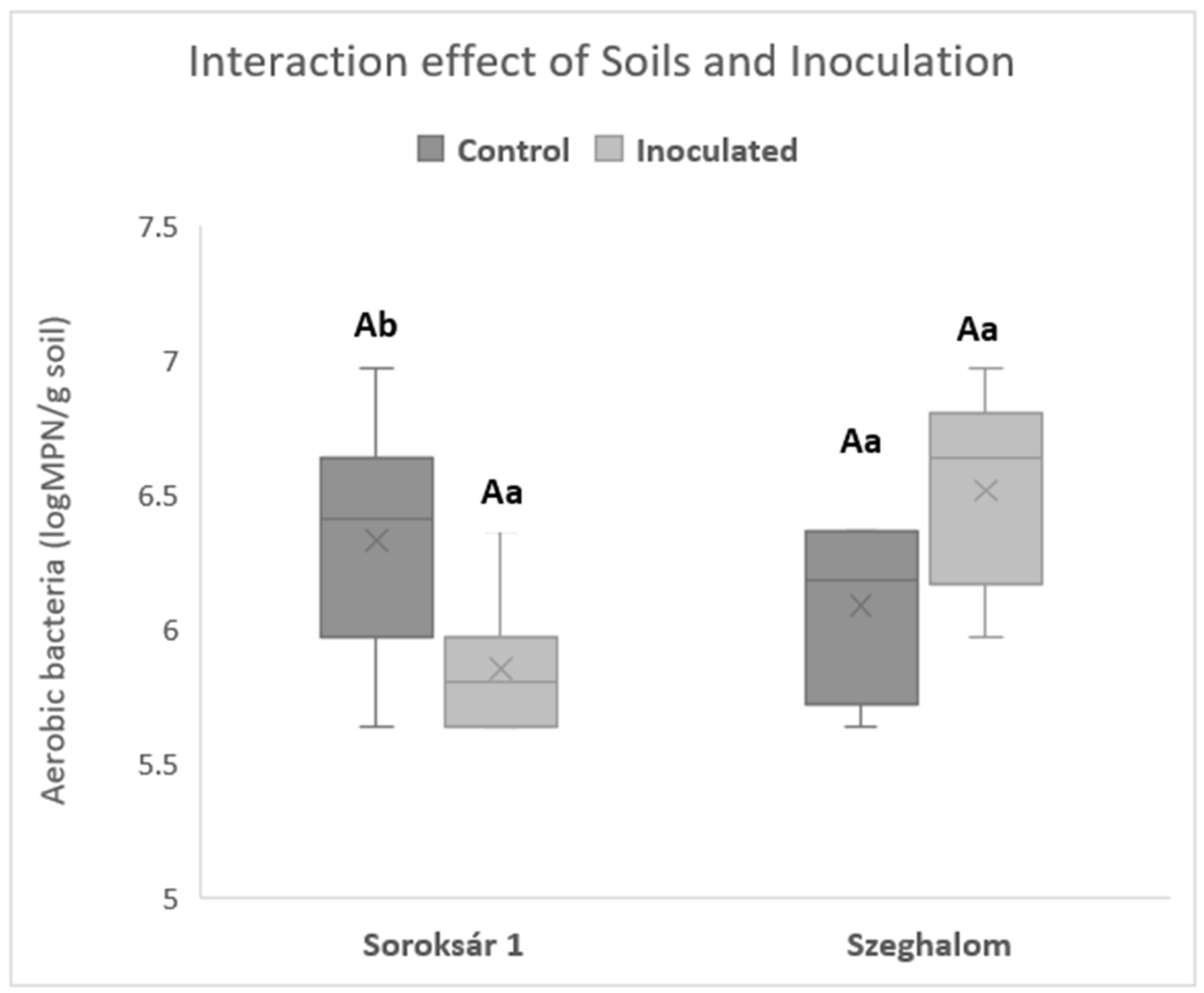
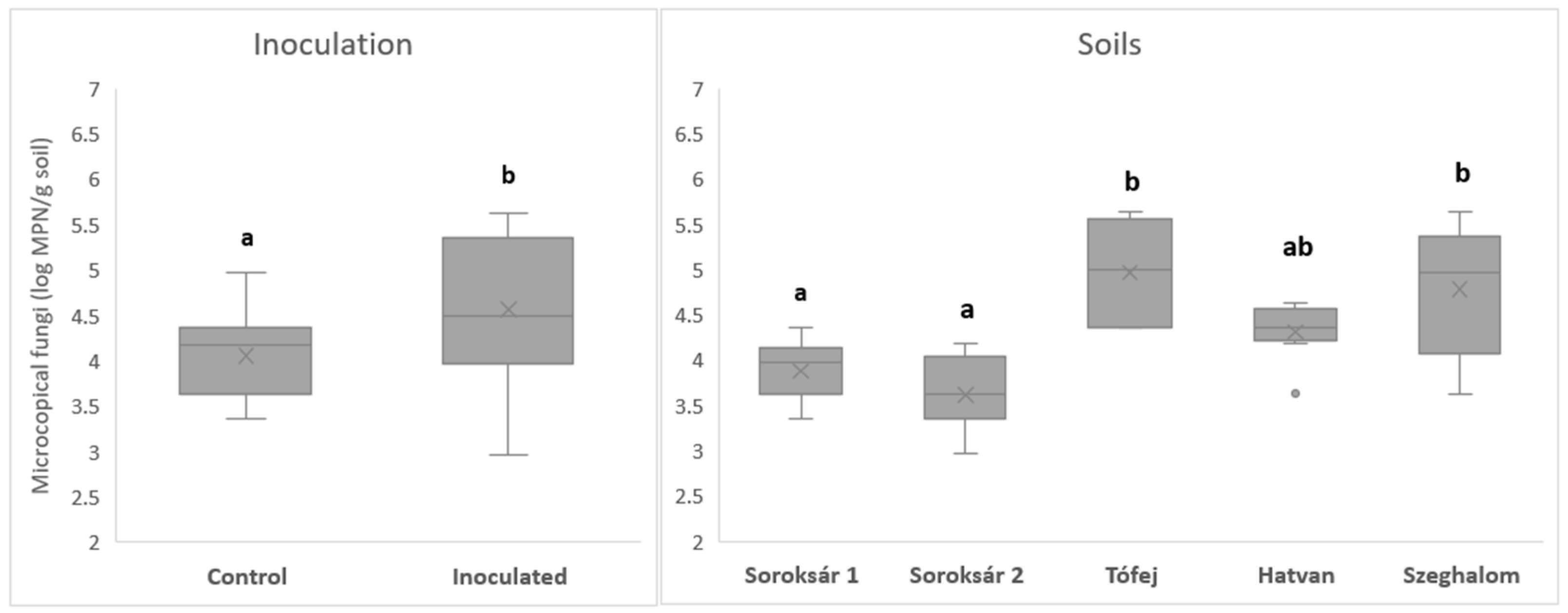
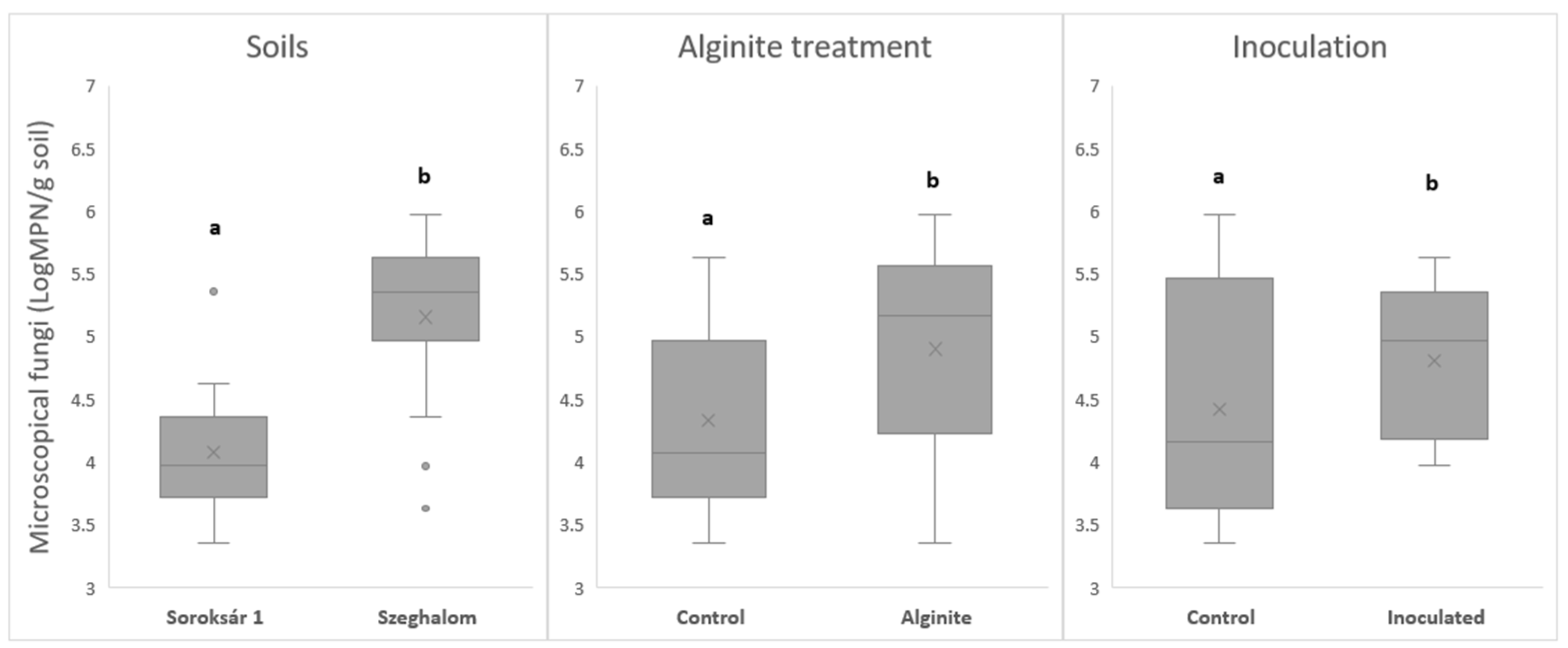
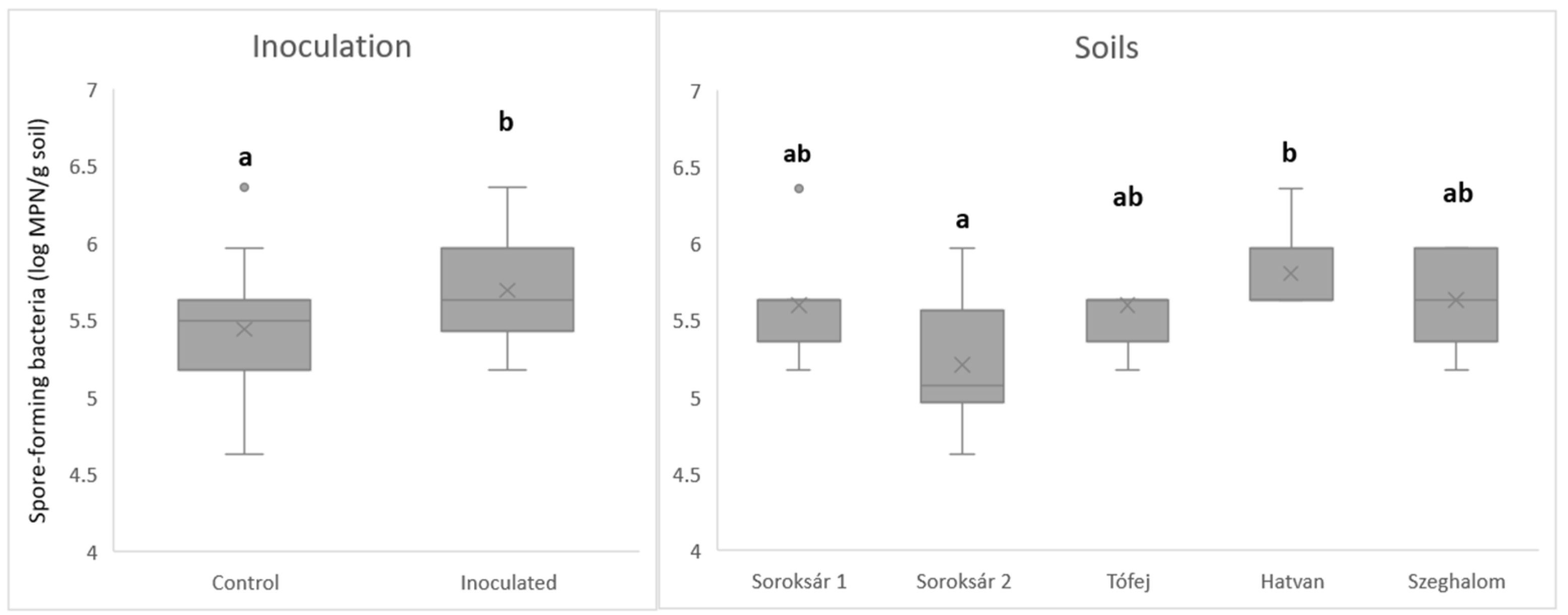

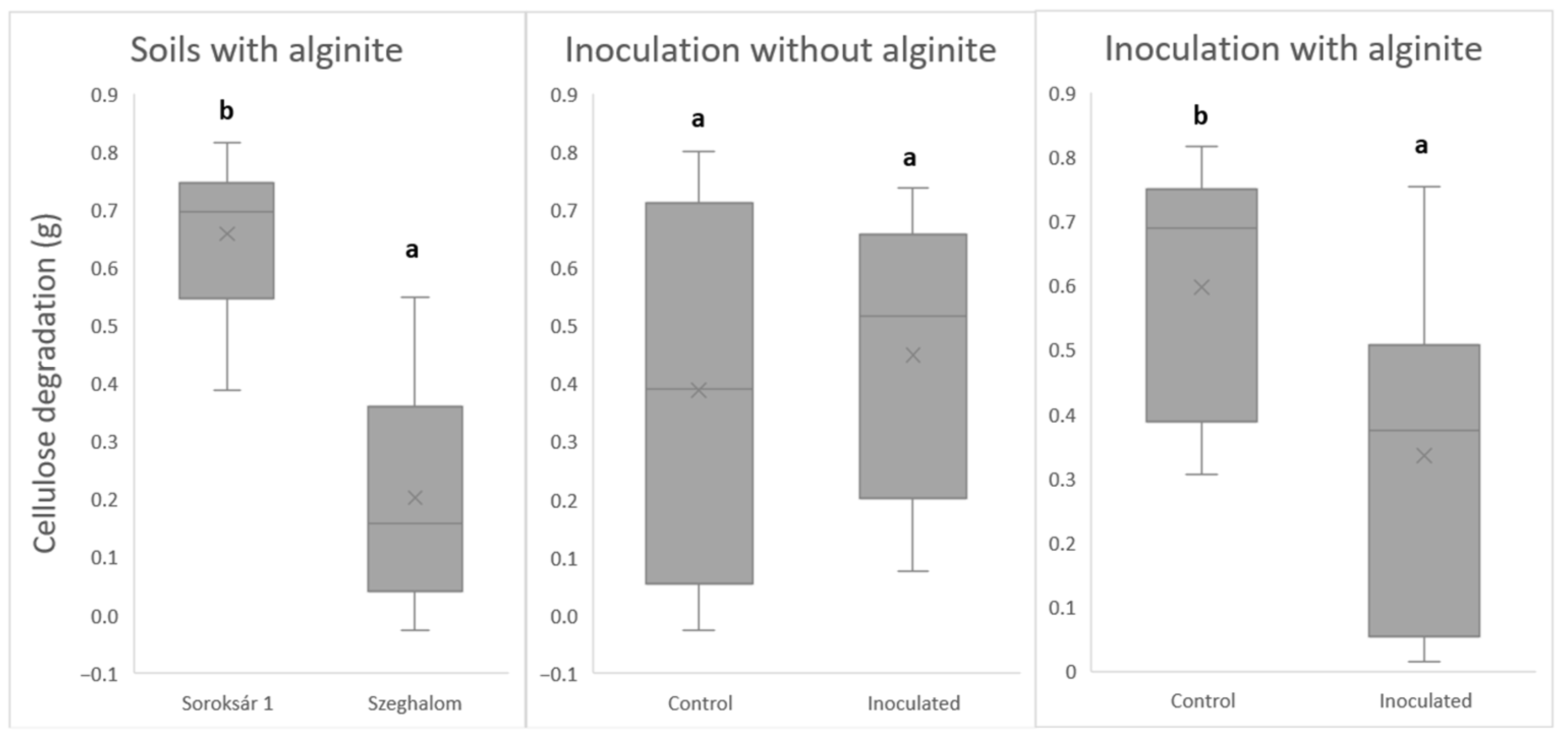
| Strain Composition | Properties, Abilities |
|---|---|
| S1—Enterobacter ludwigii | PSB, KSB, N bonding, Fusarium fungal antagonism, IAA, siderophore, and extracellular polysaccharide production [19,20,21,22] |
| S2—Bacillus subtilis | Cellulose decomposition, PSB, biocontrol [23,24,25] |
| S3—Pseudomonas fluorescens | PSB, biocontrol effect, IAA, and siderophore production [14,23] |
| S4—Kosakonia cowanii | Nitrogen bonding, PSB, KSB, and EPS production [26,27,28,29,30] |
| S5—Trichorderma harzianum | Biocontrol effect, cellulose decomposition [31,32] |
| Measured Parameters | Soroksár 1 | Soroksár 2 | Hatvan | Tófej | Szeghalom |
|---|---|---|---|---|---|
| Soil type | Arenosol | Gleysol | Chernozem | Luvisol | Gleysol |
| Texture | Sand | Clay loam | Clay loam | Clay | Clay |
| Soil plasticity (KA) | 26 | 43.4 | 49 | 54 | 57.5 |
| pH(H2O) | 7.49 | 7.42 | 7.44 | 7.50 | 7.61 |
| pH(KCl) | 6.94 | 7.13 | 6.58 | 6.74 | 6.45 |
| Water-soluble salts w/w% | 0.0317 | 0.0216 | 0.0268 | 0.0555 | 0.0665 |
| Humus content (H%): | 2.18 | 4.09 | 4.63 | 3.89 | 3.75 |
| FDA | p-Value | |
|---|---|---|
| Mustard germination% | 0.29418 | 0.5715 |
| Mustard dry biomass (g) | 0.4300 | 0.4033 |
| Perennial ryegrass dry biomass (g) | 0.1331 | 0.8015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabar, S.A.; Kotroczó, Z.; Takács, T.; Biró, B. Evaluating the Efficacy of Selected Plant Growth-Promoting Microorganisms in Optimizing Plant Growth and Soil Health in Diverse Soil Types. Agriculture 2024, 14, 1586. https://doi.org/10.3390/agriculture14091586
Pabar SA, Kotroczó Z, Takács T, Biró B. Evaluating the Efficacy of Selected Plant Growth-Promoting Microorganisms in Optimizing Plant Growth and Soil Health in Diverse Soil Types. Agriculture. 2024; 14(9):1586. https://doi.org/10.3390/agriculture14091586
Chicago/Turabian StylePabar, Sándor Attila, Zsolt Kotroczó, Tünde Takács, and Borbála Biró. 2024. "Evaluating the Efficacy of Selected Plant Growth-Promoting Microorganisms in Optimizing Plant Growth and Soil Health in Diverse Soil Types" Agriculture 14, no. 9: 1586. https://doi.org/10.3390/agriculture14091586
APA StylePabar, S. A., Kotroczó, Z., Takács, T., & Biró, B. (2024). Evaluating the Efficacy of Selected Plant Growth-Promoting Microorganisms in Optimizing Plant Growth and Soil Health in Diverse Soil Types. Agriculture, 14(9), 1586. https://doi.org/10.3390/agriculture14091586








