Photosynthetic Performance and Heterogeneous Anatomical Structure in Prunus humilis under Saline–Alkaline Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Gas Exchange Measurements
2.3. Relative Chlorophyll Content and Anatomical Analyses
2.4. Measurement of Chlorophyll Fluorescence Transients (OJIP) and Modulated Chlorophyll Fluorescence Parameters
- (1)
- Absorption flux per cross-section of leaf, ABS/CSo ≈ Fo;
- (2)
- Approximated initial slope of the fluorescence transient, Mo = 4·(Fk − Fo)/(Fm − Fo);
- (3)
- Relative variable fluorescence at the J-step, Vj = (Fj − Fo)/(Fm − Fo);
- (4)
- The maximum quantum yield of PSII, Fv/Fm = φPo = (Fm − Fo)/Fm;
- (5)
- The normalized relative variable fluorescence at the K step, Wk = (Fk − Fo)/(Fj − Fo);
- (6)
- Probability that a trapped exciton moves an electron into the electron transport chain beyond QA−, ψo = ETo/TRo = (1 − Vj);
- (7)
- Density of active reaction centers, RC/CSo = (Fv/Fm)·(Vj/Mo)·(ABS/CSo);
- (8)
- Performance index on absorption basis, PIabs = (RC/ABS)·[φPo/(1 − φPo)]·[ψo/(1 − ψo)].
2.5. Statistical Methods
3. Results
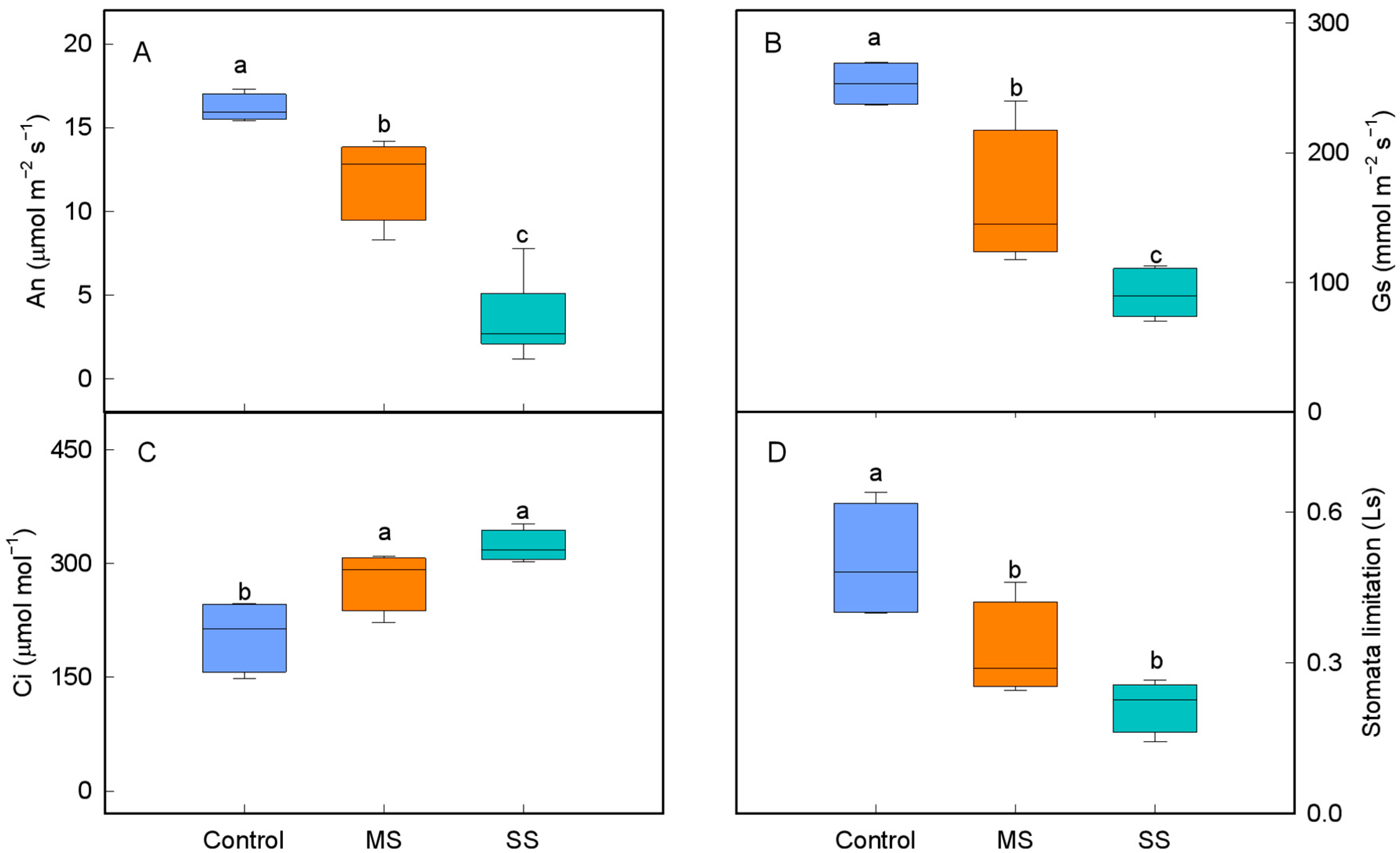
3.1. Changes in Leaf Gas Exchange Characteristics
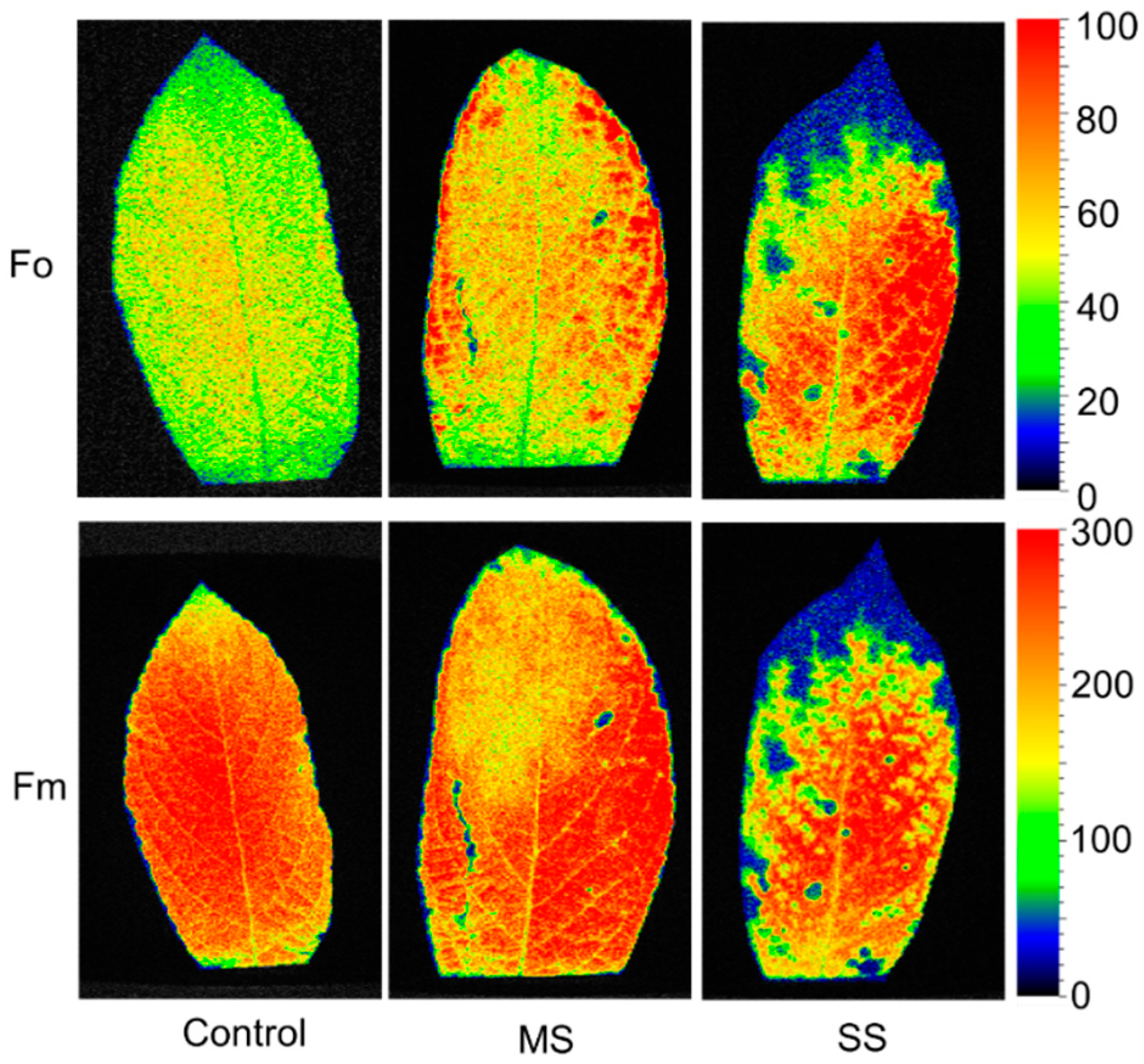
3.2. Heterogeneity in P. humilis Leaves under Saline–Alkaline Stress
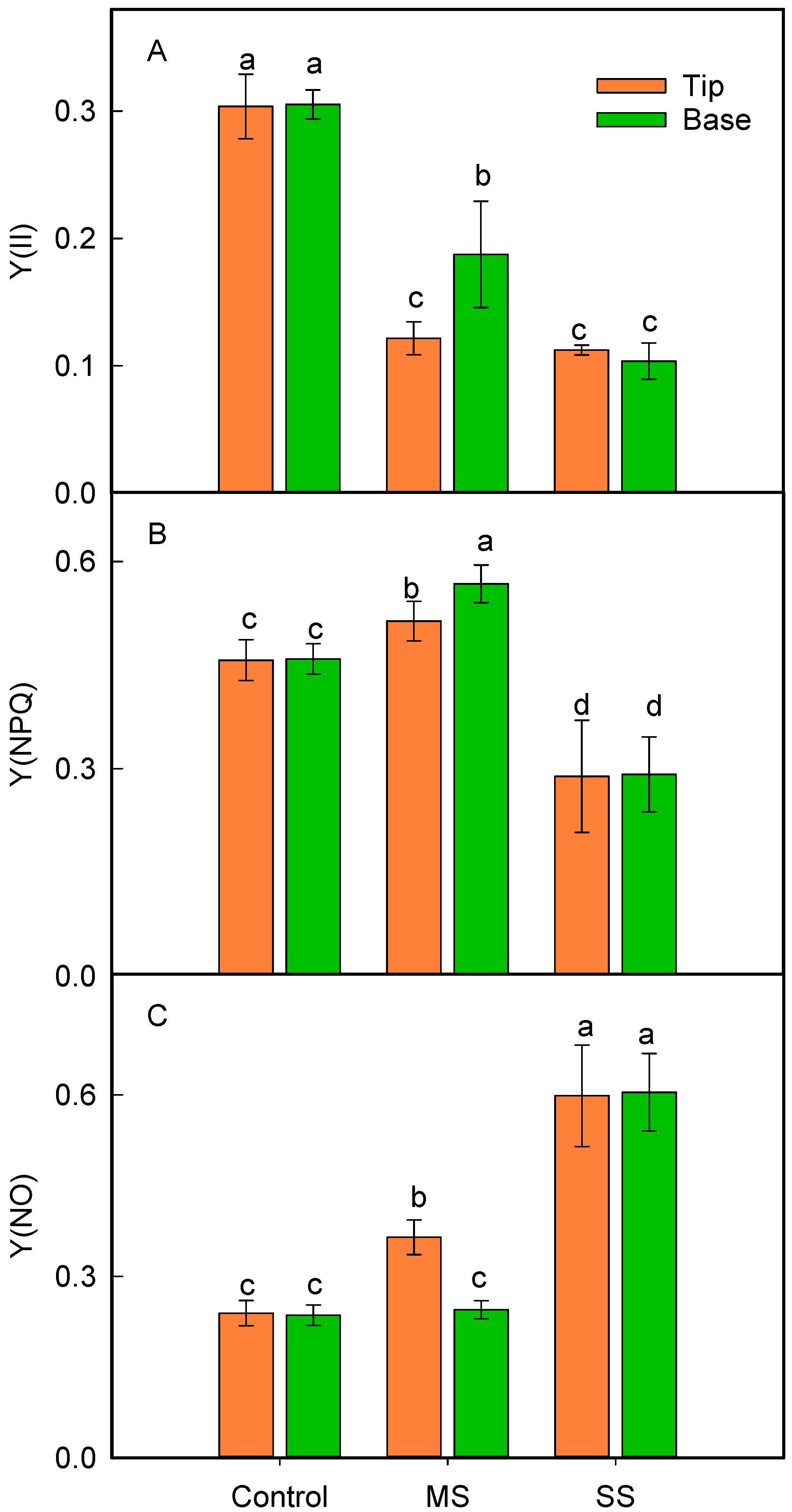
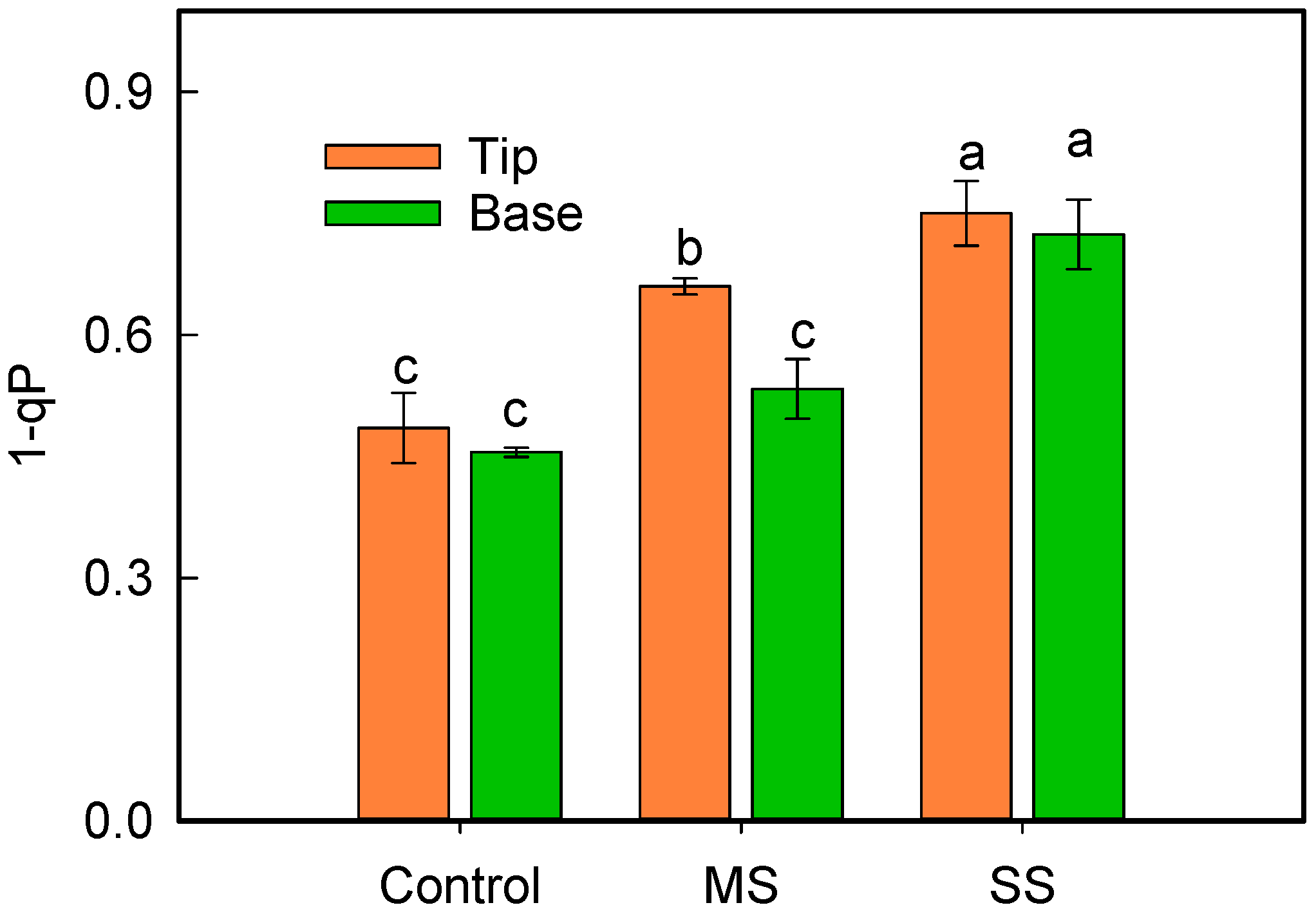
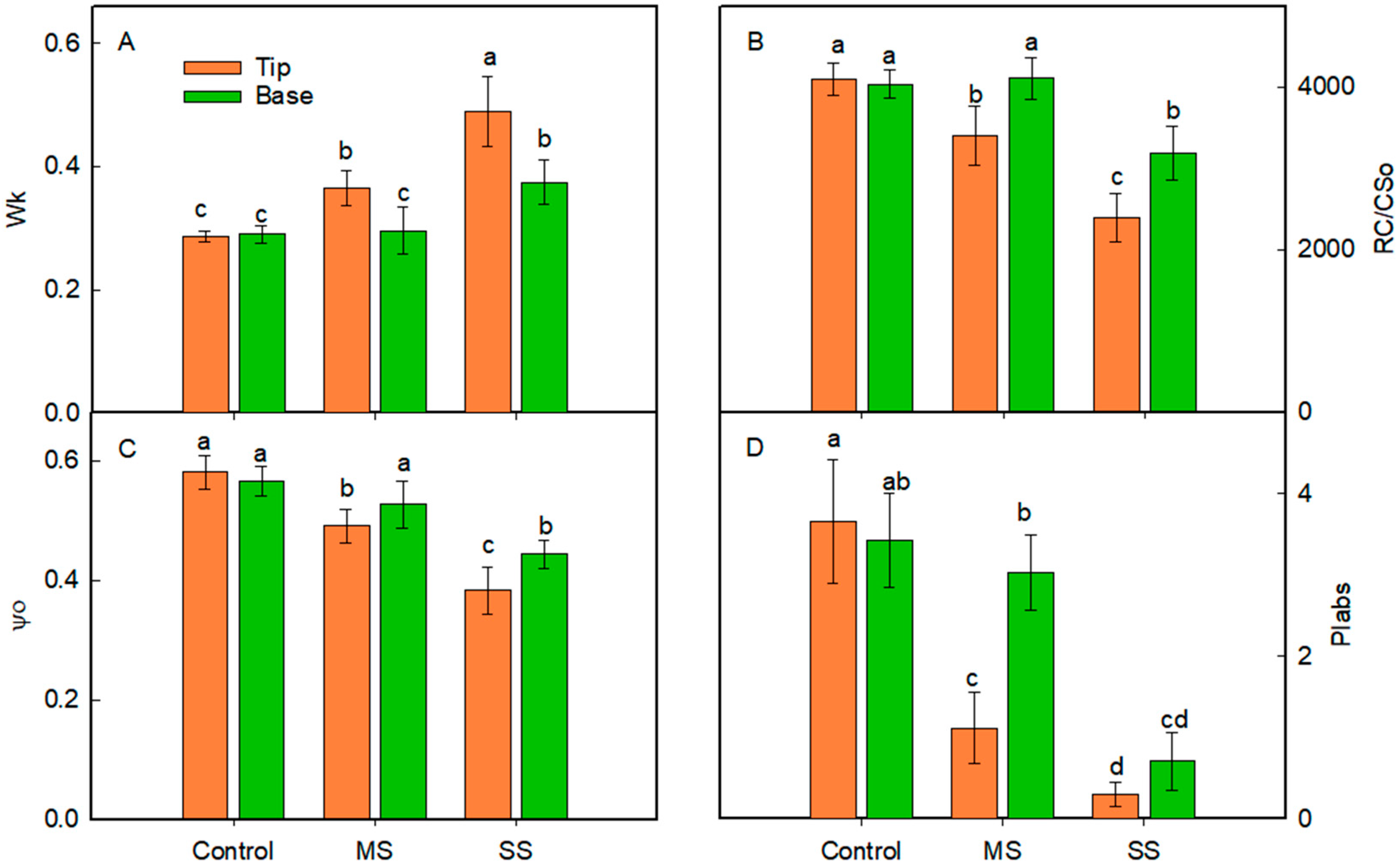
3.3. Changes in Leaf Relative Chlorophyll Content and Energy Distribution of Light Absorbed by PSII
3.4. Changes in the Chlorophyll a Fluorescence Transients
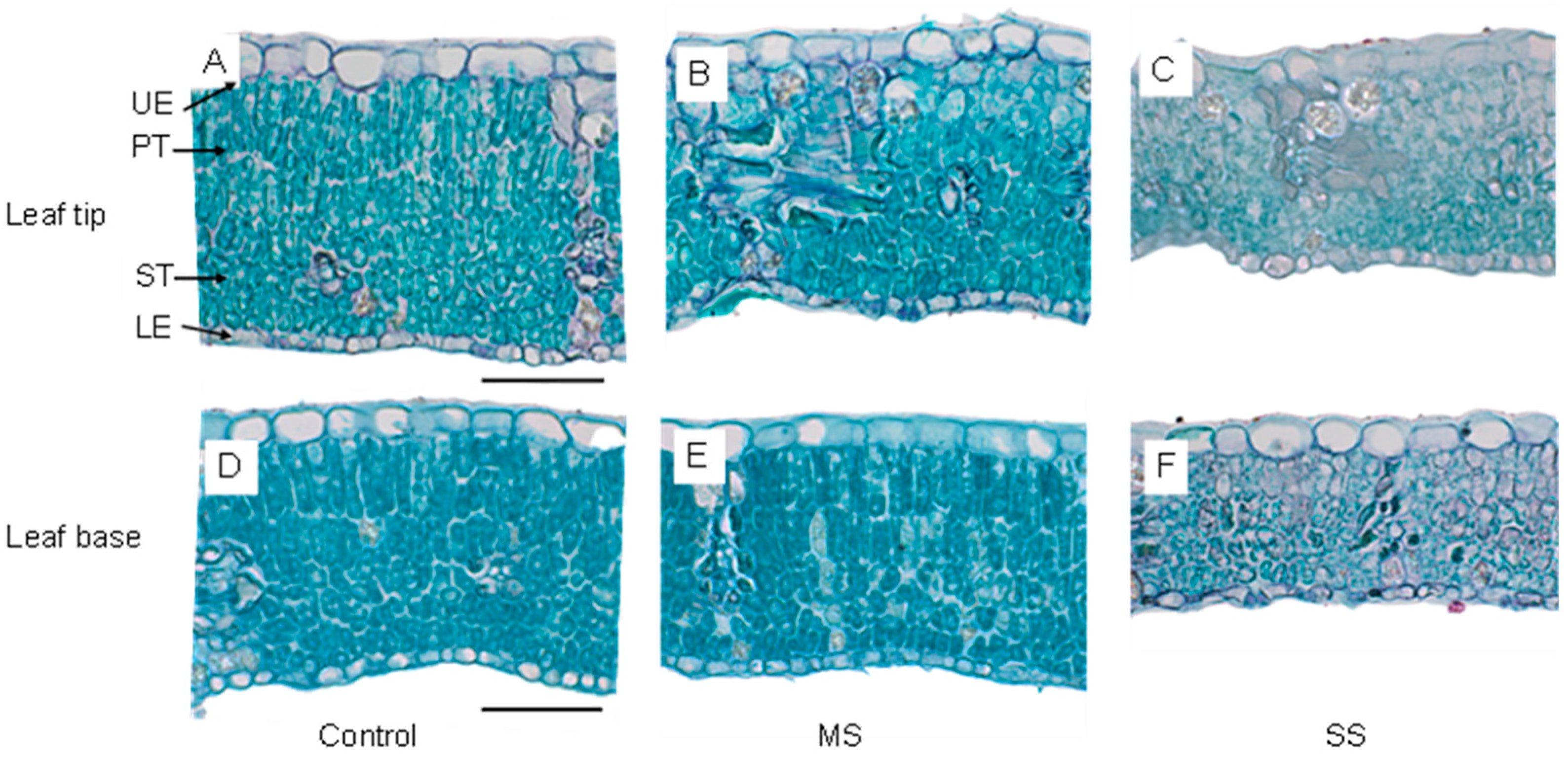
3.5. Changes in Leaf Anatomy
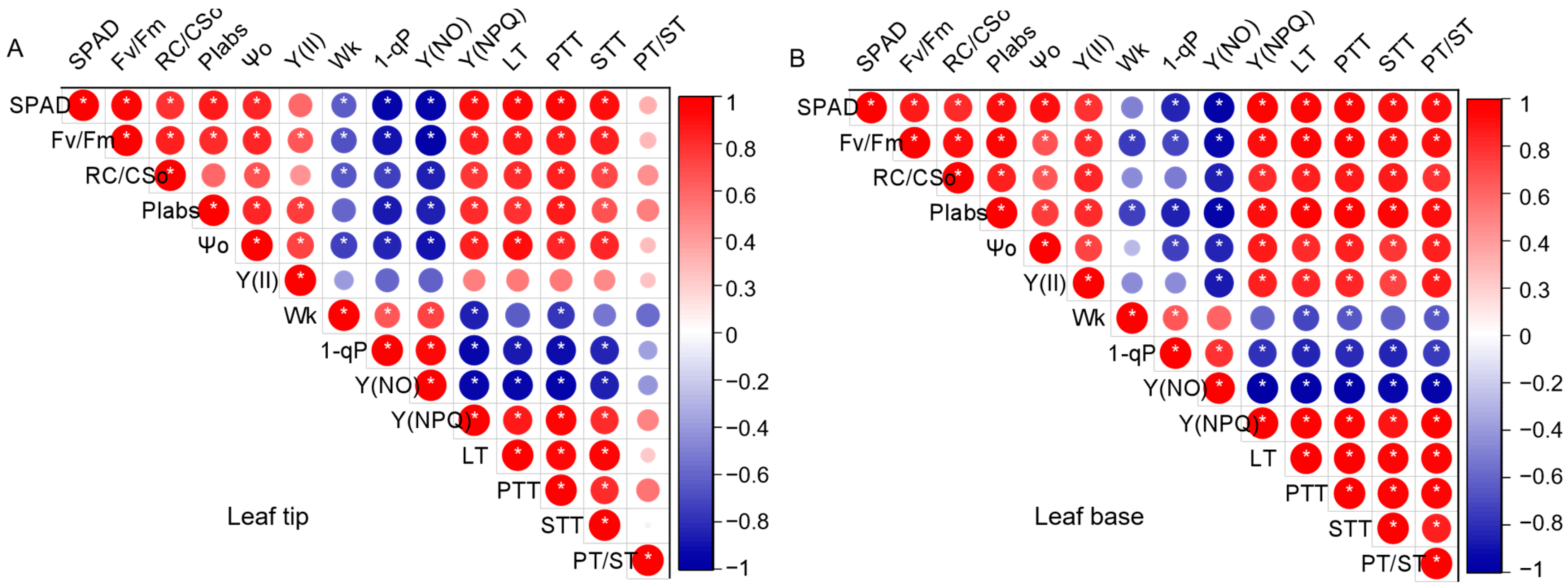
3.6. Correlation Analysis of Chlorophyll Fluorescence Parameters and Anatomical Structure Characteristics of P. humilis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Yang, H.; Chi, J. A preliminary study of selected strains of the Chinese dwarf cherry tree. China Fruits 1993, 3, 23–24. [Google Scholar]
- Song, X.S.; Shang, Z.W.; Yin, Z.P.; Ren, J.; Sun, M.C.; Ma, X.L. Mechanism of xanthophyll–cycle–mediated photoprotection in Cerasus humilis seedlings under water stress and subsequent recovery. Photosynthetica 2011, 49, 523–530. [Google Scholar] [CrossRef]
- Mu, X.; Wang, P.; Du, J.; Gao, Y.G.; Zhang, J. Comparison of fruit organic acids and metabolism-related gene expression between Cerasus humilis (Bge.) Sok and Cerasus glandulosa (Thunb.) Lois. PLoS ONE 2018, 13, e0196537. [Google Scholar] [CrossRef]
- Mu, X.P.; Aryal, N.; Du, J.M.; Du, J.J. Oil content and fatty acid composition of the kernels of 31 genotypes of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.]. J. Hortic. Sci. Biotechnol. 2015, 90, 525–529. [Google Scholar] [CrossRef]
- Chau, C.F.; Wu, S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment—Plants—Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 3–20. [Google Scholar]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef]
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of plant saline-alkaline tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Sun, L.; Yang, L.; Yang, Y.; Wang, Y.; Siddique, K.H.M.; Pang, J. Alkaline salt Inhibits seed germination and seedling growth of Canola more than neutral salt. Front. Plant Sci. 2022, 13, 814755. [Google Scholar] [CrossRef]
- Shi, D.; Wang, D. Effects of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant Soil 2005, 271, 15–26. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.T.; Shi, F.; Zhai, Y.; Xue, J.; Wang, S.; Ren, X.; Zhang, X. Acidic and akaline conditions affect the growth of tree peony plants via altering photosynthetic characteristics, limiting nutrient assimilation, and impairing ROS balance. Int. J. Mol. Sci. 2022, 23, 5094. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Wang, P.; Li, C.Y.; Shi, D.C.; Wang, D.L. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 2008, 46, 107–114. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Hu, L.; Xiang, L.; Zhang, L.; Zhou, X.; Zou, Z.; Hu, X. The photoprotective role of spermidine in tomato seedlings under salinity–alkalinity stress. PLoS ONE 2014, 9, e110855. [Google Scholar] [CrossRef]
- Xiang, L.; Hu, L.; Xu, W.; Zhen, A.; Zhang, L.; Hu, X. Exogenous gamma-aminobutyric acid improves the structure and function of photosystem II in muskmelon seedlings exposed to salinity-alkalinity stress. PLoS ONE 2016, 11, e0164847. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J. Photochem. Photobiol. B. 2018, 183, 275–287. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Salinity tolerance of quinoa (Chenopodium quinoa Willd) as assessed by chloroplast ultrastructure and photosynthetic performance. Environ. Exp. Bot. 2019, 162, 103–114. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Guo, J. Relationship between photosystem activity and ultraweak luminescence excitation in Cerasus humilis leaves under salt stress. Plant Physiol. Biochem. 2023, 196, 1032–1045. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Fan, Y.; Li, Z.; Lv, G. Relationship between photosynthetic characteristics, anatomical structure, and physiological indexes of two halophytes in different habitats. Forests 2022, 13, 2189. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ferrante, A.; Darras, A.; Ahmed, T.; Jalil, S.; Al-Ashkar, I.; Sabagh, A.E. Melatonin seed priming improves growth and physio-biochemical aspects of Zinnia elegans under salt stress. Sci. Hortic. 2024, 323, 112495. [Google Scholar] [CrossRef]
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.B.Q.; Martins, A.O.; Jarma-Orozco, A.; Bhatt, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Biochem. 2021, 168, 116–127. [Google Scholar] [CrossRef]
- Li, Q.; Wen, J.; Zhao, C.-Z.; Zhao, L.-C.; Ke, D. The relationship between the main leaf traits and photosynthetic physiological characteristics of Phragmites australis under different habitats of a salt marsh in Qinwangchuan, China. AoB Plants 2022, 14, plac054. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, C.-Z.; Wen, J.; Kang, M.; Li, X. Fractal analysis of root architecture responses of Saussurea salsa to a gradient of flooding intensity and salinity. Plant Soil 2022, 471, 669–683. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Terashima, I.; Araya, T.; Miyazawa, S.-I.; Sone, K.; Yano, S. Construction and Maintenance of the Optimal Photosynthetic Systems of the Leaf, Herbaceous Plant and Tree: An Eco-developmental Treatise. Ann. Bot. 2005, 95, 507–519. [Google Scholar] [CrossRef]
- Klich, M.G. Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environ. Exp. Bot. 2000, 44, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Czesnick, H.; Lenhard, M. Size control in plants-lessons from leaves and flowers. CSH Perspect. Biol. 2015, 7, a019190. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.P.; Lima, M.D.R.; da Silva, B.R.S.; Batista, B.L.; da Silva Lobato, A.K. Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf Anatomy. J. Plant Growth Regul. 2019, 38, 557–573. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Xiao, H.; Liu, J.; Wang, L. Variations in leaf functional traits among plant species grouped by growth and leaf types in Zhenjiang, China. J. For. Res. 2017, 28, 241–248. [Google Scholar] [CrossRef]
- Yin, Z.; Ren, J.; Zhou, L.; Sun, L.; Wang, J.; Liu, Y.; Song, X. Water deficit mechanisms in perennial shrubs Cerasus humilis leaves revealed by physiological and proteomic analyses. Proteome Sci. 2016, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Wang, F.; Wang, J.W.; Sun, L.J.; Gao, W.R.; Song, X.S. Overexpression of the ChVDE gene, encoding a violaxanthin de-epoxidase, improves tolerance to drought and salt stress in transgenic Arabidopsis. 3 Biotech 2019, 9, 197. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.W.; Sun, L.J.; Song, X.S. The molecular cloning and functional characterization of ChNAC1, a NAC transcription factor in Cerasus humilis. Plant Growth Regul. 2019, 89, 331–343. [Google Scholar] [CrossRef]
- Tianjiao, F.; Dong, W.; Ruoshui, W.; Yixin, W.; Zhiming, X.; Fengmin, L.; Yuan, M.; Xing, L.; Huijie, X.; Caballero-Calvo, A.; et al. Spatial-temporal heterogeneity of environmental factors and ecosystem functions in farmland shelterbelt systems in desert oasis ecotones. Agric. Water Manag. 2022, 271, 107790. [Google Scholar] [CrossRef]
- Cao, Q.; Li, J.; Wang, G.; Wang, D.; Xin, Z.; Xiao, H.; Zhang, K. On the spatial variability and influencing factors of soil organic carbon and total nitrogen stocks in a desert oasis ecotone of northwestern China. Catena 2021, 206, 105533. [Google Scholar] [CrossRef]
- Yang, F.; Liang, Z.-W.; Wang, Z.-C.; Chen, Y. Relationship between diurnal changes of net photosynthetic rate and influencing factors in rice under saline sodic stress. Rice Sci. 2008, 15, 119–124. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring Fast Fluorescence Transients to Address Environmental Questions: The JIP Test; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 977–980. [Google Scholar]
- Strasser, B.J. Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Kyeong, S.Y.; Ram, B.S.; Gu, L.J. Monitoring of salinity, temperature, and drought stress in grafted watermelon seedlings using chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 786309. [Google Scholar]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Huner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Hawkins, T.S.; Gardiner, E.S.; Comer, G.S. Modeling the relationship between extractable chlorophyll and SPAD–502 readings for endangered plant species research. J. Nat. Conserv. 2009, 17, 123–127. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal leaf positions for SPAD meter measurement in rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Ivanov, A.G.; Wilson, K.E.; Miskiewicz, E.; Krol, M.; Öquist, G. Energy sensing and photostasis in photoautotrophs. In Sensing, Signaling and Cell Adaptation; Storey, K.B., Storey, J.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 243–255. [Google Scholar]
- Toth, S.Z.; Schansker, G.; Kissimon, J.; Kovacs, L.; Garab, G.; Strasser, R.J. Biophysical studies of photosystem II-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.). J. Plant Physiol. 2005, 162, 181–194. [Google Scholar] [CrossRef]
- Ma, X.; Ren, X.; Gao, H.; Wu, X.; Chen, K.; Khan, R. Proteomic, transcriptomic, biochemical, and physio-anatomical analyses provide insights into energy metabolism, light usage, and photosynthesis in cigar tobacco under different light intensities. Ind. Crops Prod. 2023, 198, 116651. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Li, Q.; Jin, R.; Chen, W.; Liu, X.-L.; Kong, F.-L.; Ke, Y.-P.; Shi, H.-C.; Yuan, J.-C. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrogen tolerances. J. Integr. Agric. 2019, 18, 1246–1256. [Google Scholar] [CrossRef]
- Xiong, D.; Yu, T.; Liu, X.; Li, Y.; Peng, S.; Huang, J. Heterogeneity of photosynthesis within leaves is associated with alteration of leaf structural features and leaf N content per leaf area in rice. Func. Plant Biol. 2015, 42, 687–696. [Google Scholar] [CrossRef]
- Villadangos, S.; González, C.; Munné-Bosch, S. Photo-oxidative stress leading to oxylipin accumulation may trigger drought-induced leaf abscission in olive trees. Physiol. Plant. 2023, 175, e13941. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Spatio-temporal heterogeneity in Arabidopsis thaliana leaves under drought stress. Plant Biol. 2012, 14, 118–128. [Google Scholar] [CrossRef]
- Walter, A.; Rascher, U.; Osmond, B. Transitions in photosynthetic parameters of midvein and interveinal regions of leaves and their importance during leaf growth and development. Plant Biol. 2004, 6, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Freschi, L.; Takahashi, C.A.; Cambui, C.A.; Semprebom, T.R.; Cruz, A.B.; Mioto, P.T.; de Melo Versieux, L.; Calvente, A.; Latansio-Aidar, S.R.; Aidar, M.P.M.; et al. Specific leaf areas of the tank bromeliad Guzmania monostachia perform distinct functions in response to water shortage. J. Plant Physiol. 2010, 167, 526–533. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Haldimann, P.; Strasser, R.J. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L.). Photosynth. Res. 1999, 62, 67–83. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Glaubitz, U.; Erban, A.; Kopka, J.; Zuther, E. Differentiation of the high night temperature response in leaf segments of rice cultivars with contrasting tolerance. Int. J. Mol. Sci. 2021, 22, 10451. [Google Scholar] [CrossRef]
- Jiang, C.D.; Wang, X.; Gao, H.Y.; Shi, L.; Chow, W.S. Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-Light tolerance in sorghum. Plant Physiol. 2011, 155, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B.G.; Zhang, Y.; Zambrano, M.A.; Ainsworth, E.A. Anatomical determinants of gas exchange and hydraulics vary with leaf shape in soybean. Ann. Bot. 2023, 131, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.A.; Strable, J.; Li, S.; Scanlon, M.J. On the mechanisms of development in monocot and eudicot leaves. New Phytol. 2019, 221, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, S.; Yano, K.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Relationship between the distribution of Na and the damages caused by salinity in the leaves of rice seedlings grown under a saline condition. Plant Prod. Sci. 2002, 5, 269–274. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Bosabalidis, A.; Patakas, A.; Vemmos, S. Effects of water stress on water relations, gas exchange and leaf structure of olive tree. Acta Hortic. 2000, 537, 241–247. [Google Scholar] [CrossRef]



| Soil Characteristic | Plant Growth Characteristic | ||||
|---|---|---|---|---|---|
| EC (μs·cm−1) | pH | VWC (%) | PH (cm) | BN | |
| Control | 230 ± 98 a | 7.9 ± 0.13 a | 24.5 ± 4.95 a | 13.4 ± 3.85 a | 72.72 ± 3.83 a |
| MS | 517 ± 110 b | 8.3 ± 0.08 b | 25.2 ± 1.04 a | 11 ± 4.45 a | 58.64 ± 8.58 b |
| SS | 1055 ± 105 c | 9.1 ± 0.3 c | 25.4 ± 2.17 a | 4.83 ± 1.33 b | 41.87 ± 7.3 c |
| LT (μm) | UE (μm) | PTT (μm) | STT (μm) | LE (μm) | PT/ST | ||
|---|---|---|---|---|---|---|---|
| Control | Tip | 154.12 ± 2.79 a | 18.07 ± 1.52 a | 58.21 ± 3.232 a | 64.11 ± 2.22 a | 7.60 ± 0.67 a | 0.91 ± 0.04 a |
| Base | 136.91 ± 6.27 b | 18.14 ± 1.75 a | 52.33 ± 4.26 a | 55.8 ± 3.76 b | 7.41 ± 0.76 a | 0.94 ± 0.04 a | |
| MS | Tip | 140.68 ± 6.03 b | 15.13 ± 1.16 b | 48.61 ± 1.87 b | 67.6 ± 6.46 a | 8.09 ± 1.09 a | 0.73 ± 0.09 b |
| Base | 140.71 ± 5.97 b | 14.96 ± 1.51 b | 55.92 ± 2.02 a | 59.83 ± 3.54 bc | 7.76 ± 0.71 a | 0.94 ± 0.05 a | |
| SS | Tip | 110.10 ± 6.27 c | 15.48 ±1.17 b | 33.58 ± 1.33 c | 50.67 ± 4.54 c | 8.05 ± 1.33 a | 0.67 ± 0.06 b |
| Base | 91.91 ± 3.47 d | 14.46 ± 1.24b | 28.65 ± 0.90 | 40.48 ± 2.12 d | 7.43 ± 1.11 a | 0.71 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, X.; Shao, Q.; Wang, Q.; Wang, S.; Yu, R.; Dong, S.; Xin, Z.; Xiao, H.; Cheng, J. Photosynthetic Performance and Heterogeneous Anatomical Structure in Prunus humilis under Saline–Alkaline Stress. Agriculture 2024, 14, 1606. https://doi.org/10.3390/agriculture14091606
Sun Y, Wang X, Shao Q, Wang Q, Wang S, Yu R, Dong S, Xin Z, Xiao H, Cheng J. Photosynthetic Performance and Heterogeneous Anatomical Structure in Prunus humilis under Saline–Alkaline Stress. Agriculture. 2024; 14(9):1606. https://doi.org/10.3390/agriculture14091606
Chicago/Turabian StyleSun, Yongjiang, Xiang Wang, Qiwen Shao, Qi Wang, Siyuan Wang, Ruimin Yu, Shubin Dong, Zhiming Xin, Huijie Xiao, and Jin Cheng. 2024. "Photosynthetic Performance and Heterogeneous Anatomical Structure in Prunus humilis under Saline–Alkaline Stress" Agriculture 14, no. 9: 1606. https://doi.org/10.3390/agriculture14091606
APA StyleSun, Y., Wang, X., Shao, Q., Wang, Q., Wang, S., Yu, R., Dong, S., Xin, Z., Xiao, H., & Cheng, J. (2024). Photosynthetic Performance and Heterogeneous Anatomical Structure in Prunus humilis under Saline–Alkaline Stress. Agriculture, 14(9), 1606. https://doi.org/10.3390/agriculture14091606







