People, Palms, and Productivity: Testing Better Management Practices in Indonesian Smallholder Oil Palm Plantations †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Farmer Selection
2.3. Training and Management
2.4. Experimental Setup
2.5. Yield Recording
2.6. Vegetative Measurements; Soil and Tissue Sample Collection
2.7. Processing and Analysis of Soil Samples
2.8. Processing and Analysis of Leaf Samples
2.9. Data Analysis
3. Results
3.1. Application of Management Practices
3.2. Bunch Weight and Yield
3.3. Tissue Nutrient Concentrations
3.4. Vegetative Growth
3.5. Costs and Benefits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-FAS. Production, Supply and Distribution Online: Historical Datasets, Oilseeds; USDA Foreign Agricultural Service: Washington, DC, USA, 2021. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/downloads (accessed on 30 December 2021).
- Corley, R.H.V. How much palm oil do we need? Environ. Sci. Policy 2009, 12, 134–139. [Google Scholar] [CrossRef]
- Molenaar, J.W.; Persch-Ort, M.; Lord, S.; Taylor, C.; Harms, J. Diagnostic Study on Indonesian Oil Palm Smallholders: Developing a Better Understanding of Their Performance and Potential; International Finance Corporation: Jakarta, Indonesia, 2013; p. 84. [Google Scholar]
- Euler, M.; Krishna, V.; Schwarze, S.; Siregar, H.; Qaim, M. Oil palm adoption, household welfare, and nutrition among smallholder farmers in Indonesia. World Dev. 2017, 93, 219–235. [Google Scholar] [CrossRef]
- Vermeulen, S.; Goad, N. Towards Better Practice in Smallholder Palm Oil Production; Natural Resource Issues Series No. 5; IIED: London, UK, 2006; p. 55. [Google Scholar]
- Jelsma, I.; Schoneveld, G.C.; Zoomers, A.; van Westen, A.C.M. Unpacking Indonesia’s independent oil palm smallholders: An actor-disaggregated approach to identifying environmental and social performance challenges. Land Use Policy 2017, 69, 281–297. [Google Scholar] [CrossRef]
- Monzon, J.P.; Li Lim, Y.; Tenorio, F.A.; Farrasati, R.; Pradiko, I.; Sugianto, H.; Donough, C.R.; Edreira, J.I.R.; Rahutomo, S.; Agus, F.; et al. Agronomy explains large yield gaps in smallholder oil palm fields. Agric. Syst. 2023, 210, 103689. [Google Scholar] [CrossRef]
- Hoffmann, M.P.; Castaneda Vera, A.; Wijk, M.T.v.; Giller, K.E.; Oberthür, T.; Donough, C.; Whitbread, A.M. Simulating potential growth and yield of oil palm (Elaeis guineensis) with PALMSIM: Model description, evaluation and application. Agric. Syst. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Abood, S.A.; Lee, J.S.H.; Burivalova, Z.; Garcia-Ulloa, J.; Koh, L.P. Relative contributions of the logging, fiber, oil palm, and mining industries to forest loss in Indonesia. Conserv. Lett. 2015, 8, 58–67. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Hergoualc’h, K.; Verchot, L.V. Opportunities for reducing greenhouse gas emissions in tropical peatlands. Proc. Natl. Acad. Sci. USA 2010, 107, 19655–19660. [Google Scholar] [CrossRef]
- Smit, H.H.; Meijaard, E.; van der Laan, C.; Mantel, S.; Budiman, A.; Verweij, P. Breaking the link between environmental degradation and oil palm expansion: A method for enabling sustainable oil palm expansion. PLoS ONE 2013, 8, e68610. [Google Scholar] [CrossRef]
- Fairhurst, T.; Griffiths, W.; Rankine, I. TCCL Field Handbooks of Oil Palm Agronomy; Tropical Crop Consultants: Kent, UK, 2019. [Google Scholar]
- Woittiez, L.S.; van Wijk, M.T.; Slingerland, M.; van Noordwijk, M.; Giller, K.E. Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 2017, 83, 57–77. [Google Scholar] [CrossRef]
- Euler, M.; Hoffmann, M.P.; Fathoni, Z.; Schwarze, S. Exploring yield gaps in smallholder oil palm production systems in eastern Sumatra, Indonesia. Agric. Syst. 2016, 146, 111–119. [Google Scholar] [CrossRef]
- Martin, S.; Rieple, A.; Chang, J.; Boniface, B.; Ahmed, A. Small farmers and sustainability: Institutional barriers to investment and innovation in the Malaysian palm oil industry in Sabah. J. Rural Stud. 2015, 40, 46–58. [Google Scholar] [CrossRef]
- Woittiez, L.S.; Slingerland, M.; Rafik, R.; Giller, K.E. Nutritional imbalance in smallholder oil palm plantations in Indonesia. Nutr. Cycl. Agroecosystems 2018, 111, 73–86. [Google Scholar] [CrossRef]
- Woittiez, L.S.; Turhina, S.; Deccy, D.; Slingerland, M.; Van Noordwijk, M.; Giller, K.E. Fertiliser application practices and nutrient deficiencies in smallholder oil palm plantations in Indonesia. Exp. Agric. 2018, 55, 543–559. [Google Scholar] [CrossRef]
- Jelsma, I.; Woittiez, L.S.; Ollivier, J.; Dharmawan, A.H. Do wealthy farmers implement better agricultural practices? An assessment of implementation of good agricultural practices among different types of independent oil palm smallholders in Riau, Indonesia. Agric. Syst. 2019, 170, 63–76. [Google Scholar] [CrossRef]
- Hutabarat, S.; Slingerland, M.; Rietberg, P.; Dries, L. Costs and benefits of certification of independent oil palm smallholders in Indonesia. Int. Food Agribus. Manag. Rev. 2018, 21, 681–700. [Google Scholar] [CrossRef]
- Molenaar, J.W.; Orth, M.; Lord, S.; Meekers, P.; Taylor, C.; Darto, M.; Elson, D.; Ginting, L. Analysis of the Agronomic and Institutional Constraints to Smallholder Yield Improvement in Indonesia; AidEnvironment and Global Sustainability Associates: Amsterdam, The Netherlands, 2010; p. 74. [Google Scholar]
- Sugianto, H.; Monzon, J.P.; Pradiko, I.; Tenorio, F.A.; Lim, Y.L.; Donough, C.R.; Sunawan; Rahutomo, S.; Agus, F.; Cock, J.; et al. First things first: Widespread nutrient deficiencies limit yields in smallholder oil palm fields. Agric. Syst. 2023, 210, 103709. [Google Scholar] [CrossRef]
- Jelsma, I.; Slingerland, M.; Giller, K.E.; Bijman, J. Collective action in a smallholder oil palm production system in Indonesia: The key to sustainable and inclusive smallholder palm oil? J. Rural Stud. 2017, 54, 198–210. [Google Scholar] [CrossRef]
- Sidhu, M.; Kong, C.; Sinuraya, Z.; Kurniawan, M.; Hasyim, A. Resumption of manuring and its impact on the nutrient status, growth and yield of unfertilised oil palm. Planter 2009, 85, 675–689. [Google Scholar] [CrossRef]
- Griffiths, W.; Fairhurst, T. Implementation of best management practices in an oil palm rehabilitation project. Better Crops Int. 2003, 17, 16–19. [Google Scholar]
- Rhebergen, T.; Fairhurst, T.; Whitbread, A.; Giller, K.E.; Zingore, S. Yield gap analysis and entry points for improving productivity on large oil palm plantations and smallholder farms in Ghana. Agric. Syst. 2018, 165, 14–25. [Google Scholar] [CrossRef]
- Atta-Ankomah, R.; Danso-Mensah, K. Best management practices for sustainable oil palm production: The case of smallholder farmers’ adoption in Ghana. For. Trees Livelihoods 2022, 31, 123–138. [Google Scholar] [CrossRef]
- Rankine, I.R.; Fairhurst, T.H. Field Handbook: Oil Palm Series, Volume 3—Mature, 2nd ed.; Potash & Phosphate Institute (PPI): Singapore, 1999; Volume 3, p. 135. [Google Scholar]
- Woittiez, L.S.; Haryono, S.; Turhina, S.; Dani, H.; Dukan, T.P.; Smit, H.H. Smallholder Oil Palm Handbook Module 2: Harvesting, Grading, Transport, 3rd ed.; Wageningen University and SNV International Development Organisation: Wageningen, The Netherlands, 2016; 25p. [Google Scholar]
- Woittiez, L.S.; Haryono, S.; Turhina, S.; Dani, H.; Dukan, T.P.; Smit, H.H. Smallholder Oil Palm Handbook Module 3: Plantation Maintenance, 3rd ed.; Wageningen University and SNV International Development Organisation: Wageningen, The Netherlands, 2016; 53p. [Google Scholar]
- Chapman, G.W.; Gray, H.M. Leaf analysis and the nutrition of the oil palm (Elaeis-guineensis Jacq). Ann. Bot. 1949, 13, 415–433. [Google Scholar] [CrossRef]
- Fairhurst, T. Management of Nutrients for Efficient Use in Smallholder Oil Palm Plantations. Ph.D. Thesis, Wye College, University of London, London, UK, 1996; p. 211. [Google Scholar]
- Foster, H.L.; Prabowo, N.E. Partition and transfer of nutrients in the reserve tissues and leaves of oil palm. In Proceedings of the Workshop on Nutrient Needs in Oil Palm, Singapore, 17–18 October 2006; p. 18. [Google Scholar]
- Williams, S.E.; van Noordwijk, M.; Penot, E.; Healey, J.R.; Sinclair, F.L.; Wibawa, G. On-farm evaluation of the establishment of clonal rubber in multistrata agroforests in Jambi, Indonesia. Agrofor. Syst. 2001, 53, 227–237. [Google Scholar] [CrossRef]
- Rhebergen, T.; Zingore, S.; Giller, K.E.; Frimpong, C.A.; Acheampong, K.; Ohipeni, F.T.; Panyin, E.K.; Zutah, V.; Fairhurst, T. Closing yield gaps in oil palm production systems in ghana through best management practices. Eur. J. Agron. 2020, 115, 19. [Google Scholar] [CrossRef]
- Ollagnier, M.; Ochs, R. Management of mineral nutrition in industrial oil palm plantations. Fertilizer savings. Oléagineux 1981, 36, 409–421. [Google Scholar]
- Dubos, B.; Baron, V.; Bonneau, X.; Flori, A.; Ollivier, J. High soil calcium saturation limits the use of leaf potassium diagnosis when KCl is applied in oil palm plantations. Exp. Agric. 2017, 54, 794–804. [Google Scholar] [CrossRef]
- Woittiez, L.S. On Yield Gaps and Better Management Practices in Indonesian Smallholder Oil Palm Plantations. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019; pp. xii + 287. [Google Scholar]
- Daliparthy, J.; Barker, A.V.; Mondal, S.S. Potassium fractions with other nutrients in crops—A review focusing on the tropics. J. Plant Nutr. 1994, 17, 1859–1886. [Google Scholar] [CrossRef]
- Corrado, F.; Quencez, P.; Tailliez, B. Boron deficiency in oil palm. Symptoms and corrections. Oléagineux 1992, 47, 719–725. [Google Scholar]
- Caliman, J.P.; Southworth, A.; Jatmika, A.; Bangun, D.; Asmono, D.; Sutarta, E.S.; Pamin, K.; Guritno, P.; Prawirosukarto, S.; Wahyono, T.; et al. Effect of drought and haze on the performance of oil palm. In Proceedings of the International Oil Palm Conference ‘Commodity of the Past, Today, and the Future’, Bali, Indonesia, 23–25 September 1998; pp. 250–274. [Google Scholar]
- Breure, C.J. Preliminary results from an oil palm density x fertiliser experiment on young volcanic soils in West New Britain. In Proceedings of the International Developments in Oil Palm: Proceedings of the Malaysian International Agricultural Oil Palm Conference, Kuala Lumpur, Malaysia, 14–17 June 1976; pp. 192–207. [Google Scholar]
- Teuscher, M.; Gérard, A.; Brose, U.; Buchori, D.; Clough, Y.; Ehbrecht, M.; Hölscher, D.; Irawan, B.; Sundawati, L.; Wollni, M.; et al. experimental biodiversity enrichment in oil-palm-dominated landscapes in Indonesia. Front. Plant Sci. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Uexküll, H.v.; Henson, I.E.; Fairhurst, T. Canopy management to optimize yield. In Oil Palm Management for Large and Sustainable Yields; Fairhurst, T., Härdter, R., Eds.; Potash & Phosphate Institute of Canada, Potash & Phosphate Institute, International Potash Institute: Singapore, 2003; pp. 163–180. [Google Scholar]
- Corley, R.H.V. Ripening, harvesting and oil extraction. Planter 2001, 77, 507–524. [Google Scholar]
- Donough, C.; Witt, C.; Fairhurst, T. Yield intensification in oil palm using BMP as a management tool. In Proceedings of the International Oil Palm Conference, Yogyakarta, Indonesia, 1–3 June 2010. [Google Scholar]
- Lee, J.S.H.; Ghazoul, J.; Obidzinski, K.; Koh, L.P. Oil palm smallholder yields and incomes constrained by harvesting practices and type of smallholder management in Indonesia. Agron. Sust. Dev. 2014, 34, 501–513. [Google Scholar] [CrossRef]
- Mettauer, R.; Baron, V.; Turinah; Demitria, P.; Smit, H.; Alamsyah, Z.; Penot, E.; Bessou, C.; Chambon, B.; Ollivier, J.; et al. Investigating the links between management practices and economic performances of smallholders’ oil palm plots. A case study in Jambi province, Indonesia. Agric. Syst. 2021, 194, 103274. [Google Scholar] [CrossRef]
- Foster, H.L.; Mohammed, A.T. Variation in the fertilizer requirements of oil palm in peninsular Malaysia. I. Within the same soil series. PORIM Bull. 1988, 1–9. [Google Scholar]
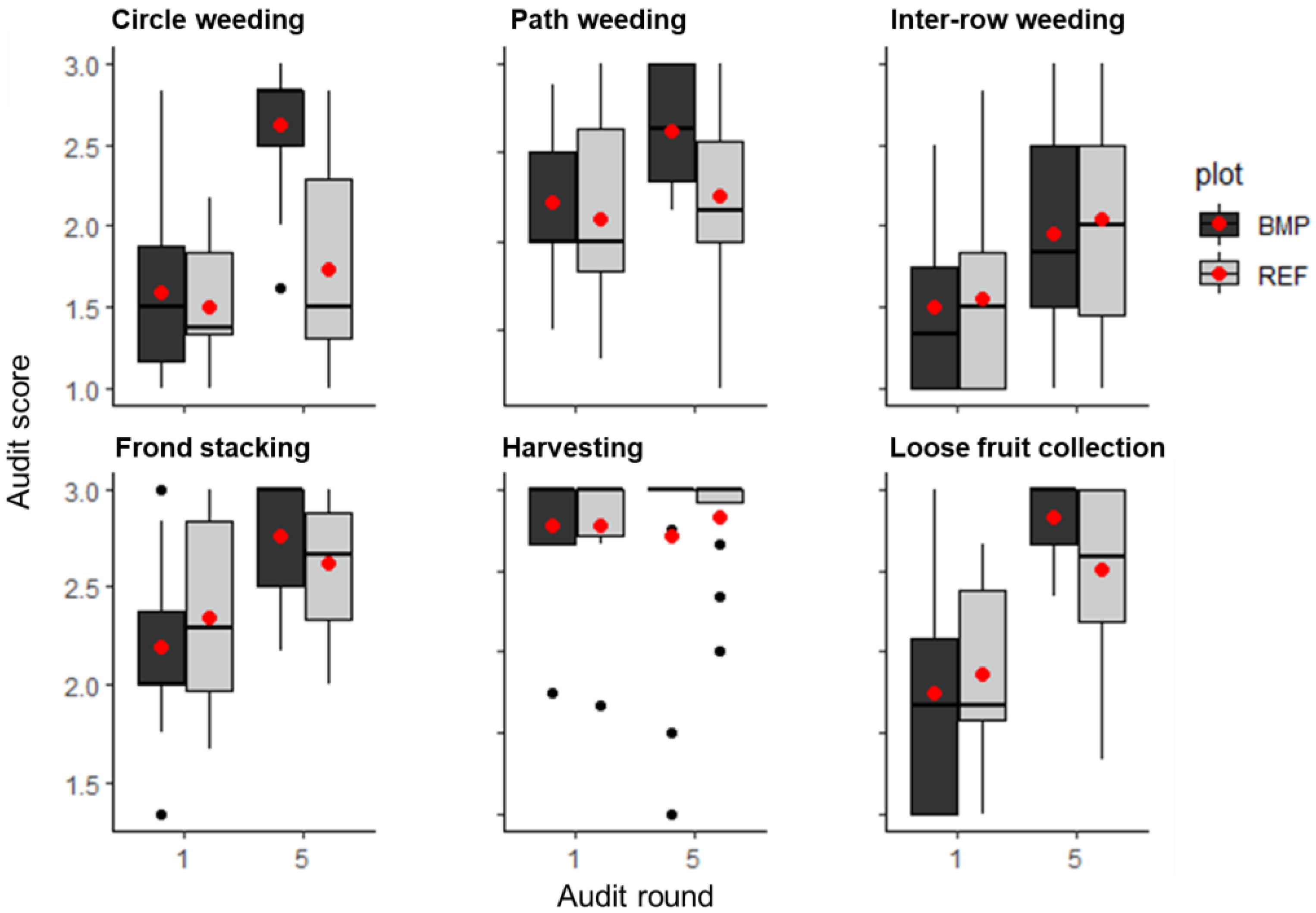

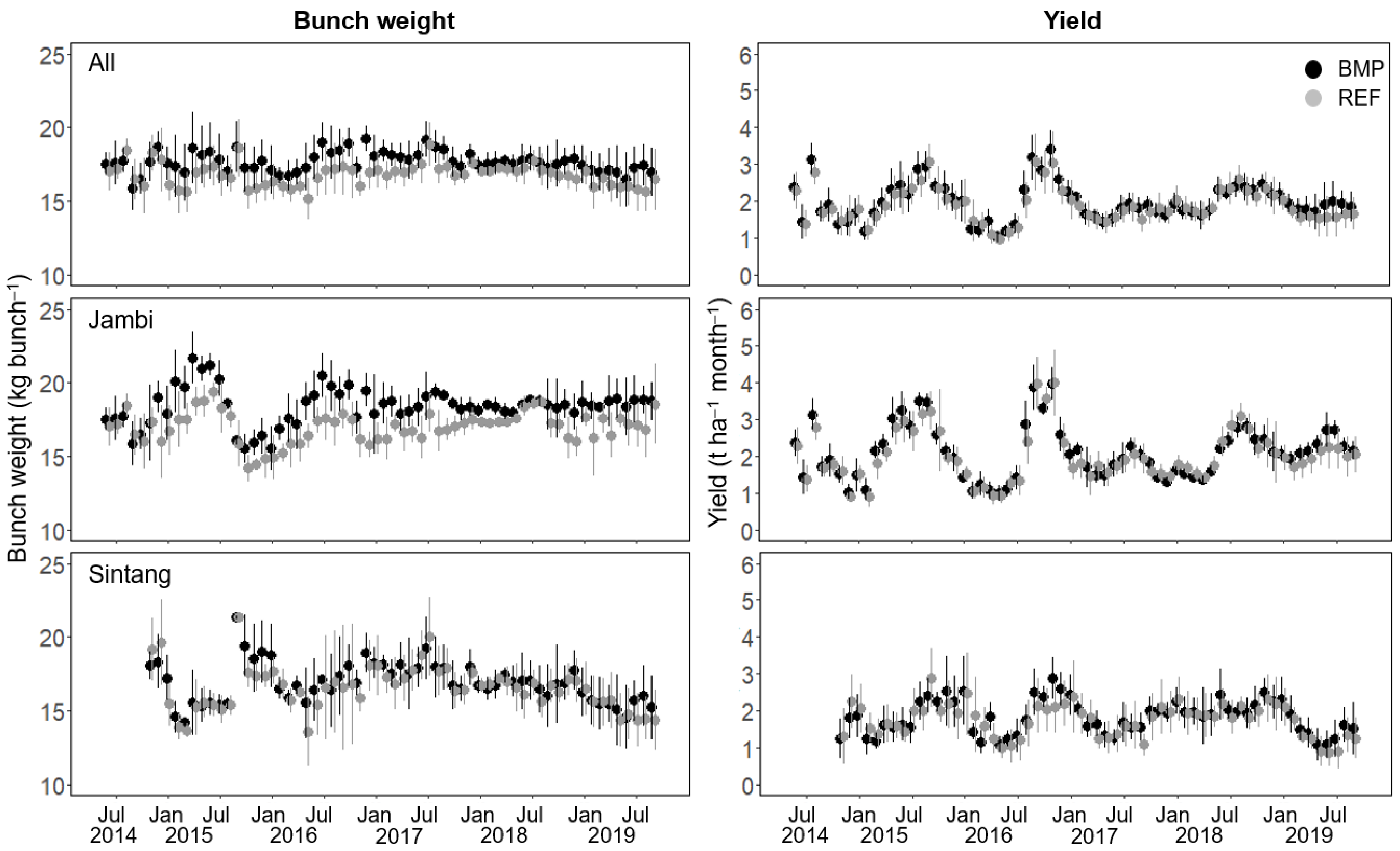
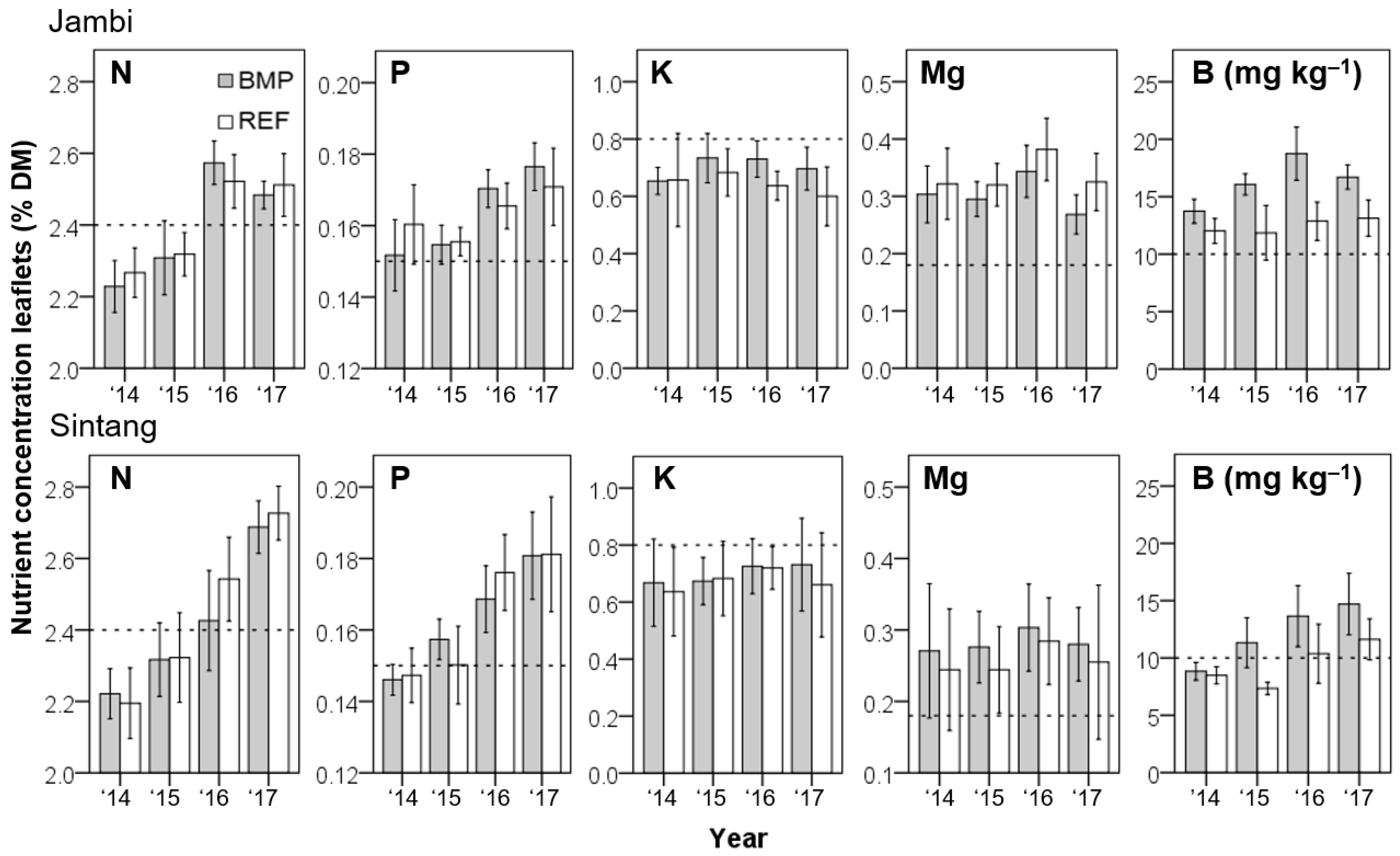
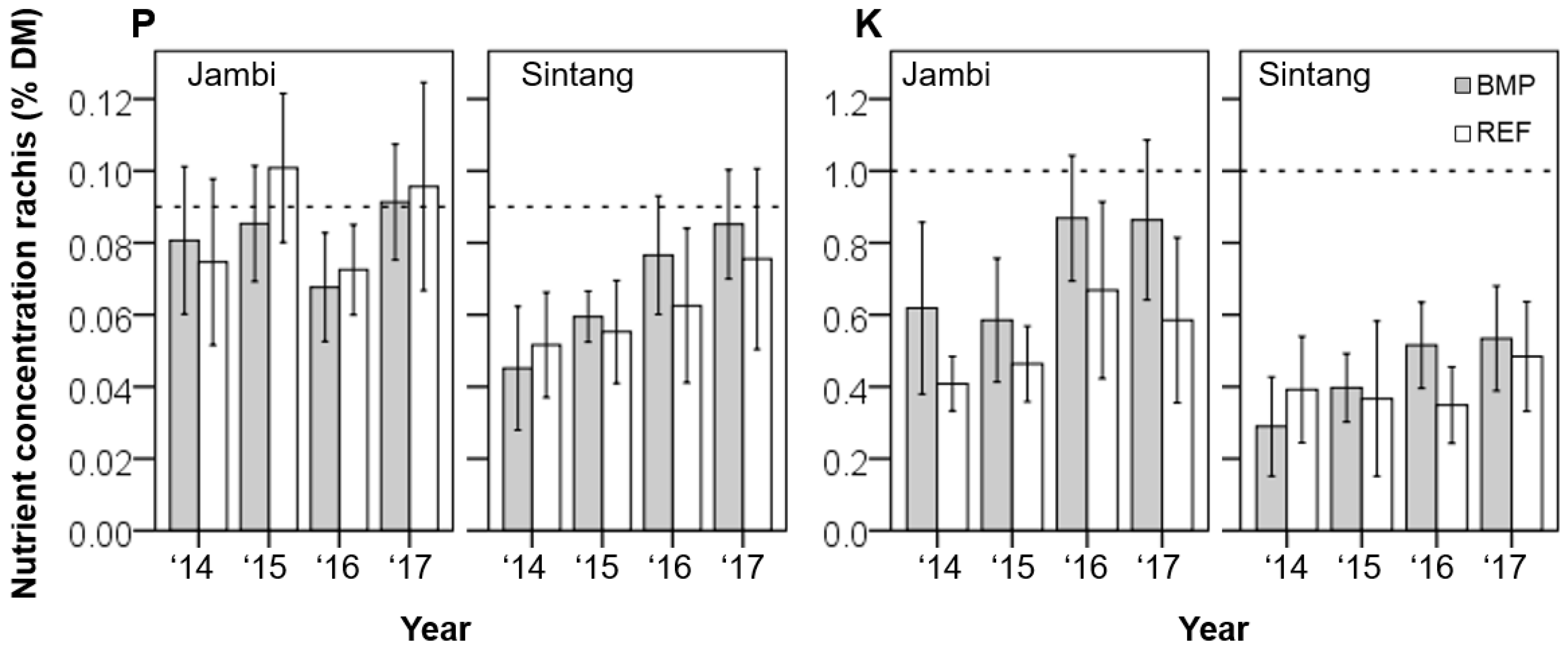

| Code | Palm Density | Soil Properties | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Texture | SOC a | pH b | N b | P b | Ca b | Mg b | K b | |||
| (palms ha−1) | (%) | (%) | (mg kg−1) | (mmol kg−1) | ||||||
| Jambi | ||||||||||
| J1 | 132 | Clay | 3.8 | 4.2 | 0.14 | 9.0 | 17.0 | 7.51 | 0.83 | 2017: Flooding in REF plot |
| J2 | 130 | Clay | 4.9 | 4.5 | 0.20 | 33.8 | 29.4 | 10.5 | 1.13 | |
| J3 | 142 | Clay | 3.1 | 4.6 | 0.14 | 7.0 | 17.5 | 8.45 | 0.93 | Full legume cover crop |
| J5 | 137 | Clay | 3.8 | 4.2 | 0.15 | 24.7 | 17.0 | 4.89 | 1.13 | 2017: Flooding in REF plot |
| J6 | 135 | Clay | 4.3 | 4.3 | 0.17 | 12.0 | 30.0 | 11.0 | 0.85 | |
| J4 c | 153 | Clay | 3.5 | 4.6 | 0.14 | 28.9 | 33.4 | 9.02 | 1.80 | |
| Sintang | ||||||||||
| S1 | 144 | Silt loam | 4.6 | 4.8 | 0.06 | 26.9 | 6.10 | 0.53 | 1.93 | Palm density BMP too high |
| S4 | 135 | Sandy loam | 4.5 | 4.9 | 0.09 | 11.5 | 4.20 | 0.90 | 1.00 | |
| S6 | 136 | Clay loam | 5.4 | 4.4 | 0.13 | 94.7 | 4.53 | 0.53 | 2.28 | Sloping |
| S7 | 135 | Clay loam | 4.6 | 4.9 | 0.10 | 73.7 | 6.45 | 0.85 | 1.95 | Sloping; eroded |
| S8 | 134 | Loam | 3.8 | 4.8 | 0.08 | 25.2 | 2.85 | 0.48 | 1.40 | Sloping |
| S3 c | 137 | Clay loam | 7.4 | 4.9 | 0.10 | 43.4 | 11.7 | 1.95 | 0.50 | |
| S3P c,d | 136 | Peat | 22.6 | 4.1 | 0.82 | 174.4 | 63.2 | 5.73 | 2.75 | Front + back of field (0.9 ha) |
| S5 c | 138 | Sandy loam | 5.2 | 4.9 | 0.06 | 12.2 | 14.1 | 1.28 | 1.15 | |
| S5P c,d | 134 | Peat | 20.5 | 4.1 | 0.92 | 73.4 | 28.3 | 2.78 | 3.18 | Centre of field (0.9 ha) |
| S2 e | 135 | Loam | 7.3 | 4.9 | 0.14 | 66.2 | 11.1 | 1.03 | 1.80 | Sloping |
| S2P d,e | 136 | Peat | 21.8 | 4.2 | 0.76 | 228.9 | 31.3 | 2.65 | 3.53 | Centre of field (0.8 ha) |
| Category | Activity | Method | Frequency |
|---|---|---|---|
| Weeding | Establishing weeded circles | Manual/mechanical/chemical | 3 rounds/year |
| Establishing harvesting paths | Manual/mechanical/chemical | 3 rounds/year | |
| Removing woody weeds | Manual/mechanical/chemical | 2 rounds/year | |
| Cutting inter-row weeds to knee height | Manual/mechanical | 2 rounds/year | |
| Pruning | Pruning to 40 leaves per palm | Manual | 2 rounds/year |
| Stacking fronds in U-box | Manual | At pruning/harvesting | |
| Harvesting | Harvesting when bunches are fully ripe (at least 1 loose fruit) | Manual | Every 10 days |
| Collecting bunches separately at roadside | Manual/with motorbike | At harvesting | |
| Collecting all loose fruits | Manual | At harvesting | |
| Counting bunches and recording bunch quality | Manual | At harvesting | |
| Recording yield per plot | Manual | At harvesting | |
| Other | Making footbridges over canals | Manual | At project start |
| Nutrient | Amount (kg palm−1 year−1) | Applied as | Remarks | |||
|---|---|---|---|---|---|---|
| 2014 a | 2015 | 2016 b | 2017 | |||
| Jambi | ||||||
| N | 0.8 | 0.9 | 0.9 | 0.9 | Urea | Two splits |
| P | 0.6 | 0.2 | 0.2 | 0.2 | Rock phosphate | One split |
| K | 1.2 | 1.5 | 1.5 | 1.5 | KCl | Two splits |
| B | 0.03 | 0.03 | 0.03 | 0.03 | Fertiliser borate | One split |
| Sintang | ||||||
| N | 0.5 | 1.2 | 3.2 | 1.2 | Urea; EFB (2016) | Two splits |
| P | 0 | 0.6 | 0.4 | 0.2 | Rock phosphate; EFB (2016) | One split |
| K | 0.7 | 1.3 | 6.7 | 1.3 | Korn Kali B; EFB (2016) | Two splits |
| Mg | 0.1 | 0.2 | 0.5 | 0.2 | Korn Kali B; kieserite; EFB (2016) | Two splits |
| B | 0.02 | 0.03 | 0.01 | 0.03 | Korn Kali B; EFB (2016) | Two splits |
| Cu | 0 | 0.05 | 0 | 0 | CuSO4 (2015) | One split, on peat |
| Zn | 0 | 0.08 | 0 | 0 | ZnSO4 (2015) | One split, on peat |
| Location | Year | Yield Change a | Change in Benefits b | Change in Costs c | Change in Profit d | Change in Profit e ($) |
|---|---|---|---|---|---|---|
| (kg ha−1 year−1) | (Million Rp ha−1 year−1) | USD | ||||
| Jambi | 2015 | 2151 | 2.58 | 2.96 | −0.38 | −25.20 |
| 2016 | 492 | 0.59 | 2.77 | −2.18 | −145.27 | |
| 2017 | 1117 | 1.34 | 2.98 | −1.64 | −109.34 | |
| 2018 f | −1010 | −1.21 | 2.90 | −4.11 | −274.16 | |
| 2019 f | 2362 | 2.83 | 2.90 | −0.07 | −4.47 | |
| Average | 1022 | 1.23 | 2.90 | −1.67 | −111.55 | |
| Sintang | 2015 | −105 | −0.13 | 4.44 | −4.57 | −304.41 |
| 2016 | 2861 | 3.43 | 1.72 | 1.71 | 114.18 | |
| 2017 | 999 | 1.20 | 0.70 | 0.50 | 33.25 | |
| 2018 f | 621 | 0.75 | 2.29 | −1.54 | −102.95 | |
| 2019 f | 1939 | 2.33 | 2.29 | 0.04 | 2.42 | |
| Average | 1263 | 1.52 | 2.29 | −0.77 | −51.64 | |
| Combined | 2015 | 1023 | 1.23 | 3.70 | −2.47 | −164.81 |
| 2016 | 1677 | 2.01 | 2.24 | −0.23 | −15.21 | |
| 2017 | 1058 | 1.27 | 1.84 | −0.57 | −38.04 | |
| 2018 f | −194 | −0.23 | 2.60 | −2.83 | −188.89 | |
| 2019 f | 2150 | 2.58 | 2.60 | −0.02 | −1.36 | |
| Average | 1143 | 1.37 | 2.60 | −1.23 | −81.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woittiez, L.S.; Slingerland, M.; Noordwijk, M.v.; Silalahi, A.J.; Heerwaarden, J.v.; Giller, K.E. People, Palms, and Productivity: Testing Better Management Practices in Indonesian Smallholder Oil Palm Plantations. Agriculture 2024, 14, 1626. https://doi.org/10.3390/agriculture14091626
Woittiez LS, Slingerland M, Noordwijk Mv, Silalahi AJ, Heerwaarden Jv, Giller KE. People, Palms, and Productivity: Testing Better Management Practices in Indonesian Smallholder Oil Palm Plantations. Agriculture. 2024; 14(9):1626. https://doi.org/10.3390/agriculture14091626
Chicago/Turabian StyleWoittiez, Lotte S., Maja Slingerland, Meine van Noordwijk, Abner J. Silalahi, Joost van Heerwaarden, and Ken E. Giller. 2024. "People, Palms, and Productivity: Testing Better Management Practices in Indonesian Smallholder Oil Palm Plantations" Agriculture 14, no. 9: 1626. https://doi.org/10.3390/agriculture14091626
APA StyleWoittiez, L. S., Slingerland, M., Noordwijk, M. v., Silalahi, A. J., Heerwaarden, J. v., & Giller, K. E. (2024). People, Palms, and Productivity: Testing Better Management Practices in Indonesian Smallholder Oil Palm Plantations. Agriculture, 14(9), 1626. https://doi.org/10.3390/agriculture14091626







