Nutrient Formulation—A Sustainable Approach to Combat PRSV and Enhance Productivity in Papaya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nutrient Formulation
2.2. Standardization of Time of Nutrient Formulation Foliar Spray on Growth, Yield, Quality and PRSV Management in Papaya
2.3. Efficacy of Nutrient Formulation on Growth, Yield, Quality and PRSV Tolerance in Commercial Papaya Varieties
2.4. Observations
2.4.1. Growth Attributes
2.4.2. Yield Attributes
2.4.3. Quality Attributes
2.4.4. Papain Attributes
2.4.5. PRSV Disease Incidence (%)
2.5. Estimation of Leaf Petiole Nutrient Content and Enzyme Activities
2.6. Estimation of Leaf Metabolites
3. Statistical Analysis
4. Results
4.1. Effect of Nutrient Formulation on Growth, Yield, Quality and Papain Activity in Papaya
4.2. Efficacy of Nutrient Formulation
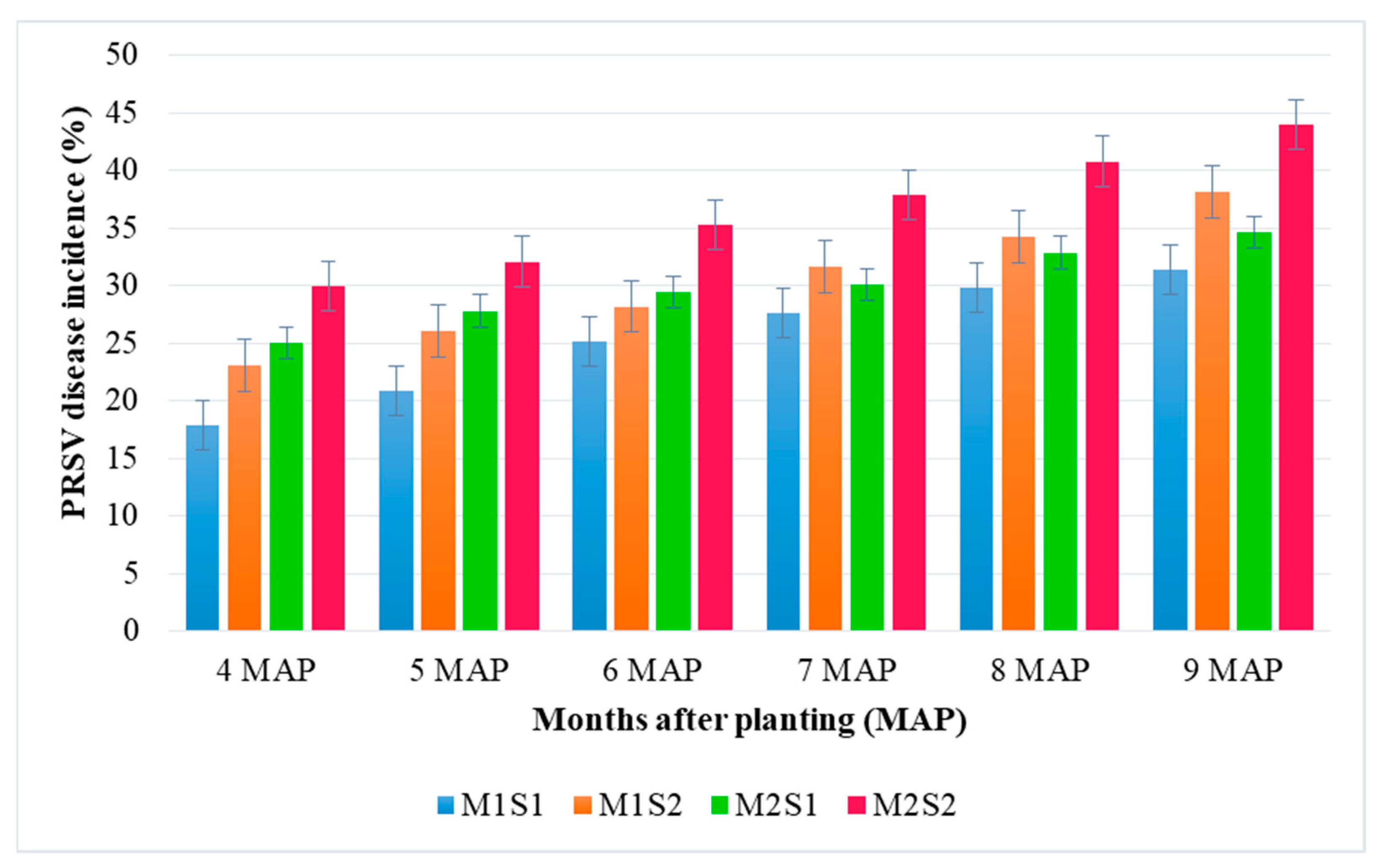
4.2.1. Effect of Nutrient Formulation on Growth, Yield, Quality and PRSV Tolerance in Commercial Varieties of Papaya
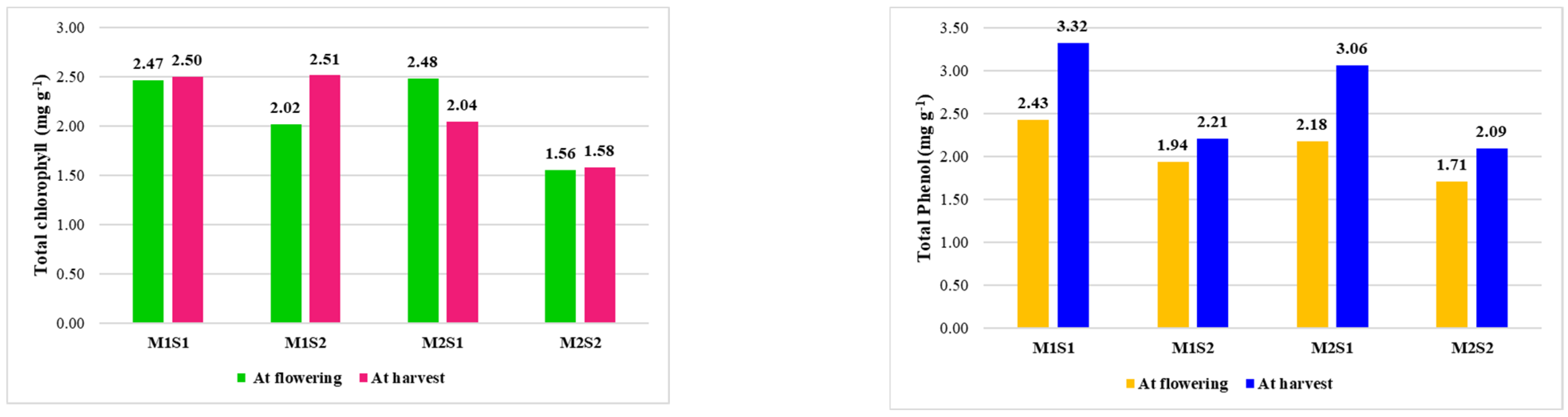
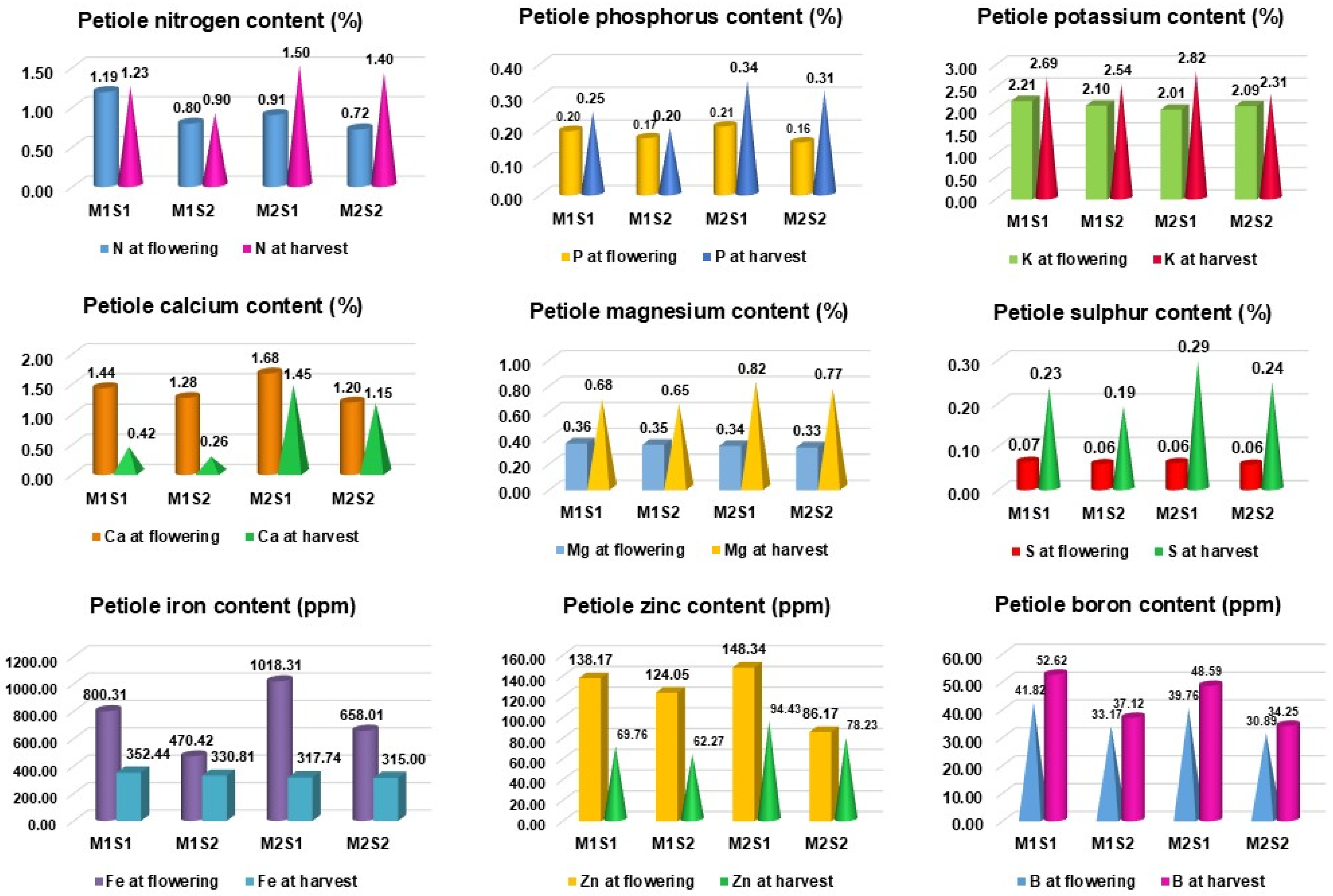
4.2.2. Effect of Nutrient Formulation on Biochemical Attributes, Petiole Nutrient Content and Enzyme Activities in Commercial Varieties of Papaya
4.2.3. Effect of Nutrient Formulation Spray on Papain Attributes
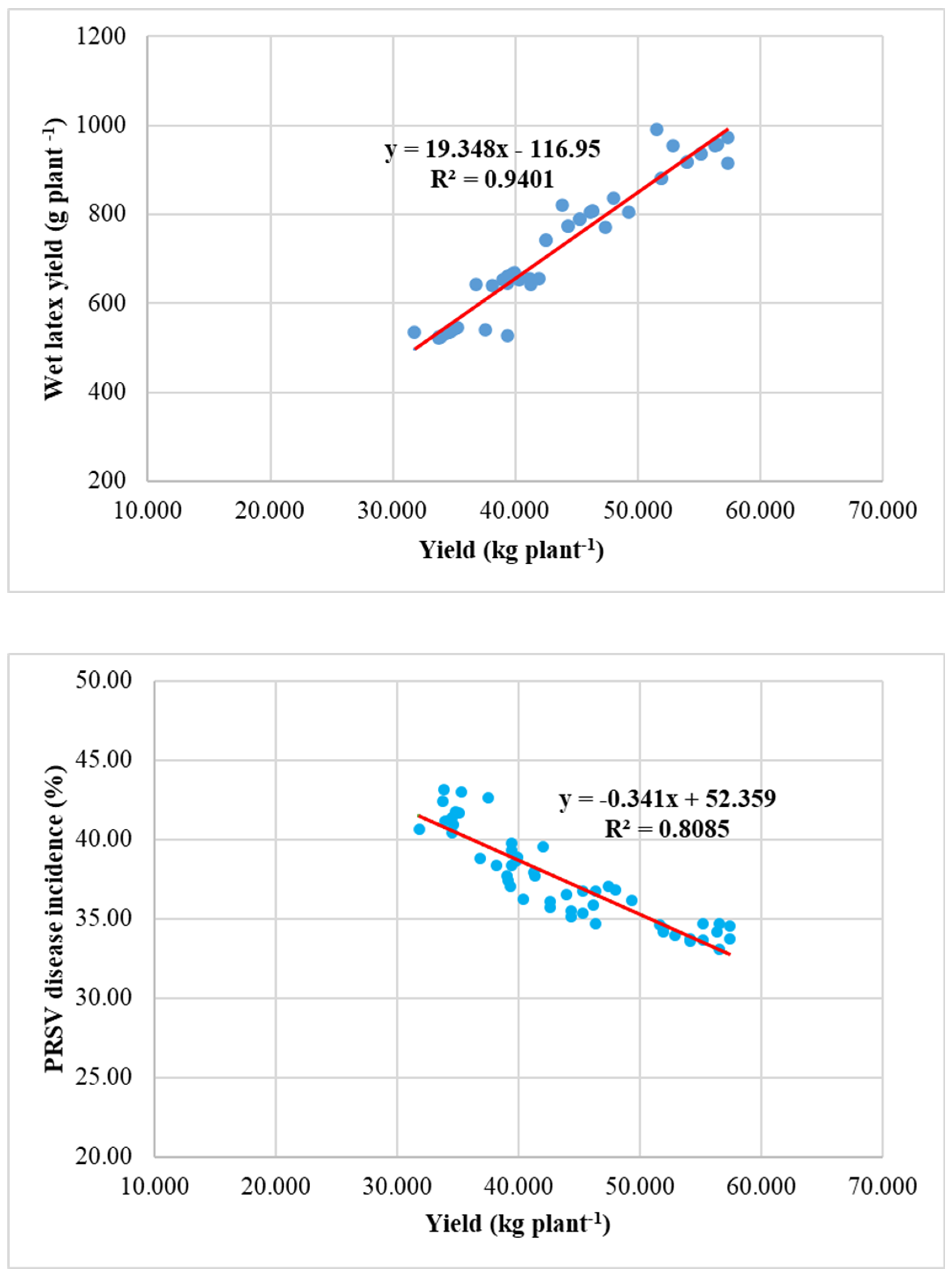
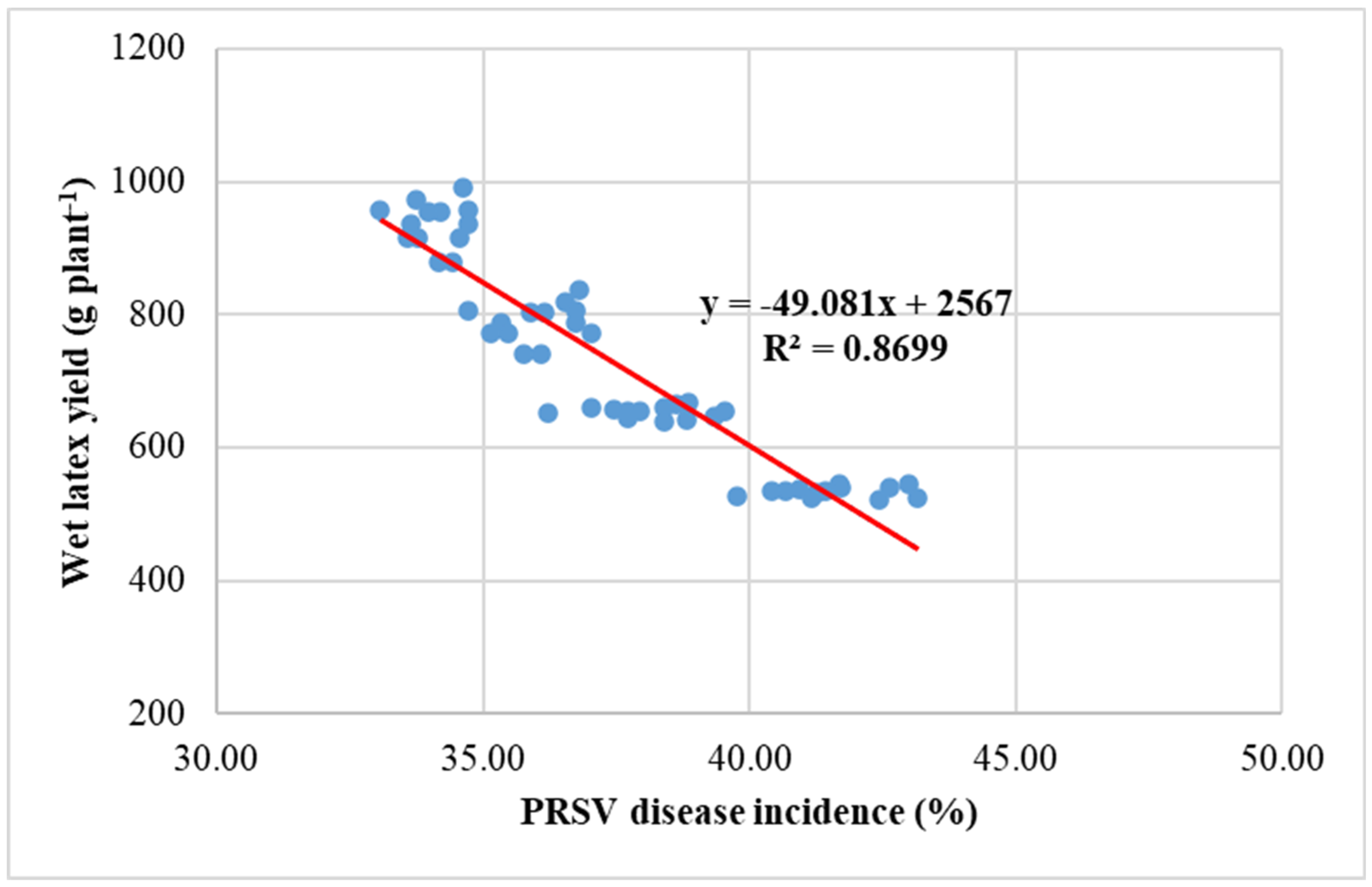
4.2.4. Relationship Between Yield, PRSV Disease Incidence and Wet Latex Yield
4.2.5. Influence of Nutrient Formulation on Leaf Metabolites
5. Discussion
5.1. Influence of Nutrient Formulation on Growth Parameters
5.2. Influence of Nutrient Formulation on Leaf Petiole Nutrient
5.3. Influence of Nutrient Formulation on Biochemical Parameters
5.4. Influence of Nutrient Formulation on Yield and Yield Attributes
5.5. Influence of Nutrient Formulation on Fruit Quality Attributes
5.6. Influence of Nutrient Formulation on Shelf Life and Papain Activity
5.7. Influence of Nutrient Formulation on PRSV Disease Incidence
5.8. Influence of Nutrient Formulation on Metabolites of Leaf
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/statistics/578039/world-papaya-production/#:~:text=This%20statistic%20depicts%20the%20production,million%20metric%20tons%20in%202021 (accessed on 4 October 2024).
- Agricultural Statistics at a Glance 2022–23. Available online: https://desagri.gov.in/wp-content/uploads/2023/05/Agricultural-Statistics-at-a-Glance-2022.pdf (accessed on 20 October 2024).
- Crop Production Guide Horticulture Crops; Directorate of Horticulture and Plantation Crops and Tamil Nadu Agricultural University: Chennai and Coimbatore, India, 2020; pp. 1–405.
- Monika, G.; Soorianathasundaram, K.; Auxcilia, J.; Chitdeshwari, T.; Kavitha, C.; Muthulakshmi, P. Effect of foliar nutrition of calcium and sulphur on growth and yield of papaya (Carica papaya L.). Int. J. Chem. Stud. 2018, 6, 765–769. [Google Scholar]
- Monika, G.; Soorianathasundaram, K.; Auxcilia, J.; Chitdeshwari, T.; Kavitha, C. Effect of foliar nutrition of calcium and sulphur on pulp quality attributes and shelf-life of papaya (Carica papaya L.). Int. J. Chem. Stud. 2018, 6, 2728–2730. [Google Scholar]
- Kalleshwaraswamy, C.; Kumar, N.K. Transmission efficiency of Papaya ringspot virus by three aphid species. Phytopathology 2008, 98, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Purcifull, D.E.; Edwardson, J.R.; Hiebert, E.; Gonsalves, D. Papaya ringspot virus. CMI/AAB Descr. Plant Viruses 1984, 292. [Google Scholar]
- Thirugnanavel, A.; Balamohan, T.; Manoranjitham, S.; Karunakaran, G. Screening of Papaya (Carica papaya L.) Cultivars for Resistance to PRSV Under Polyhouse Conditions. Madras Agric. J. 2013, 100, 287–290. [Google Scholar]
- Warghane, A.; Saini, R.; Shri, M.; Andankar, I.; Ghosh, D.K.; Chopade, B.A. Application of nanoparticles for management of plant viral pathogen: Current status and future prospects. Virology 2024, 592, 109998. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, W.; Ma, C.; White, J.C.; Zhang, Z.; Adeel, M.; Zhou, T.; Rui, Y.; Xing, B. Engineered nanomaterials suppress Turnip mosaic virus infection in tobacco (Nicotiana benthamiana). Environ. Sci. Nano 2018, 5, 1685–1693. [Google Scholar] [CrossRef]
- John, K.S.; Anju, P.S.; Suja, G.; Mathew, J.; Shivay, Y.S. Zinc nutrition in tropical tuber crops: A review. Indian J. Agron. 2019, 64, 1–10. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; AbdAllah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Debnath, S.; Sharma, S.; Sharma, P.; Purohit, J. Role of nutrients in controlling the plant diseases in sustainable agriculture. Agric. Important Microbes Sustain. Agric. 2017, 2, 217–262. [Google Scholar]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Bhamare, U.U.; Mali, Y.S.; Shaikh, A.Z. Neem: As a natural medicine. Res. J. Pharmacogn. Phytochem. 2020, 12, 245–255. [Google Scholar]
- Elmer, W.; Datnoff, L. Mineral nutrition and suppression of plant disease. In Encyclopedia of Agriculture and Food Systems; LSU: Baton Rouge, LA, USA, 2014; pp. 231–244. [Google Scholar] [CrossRef]
- Karikari, S. Estimation of leaf area in papaya (Carica papaya) from leaf measurements. Trop. Agric. 1973, 50, 346. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Ranganna, S. Manual of Analysis Fruits and Vegetables; Tata McGraw-Hill: New Delhi, India, 1977; p. 634. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Japan. Soc. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Moore, D.J. The production and processing of papain. Bull. Trop. Dev. Res. Instt. 1984. [Google Scholar]
- Dhanam, S. Studies on Papaya Ring Spot Disease. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2006. [Google Scholar]
- Bhargava, B.; Chadha, K. Leaf nutrient guide for fruit crops. In Advances in Horticulture; Chadha, K., Pareek, O., Eds.; Malhothra Publishing House: New Delhi, India, 1993; Volume 2, pp. 973–1029. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Esterbauer, H.; Schwarzl, E.; Hayn, M. A rapid assay for catechol oxidase and laccase using 2-nitro-5-thiobenzoic acid. Anal. Biochem. 1977, 77, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Brueske, C.H. Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root-knot nematode, Meloidogyne incognita. Physiol. Plant Pathol. 1980, 16, 409–414. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Workers, 2nd ed.; Indian Council of Agricultural Research: New Delhi, India, 1967. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 20 October 2024).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2020. Available online: https://CRAN.R-project.org/package=agricolae, (accessed on 20 October 2024).
- Srivastava, R.; Aragno, M.; Sharma, A. Cow dung extract: A medium for the growth of pseudomonads enhancing their efficiency as biofertilizer and biocontrol agent in rice. Indian J. Microbiol. 2010, 50, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, K. Studies on the Efficacy of Foliar Spray of Nutrient Formulation on Growth, Yield, Quality and CMD Incidence in Cassava; Tamil Nadu Agricultural University: Coimbatore, India, 2013. [Google Scholar]
- Kumar, P.; Kumar, T.; Singh, S.; Tuteja, N.; Prasad, R.; Singh, J. Potassium: A key modulator for cell homeostasis. J. Biotechnol. 2020, 324, 198–210. [Google Scholar] [CrossRef]
- Kumar, K.N.; Kavitha, C.; Muthuvel, I.; Kalarani, M.; Elaiyabharathi, T. Foliar Application of Nutrient Formulation to Enhance Growth and PRSV Tolerance in Commercial Varieties of Papaya. Int. J. Plant Soil Sci. 2023, 35, 946–953. [Google Scholar] [CrossRef]
- Manjunatha, S.; Swamy, G.; Prakash, N.; Jagadeesha, R.; Chavan, M.; Shankarappa, K. Effect of Micronutrients and Silicon on Growth and Yield of Papaya cv. Red lady. J. Agric. Res. Technol. 2014, 39, 15–20. [Google Scholar]
- Subedi, A.; Shrestha, A.; Tripathi, K.; Shrestha, B. Effect of Foliar Spray of Boron and Zinc on Growth and Yield of Papaya (Carica papaya L.) cv. Red Lady in Chitwan, Nepal. Field Crop 2019, 2, 1–6. [Google Scholar]
- Singh, D.; Ghosh, S.; Paul, P.; Suresh, C. Effect of different micronutrients on growth, yield and quality of papaya (Carica papaya L.) cv. Ranchi. Acta Hortic. 2010, 851, 351–356. [Google Scholar] [CrossRef]
- Bhalerao, P.; Patel, B. Effect of foliar application of Ca, Zn, Fe and B on growth, yield and quality of papaya var. Taiwan Red Lady. Indian J. Hortic. 2015, 72, 325–328. [Google Scholar] [CrossRef]
- Bhalerao, P.; Patel, B. Effect of foliar application of Ca, Zn, Fe and B on physiological attributes, nutrient status, yield and economics of papaya (Carica papaya L.) cv. Taiwan Red lady. Madras Agric. J. 2012, 99, 298–300. [Google Scholar]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and biological functions. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; Volume 1, pp. 269–284. [Google Scholar]
- Reddy, P.V.K.; Gowda, V.N. Influence of greenhouse cultivation on fruit quality of ‘Red Lady’papaya. In Proceedings of the International Symposium on Tropical and Subtropical Fruits, Chiang Mai, Thailand, 12 March 2014; pp. 109–114. [Google Scholar]
- Gupta, R.; Verma, N.; Tewari, R.K. Micronutrient deficiency-induced oxidative stress in plants. Plant Cell Rep. 2024, 43, 213. [Google Scholar] [CrossRef]
- Everse, J. Heme Proteins. In Heme Proteins, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 532–538. [Google Scholar] [CrossRef]
- Yue, Z.; Chen, Y.; Chen, C.; Ma, K.; Tian, E.; Wang, Y.; Liu, H.; Sun, Z. Endophytic Bacillus altitudinis WR10 alleviates Cu toxicity in wheat by augmenting reactive oxygen species scavenging and phenylpropanoid biosynthesis. J. Hazard. Mater. 2021, 405, 124272. [Google Scholar] [CrossRef] [PubMed]
- Thakker, J.N.; Patel, S.; Dhandhukia, P.C. Induction of defense-related enzymes in banana plants: Effect of live and dead pathogenic strain of Fusarium oxysporum f. sp. cubense. Int. Sch. Res. Not. 2013, 2013, 601303. [Google Scholar] [CrossRef] [PubMed]
- Scibior, D.; Czeczot, H. Katalaza–budowa, właściwości, funkcje [Catalase: Structure, properties, functions]. Postep. Hig Med. Dosw. 2006, 60, 170–180. [Google Scholar]
- Fan, H.; Yan, X.; Fu, M.; Liu, D.; Awan, A.W.; Chen, P.; Rasheed, S.M.; Gao, L.; Zhang, R. Interactive effect of biological agents chitosan, Lentinan and Ningnanmycin on papaya ringspot virus resistance in papaya (Carica papaya L.). Molecules 2022, 27, 7474. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Xie, L.; Zheng, L.; Lin, Q. Induction of systemic resistance in tobacco against Tobacco mosaic virus by Bacillus spp. Biocontrol Sci. Technol. 2011, 21, 281–292. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [PubMed]
- Jian, W.; Zhang, D.-W.; Zhu, F.; Wang, S.-X.; Zhu, T.; Pu, X.-J.; Zheng, T.; Feng, H.; Lin, H.-H. Nitrate reductase-dependent nitric oxide production is required for regulation alternative oxidase pathway involved in the resistance to Cucumber mosaic virus infection in Arabidopsis. Plant Growth Regul. 2015, 77, 99–107. [Google Scholar] [CrossRef]
- Ranasinghe, C.; De Costa, D. Field Performance of Mixtures of Pseudomonas and Bacillus spp. in Managing Papaya Ringspot Virus Disease and their Effect on Plant Defense Enzyme Activity. Trop. Agric. Res. 2020, 31, 75–85. [Google Scholar]
- Sagar, S.; Singh, A.; Bala, J.; Chauhan, R.; Kumar, R.; Bhatia, R.K.; Walia, A. Insights into Cow Dung-Based Bioformulations for Sustainable Plant Health and Disease Management in Organic and Natural Farming System: A Review. J. Soil Sci. Plant Nutr. 2023, 24, 30–53. [Google Scholar] [CrossRef]
- Raja, M.E. Importance of micronutrients in the changing horticultural scenario in India. J. Hortic. Sci. 2009, 4, 1–27. [Google Scholar] [CrossRef]
- Thakur, S.; Sinha, A.; Ghosh Bag, A. Boron-a critical element for fruit nutrition. Commun. Soil Sci. Plant Anal. 2023, 54, 2899–2914. [Google Scholar] [CrossRef]
- Babu, R.; Tripathi, V. Impact of foliar application of NAA, Zinc and Boron on growth, yield and quality parameters of Guava (Psidium guajava L.). Progress. Agric. 2022, 22, 190–194. [Google Scholar] [CrossRef]
- Moganapathi, B.; Kavitha, C.; Pugalendhi, L.; Kalarani, M.K. Effect of Nutrient Formulation on Shelf Life and Quality Attributes of Papaya (Carica papaya L.). Biol. Forum—Int. J. 2022, 14, 348–352. [Google Scholar]
- Ishwariya, R.; Sivakumar, V.; Shanmugasundaram, K.; Vanitha, K.; Seenivasan, N.; Muthuvel, I. Nutri-hormonal Manipulation for Yield and Quality Improvement in Guava (Psidium guajava L.). Int. J. Environ. Clim. Change 2023, 13, 3494–3502. [Google Scholar] [CrossRef]
- Kavitha, M.; Kumar, N.; Jeyakumar, P. Role of zinc and boron on fruit yield and associated characters in papaya cv. Co. 5. South Indian Hortic. 2000, 48, 6–10. [Google Scholar]
- Ball, J.A. Evaluation of Two Lipid-Based Edible Coatings for Their Ability to Preserve Post Harvest Quality of Green Bell peppers. Ph.D. Thesis, Faculty of the Virginia Polytecnic Institute and state University, Blacksburg, VA, USA, 1997. [Google Scholar]
- Zelená, E.; Holasová, M.; Zelený, F.; Fiedlerová, V.; Novotná, P.; Landfeld, A.; Houška, M. Effect of sulphur fertilisation on lycopene content and colour of tomato fruits. Czech J. Food Sci. 2009, 27, S80–S84. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Preharvest calcium applications inhibit some cell wall-modifying enzyme activities and delay cell wall disassembly at commercial harvest of ‘Fuji Kiku-8′ apples. Postharvest Biol. Technol. 2011, 62, 161–167. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Yan, H.; Wang, H.; Wu, D.; Grierson, D.; Chen, K. The calcium-mediated homogalacturonan pectin complexation in cell walls contributes the firmness increase in loquat fruit during postharvest storage. J. Adv. Res. 2023, 49, 47–62. [Google Scholar] [CrossRef]
- Irfan, P.; Vanjakshi, V.; Prakash, M.K.; Ravi, R.; Kudachikar, V. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Schröder, E.; Phillips, C.; Garman, E.; Harlos, K.; Crawford, C. X-ray crystallographic structure of a papain-leupeptin complex. FEBS Lett. 1993, 315, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Akino, A.; Soorianathasundaram, K.; Paramaguru, P.; Jeyakumar, P.; Muthulakshmi, P. Influence of bioregulators on latex yield and proteolytic activity of papain extracted from field-grown TNAU papaya CO. 8 under the natural incidence of papaya ring spot virus. Madras Agric. J. 2021, 108, 266–274. [Google Scholar]
- Wang, X.; Zhao, D.; Shen, L.; Jing, C.; Zhang, C. Application and mechanisms of Bacillus subtilis in biological control of plant disease. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Stress management and agricultural sustainability; Springer: Singapore, 2018; Volume 1, pp. 225–250. [Google Scholar]
- Lowery, D.T.; Isman, M.B. Antifeedant activity of extracts from neem, Azadirachta indica, to strawberry aphid, Chaetosiphon fragaefolii. J. Chem. Ecol. 1993, 19, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Deepika, S.; Manoranjitham, S.; Sendhilvel, V.; Karthikeyan, G.; Kavitha, C. Foliar nutrition enhances the host immunity against papaya ringspot virus. Pharma Innov. 2021, 10, 165–169. [Google Scholar]
- Reena, B.; Kavitha, C.; Pugalendhi, L.; Kalarani, M.; Manoranjitham, S. Effect of foliar application of nutrient formulation on growth, yield and PRSV incidence of papaya (Carica papaya L.). Biol. Forum—Int. J. 2022, 14, 53–56. [Google Scholar]
- More, P.; Agarwal, P.; Agarwal, P.K. The Jatropha leaf curl Gujarat virus on infection in Jatropha regulates the sugar and tricarboxylic acid cycle metabolic pathways. 3 Biotech 2022, 12, 275. [Google Scholar] [CrossRef]
- Chen, H.-C.; Zhang, S.-L.; Wu, K.-J.; Li, R.; He, X.-R.; He, D.-N.; Huang, C.; Wei, H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicol. Environ. Saf. 2020, 187, 109790. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Vanlerberghe, G.C. Coordination of a mitochondrial superoxide burst during the hypersensitive response to bacterial pathogen in Nicotiana tabacum. Plant Cell Environ. 2012, 35, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, F.; Gonzalez, D.H.; Welchen, E. Plant mitochondria under pathogen attack: A sigh of relief or a last breath? Mitochondrion 2014, 19, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Boubakri, H. The role of ascorbic acid in plant–pathogen interactions. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munné-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 255–271. [Google Scholar]
- Suekawa, M.; Fujikawa, Y.; Esaka, M. Physiological role of ascorbic acid recycling enzymes in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munné-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 355–373. [Google Scholar]
- Wang, S.-D.; Zhu, F.; Yuan, S.; Yang, H.; Xu, F.; Shang, J.; Xu, M.-Y.; Jia, S.-D.; Zhang, Z.-W.; Wang, J.-H. The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta 2011, 234, 171–181. [Google Scholar] [CrossRef]
- Burbidge, C.A.; Ford, C.M.; Melino, V.J.; Wong, D.C.J.; Jia, Y.; Jenkins, C.L.D.; Soole, K.L.; Castellarin, S.D.; Darriet, P.; Rienth, M. Biosynthesis and cellular functions of tartaric acid in grapevines. Front. Plant Sci. 2021, 12, 643024. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Mou, Z. Salicylic acid and defense responses in plants. In Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications; Tran, L.-S.P., Pal, S., Eds.; Springer: New York, NY, USA, 2014; pp. 191–219. [Google Scholar]
- Singh, D.P.; Moore, C.A.; Gilliland, A.; Carr, J.P. Activation of multiple antiviral defence mechanisms by salicylic acid. Mol. Plant Pathol. 2004, 5, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Dixon, R.A.; Barros, J. Lignin biosynthesis: Old roads revisited and new roads explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, L.; Zhang, H.; Du, X.; An, C.; Xia, X.; Chen, F.; Jiang, J.; Chen, S. CmMYB19 over-expression improves aphid tolerance in chrysanthemum by promoting lignin synthesis. Int. J. Mol. Sci. 2017, 18, 619. [Google Scholar] [CrossRef] [PubMed]


| Components | Features |
|---|---|
| Cow dung | Serves as a habitat for beneficial microorganisms. Upon fermentation, cow dung releases major and minor nutrients and produces an odor and volatile compounds that prevent pest and disease infestation [13] |
| Bacillus subtilis | Plant growth promoting rhizobacteria (PGPR) involved in plant growth promotion (BNF, P and K solubilization, production of siderophore and phytohormones, root colonization and increased uptake of plant nutrients) and biocontrol activity (production of antibiotics, induced systemic resistance (ISR), rhizosphere competence and root colonization) [14] |
| Neem cake | Source of primary, secondary and micronutrients, and produces various bioactive compounds like azadirachtin, nimbin, nimbinin and salannin, which have antimicrobial, antiviral and antifeedant roles against various insect vectors [15] |
| Inorganic nutrients | Adequate and efficient fertilization increases the vigor of the plant and reduces nutrient deficiency symptoms and disease development [13]. Complete and balanced fertilization is the first line of defense against plant pathogens [16] |
| Score | Symptoms |
|---|---|
| 0 | No symptoms |
| 1 | Mild mosaic or oily spots, streaks on petioles or stem, oily spots on fruits |
| 3 | Mild mosaic and oily streaks/spots on petiole or stem and ring spots on fruits |
| 5 | Oily spots/streaks on petiole (or) stem (or) ring spots on fruits |
| 7 | Oily spots/streaks on petioles, stem, (or) on fruits, (ring spots), severe mosaic or blistering on leaves and leaf deformation and severe leaf reduction or mild fruit deformation with ring spots |
| 9 | Oily spots/streaks on petiole or stem and shoestring formation or severe fruit deformation with ring spots and stunted plants |
| Treatments | Plant Height (cm) | Stem Girth (cm) | Leaf Area (cm2) | Days to First Flowering | Days to First Harvest |
|---|---|---|---|---|---|
| T1 | 181.1 a | 26.6 a | 2405.6 b | 102.09 c | 248.09 bc |
| T2 | 185.8 a | 28.6 a | 2541.2 a | 99.48 d | 244.48 c |
| T3 | 176.1 ab | 27.5 ab | 2230.6 c | 104.14 b | 251.14 ab |
| T4 | 168.4 b | 24.2 a | 2137.5 d | 108.20 a | 255.16 a |
| CD | 7.06 | 1.80 | 86.36 | 3.15 | 5.01 |
| Treatments | Number of Fruits Plant−1 | Fruit Weight (kg) | Fruit Yield (kg plant −1) | Pulp Thickness (cm) | Fruit Firmness (kg cm−2) | Shelf-Life (Days) | PRSV Incidence (%) |
|---|---|---|---|---|---|---|---|
| T1 | 31.20 a | 1.38 ab | 43.06 a | 2.63 ab | 3.08 a | 5.72 b | 35.78 (36.70) bc |
| T2 | 32.51 a | 1.40 a | 45.51 a | 2.54 a | 3.33 a | 6.21 a | 32.22 (34.56) c |
| T3 | 28.33 b | 1.32 bc | 37.40 b | 2.38 b | 2.84 b | 5.53 b | 39.24 (38.77) b |
| T4 | 27.17 c | 1.31 c | 35.59 b | 2.31 b | 2.53 c | 3.81 c | 45.79 (42.56) a |
| CD (p = 0.05) | 1.50 | 0.07 | 2.52 | 0.56 | 0.47 | 0.35 | 2.31 |
| Treatments | TSS (°Brix) | Titratable Acidity (%) | Total Sugars (%) | Ascorbic Acid (mg 100 g−1) | β Carotene (mg 100 g−1) | Lycopene (mg 100 g−1) | Wet Latex (g) | Dry Latex (g) | Papain Activity (TU g−1) |
|---|---|---|---|---|---|---|---|---|---|
| T1 | 12.08 b | 0.114 b | 12.34 ab | 45.92 b | 2.70 a | 2.19 a | 732.6 ab | 170.0 ab | 30,496.9 b |
| T2 | 12.58 a | 0.112 a | 11.92 a | 48.05 a | 2.83 a | 2.13 a | 803.5 a | 198.8 a | 33,182.1 a |
| T3 | 11.88 b | 0.115 b | 11.55 bc | 42.57 c | 2.54 b | 2.02 ab | 613.3 bc | 146.5 bc | 28,045.2 c |
| T4 | 11.80 c | 0.121 c | 11.22 c | 40.55 d | 2.17 b | 1.98 b | 550.4 c | 135.9 c | 27,545.1 d |
| CD (p = 0.05) | 0.50 | 0.003 | 0.32 | 2.10 | 0.23 | 0.09 | 80.3 | 18.29 | 1052.03 |
| Treatments | Plant Height (cm) | Stem Girth (cm) | Leaf Area (cm2) | Days to First Flowering | Days to First Harvest | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | |
| S1 | 228.38 a | 181.23 c | 204.81 a | 32.05 b | 35.88 a | 33.96 a | 2550.66 a | 2235.70 b | 2393.18 a | 122.22 b | 114.23 d | 118.22 b | 243.45 b | 221.71 d | 232.58 b |
| S2 | 198.15 b | 170.77 d | 184.46 b | 28.08 d | 30.13 c | 29.11 b | 2076.59 c | 1932.83 d | 2004.71 b | 128.02 a | 118.23 c | 123.12 a | 257.80 a | 230.76 c | 244.28 a |
| Mean | 213.27 a | 176.00 b | 194.63 | 30.07 b | 33.00 a | 31.53 | 2313.63 a | 2084.27 b | 2198.95 | 125.12 a | 116.23 b | 120.67 | 250.62 a | 226.24 b | 238.43 |
| SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | ||||||
| S | 2.97 | 6.14s | 0.57 | 1.18 | 32.50 | 67.08 | 0.66 | 1.37 | 1.66 | 3.43 | |||||
| M | 2.37 | 5.17 | 0.32 | 0.70 | 22.19 | 48.36 | 0.9 | 1.96 | 1.11 | 2.42 | |||||
| S at M | 4.20 | 8.68 | 0.81 | 1.67 | 45.96 | 94.86 | 0.94 | 1.93 | 2.35 | 4.86 | |||||
| M at S | 3.80 | 8.02 | 0.66 | 1.37 | 39.35 | 82.67 | 1.12 | 2.39 | 2 | 4.2 | |||||
| Treatments | Number of Fruits Plant−1 | Fruit Weight (kg) | Fruit Yield (kg Plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | |
| S1 | 33.65 a | 31.21 b | 32.43 a | 1.63 a | 1.46 b | 1.54 a | 54.68 a | 45.52 b | 50.10 a |
| S2 | 29.29 c | 26.92 d | 28.10 b | 1.35 c | 1.29 d | 1.32 b | 39.68 c | 34.86 d | 37.27 b |
| Mean | 31.47 a | 29.06 b | 30.27 | 1.49 a | 1.38 b | 1.43 | 47.18 a | 40.19 b | 43.68 |
| SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | ||||
| S | 0.12 | 0.26 | 0.01 | 0.03 | 0.47 | 0.97 | |||
| M | 0.02 | 0.04 | 0.02 | 0.03 | 0.43 | 0.94 | |||
| S at M | 0.18 | 0.36 | 0.02 | 0.04 | 0.66 | 1.37 | |||
| M at S | 0.13 | 0.26 | 0.02 | 0.04 | 0.64 | 1.35 | |||
| Treatments | Fruit Firmness (kg cm−2) | Pulp Thickness (cm) | Shelf Life (Days) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | |
| S1 | 3.89 a | 3.65 b | 3.77 a | 2.53 b | 3.05 a | 2.79 a | 6.58 a | 5.46 b | 6.02 a |
| S2 | 3.27 c | 2.78 d | 3.03 b | 2.32 d | 2.35 c | 2.34 b | 4.25 c | 3.69 d | 3.97 b |
| Mean | 3.58 a | 3.22 b | 3.4 | 2.42 b | 2.70 a | 2.56 | 5.42 a | 4.58 b | 5.00 |
| SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | ||||
| S | 0.01 | 0.03 | 0.01 | 0.02 | 0.12 | 0.24 | |||
| M | 0.01 | 0.01 | 0.01 | 0.01 | 0.07 | 0.14 | |||
| S at M | 0.02 | 0.04 | 0.02 | 0.03 | 0.16 | 0.34 | |||
| M at S | 0.01 | 0.03 | 0.01 | 0.02 | 0.13 | 0.28 | |||
| (a) | |||||||||
| Treatments | TSS (°Brix) | Titratable Acidity (%) | Total Sugars (%) | ||||||
| M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | |
| S1 | 12.80 b | 13.20 a | 13.00 a | 0.110 c | 0.090 d | 0.100 b | 12.30 b | 12.86 a | 12.58 a |
| S2 | 11.60 c | 11.31 d | 11.45 b | 0.124 b | 0.156 a | 0.140 a | 10.98 c | 10.66 d | 10.82 b |
| Mean | 12.20 b | 12.26 a | 12.23 | 0.117 b | 0.123 a | 0.120 | 11.64 b | 11.76 a | 11.70 |
| SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | ||||
| S | 0.05 | 0.10 | 0.001 | 0.001 | 0.048 | 0.099 | |||
| M | 0.01 | 0.01 | 0.001 | 0.001 | 0.006 | 0.014 | |||
| S at M | 0.07 | 0.15 | 0.001 | 0.001 | 0.068 | 0.140 | |||
| M at S | 0.05 | 0.10 | 0.001 | 0.009 | 0.049 | 0.101 | |||
| (b) | |||||||||
| Treatments | Ascorbic Acid (mg 100 g−1) | β-Carotene (mg 100 g−1) | Lycopene (mg 100 g−1) | ||||||
| M1 | M2 | Mean | M1 | M2 | Mean | M1 | M2 | Mean | |
| S1 | 53.48 b | 56.20 a | 54.84 a | 3.29 a | 2.96 b | 3.13 a | 2.34 a | 2.30 b | 2.32 a |
| S2 | 47.60 c | 45.51 d | 46.56 b | 2.67 c | 2.35 d | 2.51 b | 1.96 c | 1.89 d | 1.93 b |
| Mean | 50.54 b | 50.85 a | 50.70 | 2.98 a | 2.66 b | 2.82 | 2.15 a | 2.10 b | 2.12 |
| SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | SE d | CD (p = 0.05) | ||||
| S | 0.21 | 0.43 | 0.012 | 0.024 | 0.009 | 0.018 | |||
| M | 0.03 | 0.06 | 0.002 | 0.004 | 0.001 | 0.003 | |||
| S at M | 0.30 | 0.61 | 0.017 | 0.034 | 0.013 | 0.026 | |||
| M at S | 0.21 | 0.44 | 0.012 | 0.024 | 0.009 | 0.018 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnasamy, K.; Krishnan, N.K.; Balasubramaniam, M.; Balamurugan, R.; Lakshmanan, P.; Karuppasami, K.M.; Karuppannan, M.S.; Thiyagarajan, E.; Alagarswamy, S.; Muthusamy, S. Nutrient Formulation—A Sustainable Approach to Combat PRSV and Enhance Productivity in Papaya. Agriculture 2025, 15, 201. https://doi.org/10.3390/agriculture15020201
Chinnasamy K, Krishnan NK, Balasubramaniam M, Balamurugan R, Lakshmanan P, Karuppasami KM, Karuppannan MS, Thiyagarajan E, Alagarswamy S, Muthusamy S. Nutrient Formulation—A Sustainable Approach to Combat PRSV and Enhance Productivity in Papaya. Agriculture. 2025; 15(2):201. https://doi.org/10.3390/agriculture15020201
Chicago/Turabian StyleChinnasamy, Kavitha, Naveen Kumar Krishnan, Moganapathi Balasubramaniam, Reena Balamurugan, Pugalendhi Lakshmanan, Kalarani M. Karuppasami, Manoranjitham S. Karuppannan, Elaiyabharathi Thiyagarajan, Senthil Alagarswamy, and Saraladevi Muthusamy. 2025. "Nutrient Formulation—A Sustainable Approach to Combat PRSV and Enhance Productivity in Papaya" Agriculture 15, no. 2: 201. https://doi.org/10.3390/agriculture15020201
APA StyleChinnasamy, K., Krishnan, N. K., Balasubramaniam, M., Balamurugan, R., Lakshmanan, P., Karuppasami, K. M., Karuppannan, M. S., Thiyagarajan, E., Alagarswamy, S., & Muthusamy, S. (2025). Nutrient Formulation—A Sustainable Approach to Combat PRSV and Enhance Productivity in Papaya. Agriculture, 15(2), 201. https://doi.org/10.3390/agriculture15020201







