Potential of Steinernema feltiae (Nematoda: Steinernematidae) Native Populations in the Biocontrol of Lycoriella ingenua (Diptera: Sciaridae) and Their Impact on Mushroom Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Entomopathogenic nematodes
2.2. Rearing of Lycoriella ingenua
2.3. Bioassays
2.3.1. Bioassay In Vitro
2.3.2. Bioassay In Vivo
2.4. Data Analysis
3. Results
3.1. Bioassay In Vitro
3.2. Bioassay In Vivo
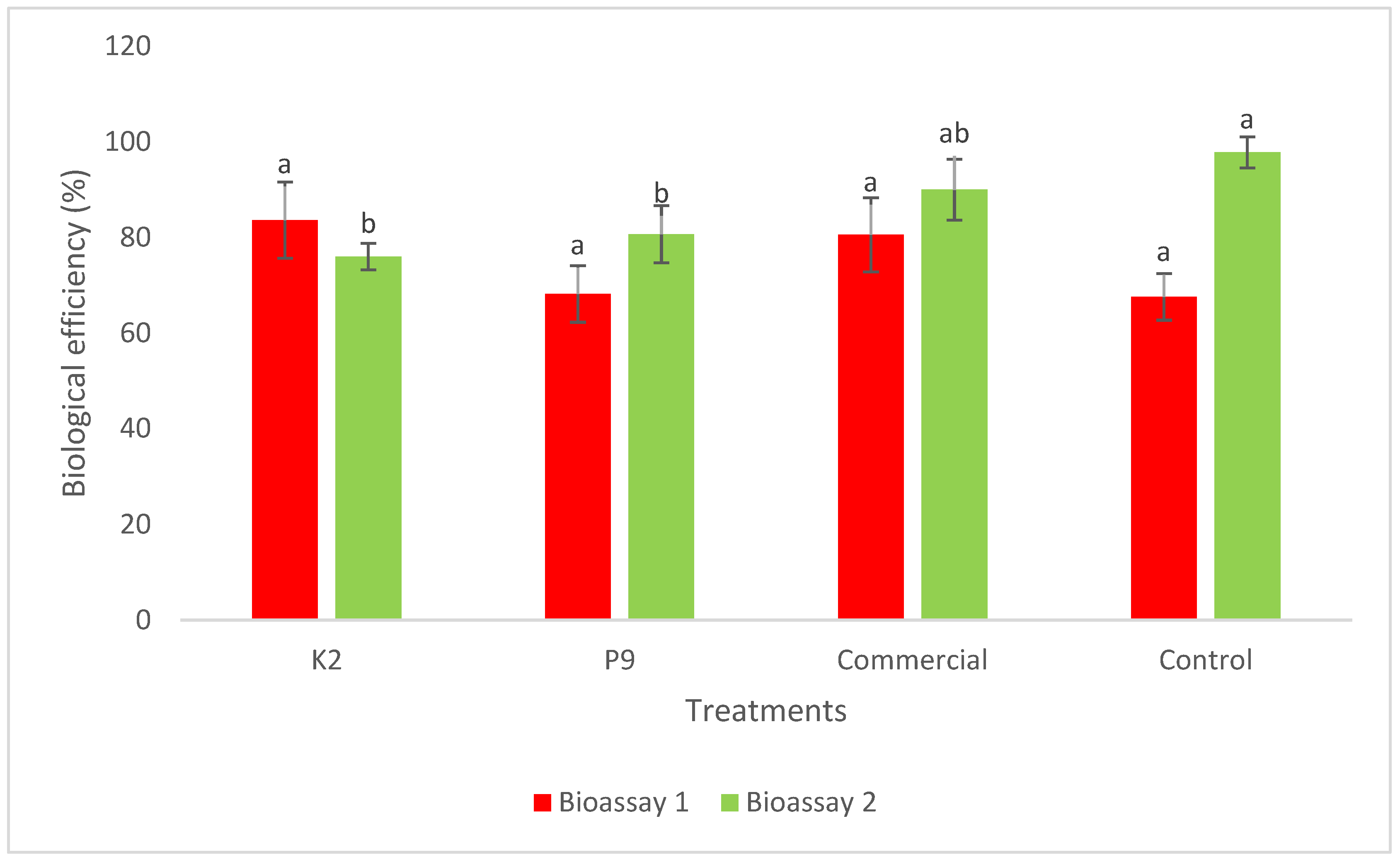
3.3. Impact on Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, I.K.; Choi, K.S.; Kim, D.H.; Choi, I.H.; Kim, L.S.; Bak, W.C.; Choi, J.W.; Shin, S.C. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manag. Sci. 2006, 62, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Erler, F.; Polat, E.; Demir, H.; Catal, M.; Tuna, G. Control of mushroom sciarid fly Lycoriella ingenua populations with insect growth regulators applied by soil drench. J. Econ. Entomol. 2011, 104, 839–844. [Google Scholar] [CrossRef]
- Rinker, D.L. Insect, mite, and nematode pests of commercial mushroom production. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 221–237. [Google Scholar] [CrossRef]
- Drobnjaković, T.; Marčić, D.; Potočnik, I.; Rekanović, E.; Prijović, M.; Milijašević-Marčić, S.; Stepanović, M. Control of mushroom sciarid fly Lycoriella inguena (Dufour) with an azadirachtin-based insecticide. Pestic. Phytomed. 2019, 34, 111–121. [Google Scholar] [CrossRef]
- Doroški, A.; Klaus, A.; Režek Jambrak, A.; Djekic, I. Food waste originated material as an alternative substrate used for the cultivation of oyster mushroom (Pleurotus ostreatus): A review. Sustainability 2022, 14, 12509. [Google Scholar] [CrossRef]
- Lewandowski, M.; Sznyk, A.; Bednarek, A. Biology and morphometry of Lycoriella ingenua (Diptera: Sciaridae). Biol. Lett. 2004, 41, 41–50. [Google Scholar]
- Binns, E.S. Field and laboratory observations on the substrates of the mushroom fungus gnat Lycoriella auripila (Diptera: Sciaridae). Ann. Appl. Biol. 1980, 96, 143–152. [Google Scholar] [CrossRef]
- Shamshad, A. The development of integrated pest management for the control of mushroom sciarid flies, Lycoriella ingenua (Dufour) and Bradysia ocellaris (Comstock), in cultivated mushrooms. Pest Manag. Sci. 2010, 66, 1063–1074. [Google Scholar] [CrossRef]
- Kielbasa, R.; Snetsinger, R. Life history of a sciarid fly, Lycoriella mali, and its injury threshold on the commercial mushroom [includes taxonomy]. PSU Bull. 1980, 833, 14. [Google Scholar]
- White, P.F. The effect of sciarid larvae (Lycoriella auripila) on the yield of the cultivated mushroom (Agaricus bisporus). Ann. Appl. Biol. 1986, 109, 11–17. [Google Scholar] [CrossRef]
- Brewer, K.K.; Keil, C.B. Permethrin resistance in Lycoriella mali (Diptera: Sciaridae). J. Econ. Entomol. 1989, 82, 17–21. [Google Scholar] [CrossRef]
- Brewer, K.K.; Keil, C.B. A mixed function oxidase factor contributing to permethrin and dichlorvos resistance in Lycoriella mali (Fitch) Diptera: Sciaridae. Pestic. Sci. 1989, 26, 29–39. [Google Scholar] [CrossRef]
- Bartlett, G.R.; Keil, C.B.O. Identification and characterization of a permethrin resistance mechanism in populations of the fungus gnat Lycoriella mali (Fitch) (Diptera: Sciaridae). Pestic. Biochem. Physiol. 1998, 58, 173–181. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Geels, F.P.; Smits, P.H.; Van Griensven, L.J.L.D. Control of the mushroom pests Lycoriella auripila (Diptera: Sciaridae) and Megaselia halterata (Diptera: Sciaridae) and Megaselia halterata (Diptera: Phoridae) by Steinernema feltiae (Nematoda: Steinernematidae) in field experiments. Ann. Appl. Biol. 1997, 131, 359–368. [Google Scholar] [CrossRef]
- Smith, J.E. Dimilin resistance in mushroom sciarids. Mushroom J. 2002, 656, 15. [Google Scholar]
- EC (European Commission). EU Pesticide Database—Active Substances, Safeners and Synergists. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 20 September 2024).
- Team of Editors. Pesticides in Agriculture and Forestry in Serbia, 22nd ed.; Plant Protection Society of Serbia: Belgrade, Serbia, 2024. (In Serbian) [Google Scholar]
- Navarro, M.J.; Gea, F.J. Entomopathogenic nematodes for the control of phorid and sciarid flies in mushroom crops. Pesqui. Agropecu. Bras. 2014, 49, 11–17. [Google Scholar] [CrossRef]
- United Nations Sustainable Development Goals (UN SDGs). Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.un.org/sustainabledevelopment/wp-content/uploads/2019/01/SDG_Guidelines_AUG_2019_Final.pdf&ved=2ahUKEwiV1OOS8eCLAxW7UMMIHZ-zLnoQFnoECBIQAQ&usg=AOvVaw0KEB_8CHsPLDf_-O85r1rc (accessed on 7 June 2024).
- Ehlers, R.-U. Forum on safety and regulations. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.-U., Shapiro-Ilan, D.I., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 107–114. [Google Scholar] [CrossRef]
- Jess, S.; Schweizer, H.; Kilpatrick, M. Mushroom applications. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.-U., Shapiro-Ilan, D.I., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 191–213. [Google Scholar] [CrossRef]
- Jess, S.; Schweizer, H. Biological control of Lycoriella ingenue (Diptera: Sciaridae) in commercial mushroom (Agaricus bisporus) cultivation: A comparison between Hypoaspis miles and Steinernema feltiae. Pest Manag. Sci. 2009, 65, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Matuska-Łyźwa, J.; Duda, S.; Dominika Nowak, D.; Kaca, W. Impact of abiotic and biotic environmental conditions on the development and infectivity of entomopathogenic nematodes in agricultural soils (review). Insects 2024, 15, 421. [Google Scholar] [CrossRef]
- Grewal, P.S.; Richardson, P.N. Effects of application rates of Steinernema feltiae (Nematoda: Steinernematidae) on biological control of the mushroom fly Lycoriella auripila (Diptera: Sciaridae). Biocontrol Sci. Technol. 1993, 3, 29–40. [Google Scholar] [CrossRef]
- Gouge, D.H.; Hague, N.G.M. The susceptibility of different species of sciarid flies to entomopathogenic nematodes. J. Helminthol. 1995, 69, 313–318. [Google Scholar] [CrossRef]
- Hay, D.B.; Fenlon, J.S. A modified binomial model that describes the infection dynamics of the entomopathogenic nematode Steinernema feltiae (Steinernematidae; Nematoda). Parasitology 1995, 111, 627–633. [Google Scholar] [CrossRef]
- Tomalak, M. Selective breeding of Steinernema feltiae (Filipjev) (Nematoda: Steinernematidae) for improved efficacy in control of a mushroom fly, Lycoriella solani Winnertz (Diptera: Sciaridae). Biocontrol Sci. Technol. 1994, 4, 187–198. [Google Scholar] [CrossRef]
- Grewal, P.S.; Richardson, I.P.N.; Collins, G.; Edmonson, R.N. Comparative effects of Steinernema feltiae (Nematoda: Steinernematidae) and insecticides on yield and cropping of the mushroom Agaricus bisporus. Ann. Appl. Biol. 1992, 121, 511–520. [Google Scholar] [CrossRef]
- Rinker, D.L.; Olthof, T.H.A.; Dano, J.; Alm, G. Effects of Entomopathogenic Nematodes on Control of a Mushroom-infesting Sciarid Fly and on Mushroom Production. Biocontrol Sci. Technol. 1995, 5, 109–120. [Google Scholar] [CrossRef]
- Hay, D.B.; Richardson, P.N. Inter- and intraspecific variation in infectivity of Steinernema spp. to larvae of the mushroom fly Lycoriella solani. Entomol. Exp. Appl. 1995, 77, 11–15. [Google Scholar] [CrossRef]
- Olthof, T.H.A.; Rinker, D.L.; Dano, J. The effect of entomophilic nematodes on a sciarid fly, Lycoriella mali, and on growth and yield of mushrooms, Agaricus bisporus. J. Nematol. 1991, 23, 543. [Google Scholar]
- Grewal, P.S.; Tomalak, M.; Keil, C.B.O.; Gaugler, R. Evaluation of a genetically selected strain of Steinernema feltiae against the mushroom sciarid Lycoriella mali. Ann. Appl. Biol. 1993, 123, 695–702. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Geels, F.P.; Rutjens, A.J.; Smits, P.H.; Van Griensven, L.J.L.D. Comparison of the efficacy of entomopathogenic nematodes for the biological control of the mushroom pests Lycoriella auripila (Sciaridae) and Megaselia halterata (Phoridae). Biocontrol Sci. Technol. 1998, 8, 277–287. [Google Scholar] [CrossRef]
- Katumanyane, A. Prospects for Using Entomopathogenic Nematodes as a Biocontrol Agent Against Fungus Gnats, Bradysia spp. (Diptera: Sciaridae) in Nursery and Glass House Crops. Masters Thesis, Stellenbosch University, Stellenbosch, South Africa, 2017; pp. 1–122. [Google Scholar]
- Ma, J.; Chen, S.; Moens, M.; Han, R.; De Clercq, P. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat, Bradysia odoriphaga. J. Pest Sci. 2013, 86, 551–561. [Google Scholar] [CrossRef]
- Katumanyane, A.; Ferreira, T.; Malan, A.P. Greenhouse application of Steinernema yirgalemense to control fungus gnats, Bradysia impatiens. BioControl 2018, 63, 729–738. [Google Scholar] [CrossRef]
- Katumanyane, A.; Ferreira, T.; Malan, A.P. Potential use of local entomopathogenic nematodes to control Bradysia impatiens (Diptera: Sciaridae) under laboratory conditions. Afr. Entomol. 2018, 26, 350–358. [Google Scholar] [CrossRef]
- Chen, C.; Ma, H.; Ma, M.; Li, J.; Zheng, S.; Song, Q.; Gu, X.; Shapiro-Ilan, D.; Ruan, W. An innovative strategy for control of fungus gnats using entomopathogenic nematodes alone or in combination with waterlogging. J. Nematol. 2021, 52, e2020-57. [Google Scholar] [CrossRef] [PubMed]
- Grujić, N.; Lozančić, S.; Nježić, B. Preliminary survey of entomopathogenic nematodes in Serbia. In Proceedings of the IX International Scientific Agricultural Symposium “Agrosym 2018”, Jahorina, Bosnia and Herzegovina, 4–7 October 2018; p. 658. [Google Scholar]
- Grujić, N.; Lozančić, S.; Paunović, D.; Nježić, B. Morphological and molecular characterisation of Steinernema feltiae population from Belgrade. In Proceedings of the VIII Congress on Plant Protecion, Zlatibor, Serbia, 25–29 November 2019; pp. 176–177. [Google Scholar]
- Grujić, N.; Tepić, S.; Živković, A.; Vasić, M.; Nježić, B. Novi nalazi entomopatogenih nematoda u Srbiji i Republici Srpskoj. Zbornik rezimea radova XIV Simpozijum o zaštiti bilja. New findings of entomopathogenic nematodes in Serbia and Republic of Serpska. In Proceedings of the XIV Symposium on Plant Protection, Zlatibor, Serbia, 22–25 November 2021; p. 64. (In Serbian). [Google Scholar]
- Grujić, N.; Nježić, B. Patogenost nekih vrsta entomopatogenih nematoda prema Phthorimaea operculella (Gelechiidae: Lepidoptera). Zbornik rezimea radova XV Savetovanje o zaštiti bilja. Pathogenicity of some species of entomopathogenic nematodes to Phthorimaea operculella (Gelechiidae: Lepidoptera). In Proceedings of the XV Conference on Plant Protection, Zlatibor, Serbia, 22–25 February 2021; p. 50. (In Serbian). [Google Scholar]
- Grujić, N.; Popović, A.; Galac, M.; Drobnjaković, T.; Nježić, B.; Tarasco, E. Pathogenicity of entomopathogenic nematodes to Phthorimaea operculella (Zeller) (Lepidoptera, Gelechideae). In Proceedings of the IX Congress on Plant Protecion, Zlatibor, Serbia, 25–28 November 2024; p. 92. [Google Scholar]
- Wiesner, A.; Gotz, P. Silica beads induce cellular and humoral immune responses in Galleria mellonella larvae and in isolated plasmatocytes, obtained by a newly adapted nylon wool separation method. J. Insect Physiol. 1993, 39, 865–876. [Google Scholar] [CrossRef]
- White, G.F. A method for obtaining infective nematode larvae from cultures. Science 1927, 66, 302–303. [Google Scholar] [CrossRef]
- Richardson, P.N. Susceptibility of mushroom pests to the insect-parasitic nematodes Steinernema feltiae and Heterorhabditis heliothidis. Ann. Appl. Biol. 1987, 111, 433–438. [Google Scholar] [CrossRef]
- Nickle, W.R.; Cantelo, W.W. Control of a Mushroom-infesting Fly, Lycoriella mali, with Steinernema feltiae. J. Nemtatol. 1991, 23, 145–147. [Google Scholar] [CrossRef]
- Grujić, N.; Drobnjaković, T.; Andjelković, N.; Luković, J.; Milijašević-Marčić, S.; Šantrić, L.; Potočnik, I.; Marčić, D. Potential of different strains of Steinernema feltiae (Filipjev) to control Lycoriella ingenue (Dufour) and impact on mushroom yield. In Proceedings of the 35th Symposium of the European Society of Nematologists, Cordoba, Spain, 15–19 April 2024; p. 258. [Google Scholar]
- Keil, C.B. Arthropod pests. In Mushroom Integrated Pest Management; Penn State University: State College, PA, USA, 2002; pp. 47–51. [Google Scholar]
- Tomalak, M.; Lipa, J.J. Factors affecting entomophilic activity of Neoaplectana feltiae in mushroom compost. Entomol. Exp. Appl. 1991, 59, 105–110. [Google Scholar] [CrossRef]
- Menzel, F.; Mohrig, W. Revision der paläarktischen Trauermücken (Diptera: Sciaridae) A revision of the Palaearctic black fungus gnats (Diptera: Sciaridae). In Studia Dipterologica—Suppl.6; Ampyx-Verlag: Halle, Germany, 1999; pp. 1–761. (In German) [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Tello, J.C. Reduced sensitivity of the mushroom pathogen Verticillium fungicola to prochloraz-manganese in vitro. Mycol. Res. 2005, 109, 741–745. [Google Scholar] [CrossRef]
- Chrysay-Tokousbalides, M.; Kastanias, M.A.; Philippoussis, A.; Diamantopoulou, P. Selective fungitoxicity of famaxadone, tebuconazole and trifloxystrobin between Verticillium fungicola and Agaricus bisporus. Crop Prot. 2007, 26, 469–475. [Google Scholar] [CrossRef]
- Reeb, J.E.; Milota, M.R. Moisture content by the oven-dry method for industrial testing. In Proceedings of the 50th Annual Dubois Meeting of the Western Dry Kiln Association, Portland, OR, USA, May 1999; pp. 66–74. Available online: https://ir.library.oregonstate.edu/concern/conference_proceedings_or_journals/fq977v782 (accessed on 10 July 2024).
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: Cambridge, UK, 1971; pp. 1–333. [Google Scholar]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2017; p. 212. [Google Scholar] [CrossRef]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 7. Available online: https://www.statsoft.de/en/data-science-applications/tibco-statistica/ (accessed on 12 September 2024).
- Grewal, P.S.; Peters, A. Formulation and Quality. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.-U., Shapiro-Ilan, D.I., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 79–90. [Google Scholar] [CrossRef]
- Shamshad, A.; Clift, A.D.; Mansfield, S. Toxicity of six commercially formulated insecticides and biopesticides to third instar larvae of mushroom sciarid, Lycoriella ingenua Dufour (Diptera: Sciaridae), in New South Wales, Australia. Austral. J. Entomol. 2008, 47, 256–260. [Google Scholar] [CrossRef]
- Jess, S.; Kilpatrick, M. An integrated approach to the control of Lycoriella solani (Diptera:Sciaridae) during production of the cultivated mushroom (Agaricus bisporus). Pest Manag. Sci. 2000, 56, 477–485. [Google Scholar] [CrossRef]
- Jess, S.; Bingham, J.F.W. Biological control of sciarid and phorid pests of mushroom with predatory mites from the genus Hypoaspis (Acari: Hypoaspidae) and the entomopathogenic nematode Steinernema feltiae. Bull. Entomol. Res. 2004, 94, 159–167. [Google Scholar] [CrossRef]
- Navarro, M.J.; Carrasco, J.; Gea, F. Chemical and Biological Control of Diptera in Spanish Mushroom Crops. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; pp. 549–556. [Google Scholar]
- Shapiro-Ilan, D.I.; Brown, I.; Lewis, E.E. Freezing and desiccation tolerance in entomopathogenic nematodes: Diversity and correlation of traits. J. Nematol. 2014, 46, 27–34. [Google Scholar] [PubMed]
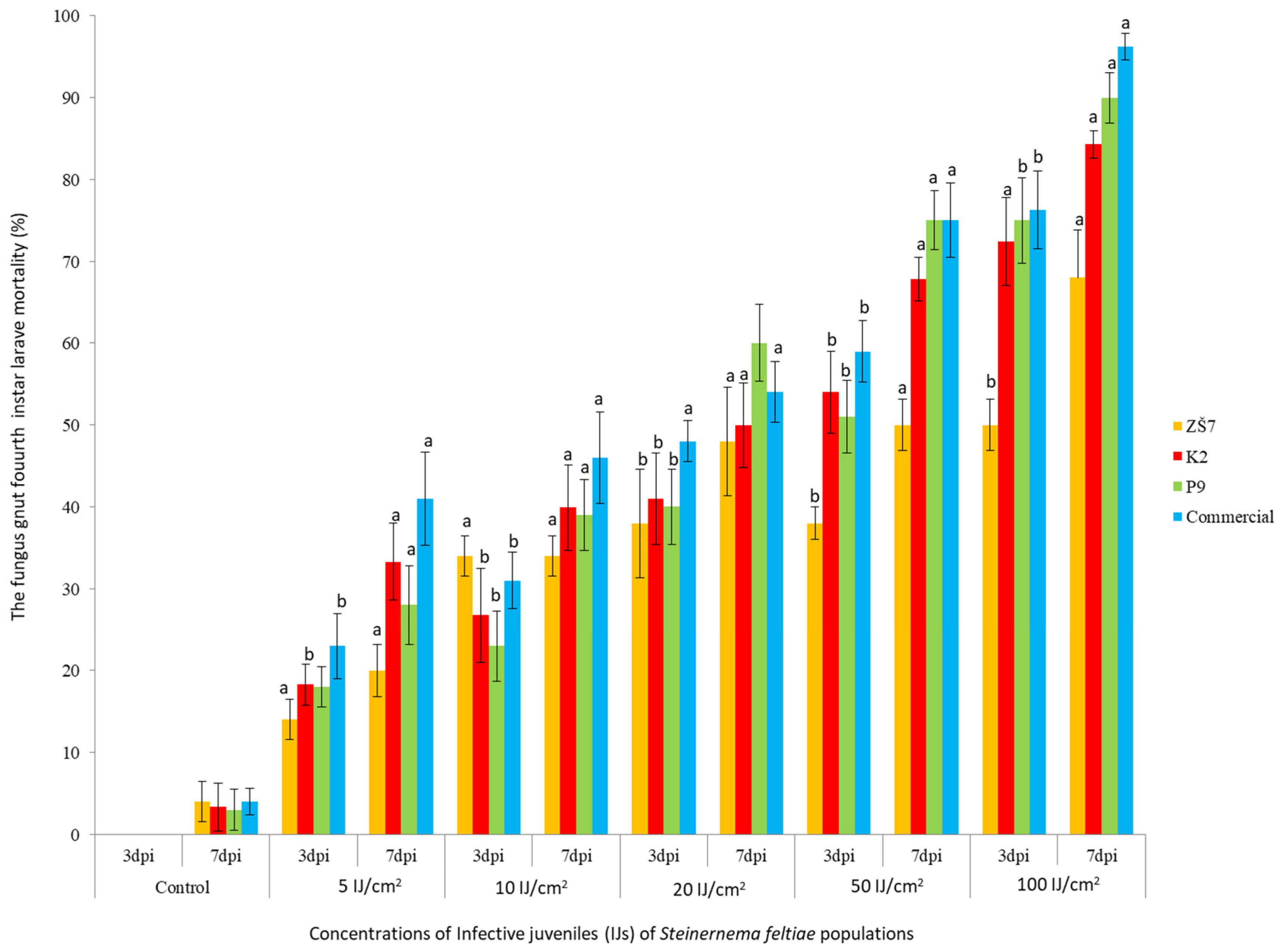

| EPN Population | n | LC50 (IJ/cm2) (95% CLs) | LC90 (IJ/cm2) (95% CLs) | Slope (±SE) | χ2 | df |
|---|---|---|---|---|---|---|
| K2 | 600 | 18.47 c (13.60–25.87) | 121.32 (68.02–378.86) | 1.57 (±0.28) | 1.983 | 3 |
| P9 | 600 | 15.77 b (11.56–20.68) | 102.68 (67.78–198.04) | 1.58 (±0.21) | 1.944 | 3 |
| Commercial | 600 | 11.48 a (25.65–229.00) | 83.68 (37.32–178.94) | 1.48 (±0.16) | 11.546 | 3 |
| Treatments | K2 | P9 | Commercial | Control | Anova Parameters | |||
|---|---|---|---|---|---|---|---|---|
| No of Adult Flies | Control Efficacy (%) | No of Adult Flies | Control Efficacy (%) | No of Adult Flies | Control Efficacy (%) | No of Adult Flies | ||
| 8 DAT | 1.00 ± 0.68 b | 70.0 | 1.67 ± 0.80 b | 50.0 | 0.33 ± 0.33 b | 90.0 | 3.33 ± 0.84 a | F3,20 = 13.812 p < 0.005 |
| 15 DAT | 3.00 ± 0.45 b | 76.9 | 2.33 ± 0.61 b | 82.0 | 1.33 ± 0.84 b | 89.7 | 13.00 ± 1.12 a | F3,20 = 15.011 p < 0.005 |
| 22 DAT | 5.33 ± 0.40 b | 51.5 | 5.33 ± 0.42 bc | 51.5 | 2.00 ± 0.42 c | 81.8 | 11.00 ± 0.85 a | F3,20 = 23.49 p < 0.001 |
| 29 DAT | 10.33 ± 0.61 b | 48.3 | 8.67 ± 0.66 b | 56.7 | 5.00 ± 0.73 c | 75.0 | 20.00 ± 0.73 a | F3,20 = 20.849 p < 0.001 |
| 36 DAT | 12.33 ± 0.61 b | 61.9 | 10.67 ± 0.67 b | 67.0 | 7.33 ± 0.45 c | 77.3 | 32.33 ± 0.95 a | F3,20 = 88.075 p < 0.001 |
| Treatments | K2 | P9 | Commercial | Control | Anova Parameters | |||
|---|---|---|---|---|---|---|---|---|
| No of Adult Flies | Control Efficacy (%) | No of Adult Flies | Control Efficacy (%) | No of Adult Flies | Control Efficacy (%) | No of Adult Flies | ||
| 8 DAT | 0.67 ± 0.33 b | 81.82 | 1.67 ± 0.54 b | 68.18 | 0.33 ± 0.21 b | 90.01 | 3.67 ± 0.42 a | F3,20 = 9.417 p < 0.001 |
| 15 DAT | 2.17 ± 0.75 b | 85.23 | 1.67 ± 0.80 b | 88.64 | 0.67 ± 0.42 b | 95.45 | 14.67 ± 0.49 a | F3,20 = 28.084 p < 0.001 |
| 22 DAT | 2.83 ± 0.31 b | 85.83 | 2.00 ± 0.26 c | 90.00 | 1.67 ± 0.21 c | 91.67 | 20.00 ± 0.58 a | F3,20 = 325.809 p < 0.001 |
| 29 DAT | 8.33 ± 0.42 b | 66.44 | 6.50 ± 0.22 c | 73.82 | 5.33 ± 0.21 d | 78.52 | 24.83 ± 0.54 a | F3,20 = 496.10 p < 0.001 |
| 36 DAT | 11.83 ± 0.54 b | 62.83 | 10.67 ± 0.47 b | 66.49 | 7.33 ± 0.49 c | 76.96 | 31.83 ± 0.65 a | F3,20 = 283.262 p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobnjaković, T.; Grujić, N.; Luković, J.; Anđelković, N.; Potočnik, I.; Milijašević-Marčić, S.; Šantrić, L.; Popović, A.; Marčić, D. Potential of Steinernema feltiae (Nematoda: Steinernematidae) Native Populations in the Biocontrol of Lycoriella ingenua (Diptera: Sciaridae) and Their Impact on Mushroom Production. Agriculture 2025, 15, 537. https://doi.org/10.3390/agriculture15050537
Drobnjaković T, Grujić N, Luković J, Anđelković N, Potočnik I, Milijašević-Marčić S, Šantrić L, Popović A, Marčić D. Potential of Steinernema feltiae (Nematoda: Steinernematidae) Native Populations in the Biocontrol of Lycoriella ingenua (Diptera: Sciaridae) and Their Impact on Mushroom Production. Agriculture. 2025; 15(5):537. https://doi.org/10.3390/agriculture15050537
Chicago/Turabian StyleDrobnjaković, Tanja, Nikola Grujić, Jelena Luković, Nikola Anđelković, Ivana Potočnik, Svetlana Milijašević-Marčić, Ljiljana Šantrić, Angelina Popović, and Dejan Marčić. 2025. "Potential of Steinernema feltiae (Nematoda: Steinernematidae) Native Populations in the Biocontrol of Lycoriella ingenua (Diptera: Sciaridae) and Their Impact on Mushroom Production" Agriculture 15, no. 5: 537. https://doi.org/10.3390/agriculture15050537
APA StyleDrobnjaković, T., Grujić, N., Luković, J., Anđelković, N., Potočnik, I., Milijašević-Marčić, S., Šantrić, L., Popović, A., & Marčić, D. (2025). Potential of Steinernema feltiae (Nematoda: Steinernematidae) Native Populations in the Biocontrol of Lycoriella ingenua (Diptera: Sciaridae) and Their Impact on Mushroom Production. Agriculture, 15(5), 537. https://doi.org/10.3390/agriculture15050537






