Abstract
Over the last few decades, the productivity of sows has improved tremendously, especially in terms of litter size. Colostral immunoglobulins (Igs) are essential for the early protection of piglets against pathogens. We hypothesized that with the increase in sows’ productivity, the Ig content of colostrum has declined. The research results reviewed confirmed a negative trend in the case of IgG and IgA. The sows’ blood IgG and IgM concentrations decreased from late gestational levels, confirming the transfer from serum (with most of them not synthesized in the mammary gland). This connection was also confirmed by our correlation analyses. Colostrum Ig levels correlate well with the piglets’ blood Ig concentrations, proving the importance of colostral Ig intake. The most effective feed supplements are antioxidants and arginine to improve the Ig content of the blood (about 30 to 70%) and colostrum (about 10 to 70%). Pre- and probiotics and other feed supplements express only a modest (about +10 to 20%) but positive effect.
1. Introduction
Over the last fifty years, progress in genetic selection has led to a significant increase in litter size. Modern genotypes produce large litters (18–19 piglets or more per farrowing on farms on average), and the individual bodyweight of piglets at birth is inversely proportional to the size of the litter [1]. Therefore, the number of low-birth-weight piglets has increased over the years [2]. It is evident that with the decrease in birth weight, pre-weaning loss is increasing [3]. One important reason is that with the increased litter size, the colostrum yield per liveborn piglet decreases [4]. Therefore, one of the cornerstones of high-production pig farms is to keep piglets born with a lower bodyweight alive [5]. Piglets that do not have an acceptable amount of quality colostrum are 4–5 times more likely to die. Unfortunately, modern high-productivity hybrids have lower protein content in their milk after farrowing compared to conventional genotypes [6]. Also, the duration of parturition has increased in modern high-productivity sows (from 156 to 262 min), so piglets can still be in the uterus 6 h after the onset of parturition; therefore, their colostrum intake can be limited and fewer immunoglobulins (Igs) can be transferred due to the rapid decline in colostrum [7]. The lower colostrum intake and the impaired quality of colostrum in these high-productivity hybrids result in their piglets being more susceptible to diseases [8]. Ensuring that piglets receive adequate amounts of quality colostrum not only enhances their survival rates but also lays the foundation for their future growth and productivity, making it a key focus for swine producers and animal health professionals.
Early studies with 125I-labelled immunoglobulins revealed that about 90% of them in colostrum are of serum origin, and only the remaining 10% are synthesized in the mammary gland [9]. Therefore, the immunological status of the sow, especially during late gestation, plays a very important role.
Our hypothesis is that with the genetic progress in litter size and milk production, the immunoglobulin content of colostrum may have changed (declined). This could be due to a possible physiological limit of Ig production and less available nutrients for Ig synthesis because of the greater need of fetuses’ and mammary gland development. We aimed to analyze the available publications over the last four decades to calculate the trend and analyze the possible influencing factors. Since it seems that colostrum Ig content is highly dependent on serum, we summarized the effects of various feed supplements on serum and colostrum Ig levels based on the available publications.
2. Materials and Methods
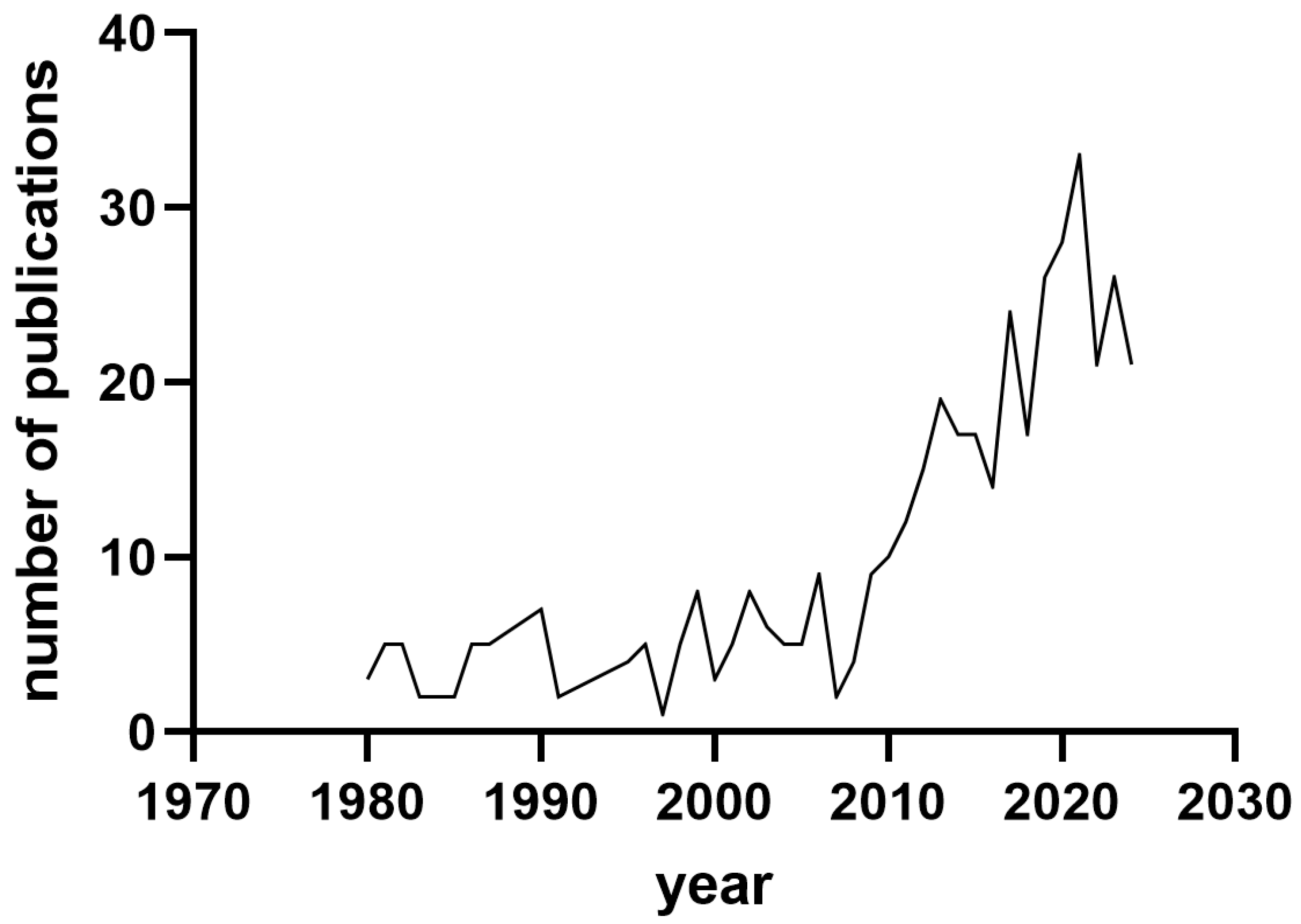
Potential publications were identified using the Publish or Perish tool [10] through keywords including sow, colostrum, milk, and immunoglobulin. The maximum number of papers was set to 500. Figure 1 shows the distribution of the papers dealing with the searched topic during the period of 1980–2024. It seems that researchers were more active in the fields of sow colostrum and milk in the period of 2010–2024.
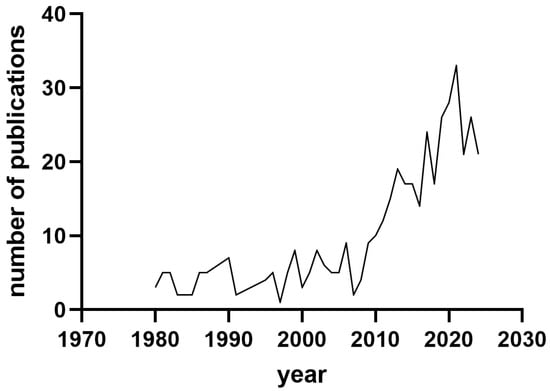
Figure 1.
Distribution of publications studying the composition of sows’ colostrum and milk between 1980 and 2024.
The extracted papers were evaluated using the Rayyan tool [11] with the following criteria:
- -
- Keywords: sow AND colostrum AND milk AND immunoglobulin
- -
- English language
- -
- Only journal articles
- -
- The period of 1980–2024
A total of 46 eligible papers were selected. For the correlation and regression analyses, we reviewed the publications and excluded those where no serum/plasma Ig content was measured or no numerical result (only figure) was presented (17); review papers (4) and irrelevant publications were disregarded as well (3). The process resulted in 22 publications. In addition, we also reviewed the references of these articles, and 34 other publications were identified, resulting in the inclusion of 56 manuscripts. Only the data (IgG, IgA, and IgM in colostrum, milk, and serum/plasma) of the control groups from these publications were compiled in MS Excel; in every row, only the data originating from the same time point were placed. The database consisted of 175 records, which were used in the correlation and regression analyses. When the data were graphically reviewed, some obvious “outliers” were seen. As these data were already group averages from a trial (not a single measurement error), we decided not to exclude them.
All statistical analyses were performed using GraphPad Prism 10.4.0. (GraphPad Software Inc., San Diego, CA, USA). A p-value of less than 0.05 was considered statistically significant. The relationship between colostrum IgG, IgA, and IgM concentrations and the independent variables (sow serum and pig serum) was evaluated using Pearson (r) correlation coefficients and linear regression.
3. The Role of Immunoglobulins and Their Presence in Sow Milk and Colostrum
Immunoglobulins (Ig), also known as antibodies, are glycoproteins produced by plasma cells in response to antigens. They play an important role in the immune system by recognizing and neutralizing toxins and pathogens like bacteria and viruses. Each Ig has a unique region that binds specifically to a particular antigen. There are five main types of Igs (IgG, IgA, IgM, IgE, and IgD) in sows, and each has unique functions and properties [12]. IgA is found mainly in mucosal areas, such as the gut, respiratory tract, and urogenital tract but also in saliva, tears, and breast milk. It plays an important role in mucosal immunity by preventing pathogen adherence and invasion and protecting mucosal surfaces [13]. IgM is located in the blood and lymphatic fluids. This is the predominant antibody produced in response to an infection. It is highly effective in forming complexes with different antigens, leading to pathogen lysis [14]. IgG is present in the blood and extracellular fluids. IgG provides the major part of antibody-based immunity, synthesized in the secondary immune response to pathogens [15]. In sow milk, IgA, IgM, and IgG are the main types [16] and are synthesized locally in the mammary gland [17]. These antibodies are essential for the development of the piglet’s immune system because they provide passive immunity until the piglet’s own immune system matures.
Concentrations of Igs are the highest in colostrum for the first hours postpartum and constantly decline after 6 h; however, the decrease in IgG concentration is faster than the decrease in IgA and IgM concentrations [6,18,19]. Colostrum also has higher concentrations of dry matter, proteins, vitamins, minerals (e.g., zinc, copper, iron, and iodine), and hormones; however, lactose and fat are present in lower concentrations compared to those in mature milk [18,20,21,22]. The milk reaches its mature composition between the seventh and tenth day of lactation and then remains almost stable until the rest of lactation [6].
The epitheliochorial placenta of the sow does not allow the transfer of maternal antibodies and immune cells to the fetus; therefore, piglets rely on their natural immune system and the passive immunity received from the sow via the colostrum [20]. Transfer of Igs from sow plasma to the mammary glands starts before farrowing (approximately 10 days pre-farrowing), increases 3–4 days before farrowing, and is maximal on the farrowing day [23]. Therefore, colostrum synthesis is not finished fully before the parturition of sows. The neonatal fragment crystallizable (Fc) receptor for IgGs (known as FcRn) is expressed by mammary glands and most probably mediates IgG transfer from the plasma into the mammary gland and colostrum [24]. IgA and IgM are transferred into the colostrum by the polymeric Ig receptor (pIgR) located in the mammary gland. FcRn is present in gut epithelial cells, but the absorption of colostral Igs in piglets may not be regulated by just this receptor. IgG, IgM, and IgA are transferred to enterocytes via selective transcytosis stimulated by colostral factors [17]. The period of protection of piglets by maternal antibodies depends on the timing of gut closure [25], which is about 24–36 h postpartum. Studies show that it is not the amount of Igs but rather nutrients (e.g., glucose and protein) and other hormones that influence closure [26].
Mortality of piglets in the first few days is mostly caused by inadequate colostrum intake. The recommendation for colostrum intake is 200–250 g per piglet [20], which influences piglet survival before weaning [27]. Unfortunately, the amount of colostrum is limited in large litters; therefore, some piglets may not obtain enough. A lower birth weight (<1.0 kg), especially in IUGR (intrauterine growth retarded) piglets, can result in even lower colostrum intake, which increases mortality. A recent study [28] concluded that if the piglet’s birth weight is greater than 1.5 kg, then its subsequent performance is not influenced. In a study with more than 500 sows [29], piglets with a birth weight lower than 0.6 kg had the highest mortality rate, and in those with a birth weight above 1.4 kg, the mortality rate was the lowest. Piglets with a birth weight above 1.3 kg had the same probability of death, regardless of colostrum intake. However, piglets with a birth weight of 1.2–1.3 kg and 1.1–1.2 kg had the same probability of death only when their colostrum intake reached 200 and 250 g/day, respectively [30]. Generally, low-birth-weight piglets are below 1.0 kg in weight, and to determine whether they are IUGR, a morphological examination is needed [31]. Several other factors can influence colostrum consumption, such as the health status of the sow, the number of parity, environmental conditions [32], changes in reproductive hormones, genetics, and nutrition [22]. Therefore, we need to use all measures available to maximize colostrum and Ig intake, including rearing technology (such as split suckling) and nutritional interventions (to improve colostrum quality) [7].
4. Factors Other than Nutrition That Can Affect the Ig Content in Colostrum
Several studies have found that primiparous sows have lower levels of Igs in their colostrum and milk compared to multiparous sows [33,34,35], while other experiments have revealed no connection between sow parity and Ig content [22,36,37]. Extreme temperature, whether hot or cold, produces stress in sows, which affects production, reproductive performance, milk production, immune function, and the serum levels of Igs during gestation and lactation [38,39,40]. Heat stress, for example, has been shown to reduce feed intake and alter hormonal balances, potentially leading to a decrease in Ig levels in colostrum [41,42]. Proper management practices such as optimal housing conditions and hygiene may help maintain the quality of sow milk. Sows kept in a traditional open-housing system had higher Ig content in their colostrum than sows kept in an evaporative cooling-housing system [43]; however, Zhao et al. [44] did not detect a housing effect during gestation. High social stress due to overcrowding or aggressive behavior can also negatively impact sow health [45]. Several studies have also reported that breed and genotype can influence the composition of colostrum [21,27,35,39,46,47,48,49]. Iberian sows had lower crude protein, fat, and lactose content in their colostrum than German Landrace sows [35,46]. Farmer and Quesnel, ref. [39], found that Duroc pigs have higher protein and IGF (insulin-like growth factor) content than Landrace pigs. Meishan sows have less lactose than European White breeds [47]; however, lactose content was greater in the colostrum of the Yorkshire genotype compared to the Belgian Landrace/Pietrain and Duroc lines [48]. The concentration of IgA in colostrum was higher in the Hampshire and Landrace x Yorkshire sows; however, it was lower in the Landrace and Yorkshire purebreds [49]. The concentration of IgGs was greater in the Large White than in the Large White x Landrace [27]. The Duroc breed has the highest IgG and IgA levels in its colostrum compared to Landrace and Large White sows [21]. These differences between genotypes may indicate the significance of selection to improve the composition and yield of colostrum and milk.
5. Changes in Ig Concentrations in Colostrum Between 1980 and 2024
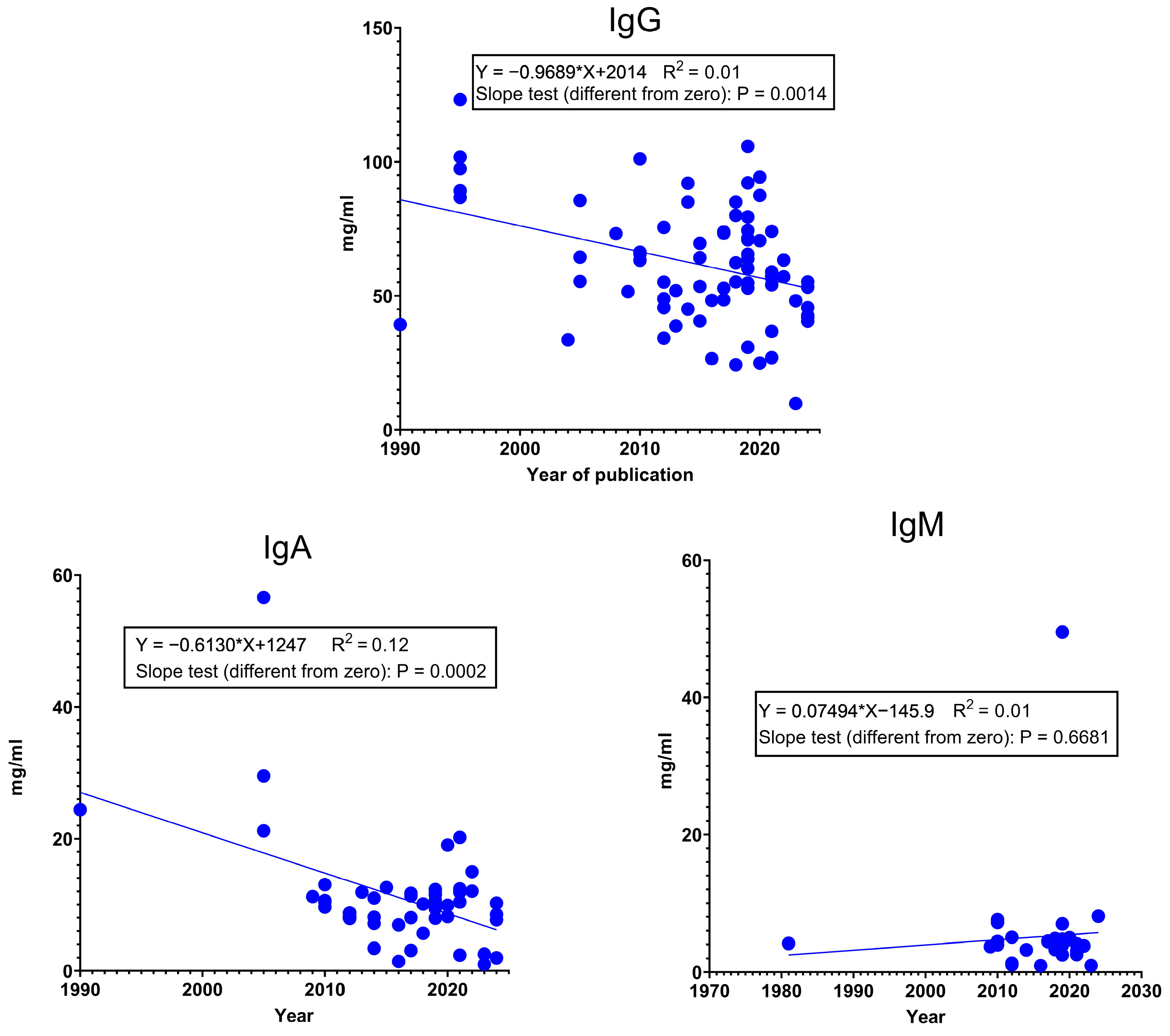
Since the productivity of sows has changed quite a lot during the last forty years, we hypothesized that this might have had an effect on colostrum Ig content. The p-value of IgG and IgM in the slope test indicates that the slope is significantly different from zero, and since the slope (a) has a negative value, it justifies our hypothesis. Figure 2 also illustrates that the measured values show a very diffuse pattern. This suggests that the immunoglobulin content of colostrum is affected by several other factors (e.g., genotype, parity, age, health status, nutrition, farm management, and environment), and this can result in large differences. Some of them are discussed in Section 4. However, we would like to mention another one. The main methodologies for measuring the Ig content of colostrum are BRIX, RID (radial immunodiffusion), and ELISA (Enzyme-Linked Immunosorbent Assay) [50]. The BRIX refractometer is an on-farm method because it is fast, easy to use, and not expensive and requires only a small amount of sample. The RID assay is the best method for direct measurements; however, it is very expensive and time-consuming and requires special equipment and skills. ELISA is more cost-effective and can measure a large number of samples at one time [46]. The widely used ELISA tests have a nanogram sensitivity [51,52], which means that the colostrum and milk samples need to be diluted before analysis, and there are big differences in dilution ratios (for instance: Davis et al. [53] and [54]: 1:200,000 for IgA, 1:500,000 for IgG, and 1:25,000 for IgM; Marciag et al. [55]: 1:500,000 for IgG and 1:30,000 for IgA; Llsley and Miller, [56]: colostrum 1:250,000 for IgG and 1:100,000 for IgA and milk 1:2500 for IgG and 1:20,000 for IgA). Most publications report colostrum and milk Ig concentrations in mg/mL, but some report them in µg/mL or ng/mL. In Figure 2, and also in Figure 3, several “outlier” data can be observed; however, since these data already group averages, we did not remove them from the analysis. These data could be a result of a systematic analysis error or differences in methodologies, which warrants standardization.
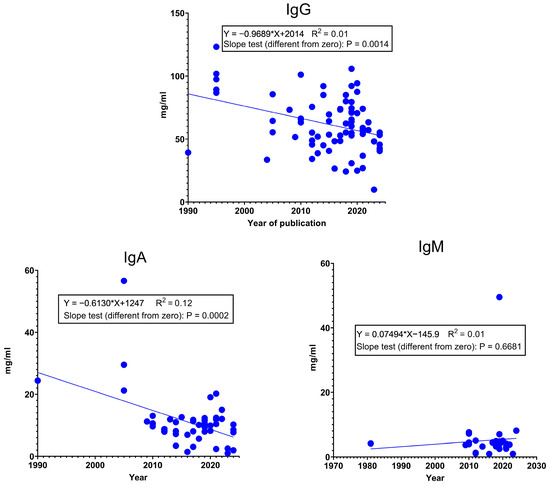
Figure 2.
Changes in the IgG, IgA, and IgM concentration (mg/mL) in colostrum between 1980 and 2024. Data from [4,19,25,32,33,40,43,50,51,53,54,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89].
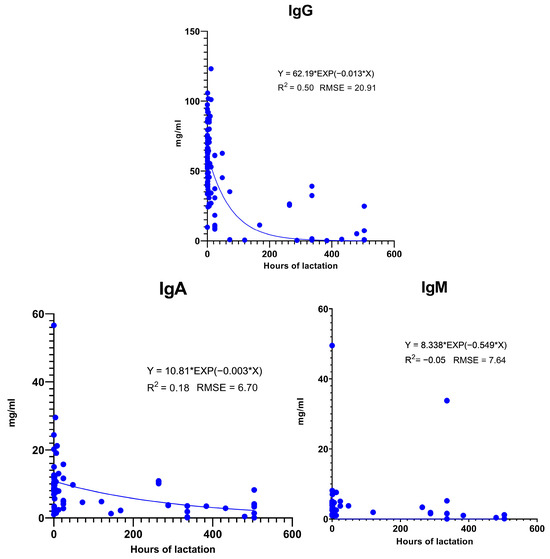
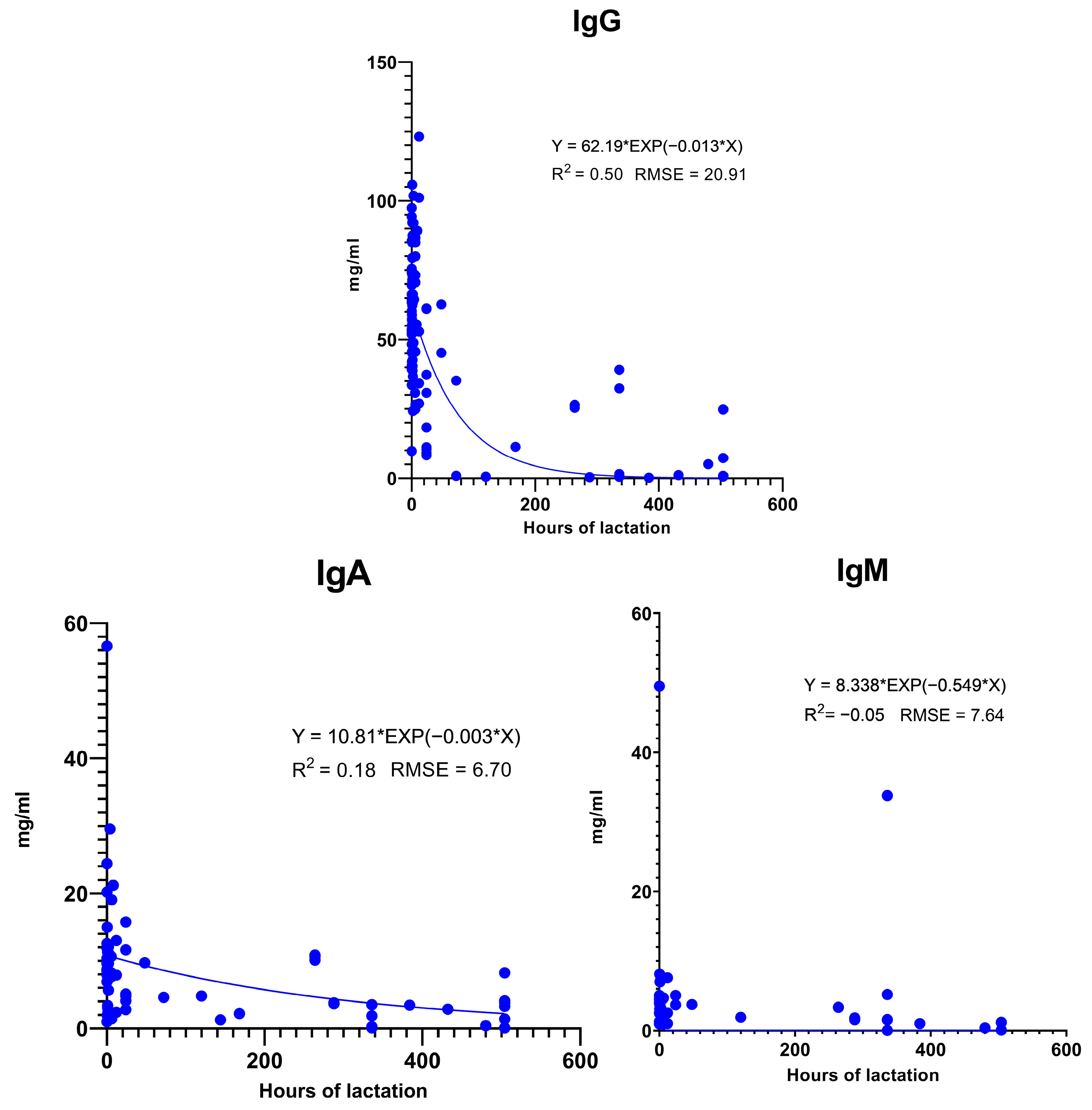
Figure 3.
Changes in Ig concentration in colostrum and milk during lactation. Data from [4,19,25,32,33,40,43,50,51,53,54,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89].
All three Igs exhibit significant variance in colostrum concentration at farrowing (Figure 3). Only the analysis of IgG shows a considerable coefficient of determination with the exponential curve fitted to the time elapsed from farrowing (0 h). The exponential curve results in 62.19 mg/mL IgG at farrowing, while by 12 h, it is 53.07 mg/mL, and by 24 h, it results in a 45.29 mg/mL concentration. This means that in 24 h, the IgG concentration decreased by only 27%. However, since there are several relatively strong research results, which affect the parameters of the calculated equation, these results should be interpreted with caution. In real life, at a specific farm, the Ig content in colostrum may decline more rapidly. Therefore, it is still very important to maximize colostrum intake on the first day.
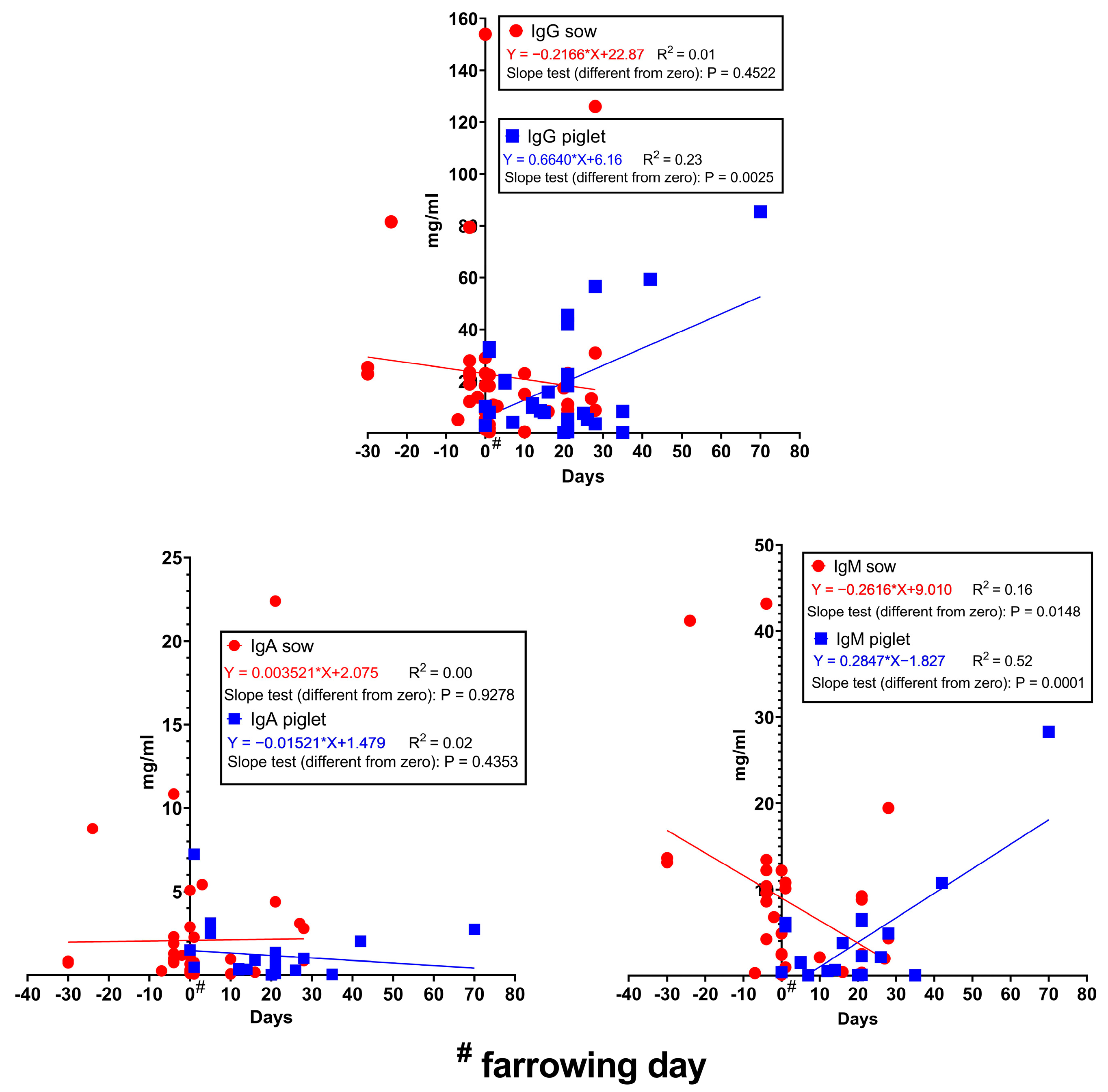
The concentration of immunoglobulins in the serum/plasma of sows and piglets can be seen in Figure 4. Due to some extreme published values, only piglet IgM values have a notable R2 value (0.52) over the lactation period. The slope test results indicate that the IgM concentration decreases from the concentrations measured before farrowing, while the IgG and IgM levels in the piglets’ blood increase during the lactation period. The IgG and IgM concentrations in the colostrum show a significant correlation with the blood level during late gestation (Table 1). IgA and IgM concentrations in the colostrum correlate very well with the sow’s blood concentrations at farrowing and during late lactation and also with milk IgA and IgM levels, respectively. This result justifies the findings of [90] in that the colostrum Ig content is mainly of serum origin. Since immune status during colostrogenesis is an important factor, nutritional interventions that can improve serum/plasma Ig levels, especially IgG, can have a positive effect on piglet health and growth. As presented in Table 1, piglets’ serum/plasma Ig levels have a moderate-to-good significant correlation or tended to have a significant correlation with the colostrum Ig concentration; this underlines the importance of colostral Ig content.
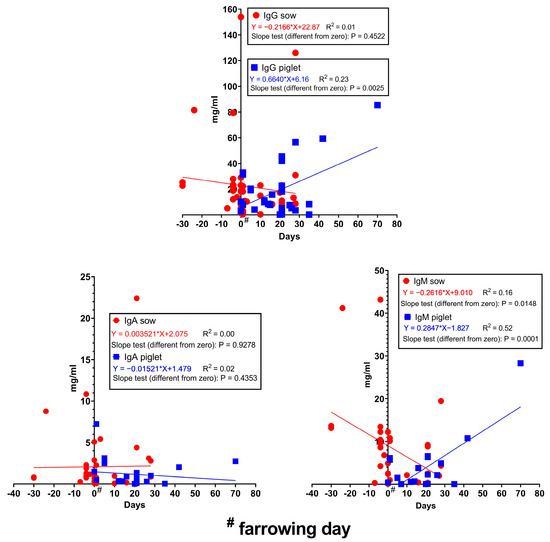
Figure 4.
Changes in Ig concentrations in the serum/plasma of sows and piglets during gestation and lactation. Data from [32,52,56,57,58,61,62,63,64,71,72,75,78,80,87,88,91,92,93,94,95,96,97,98,99,100,101,102].

Table 1.
Correlation between colostrum immunoglobulin concentrations and the serum/plasma of sows and piglets and milk.
6. Feed Supplements and Their Effects on Colostrum and Milk Composition and Sow and Piglet Performance
We prioritized studies only about feed additives in the diets of gestating and/or lactating sows, which affect colostrum composition. Research mostly focused on supplementation with amino acids, dietary antioxidants, pre- and probiotics, and other less-tested substances.
6.1. Amino Acids
6.1.1. Mode of Action
Amino acids are essential for protein synthesis but also play an important role in gene expression. They are also substrates for many biologically active substances (e.g., polyamides, glutathione, hormones, and nucleic acids), hence the term functional amino acids [103]. Essential amino acids must be supplemented by nutrition because they cannot be synthesized or are inadequately synthesized in sows. Amino acids can benefit pregnant and lactating sows by improving fetal growth, neonatal health, and lactation performance [103]. Protein deficiency decreases immune function and increases the possibility of disease outbreaks. Arginine, cysteine, and glutamine have important roles in the immune system [104].
6.1.2. Most Pronounced Effects
Dietary supplementation of arginine (1%) improves the immune cell population (increased number of white blood cells, neutrophils, and basophils) of sows [105]. Arginine is also necessary for the growth of the placenta and the development of the fetus [106]. It stimulates the secretion of prolactin and growth hormones, and it is necessary for the growth of mammary glands [107,108]. In studies in the literature, arginine supplementation improved the weight of the litter [106,109,110] and increased the number of piglets born alive [100], impacted the fat and protein content of milk [108], and also increased the immune reaction (shown by the increased Ig level in the serum) [100,111,112]. Arginine in the diet improved sow production performance and intestinal microbiota [95]; however, Krogh et al. [113] did not find any change.
Glutamate is a peptide-binding amino acid. It can act as a substrate and activator in protein synthesis and improve sow milk production. Lactating sows may not obtain enough glutamate to promote milk production; therefore, supplementation can be beneficial. In one study, glutamine and glutamate supplementation in sows resulted in higher fat content in colostrum, which could be beneficial for suckling piglets [114]. Monosodium glutamate in sow nutrition improved daily milk production, increased the protein and fat content of milk during late gestation and lactation, and also improved the growth of piglets [92,115] (Table 2).
Serin is required for the biosynthesis of selenocysteine and can directly promote the synthesis of selenoproteins [116] and regulate selenoprotein synthesis in young pigs [117]. Late gestation and lactating diets supplemented with serine increased the selenoprotein P (sepp1) and selenium content in the milk of lactating sows and resulted in higher bodyweight of the piglets and also improved the Se status of both sows and piglets [118].

Table 2.
Amino acid supplementation and its effects on Ig levels in sows’ colostrum, milk, and serum.
Table 2.
Amino acid supplementation and its effects on Ig levels in sows’ colostrum, milk, and serum.
| Changes in Ig Concentration (+/− % Changes Compared to the Control) | ||||||
|---|---|---|---|---|---|---|
| Treatment in Feed | Feeding Protocol | Colostrum | Milk | Serum/Plasma Sow or Piglet | References | Effects in General |
| L-Arg 1% supplementation | From day 30 of gestation until day 90 or day 114 | Sow plasma d −24 IgG, IgM +83; +56 d −4 IgG, IgM +60; +50 | [111] | ↑ Immune system | ||
| L-Arg 0.5% and 1% supplementation | From day 85 of gestation until farrowing | IgG 0.5%: −13 1%: +36 | [112] | ↑ Live born piglets and piglets’ birth weight ↑ IgG in colostrum with 1% suppl. | ||
| L-Arg 0.08%, 0.16%, 0.24%, and 0.32% supplementation | From day 30 of gestation until farrowing | Sow plasma d −24 IgG, IgA, IgM 0.08%: +29; +64; +6 0.16%: +21; +49; −6 0.24%: +10; +75; +12 0.32%: +9; +32; +27 d −4 IgG, IgA, IgM 0.08%: +13; +15; +4 0.16%: +9; +7; +15 0.24%: +39; +11; +7 0.32%: +21; +3; +9 | [100] | ↑ Plasma Arg ↑ Born alive ↑ Milk quality | ||
| BCCA: L-Val, L-Ile, and L-Leu at 9, 4.5, and 9 g/day; Arg: 22.5 g/day; BCCA + Arg | From day 100 of gestation until weaning (d27) | IgG, IgA, IgM BCCA: +12; +37; +75 Arg: +42; +61; +42 BCCA +Arg: −16; −30; +51 | IgG, IgA, IgM BCCA: −16; +18; −16 Arg: +4; +13; +32 BCCA+Arg: −8; +99; +186 | Sow serum IgG, IgA, IgM d −4 BCCG: +36; +21; +13 Arg: +44; +23; +7 BCCG +Arg: +82; −11; −5 d 10 BCCG: −28; +131; +24 Arg: +21; +122; +9 BCCG +Arg: −15; +75; +5 d 27 BCCG: +6; −33; +27 Arg: −13; −20; +21 BCCG +Arg: +2; −47; +7 | [95] | ↑ Sow productive performance ↑ Intestinal microbiota |
| Arg 25 g/day | From day 30 of gestation until weaning (d28) | [113] | No change | |||
| Arg 0.72%, 1%, and 1.5% in the diet | From day 70 of gestation until farrowing | [109] | ↑ Litter and piglet weight gain | |||
| L-Arg 0.5%, 1%, and 1.5% supplementation | During lactation | [108] | ↑ Piglet weight gain ↑ Milk composition | |||
| L-Arg 25.5 g/day supplementation | From d77 of gestation until farrowing | [110] | ↓ Within-litter variation | |||
| L-Arg 0.5% and 1% supplementation | During lactation | [107] | ↑ Plasma Arg level ↑ Weight gain of piglets | |||
| Total Val–Lys ratio of 0.63, 0.73, and 0.93 | From d75 of gestation until farrowing | [119] | ↑ Daily weight gain of piglets ↑ Prolactin in plasma | |||
| Glutamate 2% supplementation | From one week before farrowing until day 21 of lactation | [92] | ↑ Milk production ↑ Growth of piglets | |||
| Glutamate 1% and 2% supplementation | During lactation | [115] | ↑ Milk synthesis ↑ Piglet growth | |||
| Glutamine and glutamate 1.5% supplementation | From one week before farrowing until day 21 of lactation | [114] | ↑ Glutamine content of milk ↑ Fat content in colostrum and milk | |||
| 0.137%, 0.275%, and 0.55% serine supplementation | From day 110 of gestation until weaning | [118] | ↑Improved Se status of sows and piglets ↑ Piglet weight | |||
↑ the parameter increased; ↓ the parameter decreased.
6.1.3. Effectiveness
Amino acid supplementation has a clear positive effect on the sow’s Ig concentration in the blood during late gestation (Table 2) (+35, +26, and +15 percentages on average for IgG, IgA, and IgM, respectively). This positive effect remains only for IgA during the late lactation period (about a 38% increase). Elevated serum/plasma Ig levels in sows during late pregnancy have a more pronounced effect on IgA and IgM concentrations in both colostrum and milk (+12, +23, and +56 and −7, +43, and +67 percentages on average in colostrum and milk for IgG, IgA, and IgM, respectively). The data gathered suggest that arginine alone in about 1% supplementation can provide a beneficial effect. When supplementation is applied from the early period of gestation, lower doses may also be effective.
6.2. Dietary Antioxidants (Vitamin E, Vitamin C, Vitamin A, and Se)
6.2.1. Mode of Action
Vitamins and trace minerals are important in scavenging oxygen free radicals. Reactive oxygen species (ROS) and oxidation in sows or piglets have long-lasting effects on the offspring and the growth of piglets during lactation and post-weaning; therefore, it is necessary to decrease oxidative damage with supplementation.
Vitamin E is one of the most effective lipid-soluble chain-break antioxidants and is present in cell membranes. It can decrease lipid peroxidation caused by ROS and free radicals and maintain cell membrane integrity. Vitamin C can also decrease the damage caused by free radicals in cells and regenerate vitamin E. Selenium is a well known biological antioxidant that can provide a beneficial antioxidant status and improve humoral immune function (IgG and IgA). Piglets are born with low vitamin E concentrations due to the limited placental transfer [87]. Prenatal vitamin E and Se supply can provide an optimal antioxidant status for the piglet at birth; postnatal addition can provide the optimal antioxidant status during lactation and after weaning [120]. Vitamin A is essential for epithelial cell differentiation and mucus production and acts as the first line of defense against pathogens. β-carotene is the precursor of vitamin A and has antioxidant activity. Carotenoid supplementation in diets has beneficial effects on the immune system, enhances humoral activity, and increases resistance to disease. Vitamin A and β-carotene supplementation also enhance the reproductive performance of sows, resulting in more piglets being born [121].
6.2.2. Most Pronounced Effects
Inadequate amounts of vitamin E and selenium may result in increased sensitivity to diseases and morbidity because selenium deficiency blocks the function of neutrophils while vitamin E deficiency both impacts lymphocytes and neutrophils [122,123]. Vitamin E and vitamin C supplementation during gestation improved total Ig and IgG concentration in the serum of piglets; however, there was no effect on colostrum [87]. An increased amount of vitamin E and/or selenium in sow feed improves the fat content of the milk, the colostrum composition, and humoral and cell immunity (increased IgG and IgA). It also increases the number of born-alive piglets and the weaning weight. It can regulate the microbiota and antioxidant activity in both sows and piglets [120,124,125]. It can be concluded that supplementation of the sow’s diet with antioxidant vitamins above requirements has the potential to increase IgG concentrations (Table 3).
Oxidized β-carotene has immune-modulating activity by enhancing the immune system (increasing the production of cytokines and phagocyte activity) and decreasing inflammation [126] (Table 3). The immunological activity and the positive effect on animal health lead to the use of it in the sow diet. Dietary fully oxidized β-carotene supplementation improved IgG, IgM, and IgA levels in colostrum and lactose, IgG, and IgM concentrations in milk [89,121].
Selenium is an essential trace element for animal health; however, it can act as a toxic substance. Se deficiency or overdose can cause damage in animals; therefore, it is very important to find nutritional strategies to improve Se bioavailability. It can be conjugated into amino acids, act as a cofactor for antioxidant enzymes, and is involved in the immune system. There is a relationship between selenium intake and selenium status in lactating sows because the plasma selenoprotein P and selenium content of milk increased through increased selenium supplementation [118]. Selenium in organic (yeast) and inorganic (sodium selenite) form can be supplemented into a sow’s diet during gestation and lactation. A study conducted by Gelderman and Clapper, ref. [127], demonstrates the effect of Se sources on the passive transfer of Igs; however, the Ig concentrations were not affected in the sow but were affected in the piglets. Antioxidant enzyme activity (glutathione peroxidase) and the dietary selenium level increased in sow and piglet serum. Se status, antioxidant capacity, and immunoglobulin transfer were enhanced, piglet growth increased, and also the barrier function of the small intestine improved, according to Xiong et al. [63]. There was no significant difference in colostrum selenium content; however, the milk was influenced by selenium supplementation [128]. Organic selenium intake during gestation increased the number of total born piglets, shortened the birth interval, improved antioxidant capacity during parturition, and increased piglet growth performance [129] (Table 3).

Table 3.
Dietary antioxidant supplementation and its effects on Ig levels in the sows’ colostrum, milk, and blood.
Table 3.
Dietary antioxidant supplementation and its effects on Ig levels in the sows’ colostrum, milk, and blood.
| Changes in Ig Concentration (+/− % Changes Compared to the Control) | ||||||
|---|---|---|---|---|---|---|
| Treatment in Feed | Feeding Protocol | Colostrum | Milk | Serum/Plasma Sow or Piglet | References | Effects in General |
| 250 IU/kg vit. E | From day 107 of gestation until day 21 of lactation | IgG, IgA +20; +12 | IgG, IgA +8; +8 | Sow plasma IgG, IgA d 0: +9; +9 d 21: +7; +20 Piglet plasma IgG, IgA d 21: +11; +9 | [62] | ↑ Colostrum, milk, and plasma tocopherol levels ↑ Milk composition ↑ Humoral immune function and antioxidant activity ↑ Weight of piglets at weaning |
| 500 mg/kg vit. E, 10 g/day vit. C, and in combination | From the beginning of pregnancy until weaning | IgG Vi tE: −17 Vit C: −14 E+C: +6 | IgA Vit E: −39 Vit C: −22 E+C: −12 | Piglet plasma d 21 IgG Vit E: −6 Vit C: +3 E+C: +31 | [87] | ↑ Alpha-tocopherol in colostrum and milk and piglet tissues and plasma at weaning Combined treatment is more beneficial |
| 4 or 8 mg/kg β-carotene | From day 85 of gestation until day 21 of lactation | IgG, IgA, IgM 4 mg: +8; +82; +72 8 mg: +23; +114; +77 | IgG, IgA, IgM 4 mg: +35; +31; +113 8 mg: +60; +12; +134 | [89] | ↓ Inflammatory markers in colostrum and milk ↑ Lactose concentration in milk ↑ Litter and piglet weight at weaning | |
| 80 ppm of oxidized β-carotene for parity of 1, 2, and 3+ sows | From day 60 of gestation until the end of lactation | IgG, IgA P1: +32; +1 P2: +18; +24 P3+: −25; −24 | IgG, IgA P1: +127; +35 P2: −48; −19 P3+: +30; +24 | Sow plasma IgG, IgM d −54 P1: −63; −67 P2: −66; −63 P3+: −56; −58 d 0 P1: −15; +19 P2: +52; +24 P3+: +18; −12 d 21 P1: −25; +1 P2: −13; +24 P3+: +86; +3 | [121] | No effect on reproductive performance No effect on colostrum and milk composition |
| Combination of heat stress (HS) and active cooling (AC) in a farrowing unit with 0.30 (control) or 1.2 mg/kg organic Se supplementation | From day 85 of gestation until day 21 of lactation | IgG, IgA, IgM AC: +33; +40; +58 HS: +15; +15; +100 | IgG, IgA, IgM AC: +164; +74; +53 HS: +126; +53; +26 | Sow plasma d 0 IgG, IgA, IgM AC: +21; +3; +30 HS: +16; − 11; +6 d 21 IgG, IgA, IgM AC: +44; +127; +104 HS: +130; +40; +52 Piglet plasma d 1 IgG, IgA, IgM AC: +105; +21; +20; HS: +37; +57; +35 d 21 IgG, IgA, IgM AC: +99; +37; − 6 HS: +130; +99; +60 | [125] | ↑ Pre-weaning survival ↑ Colostrum and milk composition ↑ Matrnal selenium and antioxidant status ↑ Ig transfer irrespective of climatic conditions |
| 0.3 mg/kg Se as an inorganic source (control) or 0.2 mg/kg as an organic source | From day 85 of gestation until day 23 of lactation | IgG, IgA, IgM +11; +24; +13 | IgG, IgA, IgM +18; +12; +36 | Sow plasma IgG, IgA, IgM d 1: +3; +17; +28 d 21: +1; +85; +1 Piglet plasma IgG, IgA, IgM d 1: +2; +15; +39 d 21: +15; +30; +14 | [63] | ↑ Piglet growth during the first week of lactation ↑ Se status, antioxidant capacity, and immunoglobulin transfer ↑ microbiota ↑ Small intestinal barrier function |
| 0.15 or 0.30 mg/kg Se in inorganic or organic form | From day 6 pre-farrowing until day 3 postpartum | [128] | ↑ The organic source results in higher milk Se content ↑ The inorganic source has higher biological activity of glutathione peroxidase | |||
| 0.3 mg/kg Se as an organic and inorganic source | During gestation | [129] | ↑ Increased litter size ↑ Sow and piglet antioxidant capacity ↑ Piglet growth | |||
| 0.3 ppm inorganic or organic Se | From 60 days before breeding until farrowing | Data in the figure | Data in the figure | Data in the figure | [127] | ↑ Passive transfer of immunoglobulins |
| 0.30 mg/kg inorganic or organic Se in combination with 30 IU/kg or 90 IU/kg vit. E | From day 1 of gestation until 21 d postpartum | [124] | ↑ Antioxidant status of serum and milk with organic Se ↑ Milk composition with organic Se No beneficial effect of elevated vit. E supplementation | |||
↑ the parameter increased; ↓ the parameter decreased.
6.2.3. Effectiveness
Only one study with β-carotene supplementation measured blood immunoglobulin content during gestation, but at the beginning of the study (Table 3). The experimental groups had considerably lower Ig content at the start, and the β-carotene supplementations made it possible to mitigate this in part by the time of farrowing. As the treatments lasted until the end of lactation, late lactation blood samples showed markedly higher Ig concentrations compared to the control groups (+33, +68, and +31 percent change compared to the control group on average for IgG, IgA, and IgM, respectively). The various antioxidant supplementations were able to increase the Ig concentration in the colostrum and milk (+9, +32, and +64 and +58, +13, and +72 percent change on average in the colostrum and milk for IgG, IgA, and IgM, respectively). These positive changes resulted in elevated Ig levels in the piglet’s blood before weaning (+40, +44, and +23 percent change compared to the control group on average for IgG, IgA, and IgM, respectively). Despite the general positive effect of vitamin E supplementation on antioxidant status and milk composition, the analyses of the research results do not show a clear positive effect on Ig concentrations in the milk or blood. Selenium supplementation in organic form is more effective, and most of the results indicate a profound positive response in Ig concentrations.
6.3. Pre- and Probiotics (Dietary Fiber, MOS, Yeast, Bacteria, Enzymes, and Sugar Beet Pulp)
6.3.1. Mode of Action
Probiotics are live microorganisms (bacteria and yeast); however, prebiotics are non-living, non-digestible food components that are nutrients for gut bacteria. Microbial colonization of the gut after birth plays an important role in enhancing the immune system. The first donor of fecal microbiota to piglets is the sow [120]. The connection between the microbiota and health status of the sow has been one of the most researched areas of the present day. The microbiota is a community of microorganisms that have a connection with the host metabolism. In sows, several factors can affect the composition of the microbiota, like antibiotics, genotype, feed additives, pathogens, and stress. In pigs, the modulation of the microbiota can prevent diseases and reduce the use of antibiotics [130]. Gut microbes help to maintain nutrient metabolism and health status (protection against pathogens) and also improve immune status [131,132].
6.3.2. Most Pronounced Effects
The effects of supplementation with pre– and probiotics on sow nutrition are summarized in Table 4. Dietary fiber (alfalfa meal, beet pulp, and soybean hulls) can regulate the gut microbiota, decrease inflammatory responses, and also improve performance parameters in sows [133]. Dietary fiber during gestation and lactation improved the behavior of sows and the colostrum intake of low-birth-weight piglets [60]; moreover, the fat content of the colostrum increased [19]. Changes in the microbiota transfer from the mother to piglets by supplementing resistant starch (pea starch) were only slightly affected; however, milk composition was altered [69].
Sugar beet pulp (SBP) contains high levels of soluble fiber (e.g., pectin and glucan). It can be fermented in the hindgut, has prebiotic properties, and improves microbiota and animal health [134]. The research results showed that the IgG and IgM levels in colostrum were not affected, but IgA increased [135]. The expression of proinflammatory cytokines (TNF-α and IL-6) was downregulated while anti-inflammatory cytokine (IL-10) was upregulated by SBP supplementation. These findings suggest that dietary fermentable fiber may minimize inflammation in piglets and enhance their immune response by increasing the quantity of cytokinins and antibodies [61].
Mannan oligosaccharides (MOSs) are complex sugars that consist of mannose [96]. They are prebiotics with immunomodulatory properties [136]. Dietary MOS supplementation can be used in sow diets to improve growth performance (BW at weaning and ADG during lactation) and increase disease resistance capacity in piglets. MOSs had no effect on IgA or IgG concentrations in colostrum; however, they increased the IgM level [88]. Czech et al. [80] found that MOS supplementation for 4 weeks in the gestational sow diet increased IgG and IgM content in colostrum, while Hung and Lindemann [96] detected no difference despite supplementation during the entire gestation period. Chitosan oligosaccharides during gestation and lactation improved piglet growth [83], increased IgM levels in colostrum and umbilical cord blood, and also increased IgG, IgA, and IgM levels in serum of piglets [97]. Isomaltooligosaccharide (IMO) and Bacillus (B. subtilis and B. licheniformis) supplementation improved piglet birth weight and the antioxidant capacity of the placenta and increased the levels of growth hormones during late gestation [137].
A soybean isoflavone (SI) and astragalus polysaccharide (APS) mixture improved the colostrum components, serum antioxidant capacity, and immune and hormone levels of sows during lactation. This mixture also increased the feed intake and lactation yield of sows [102].
Supplementation of Saccharomyces cerevisiae yeast (obtained through the synthesis of a large amount of vitamin B) influences the healthy gut microbiome, improving immune response and performance, according to Simon et al. [138]. Sun et al. [139] also found that Saccharomyces cerevisiae boulardii improved immune status through the higher Ig concentration. Different forms of yeast (live yeast and superfine yeast powder) may improve the antioxidant capacity of sows and improve intestinal immunity by increasing mucosal IgA secretion in piglets [140]. Yeast supplementation during gestation and lactation improved colostrum production and sow physiology [83] and increased IgG levels in the colostrum and plasma of piglets [90]. It also increased IgG and IgA levels in the serum of sows and piglets [91] and increased IgG levels in the milk and colostrum; however, it maintained IgA levels in the milk [141]. According to Peng et al. [98], yeast supply in the sow diet enhanced colostrum composition and liver function and decreased the number of stillborn and low-body-weight piglets. The abundance of beneficial microbes increased in both sows and piglets [79]. The duration and frequency of neonatal diarrhea were reduced by Saccharomyces cerevisiae var. boulardii supplementation in piglets [93,141,142,143]. The results showed that supplementation of probiotic bacterial cells (EM Bokashi) significantly affected the immunological quality of sow milk and colostrum. This emphasizes the protective potential of colostrum and indicates immune reactions that protect the sow and piglets against infections [144] (Table 4).

Table 4.
Pre- and probiotic supplementation and their effects on Ig levels in sows’ colostrum, milk, and serum.
Table 4.
Pre- and probiotic supplementation and their effects on Ig levels in sows’ colostrum, milk, and serum.
| Changes in Ig Concentration+/− % Changes Compared to the Control | ||||||
|---|---|---|---|---|---|---|
| Treatment in Feed | Feeding Protocol | Colostrum | Milk | Serum/Plasma Sow or Piglet | References | Effects in General |
| Elevated (23.4%) total dietary fiber | From day 92 of gestation until farrowing | IgG, IgA +1; −38 | IgA −24 | [60] | ↑ Colostrum intake of low-birth-weight piglets ↑ Sow behavior | |
| Elevated (19.3 to 21.7%) total dietary fiber | From day 90 of gestation until farrowing | IgG −16 | [19] | ↑ Arterial acetate and colostral fat | ||
| 33% digestible (corn) starch and 33% resistant (pea) starch | From day 88 of gestation until weaning (d28) | IgG −6 | [69] | Changed microbiota ↑ Milk composition | ||
| Sugar beet pulp: 20% and 10% Wheat bran: 30% and 15% (gestation and lactation) | From day 86 of gestation until day 21 of lactation | IgG, IgA, IgM SBP20%: +6; +15; +7 SBP10%: +2; +9; +6 | IgG, IgA, IgM SBP20%: +1; +4; +3 SBP10%: 0; −2; 0 | Piglet serum d 21 IgG, IgA, IgM SBP20%: 0; −4; +1 SBP10%: −6; −3; +5 | [61] | ↑ Milk quality with SBP ↑ Growth performance of piglets with SBP ↑ Intestinal barrier function with SBP |
| 400 mg/kg MOS | From day 86 of gestation until weaning (d20) | IgG, IgA, IgM +4; −1; +51 | IgG, IgA, IgM −1; −2; +8 | Piglet serum d 20 IgG, IgA, IgM +15; +13; −2 | [88] | ↓ Weaning to the estrus interval ↑ Growth and immunity of piglets |
| 8 g/sow/day MOS in two experiments | From 4 weeks prepartum until 4 weeks postpartum | IgG, IgA, IgM exp. 1: +25; −1; +25 exp. 2: +14; +6; +13 | Sow plasma d −4 IgG, IgA, IgM exp. 1: +14; +11; +1 exp. 2: +9; 0; −15 d 0 exp. 1: +5; +5; +16 exp. 2: +25; +1; +31 d 21 exp. 1: +6; +18; +11 exp. 2: +31; +17; +38 Piglet plasma d 0 exp. 1: +29; +9; +21 exp. 2: +18; −2; +21 d 21 exp. 1: +8; +10; +2 exp. 2: +21; 0; 0 | [80] | ↑ Survival of piglets ↑ Bodyweight gain in piglets | |
| 0.2% MOS | From day 102 of gestation and during lactation | IgG, IgA, IgM +12; +10; +2 | IgG, IgA, IgM +30; +19; +24 | Sow serum d −2 IgG, IgA, IgM +5; +2; +26 d 16 IgG, IgA, IgM −1; +18; +37 Piglet serum d 16 IgG, IgA, IgM −5; −6; +9 | [96] | ↑ Reproductive performance ↑ Bodyweight of piglets |
| Chitosan oligosaccharide 100 mg/kg | From day 85 of gestation and during lactation (d 21) | IgG, IgA, IgM +23; +2; +46 | IgG, IgA, IgM +29; +8; +2 | Sow serum d 1 IgG, IgA, IgM +31; +28; +27 d 21 +10; +19; +15 | [83] | ↑ Piglet growth |
| Chitosan oligosaccharide 30 mg/kg | From day 86 of gestation and during lactation (d 20) | IgG, IgA, IgM +8; −8; +39 | IgG, IgA, IgM +2; +2; +8 | Piglet serum d 0 IgG, IgA, IgM +3; +1; +13 d 20 IgG, IgA, IgM +18; +19; +13 | [97] | No effect on sow reproductive performance ↑ Humoral and innate immunity and anti-inflammatory ability |
| Yeast derivative 2 g/kg | During gestation | IgG, IgA, IgM +5; −13; −12 | [74] | ↑ Colostrum production ↑ Sow physiology | ||
| Yeast 106 CFU or 107 CFU/g diet in lactation (L) feed or gestation (G) feed | During gestation and lactation (21 d) | IgG G-/L106: +10 G-/L107: +41 G106/L106: +51 G107/L107: +133 | . | Sow plasma d 1 IgG, IgA G-/L106: −25; −10 G-/L107: −1; −8 G106/L106: −20; −29 G107/L107: −11; −46 d 21 IgG, IgA G-/L106: +19; −6 G-/L107: −9; +2 G106/L106: −1; −28 G107/L107: +45; −40 Piglet plasma d 1 IgG, IgA G-/L106: +51; +29 G-/L107: +27; +28 G106/L106: +43; +9 G107/L107: +162; +10 d 21 IgG, IgA G-/L106: +64; +27 G-/L107: +14; +9 G106/L106: +62; −18 G107/L107: +108; +55 | [94] | No effect on sow reproductive performance ↓ Weaning to estrus interval |
| Live yeast 1 g/kg (1010 CFU/g) | From day 90 of gestation and during lactation (d 21) | Sow plasma d 1 IgG, IgM +47; +3 | [98] | ↑ Colostrum composition and liver function ↓ Number of stillborn and low-BW piglets | ||
| Live yeast 0.1 g/kg (1010 CFU/g) | From day 28 gestation until weaning (d 28) | IgG, IgA +2; −8 | IgA +29 | [79] | ↑ Beneficial microbes in sows and piglets | |
| Live yeast 425 mg/kg (1.5 × 1011 CFU/kg) | From day 60 of gestation until weaning (d 28) | Sow serum d 0 IgG, IgA, IgM +9; +70; +82 d 28 IgG, IgA, IgM +24; +89; +31 Piglets serum d 28 IgG, IgA, IgM +33; +52; +49 | [91] | ↑ Immunity of sows and piglets | ||
| Live yeast 1 g/kg (1010 CFU/g) | From day 94 of gestation until weaning (d 28) | IgA +103 | . | Sow serum d 0 IgA +5 Piglet serum IgA data in the Figure | [93] | ↓ Duration and severity of post-weaning diarrhea |
| Live yeast strains (4) in 0.05% or 0.5% | From day 80 gestation until weaning (d 18) | Data in the figure | Data in the figure | Data in the figure | [141] | No effect on sow and piglet weight ↑ IgG milk and colostrum ↑ IgA in milk maintained |
| Soybean isoflavone and astragalus polysaccharide mixture: 100, 200, and 300 mg/kg | From day 107 of gestation until weaning (d 21) | Sow serum d 1 IgG, IgA 100 mg: +1; +2 200 mg: +6; +9 300 mg: +6; +6 d 10 IgG, IgA 100 mg: +9; +1 200 mg: +17; +10 300 mg: +13; +3 d 21 IgG, IgA 100 mg: +2; +2 200 mg: +20; +10 300 mg: +12; +6 | [102] | ↑ Lactation yield ↑ Antioxidant capacity ↑ Hormone levels ↑ Health status | ||
| Clostridium butyricum: 10 g/sow/day | From 1 week before farrowing until weaning (d 29) | IgG, IgA −3; +5 | [70] | ↑ Lipidomic and metabolomic profile of milk | ||
| C. butyricum: 0.1%, 0.2%, or 0.4% | From day 90 of gestation until weaning (d 21) | IgG, IgM +6; +5 +9; +22 +17; +25 | IgG, IgM +5; +9 +14; +25 +13; +28 | [59] | ↓ Duration of farrowing ↑ Growth performance and antioxidant status of piglets | |
| xylanase and xylo-oligosaccharide: 100 mg/kg | From day 85 of gestation during the lactation period | IgG, IgA, IgM +7; −3; +11 | IgG, IgA, IgM 0; +11; +9 | Sow plasma d 0 IgG, IgA, IgM +3; 0; +6 d 28 IgG, IgA, IgM +14; +10; +1 | [71] | ↑ Weight gain of piglets ↓ Oxidative stress of sows |
↑ the parameter increased; ↓ the parameter decreased.
Clostridium butyricum probiotic feed additive significantly improved sow body condition, reduced the incidence of diarrhea in piglets, and impacted the fatty acid profile of colostrum and milk [70]. The duration of farrowing decreased and the growth performance and antioxidant status of piglets were improved by C. butyricum, according to Cao et al. [59].
In recent years, a so-called “stimbiotic” (a combination of xylanase and xylo-oligosaccharide) has been proven to improve farm animal performance, gut health and the immune system (Table 4). The stimbiotic supplementation works by extracting extra energy from dietary fiber, therefore increasing fiber utilization in the intestine and promoting the production of short-chain fatty acids and also enhancing growth rates and the gut health of the animal. The IgG, IgM, and IgA concentration in the plasma, colostrum, and milk increased during lactation. Stimbiotics also reduced oxidative stress in sows [71].
Fermented feed is widely used in pig nutrition. Fermented feed supplemented with Bacillus subtilis and Enterococcus faecium (Table 4) improved the performance of sows and piglets [145]. Milk yield and quality were enhanced, and the IgG and IgM levels also increased in the serum. Moreover, the microbial metabolic functions and gut microbiota of the sows significantly improved.
6.3.3. Effectiveness
The mainly late gestational treatments resulted in slightly higher Ig concentrations in sows’ pre-farrowing blood samples compared to those in the control groups (+9, +4, snf +4 percent change compared to the control group on average for IgG, IgA, and IgM, respectively), which improved somewhat by late lactation (+13; +5; +22 percent change compared to the control group on average for IgG, IgA, and IgM, respectively) (Table 4). The various pre- and probiotic supplementations were made it possible to increase the Ig concentration in the colostrum and milk (+16, +8, and +21 and +9, +5, and +12 percent change on average in the colostrum and milk for IgG, IgA, and IgM, respectively), but the results were variable. These positive changes resulted in moderately elevated Ig levels in the piglets’ blood before weaning (+27, +13, and +10 percent change compared to the control group on average for IgG, IgA, and IgM, respectively). Fiber supplementation, even in the form of highly fermentable fiber, has little if no positive effect. Prebiotic oligosaccharides and probiotics have mainly positive effects on Ig concentrations but only at a modest level. When live microorganisms are supplemented, the CFU/g concentration and the inclusion rate play an important role in effectiveness; therefore, a specific review of this field could possibly provide a clearer picture.
6.4. Other Feed Additives (Omega-3 Fatty Acids, Plants, and Plant Extracts)
6.4.1. Mode of Action and Most Pronounced Effects
Supplementing sow diets with omega-3 fatty acids can improve the fatty acid profile of sow milk, promoting brain development and overall health in piglets (Table 5). Dietary omega-3 polyunsaturated fatty acids (PUFAs) play an essential role in lipid metabolism and can reduce inflammation. Supplementation in late gestation with immune-modulating compounds may reduce the adverse effects of maternal stress on the developing fetus.
The effects of sunflower oil, fish oil, and rapeseed oil supplementation in the diet of sows have changed the n-6 to n-3 ratio in the immune cells in progeny and influenced the eicosanoid synthesis ex vivo. Also, the concentration of fatty acids in sow milk has a connection with the dietary treatments. It is known that omega-3 PUFAs, such as docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids, have an immune-modulating and anti-inflammatory nature [146]. Protected fish oil is resistant to oxidation and can enhance the concentration of DHA and EPA acid in colostrum and milk. Protected fish oil can be used to increase the concentration of n-3 PUFAs in the diet. The results showed that the growth of the piglets improved, DHA concentration in the colostrum increased, and the acute stress response in the pigs was reduced by supplementation during late gestation and lactation. A late gestation diet of protected fish oil enhanced the phospholipid profile and reduced stress and inflammation in post-weaning [147]. Fish oil and soybean oil supplementation increased the weaning survival rate and weaning weight [76]. However, Leonard et al. [57] found that fish oil supplementation decreased IgA concentrations in sow serum. Olive oil supplementation during late gestation and lactation has more beneficial effects on sows than fish oil supplementation [148]. However, IgG and IgA content in colostrum increased when using palm and sunflower oil supplementation compared with olive and fish oil [149]. IgG and IgA concentrations increased in the serum with soybean oil supplementation, although there were no significant differences in litter performance and the immunological variables of colostrum and milk compared to coconut and palm oil [68]. Increased linoleic acid and α-linoleic acid supplementation from soybean oil improved milk composition, litter size, and weaning weight; however, they did not affect piglet survivability or sow performance [150]. Conjugated linoleic acid supplementation during gestation and lactation increased lysozyme levels [64].
Resin acid-enriched compositions (RACs) contain tall oil fatty acid with an active component of resin acid. They can improve the microbial population, intestinal health, colostrum nutritional composition, and Ig content (Table 5). The IgG levels were significantly higher in the treatment groups; however, the IgA and IgM levels were not influenced [73].
Lysophospholipids are modified phospholipids that generate important secondary messengers with important physiological functions [151]. They increase the hydrophilic properties of the compound, so this could be used to enhance the capacity of fat emulsification and increase the utilization of dietary fat [152]. Supplementation of dietary lysophospholipids in the sows’ feed increased the IgG concentration and changed the fatty acid composition of their milk; however, it did not affect the IgA content [86] (Table 5)
Pinecone oil contains α-pinene, β-pinene, and limonene, which improve the performance parameters of piglets (daily gain and weaned bodyweight), milk composition (lactose and fat content increased), and maternal stress (decreased cortisol concentration) [153]. Garcinol is the main medicinal component of the dried fruit rind of Garcinia indica and is an excellent antioxidant and anti-inflammatory plant extract [154]. Garcinol supplementation significantly decreased oxidative stress in sows and increased IgA and IgG levels in both the plasma and colostrum; however, it had no effect on performance [155]. Resveratrol is a plant phenol that can provide protection against oxidative stress and inflammation; therefore, it is beneficial in improving the reproductive performance of sows [156]. Glycitein is a major soy isoflavone. Dietary supplementation of glycitein improved the antioxidant status of sows during late pregnancy and lactation; protein and fat content increased in both colostrum and milk, and the performance of the piglets was better (increased litter weight and daily gain) [157]. Brown seaweed is a rich source of bioactive compounds and it can promote animal health and antioxidant status. Supplementation of seaweed positively enhanced Ig levels in the colostrum and serum of piglets [57], improved the humoral immune response in piglets [58]. and enhanced pro-inflammatory TNF- α mRNA expression in lipopolysaccharide (LPS)-stimulated ileum tissue [52]. However, supplementation during post-weaning only improved the growth rate of piglets, but no significant effect on oxidative status was found [158] (Table 5).
Ginger extract supplementation during late gestation and lactation significantly increased the concentration of antioxidant and phenolic compounds in the plasma and also increased the IgG concentration in the colostrum and plasma of both the sows and piglets. Ginger extract in feed can influence the immune function of piglets by increasing the antioxidant levels in the plasma and the Ig concentration in the colostrum [78]. Andrographis paniculata (A. paniculata) is a medicinal plant that is native to Asia. Extracts from the plant have antifungal, antibacterial, antiviral, antimalarial, antipyretic, anti-inflammatory, hepatoprotective, anticancer, and immunostimulatory properties. Supplementation during gestation and lactation improved the antioxidant capacity of the colostrum [82]. Dietary saponins extracted from the plant Quillaja saponaria reduced the number of stillborn piglets and improved their performance; however, they had a negative effect on the utilization of feed [56]. Magnesium (Mg) is a macro-mineral, and, among its many functions, it is necessary for the immune system. MgSO4 supplementation during late gestation and lactation increased the IgG content in the plasma and the IgA content in the colostrum and milk; however, the fat content of the colostrum decreased [75].

Table 5.
Other feed additives and their effects on Ig levels in the sows’ colostrum, milk, and serum.
Table 5.
Other feed additives and their effects on Ig levels in the sows’ colostrum, milk, and serum.
| Changes in Ig Concentration (+/− % Changes Compared to the Control) | ||||||
|---|---|---|---|---|---|---|
| Treatment in Feed | Feeding Protocol | Colostrum | Milk | Serum/Plasma Sow or Piglet | References | Effects |
| Seaweed extract (SWE) 10 g/day or fish oil (FO): 100 g/day | From day 109 of gestation until weaning (d 26) | IgG, IgA, IgM SWE: +10; −15; +14 FO: +1; 0; −11 | IgG, IgA, IgM SWE: +12; −1; −15 FO: +15; +7; +18 | Piglet serum d 5 IgG, IgA, IgM SWE: +19; +25; +2 FO: +8; −19; −11 d 15 IgG, IgA, IgM SWE: +21; −13; +17 FO: −3; +29; +4 | [57] | ↑ Lymphocyte phagocytosis at weaning ↑ Leukocyte phagocytosis at weaning (FO) No effect on piglet performance |
| Seaweed extract (SWE) 1.8 g/day or 100 g/day fish oil (FO) | From day 109 of gestation until weaning (d 24) | IgG, IgA +10; −15 +1; 0 | IgG, IgA +12; −1 +15; +7 | Piglet serum d 5 IgG, IgA, IgM SWE: +19; +25; +2 FO: +8; −19; −11 d 15 IgG, IgA, IgM SWE: +21; −13; +17 FO: −3; +29; +4 | [58] | ↑ Humoral immunne response in piglets No effect on piglet performance |
| Seaweed extract (SWE) 10 g/day | From day 107 of gestation until weaning (d 26) | IgG, IgA, IgM +12; +45; +7 | IgG, IgA, IgM +7; +11; +7 | Piglet serum IgG, IgA, IgM d 14: +21; +9; +22 d 26: +5; 0; −2 | [52] | ↑ Pro-inflammatory TNF-α mRNA expression in LPS-stimulated ileum tissue at weaning |
| 3% soybean, coconut, palm, and mixed oil | From day 107 of gestation until day 21 of lactation | There was no control group; however, Ig levels were measured in the colostrum, milk, and plasma of sows | [72] | Oil source has no effect on sow or litter performance and milk composition ↑ Colostrum fat and plasma immunoglobulin level in sows and piglets when soybean oil is fed | ||
| Palm oil 3.2/4.1%, fish oil 3.0/3.9%, and soybean oil 2.9/3.8% | From day 90 of gestation until weaning (d17) | IgG, IgM PO: −8; +9; FO: +49; +36 SO: −7; −4 | IgG, IgM PO: −14; +14 FO: +16; +33 SO: −6; +4 | [76] | ↑ Weaning survival rate and weaning weight with FO and SO | |
| Pinecone oil: 200 or 400 mg/kg | From day 107 of gestation until day 21 of lactation | [153] | ↓ Maternal stress ↑ Milk quality | |||
| Conjugated linoleic acid 0.5% | From 106 days of gestation until 21 days of lactation | IgG +47 | Sow serum IgG d 2: +93 d 10: +42 d 20: +44 Piglet serum IgG d 15: +9 | [64] | ↑ Lysozyme levels | |
| Resin acid: 5 g/day | From 1 week before farrowing until weaning (d21 or d28) in three herds | IgG, IgA, IgM +22; +1; +15 +17; −11; +4 +18; −; − | [73] | ↑ Colostrum production | ||
| Lysophospholipids 0.05% | From day 110 of gestation until weaning | IgG, IgA +17; +4 | IgG, IgA +70; +2 | [86] | ↑ Intestinal health of piglets | |
| Garnicol: 200 mg/kg or 600 mg/kg | From day 90 of gestation until farrowing | IgG, IgA 200 mg: +15; +80 600 mg: +18; +166 | [155] | Alleviates bile acid disorder ↑ Maternal immune and oxidative status ↑ Milk composition | ||
| Resveratrol: 300 mg/kg diet | From day 20 after breeding through to gestation and lactation | [156] | ↑ Antioxidant status in placenta and milk ↑ Weaning weight | |||
| Glycitein: 15 mg/kg, 30 mg/kg, and 45 mg/kg | From day 85 of gestation until day 18 of lactation | [157] | ↑ Antioxidant capacity of plasma and milk ↑ Milk composition ↑ Piglet growth | |||
| Ginger extract: 0.25% and 0.5% | From 30 d before farrowing until d28 postpartum | IgG 0.25%: +15 0.5%: +17 | Sow plasma d 0 IgG 0.25%: −3 0.5%: +12 d 28 IgG 0.25%: +11 0.5%: +20 Piglet plasma d 28 IgG 0.25%: +2 0.5%: +16 | [78] | ↑ Antioxidant capacity of colostrum | |
| A. paniculata 250 ppm or 1000 ppm | From 6 d before farrowing until d25 postpartum | IgG −12 0 | [82] | ↓ Longissimus muscle loss | ||
| Dietary saponins, extracted from the plant Quillaja saponaria, 2.5 g/day | Between days 72 and 93 of gestation | IgG, IgA −15; +25 | IgG, IgA −13; −2 | Sow serum IgG, IgA d 0: +77; +3 d 3: +5; −13 d 21: +20; +10 | [56] | ↓ Performance of suckling piglets ↓ Piglet stillborn incidence |
| 200, 400, or 600 mg/kg MgSO4 supplementation | From 90 days of gestation until 21 days of lactation | IgG, IgA, IgM 200 mg: +6; +20; +14 400 mg: +16; +23; +22 600 mg: +13; +12; +8 | IgG, IgA, IgM 200 mg: −1; +3; 0 400 mg: +3; +5; +2 600 mg: +7; +11; −1 | Sow plasma d −7 IgG, IgA, IgM 200 mg: +3; +4; +20 400 mg: +39; +4; +17 600 mg: +15; 0; +20 d 0 IgG, IgA, IgM 200 mg: +5; 0; −3 400 mg: +38; +19; +3 600 mg: +54; +14; −3 d 21 IgG, IgA, IgM 200 mg: +2; +25; +17 400 mg: +47; +15; +6 600 mg: +28; +20; −3 | [75] | ↓ Survival percentage and litter weight at weaning ↓ Colostrum fat content ↑ Fecal moisture content |
| β-hydroxy β-methyl butyrate 15 mg/kg | For 15 days prior to parturition in three experiments | IgG, IgA, IgM exp 1: +35; +4; +8 exp 2: +8; +4; +10 exp 3: −4; +5; +12 | [53] | ↑ Total live-born litter weight | ||
| β-hydroxy β-methyl butyrate 5, 15, or 45 mg/kg | For 15 days prior to parturition | IgG, IgA, IgM 5 mg/kg: +6; −5; −3 15 mg/kg: +20; +10; +11 45 mg/kg: +44; +10; 0 | [54] | ↑ Total live-born litter weight and piglet birth weight in a quadratic response ↑ Colostrum intake and yield | ||
| Sodium butyrate 500 mg/kg | One month before mating until d 7 of lactation | IgG, IgA +9; +30 | [66] | ↑ Piglet growth | ||
| Fermented rapeseed meal 4% (8% in the transition period) | During gestation and lactation (d 28) | IgG, IgA, IgM +32; +17; +2 | [85] | ↑ Production parameters ↑ Nutrient digestibility ↑ Gut microbiota | ||
↑ the parameter increased; ↓ the parameter decreased.
6.4.2. Effectiveness
The treatments were mainly applied in the lactation diet, fed starting several days before expected farrowing, which resulted in moderately higher Ig concentrations in the sows pre-farrowing and late lactation blood samples compared to the control group (+19, +3, and +19 and +25, +18, and +7 percent change compared to the control group on average for IgG, IgA, and IgM, respectively) (Table 5). The various supplementations were able to somewhat increase the Ig concentration in the colostrum and milk (+13, +17, and +9 and +8, +4, and +7 percent change on average in the colostrum and milk for IgG, IgA, and IgM, respectively), but only slightly, and the results were variable. These positive changes resulted in slightly elevated Ig levels in the piglets’ blood before weaning (+10, +7, and +10 percent change compared to the control group on average for IgG, IgA, and IgM, respectively). Supplementation of a sow’s diet with high-polyunsaturated-fatty-acid-content supplements mainly has a consistent positive effect on Ig concentrations. This draws attention to evaluating their need in this sense. Plant extracts have a positive effect on production parameters, but it seems that they are less effective in terms of the Ig content of the milk and blood. MgSO4, butyrate, and fermented feeds have been proven to have a positive effect on immune status. Thus, further research is encouraged to improve our understanding related to the mode of action.
7. General Discussion
One limitation of our approach is that the Ig content of the colostrum and milk shows considerable variability even within the average values of research results. This suggests that a number of factors are involved, of which a few have been discussed in this review; however, many more remain unknown. This could not only affect the trends we found but also the Ig changes in the colostrum and milk over time. In the relevant sections, we have already stressed that the received equations predict an optimistic (small) decline in Ig levels. Therefore, comparing farm-specific equations, where only the within-farm variance exists, could provide a more precise estimation of the declining characteristics of immunoglobulins.
There is considerable evidence of a synergistic effect between vitamin E and selenium and some effects between vitamin E and arginine, but mainly in the reduction in oxidative stress and enhancing immune function. The possible synergistic effect of these substances on Ig production still requires further research. Another form of combination besides parallel feeding could be the formation of complex molecules. Seleno-arginine is one such complex molecule, and some research results indicate its positive effect on the immune and antioxidant systems [159,160]. Therefore, another area of further research could be to test the effect of seleno-arginine on Ig content in the serum and colostrum.
8. Conclusions
The analyses of the results of the published manuscripts suggest a negative colostrum IgG and IgA content trend over the years. Colostrum Ig content is mainly determined by the serum Ig content before farrowing. Therefore, the aim of late gestational feeding should be to improve the immune status of sows. The significant variance, even in the published studies, averages in the colostrum, milk, and blood Ig content indicates that considerable variance exists in sows; therefore, this area deserves further research. The most effective nutritional interventions to improve sow serum and colostrum Ig content are the following: vitamin E (250 IU/kg), organic Se (0.2 mg/kg), and arginine (1%). Pre- and probiotics and other feed additives have a moderate but mainly positive effect.
Author Contributions
Conceptualization, C.S. and M.H.; methodology, C.S., L.C., B.K., T.P. and K.P.; formal analysis, M.H.; investigation, A.D.S.V.O., J.K.L., M.H., B.C., B.K., T.P., L.C., K.P. and G.G.; resources, C.S.; data curation, M.H.; writing—original draft preparation, M.H. and C.S.; writing—review and editing, C.S., A.D.S.V.O., J.K.L., L.C., B.K., T.P., K.P., M.H., B.C., R.K., G.C. and G.G.; visualization, C.S. and M.H.; supervision, C.S.; project administration, C.S.; funding acquisition, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
The project was financed by the National Research, Development and Innovation Fund under project number 2020-1.1.2-PIACI-KFI-2021-00258. The publication is supported by the University of Debrecen Program for Scientific Publication. B. Csernus supported by the ÚNKP-23-4 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
Authors Rozália Kasza and Gábor Czakó were employed by the company CLA-Pig Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2003, 78, 63–70. [Google Scholar] [CrossRef]
- Islas-Fabila, P.; Roldán-Santiago, P.; de la Cruz-Cruz, L.A.; Limón-Morales, O.; Dutro-Aceves, A.; Orozco-Gregorio, H.; Bonilla-Jaime, H. Importance of Selected Nutrients and Additives in the Feed of Pregnant Sows for the Survival of Newborn Piglets. Animals 2024, 14, 418. [Google Scholar] [CrossRef]
- Gormley, A.; Jang, K.B.; Garavito-Duarte, Y.; Deng, Z.; Kim, S.W. Impacts of Maternal Nutrition on Sow Performance and Potential Positive Effects on Piglet Performance. Animals 2024, 14, 1858. [Google Scholar] [CrossRef]
- Decaluwé, R.; Maes, D.; Cools, A.; Wuyts, B.; De Smet, S.; Marescau, B.; De Deyn, P.P.; Janssens, G.P.J. Effect of peripartal feeding strategy on colostrum yield and composition in sows1. J. Anim. Sci. 2014, 92, 3557–3567. [Google Scholar] [CrossRef]
- Theil, P.K.; Krogh, U.; Bruun, T.S.; Feyera, T. Feeding the modern sow to sustain high productivity. Mol. Reprod. Dev. 2023, 90, 517–532. [Google Scholar] [CrossRef]
- Theil, P.K.; Hurley, W.L. Theil, P.K.; Hurley, W.L. The protein component of sow colostrum and milk. In Milk proteins: From Structure to Biological Properties and Health Aspects; Gigli, I., Ed.; IntechOpen: Rijeka, Croatia, 2016; pp. 183–198. [Google Scholar] [CrossRef]
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54, 12–21. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhang, Y.; Lv, Y.; Heng, J.; Min, T.; Li, L.; Guan, W. Recent progress of porcine milk components and mammary gland function. J. Anim. Sci. Biotechnol. 2018, 9, 77. [Google Scholar] [CrossRef]
- Curtis, J.; Bourne, F.J. Half-lives of immunoglobulins IgG, IgA and IgM in the serum of new-born pigs. Immunology 1973, 24, 147–155. [Google Scholar]
- Harzing, A.W. Publish or Perish. 2007. Available online: http://www.harzing.com/pop.htm (accessed on 2 October 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Patel, A.; Jialal, I. Biochemistry, Immunoglobulin A. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551516/ (accessed on 8 October 2024).
- Sathe, A.; Cusick, J.K. Biochemistry, Immunoglobulin M. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555995/ (accessed on 8 October 2024).
- Vaillant, A.A.J.; Jamal, Z.; Patel, P.; Ramphul, K. Immunoglobulin. 2018. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513460/ (accessed on 8 October 2024).
- Hurley, W.L.; Theil, P.K. Immunoglobulins in mammary secretions. In Advanced Dairy Chemistry, Volume 1A: Proteins: Basic Aspects, 4th ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer Science: New York, NY, USA, 2013; pp. 275–294. [Google Scholar]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 33, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L. Composition of sow colostrum and milk. In The Gestating and Lactating Sow; Wageningen Academic: Wageningen, The Netherlands, 2015; pp. 193–229. [Google Scholar] [CrossRef]
- Feyera, T.; Zhou, P.; Nuntapaitoon, M.; Sørensen, K.U.; Krogh, U.; Bruun, T.S.; Purup, S.; Jørgensen, H.; Poulsen, H.D.; Theil, P.K. Mammary metabolism and colostrogenesis in sows during late gestation and the colostral period1. J. Anim. Sci. 2019, 97, 231–245. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Amatucci, L.; Luise, D.; Correa, F.; Bosi, P.; Trevisi, P. Importance of breed, parity and sow colostrum components on litter performance and health. Animals 2022, 12, 1230. [Google Scholar] [CrossRef]
- LE Dividich, J.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 2005, 143, 469–485. [Google Scholar] [CrossRef]
- Huang, S.-C.; Hu, Z.; Hasler-Rapacz, J.; Rapacz, J. Preferential mammary storage and secretion of immunoglobulin gamma (IgG) subclasses in swine. J. Reprod. Immunol. 1992, 21, 15–28. [Google Scholar] [CrossRef]
- Schnulle, P.; Hurley, W. Sequence and expression of the FcRn in the porcine mammary gland. Veter. Immunol. Immunopathol. 2003, 91, 227–231. [Google Scholar] [CrossRef]
- Kielland, C.; Rootwelt, V.; Reksen, O.; Framstad, T. The association between immunoglobulin G in sow colostrum and piglet plasma1. J. Anim. Sci. 2015, 93, 4453–4462. [Google Scholar] [CrossRef]
- Werhahn, E.; Klobasa, F.; Butler, J. Investigation of some factors which influence the absorption of IgG by the neonatal piglet. Veter. Immunol. Immunopathol. 1981, 2, 35–51. [Google Scholar] [CrossRef]
- Quesnel, H. Colostrum production by sows: Variability of colostrum yield and immunoglobulin G concentrations. Animal 2011, 5, 1546–1553. [Google Scholar] [CrossRef]
- Lanferdini, E.; Andretta, I.; Fonseca, L.; Moreira, R.; Cantarelli, V.; Ferreira, R.; Saraiva, A.; Abreu, M. Piglet birth weight, subsequent performance, carcass traits and pork quality: A meta-analytical study. Livest. Sci. 2018, 214, 175–179. [Google Scholar] [CrossRef]
- Zotti, E.; Resmini, F.A.; Schutz, L.G.; Volz, N.; Milani, R.P.; Bridi, A.M.; Alfieri, A.A.; da Silva, C.A. Impact of piglet birthweight and sow parity on mortality rates, growth performance, and carcass traits in pigs. Rev. Bras. Zootec. 2017, 46, 856–862. [Google Scholar] [CrossRef]
- Ferrari, C.; Sbardella, P.; Bernardi, M.; Coutinho, M.; Vaz, I.; Wentz, I.; Bortolozzo, F. Effect of birth weight and colostrum intake on mortality and performance of piglets after cross-fostering in sows of different parities. Prev. Veter. Med. 2014, 114, 259–266. [Google Scholar] [CrossRef]
- Van Ginneken, C.; Ayuso, M.; Van Bockstal, L.; Van Cruchten, S. Preweaning performance in intrauterine growth-restricted piglets: Characteristics and interventions. Mol. Reprod. Dev. 2023, 90, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Martínez-Miró, S.; López, M.J.; Madrid, J.; Hernández, F. Effect of parity on reproductive performance and composition of sow colostrum during first 24 h postpartum. Animals 2020, 10, 1853. [Google Scholar] [CrossRef]
- Hasan, S.; Orro, T.; Valros, A.; Junnikkala, S.; Peltoniemi, O.; Oliviero, C. Factors affecting sow colostrum yield and composition, and their impact on piglet growth and health. Livest. Sci. 2019, 227, 60–67. [Google Scholar] [CrossRef]
- Cabrera, R.A.; Lin, X.; Campbell, J.M.; Moeser, A.J.; Odle, J. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J. Anim. Sci. Biotechnol. 2012, 3, 42. [Google Scholar] [CrossRef]
- Klobasa, F.; Butler, J.E. Absolute and relative concentrations of immunoglobulins G, M, and A, and albumin in lacteal secretion of sows of different lactation numbers. Am. J. Vet. Res. 1987, 48, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.R.; Dunshea, F.R.; Cottrell, J.J.; Wijesiriwardana, U.A.; Pluske, J.R. Primiparous and multiparous sows have largely similar colostrum and milk composition profiles throughout lactation. Animals 2019, 9, 35. [Google Scholar] [CrossRef]
- Devillers, N.; Farmer, C.; Le Dividich, J.; Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef]
- Amavizca-Nazar, A.; Montalvo-Corral, M.; González-Rios, H.; Pinelli-Saavedra, A. Hot environment on reproductive performance, immunoglobulins, vitamin E, and vitamin A status in sows and their progeny under commercial husbandry. J. Anim. Sci. Technol. 2019, 61, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Peng, Y.; Zhang, Y.; Liu, Y.; Liu, Y.; Yin, Y. Research progress on anti-stress nutrition strategies in swine. Anim. Nutr. 2023, 13, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Black, J.; Mullan, B.; Lorschy, M.; Giles, L. Lactation in the sow during heat stress. Livest. Prod. Sci. 1993, 35, 153–170. [Google Scholar] [CrossRef]
- Machado-Neto, R.; Graves, C.N.; Curtis, S.E. Immunoglobulins in Piglets from Sows Heat-Stressed Prepartum. J. Anim. Sci. 1987, 65, 445. [Google Scholar] [CrossRef]
- Nuntapaitoon, M. Colostrum and milk in sow. In Milk Protein-New Research Approaches; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.-J.; Kim, S.W. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J. Anim. Sci. 2013, 91, 5848–5858. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, S. Impacts of sow behaviour on reproductive performance: Current understanding. J. Appl. Anim. Res. 2023, 51, 256–264. [Google Scholar] [CrossRef]
- Aguinaga, M.A.; Gómez-Carballar, F.; Nieto, R.; Aguilera, J.F. Production and composition of Iberian sow’s milk and use of milk nutrients by the suckling Iberian piglet. Animal 2011, 5, 1390–1397. [Google Scholar] [CrossRef]
- Zou, S.; McLaren, D.; Hurley, W. Pig colostrum and milk composition:comparisons between Chinese Meishan and US breeds. Livest. Prod. Sci. 1992, 30, 115–127. [Google Scholar] [CrossRef]
- Farmer, C.; Charagu, P.; Palin, M.F. Influence of genotype on metabolic variables, colostrum and milk composition of primiparous sows. Can. J. Anim. Sci. 2007, 87, 511–515. [Google Scholar] [CrossRef]
- Inoue, T. Possible factors influencing the immunoglobulin M concentration in swine colostrum. Am. J. Veter. Res. 1981, 42, 1429–1432. [Google Scholar] [CrossRef]
- Souza, A.; Bombassaro, G.; Fonseca, F.; Lopes, L.; Maciag, S.; Volpato, F.; Bastos, A. A comparative evaluation of methods for estimating the colostrum quality in sows. Arq. Bras. Med. Veter. Zootec. 2021, 73, 1047–1057. [Google Scholar] [CrossRef]
- Jackson, J.R.; Hurley, W.L.; Easter, R.A.; Jensen, A.H.; Odle, J. Effects of Induced or Delayed Parturition and Supplemental Dietary Fat on Colostrum and Milk Composition in Sows112. J. Anim. Sci. 1995, 73, 1906–1913. [Google Scholar] [CrossRef]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J. Anim. Sci. 2012, 90, 505–514. [Google Scholar] [CrossRef]
- Davis, H.; Jagger, S.; Toplis, P.; Miller, H. Feeding β-hydroxy β-methyl butyrate to sows in late gestation improves litter and piglet performance to weaning and colostrum immunoglobulin concentrations. Anim. Feed. Sci. Technol. 2021, 275, 114889. [Google Scholar] [CrossRef]
- Davis, H.E.; Jagger, S.; Toplis, P.; Miller, H.M. Dietary β-hydroxy β-methyl butyrate supplementation of sows improves litter performance and colostrum production in a dose-dependent manner. Anim. Feed. Sci. Technol. 2022, 294, 115486. [Google Scholar] [CrossRef]
- Maciag, S.; Volpato, F.; Bombassaro, G.; Forner, R.; Oliveira, K.P.; Bovolato, A.L.C.; Lopes, L.; Bastos, A.P. Effects of freezing storage on the stability of maternal cellular and humoral immune components in porcine colostrum. Veter. Immunol. Immunopathol. 2022, 254, 110520. [Google Scholar] [CrossRef]
- Llsley, S.E.; Miller, H.M. Effect of dietary supplementation of sows with quillaja saponins during gestation on colostrum composition and performance of piglets suckled. Anim. Sci. 2005, 80, 179–184. [Google Scholar] [CrossRef]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J. Anim. Sci. 2010, 88, 2988–2997. [Google Scholar] [CrossRef]
- Leonard, S.; Sweeney, T.; Bahar, B.; Pierce, K.; Lynch, B.; O’Doherty, J. The effects of maternal dietary supplementation with seaweed extract and fish oil on the humoral immune response and performance of suckling piglets. Livest. Sci. 2010, 134, 211–214. [Google Scholar] [CrossRef]
- Cao, M.; Wu, Q.J.; Zhang, P.; Li, W.T.; Mao, Z.Y.; Wu, D.M.; Jiang, X.M.; Zhuo, Y.; Fang, Z.F.; Che, L.Q.; et al. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota1. J. Anim. Sci. 2019, 97, 3426–3439. [Google Scholar] [CrossRef]
- Loisel, F.; Farmer, C.; Ramaekers, P.; Quesnel, H. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J. Anim. Sci. 2013, 91, 5269–5279. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Liu, H.; Liu, S.; He, T.; Piao, X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Su, G.; Shi, B.; Shan, A. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2017, 84, 8–13. [Google Scholar] [CrossRef]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal Selenium-Enriched Yeast Supplementation in Sows Enhances Offspring Growth and Antioxidant Status through the Nrf2/Keap1 Pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef]
- Bontempo, V.; Sciannimanico, D.; Pastorelli, G.; Rossi, R.; Corino, C.; Rosi, F. Dietary conjugated linoleic acid positively affects immunologic variables in lactating sows and piglets. J. Nutr. 2004, 134, 817–824. [Google Scholar] [CrossRef]
- Göransson, L. The effect on late pregnancy feed allowance on the composition of the sow’s colostrum and milk. Acta Veter. Scand. 1990, 31, 109–115. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, M.; Guo, H.; Jia, Y.; Yang, X.; Zhao, R. Effects of sodium butyrate supplementation on reproductive performance and colostrum composition in gilts. Animal 2016, 10, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Juthamanee, P.; Suwimonteerabutr, J.; Tummaruk, P. The influence of parity, body condition, litter size and carbetocin administration on colostrum production and immunoglobulin levels in highly productive sows within a tropical environment. Trop. Anim. Health Prod. 2024, 56, 74. [Google Scholar] [CrossRef]
- Rolinec, M.; Bíro, D.; Gálik, B.; Šimko, M.; Juráček, M. Immunoglobulins in colostrum of sows with porcine reproductive and respiratory syndrome-PRRS. J. Cent. Eur. Agric. 2012, 13, 303–311. [Google Scholar] [CrossRef]
- Leblois, J.; Massart, S.; Soyeurt, H.; Grelet, C.; Dehareng, F.; Schroyen, M.; Li, B.; Wavreille, J.; Bindelle, J.; Everaert, N. Feeding sows resistant starch during gestation and lactation impacts their faecal microbiota and milk composition but shows limited effects on their progeny. PLoS ONE 2018, 13, e0199568. [Google Scholar] [CrossRef] [PubMed]
- Ruampatana, J.; Suwimonteerabutr, J.; Homyog, K.; Mekboonsonglarp, W.; Kanjanavaikoon, K.; Van der Veken, W.; Poonyachoti, S.; Feyera, T.; Settachaimongkon, S.; Nuntapaitoon, M. Clostridium butyricum Probiotic Feed Additive: Modulation of Sow Milk Metabolomics and Mitigation of Pre-Weaning Piglet Diarrhea. Animals 2024, 14, 2098. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.-N.; Sun, W.-J.; Cordero, G.; Hasan, S.; Bontempo, V.; Xiao, J.-F.; Li, Y.-P.; Pi, Y.; Li, X.-L.; et al. Effects of Dietary Supplementation of Stimbiotics to Sows on Lactation Performance, Immune Function, and Anti-Inflammatory and Antioxidant Capacities during Late Gestation and Lactation. Veter. Sci. 2024, 11, 53. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, C.; Zhao, X.; Shi, B.; Shan, A. Effects of fat sources in sow on the fatty acid profiles and fat globule size of milk and immunoglobulins of sows and piglets. Anim. Feed. Sci. Technol. 2017, 234, 217–227. [Google Scholar] [CrossRef]
- Hasan, S.; Saha, S.; Junnikkala, S.; Orro, T.; Peltoniemi, O.; Oliviero, C. Late gestation diet supplementation of resin acid-enriched composition increases sow colostrum immunoglobulin G content, piglet colostrum intake and improve sow gut microbiota. Animal 2019, 13, 1599–1606. [Google Scholar] [CrossRef]
- Hasan, S.; Junnikkala, S.; Peltoniemi, O.; Paulin, L.; Lyyski, A.; Vuorenmaa, J.; Oliviero, C. Dietary supplementation with yeast hydrolysate in pregnancy influences colostrum yield and gut microbiota of sows and piglets after birth. PLoS ONE 2018, 13, e0197586. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.X.; Cheng, S.Y.; Liu, S.T.; Shi, B.M.; Shan, A.S. Dietary supplementation of magnesium sulfate during late gestation and lactation affects the milk composition and immunoglobulin levels in sows. Asian-Australas. J. Anim. Sci. 2014, 27, 1469–1477. [Google Scholar] [CrossRef]
- Jin, C.; Fang, Z.; Lin, Y.; Che, L.; Wu, C.; Xu, S.; Feng, B.; Li, J.; Wu, D. Influence of dietary fat source on sow and litter performance, colostrum and milk fatty acid profile in late gestation and lactation. Anim. Sci. J. 2017, 88, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Maneetong, P.; Srisang, C.; Sunanta, N.; Muchalintamolee, P.; Pearodwong, P.; Suwimonteerabutr, J.; De Rensis, F.; Tummaruk, P. Postpartum prostaglandin F2α administration affects colostrum yield, immunoglobulin G, and piglet performance. Anim. Biosci. 2020, 34, 833. [Google Scholar] [CrossRef]
- Lee, S.D.; Kim, J.H.; Jung, H.J.; Kim, I.C.; Kim, S.B.; Lim, S.Y.; Jung, W.S.; Lee, S.-H.; Kim, Y.J. The effect of ginger extracts on the antioxidant capacity and IgG concentrations in the colostrum and plasma of neo-born piglets and sows. Livest. Sci. 2013, 154, 117–122. [Google Scholar] [CrossRef]
- Le Flocʹh, N.; Achard, C.S.; Eugenio, F.A.; Apper, E.; Combes, S.; Quesnel, H. Effect of live yeast supplementation in sow diet during gestation and lactation on sow and piglet fecal microbiota, health, and performance. J. Anim. Sci. 2022, 100, skac209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Czech, A.; Grela, E.R.; Mokrzycka, A.; Pejsak, Z. Efficacy of mannanoligosaccharides additive to sows diets on colostrum, blood immunoglobulin content and production parameters of piglets. Pol. J. Vet. Sci. 2010, 13, 525–531. [Google Scholar]
- Miró, S.M.; Naranjo, S.; Madrid, J.; López, M.J.; Sánchez, C.J.; Segura, M.M.; Hernández, F. Evaluation of immunoglobulin G absorption from goat colostrum by newborn piglets. Animals 2020, 10, 637. [Google Scholar] [CrossRef]
- Tummaruk, P.; Petchsangharn, K.; Shayutapong, K.; Wisetsiri, T.; Krimtum, P.; Kaewkaen, S.; Taechamaeteekul, P.; Dumniem, N.; Suwimonteerabutr, J.; De Rensis, F. Effect of Andrographis paniculata supplementation during the transition period on colostrum yield, immunoglobulin G, and postpartum complications in multiparous sows during tropical summer. Anim. Biosci. 2024, 37, 862–874. [Google Scholar] [CrossRef]
- Wan, J.; Xu, Q.; He, J. Maternal chitosan oligosaccharide supplementation during late gestation and lactation affects offspring growth. Ital. J. Anim. Sci. 2018, 17, 994–1000. [Google Scholar] [CrossRef]
- Luise, D.; Correa, F.; Fusco, L.; Bosi, P.; Trevisi, P. Productive effects of a colostrum-oriented amino acid dietary supply for sows in transition from gestation to lactation. Ital. J. Anim. Sci. 2021, 20, 1837–1850. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef]
- Jang, K.B.; Purvis, J.M.; Kim, S.W. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiome of offspring. J. Anim. Sci. 2020, 98, skaa227. [Google Scholar] [CrossRef] [PubMed]
- Pinelli-Saavedra, A.; de la Barca, A.C.; Hernández, J.; Valenzuela, R.; Scaife, J. Effect of supplementing sows’ feed with α-tocopherol acetate and vitamin C on transfer of α-tocopherol to piglet tissues, colostrum, and milk: Aspects of immune status of piglets. Res. Veter. Sci. 2008, 85, 92–100. [Google Scholar] [CrossRef]
- Duan, X.; Chen, D.; Zheng, P.; Tian, G.; Wang, J.; Mao, X.; Yu, J.; He, J.; Li, B.; Huang, Z.; et al. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim. Feed. Sci. Technol. 2016, 218, 17–25. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Zhang, Y.; Lv, Y.; Qiao, H.; Tian, M.; Cheng, L.; Chen, F.; Zhang, S.; Guan, W. Effects of maternal supplementation with fully oxidised β-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets. Br. J. Nutr. 2020, 125, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.; Bourne, F. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochim. Biophys. Acta (BBA)-Protein Struct. 1971, 236, 319–332. [Google Scholar] [CrossRef]
- Xia, T.; Yin, C.; Comi, M.; Agazzi, A.; Perricone, V.; Li, X.; Jiang, X. Live Yeast Supplementation in Gestating and Lactating Primiparous Sows Improves Immune Response in Dams and Their Progeny. Animals 2022, 12, 1315. [Google Scholar] [CrossRef]
- Li, T.X.; Kim, I.H. Supplementing Monosodium Glutamate in Sow Diets Enhances Reproductive Performance in Lactating Sows and Improves the Growth of Suckling Piglets. Animals 2024, 14, 1714. [Google Scholar] [CrossRef]
- Trckova, M.; Faldyna, M.; Alexa, P.; Zajacova, Z.S.; Gopfert, E.; Kumprechtova, D.; Auclair, E.; D’Inca, R. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 2014, 92, 767–774. [Google Scholar] [CrossRef]
- Jang, Y.; Kang, K.; Piao, L.; Jeong, T.; Auclair, E.; Jonvel, S.; D’Inca, R.; Kim, Y. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Livest. Sci. 2012, 152, 167–173. [Google Scholar] [CrossRef]
- Luise, D.; Correa, F.; Stefanelli, C.; Simongiovanni, A.; Chalvon-Demersay, T.; Zini, M.; Fusco, L.; Bosi, P.; Trevisi, P. Productive and physiological implications of top-dress addition of branched-chain amino acids and arginine on lactating sows and offspring. J. Anim. Sci. Biotechnol. 2023, 14, 40. [Google Scholar] [CrossRef]
- Ifen, H.; Lindemann, M.D. Benefits of mannan oligosaccharides (MOS) for sows and weanling pigs. In Proceedings of the 2009 Midwest Swine Nutrition Conference, Indianapolis, IN, USA, 10 September 2009. [Google Scholar]
- Duan, X.; Tian, G.; Chen, D.; Yang, J.; Zhang, L.; Li, B.; Huang, L.; Zhang, D.; Zheng, P.; Mao, X.; et al. Effects of diet chitosan oligosaccharide on performance and immune response of sows and their offspring. Livest. Sci. 2020, 239, 104114. [Google Scholar] [CrossRef]
- Peng, X.; Yan, C.; Hu, L.; Huang, Y.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; Zhuo, Y.; et al. Live yeast supplementation during late gestation and lactation affects reproductive performance, colostrum and milk composition, blood biochemical and immunological parameters of sows. Anim. Nutr. 2020, 6, 288–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jurgens, M.H.; Rikabi, R.A.; Zimmerman, D.R. The effect of dietary active dry yeast supplement on performance of sows during gestation-lactation and their pigs. J. Anim. Sci. 1997, 75, 593–597. [Google Scholar] [CrossRef]
- Wen, X.; Jiang, Z.; Yang, X.; Xiao, H.; Gao, K.; Wang, L. Effect of Dietary Standardized Ileal Digestible Arginine to Lysine Ratio on Reproductive Performance, Plasma Biochemical Index, and Immunity of Gestating Sows. Animals 2024, 14, 2688. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Li, X.G.; Kong, X.; Huang, R.; Ruan, Z.; Yao, K.; Deng, Z.; Xie, M.; Shinzato, I.; Yin, Y.; et al. Dietary l-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 2009, 37, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.; Wang, S.; Zhang, X.; Hou, J.; Xu, F.; Wang, Z.; Xu, L.; Diao, X. Effects of soybean isoflavone and astragalus polysaccharide mixture on colostrum components, serum antioxidant, immune and hormone levels of lactating sows. Animals 2021, 11, 132. [Google Scholar] [CrossRef]
- Kim, S.W.; Mateo, R.D.; Yin, Y.-L.; Wu, G. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Australas. J. Anim. Sci. 2006, 20, 295–306. [Google Scholar] [CrossRef]
- Wu, G.; Knabe, D.A.; Kim, S.W. Arginine nutrition in neonatal pigs. J. Nutr. 2004, 134, 2783S–2790S. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Mateo, R.D.; Wu, G.; Carroll, J.A.; Shinzato, I. Dietary L-arginine supplementation affects immune status of pregnant gilts. FASEB J. 2006, 20, A424. [Google Scholar] [CrossRef]
- Liu, X.D.; Wu, X.; Yin, Y.L.; Liu, Y.Q.; Geng, M.M.; Yang, H.S.; Blachier, F.; Wu, G.Y. Effects of dietary l-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 2012, 42, 2111–2119. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, C.-Y.; Gao, K.-G.; Wang, L.; Chen, Z.; Ma, X.-Y.; Jiang, Z.-Y. Dietary arginine supplementation in multiparous sows during lactation improves the weight gain of suckling piglets. J. Integr. Agric. 2017, 16, 648–655. [Google Scholar] [CrossRef]
- Moreira, R.H.R.; Lanferdini, E.; Fonseca, L.d.S.; Chaves, R.F.; Garbossa, C.A.P.; Saraiva, A.; Nogueira, E.T.; de Abreu, M.L.T. Arginine improves nutritional quality of sow milk and piglet performance. Rev. Bras. Zootec. 2018, 47, e20170283. [Google Scholar] [CrossRef]
- Hong, J.; Fang, L.H.; Jeong, J.H.; Kim, Y.Y. Effects of L-arginine supplementation during late gestation on reproductive performance, piglet uniformity, blood profiles, and milk composition in high prolific sows. Animals 2020, 10, 1313. [Google Scholar] [CrossRef]
- Quesnel, H.; Quiniou, N.; Roy, H.; Lottin, A.; Boulot, S.; Gondret, F. Supplying dextrose before insemination and L-arginine during the last third of pregnancy in sow diets: Effects on within-litter variation of piglet birth weight. J. Anim. Sci. 2014, 92, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Yang, P.; Fang, Z.; Lin, Y.; Wu, D. Effects of dietary arginine supplementation on reproductive performance and immunity of sows. Czech J. Anim. Sci. 2013, 58, 167–175. [Google Scholar] [CrossRef]
- Nuntapaitoon, M.; Muns, R.; Theil, P.K.; Tummaruk, P. I-arginine supplementation in sow diet during late gestation decrease stillborn piglet, increase piglet birth weight and increase immunoglobulin G concentration in colostrum. Theriogenology 2018, 121, 27–34. [Google Scholar] [CrossRef]
- Krogh, U.; Oksbjerg, N.; Purup, S.; Ramaekers, P.; Theil, P.K. Colostrum and milk production in multiparous sows fed supplementary arginine during gestation and lactation. J. Anim. Sci. 2016, 94 (Suppl. 3), 22–25. [Google Scholar] [CrossRef]
- de Aquino, R.S.; Junior, W.D.; Manso, H.; Filho, H.M.; Kutschenko, M.; Nogueira, E.; Watford, M. Glutamine and glutamate (AminoGut) supplementation influences sow colostrum and mature milk composition. Livest. Sci. 2014, 169, 112–117. [Google Scholar] [CrossRef]
- Rezaei, R.; Gabriel, A.S.; Wu, G. Dietary supplementation with monosodium glutamate enhances milk production by lactating sows and the growth of suckling piglets. Amino Acids 2022, 54, 1055–1068. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, L.-C.; Liu, Y.-Q.; Lu, J.-X.; Han, F.; Huang, Z.-W. The Synergistic effect of serine with selenocompounds on the expression of SelP and GPx in HepG2Cells. Biol. Trace Element Res. 2016, 173, 291–296. [Google Scholar] [CrossRef]
- Long, J.; Liu, Y.; Zhou, X.; He, L. Dietary serine supplementation regulates selenoprotein transcription and selenoenzyme activity in pigs. Biol. Trace Element Res. 2021, 199, 148–153. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, Y.; Liu, Y.; He, L.; Zhou, X.; Yin, Y. Serine supplementation in the diets of late gestating and lactating sows improves selenium nutritional status in sows and their offspring. Biol. Trace Element Res. 2022, 200, 609–614. [Google Scholar] [CrossRef]
- Che, L.; Xu, M.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Jiang, Z. Effects of dietary valine supplementation during late gestation on the reproductive performance and mammary gland development of gilts. J. Anim. Sci. Biotechnol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and feeding strategies in early life to increase piglet performance and welfare around weaning: A review. Animals 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Elefson, S.K.; Ross, J.W.; Rademacher, C.J.; Greiner, L.L. Evaluation of oxidized beta-carotene on sow and piglet immune systems, sow reproductive performance, and piglet growth. J. Anim. Sci. 2023, 101, skad066. [Google Scholar] [CrossRef]
- Sivertsen, T.; Vie, E.; Bernhoft, A.; Baustad, B. Vitamin E and selenium plasma concentrations in weanling pigs under field conditions in Norwegian pig herds. Acta Veter. Scand. 2007, 49, 1. [Google Scholar] [CrossRef]
- Wuryastuti, H.; Stowe, H.D.; Bull, R.W.; Miller, E.R. Effects of vitamin E and selenium on immune responses of peripheral blood, colostrum, and milk leukocytes of sows. J. Anim. Sci. 1993, 71, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, J.H.; Guan, W.T.; Chen, F.; Wang, C.X.; Zhang, Y.Z.; Lv, Y.T.; Lin, G. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 2016, 221, 111–123. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Guan, W.; Song, H.; Tian, M.; Cheng, L.; Shi, K.; Song, J.; Chen, F.; Zhang, S.; et al. Increasing selenium supply for heat-stressed or actively cooled sows improves piglet preweaning survival, colostrum and milk composition, as well as maternal selenium, antioxidant status and immunoglobulin transfer. J. Trace Elem. Med. Biol. 2019, 52, 89–99. [Google Scholar] [CrossRef]
- Johnston, J.B.; Nickerson, J.G.; Daroszewski, J.; Mogg, T.J.; Burton, G.W. Biologically active polymers from spontaneous carotenoid oxidation: A new frontier in carotenoid activity. PLoS ONE 2014, 9, e111346. [Google Scholar] [CrossRef]
- Gelderman, A.; Clapper, J. Effects of inorganic or organic selenium on immunoglobulins in swine. J. Anim. Sci. Biotechnol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C. Effect of organic and inorganic selenium sources and levels on sow colostrum and milk selenium content. J. Anim. Sci. 2000, 78, 100–105. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Li, S.; Yan, H.; Qin, B.; Li, Z.; Zhao, L.; Che, L.; Fang, Z.; Xu, S.; et al. Effect of maternal organic selenium supplementation during pregnancy on sow reproductive performance and long-term effect on their progeny. J. Anim. Sci. 2020, 98, skaa366. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Poor, A.P.; Muro, B.B.; Carnevale, R.F.; Leal, D.F.; Garbossa, C.A.; Moreno, A.M.; Almond, G. The sow microbiome: Current and future perspectives to maximize the productivity in swine herds. J. Swine Health Prod. 2022, 30, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef] [PubMed]
- Gaukroger, C.; Edwards, S.; Walshaw, J.; Nelson, A.; Adams, I.; Stewart, C.; Kyriazakis, I. Shifting sows: Longitudinal changes in the periparturient faecal microbiota of primiparous and multiparous sows. Animal 2020, 15, 100135. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of dietary fiber from different sources during pregnancy alters sow gut microbiota and improves performance and reduces inflammation in sows and piglets. MSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.M.; Aguirre, M.; Venema, K.; Bosch, G.; Gruppen, H.; Schols, H.A. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agric. Food Chem. 2014, 62, 1079–1087. [Google Scholar] [CrossRef]
- Wang, L.; Beltranena, E.; Zijlstra, R. Diet nutrient digestibility and growth performance of weaned pigs fed sugar beet pulp. Anim. Feed. Sci. Technol. 2016, 211, 145–152. [Google Scholar] [CrossRef]
- Rekiel, A.; Bielecki, W.; Cichowicz, M.; Więcek, J.; Kulisiewicz, J. Effect of chosen feed additives on the histology of mucosa in fatteners’ intestines. Med. Weter. 2008, 64, 339–343. [Google Scholar]
- Gu, X.; Li, H.; Song, Z.; Ding, Y.; He, X.; Fan, Z. Effects of isomaltooligosaccharide and Bacillus supplementation on sow performance, serum metabolites, and serum and placental oxidative status. Anim. Reprod. Sci. 2019, 207, 52–60. [Google Scholar] [CrossRef]
- Simon, O.; Jadamus, A.; Vahjen, W. Probiotic feed additives—Effectiveness and expected modes of action. J. Anim. Feed. Sci. 2001, 10 (Suppl. 1), 51–67. [Google Scholar] [CrossRef]
- Sun, H.; de Laguna, F.B.; Wang, S.; Liu, F.; Shi, L.; Jiang, H.; Hu, X.; Qin, P.; Tan, J. Effect of Saccharomyces cerevisiae boulardii on sows’ farrowing duration and reproductive performance, and weanling piglets’ performance and IgG concentration. J. Anim. Sci. Technol. 2022, 64, 10–22. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, L.; Wei, S.-Y.; Chen, Z.; Ma, X.-Y.; Zheng, C.-T.; Jiang, Z.-Y. Effect of yeast Saccharomyces cerevisiae supplementation on serum antioxidant capacity, mucosal sIgA secretions and gut microbial populations in weaned piglets. J. Integr. Agric. 2017, 16, 2029–2037. [Google Scholar] [CrossRef]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D’inca, R.; Auclair, E.; Salmon, H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Veter. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Carroll, J.A.; Ballou, M.A.; Starkey, J.D.; Sparks, J.C. Oral administration of Saccharomyces cerevisiae boulardii reduces mortality associated with immune and cortisol responses to Escherichia coli endotoxin in pigs. J. Anim. Sci. 2011, 89, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hancox, L.R.; Le Bon, M.; Richards, P.J.; Guillou, D.; Dodd, C.E.R.; Mellits, K.H. Effect of a single dose of Saccharomyces cerevisiae var. boulardii on the occurrence of porcine neonatal diarrhoea. Animal 2015, 9, 1756–1759. [Google Scholar] [CrossRef]
- Jarosz, Ł.; Ciszewski, A.; Marek, A.; Grądzki, Z.; Kaczmarek, B.; Rysiak, A. The effect of feed supplementation with EM Bokashi® multimicrobial probiotic preparation on selected parameters of sow colostrum and milk as indicators of the specific and nonspecific immune response. Probiotics Antimicrob. Proteins 2021, 14, 1029–1041. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Xu, B.; Hao, L.; Su, W.; Jin, M.; Wang, Y. Bacillus subtilis and Enterococcus faecium co-fermented feed regulates lactating sow’s performance, immune status and gut microbiota. Microb. Biotechnol. 2020, 14, 614–627. [Google Scholar] [CrossRef]
- Lauridsen, C.; Stagsted, J.; Jensen, S.K. n−6 and n−3 fatty acids ratio and vitamin E in porcine maternal diet influence the antioxidant status and immune cell eicosanoid response in the progeny. Prostaglandins Other Lipid Mediat. 2007, 84, 66–78. [Google Scholar] [CrossRef]
- McAfee, J.M.; Kattesh, H.G.; Lindemann, M.D.; Voy, B.H.; Kojima, C.J.; Sanchez, N.C.B.; Carroll, J.A.; Gillespie, B.E.; Saxton, A.M. Effect of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation to lactating sows on growth and indicators of stress in the postweaned pig. J. Anim. Sci. 2019, 97, 4453–4463. [Google Scholar] [CrossRef]
- Shen, Y.; Wan, H.; Zhu, J.; Fang, Z.; Che, L.; Xu, S.; Lin, Y.; Li, J.; Wu, D. Fish oil and olive oil supplementation in late pregnancy and lactation differentially affect oxidative stress and inflammation in sows and piglets. Lipids 2015, 50, 647–658. [Google Scholar] [CrossRef]
- Laws, J.; Amusquivar, E.; Laws, A.; Herrera, E.; Lean, I.; Dodds, P.; Clarke, L. Supplementation of sow diets with oil during gestation: Sow body condition, milk yield and milk composition. Livest. Sci. 2009, 123, 88–96. [Google Scholar] [CrossRef]
- Holen, J.P.; Woodworth, J.C.; Tokach, M.D.; Goodband, R.D.; DeRouchey, J.M.; Gebhardt, J.T.; DeDecker, A.E.; Martinez, X. Evaluation of essential fatty acids in lactating sow diets on sow reproductive performance, colostrum and milk composition, and piglet survivability. J. Anim. Sci. 2022, 100, skac167. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147-148, 237–250. [Google Scholar] [CrossRef]
- Zhang, Q.; Cho, S.; Kim, B.; Kim, I.H. Pinecone oil supplemented to multiparous sows from 107 days prenatal to 21 days postpartum improves reproductive performance and milk composition and affects serum parameters. J. Anim. Physiol. Anim. Nutr. 2024, 108, 226–233. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Saito, M.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Free Radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000, 48, 2320–2325. [Google Scholar] [CrossRef]
- Wang, T.; Huang, L.; Xia, C.; Zhou, Y.; Yao, W.; Zhang, L.; Huang, F. Dietary supplementation with garcinol during late gestation alleviates disorders of bile acid metabolism and improves the performance of sows and newborn piglets. J. Anim. Sci. 2023, 101, skad352. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Hu, Y.J.; Gao, K.G.; Zheng, C.T.; Wu, Z.J.; Yang, X.F.; Wang, L.; Ma, X.Y.; Zhou, A.G.; Jiang, Z.J. Effect of dietary supplementation with glycitein during late pregnancy and lactation on antioxidative indices and performance of primiparous sows. J. Anim. Sci. 2015, 93, 2246–2254. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Agazzi, A.; Comi, M.; Bontempo, V.; Guido, I.; Panseri, S.; Sauerwein, H.; Eckersall, P.D.; Burchmore, R.; Savoini, G. Effects of low ω6:ω3 ratio in sow diet and seaweed supplement in piglet diet on performance, colostrum and milk fatty acid profiles, and oxidative status. Animals 2020, 10, 2049. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Liu, A.-J.; Zhang, G.-R.; Lang, J. Effect of seleno-arginine on cellular immunological function in D-gal aging mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2007, 23, 1126–1129. [Google Scholar]
- Liu, A.; Ma, Y.; Zhu, Z. Protective effect of selenoarginine against oxidative stress in D-galactose-induced aging mice. Biosci. Biotechnol. Biochem. 2009, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).