Abstract
Rice is a vital staple crop for global food security, yet a worldwide comprehensive assessment of pests and diseases remains lacking. This study aims to (1) identify globally reported pests and diseases, (2) analyze their distribution patterns, and (3) assess their impact on rice productivity. A literature-based assessment with an initial pool of 15,969 articles from three online databases (PubMed, WOS, and CAB Abstract) resulted in 871 articles for analysis. The findings highlight a regional focus on Africa and Asia, where rice is predominantly produced. Pest occurrence varies across continents, with Diopsis, Maliarpha, and Chilo being prevalent in Africa, while Nilaparvata, Scirpophaga, Sogatella, and Chilo dominate in Asia. Key pathogens differ across regions, with Pyricularia, Xanthomonas, and Sobemovirus in Africa, while Fusarium and Bipolaris are common in Asia. Major yield losses are attributed to Pyricularia (Blast disease), Bipolaris (Brown Spot), Fusarium (Bakanae), and Sobemovirus (Rice Yellow Mottle Virus). The lack of data from major rice producers like Myanmar highlights reporting gaps, urging future research. This study enhances the global understanding of rice pest and disease distribution and their impacts on productivity. It could also support early warning systems and assess the effectiveness of control methods in the context of climate change.
1. Introduction
Rice is one of the world’s most important staple crops [1,2,3], ranking as the third most produced cereal worldwide, following corn and wheat [4]. It constitutes a major component of the daily diet for nearly half of the global population [5,6]. Only two species are produced for human consumption: Oryza sativa L. (Asian rice) and Oryza glaberrima Steud (African rice). Oryza sativa was initially cultivated in Southeast Asia, somewhere in India, Myanmar, Thailand, North Vietnam, or China, between 8000 and 15,000 years ago [5]. In contrast, O. glaberrima is thought to have been domesticated in West Africa from its wild ancestor Oryza barthii A. Chev., approximately 3000 years ago, by communities residing in the Niger River floodplains [5,7]. Presently, it is still grown in parts of West Africa [7,8,9] and Suriname [10,11]. Geographically, rice cultivation covers approximately 118 countries [12,13], predominantly in Asia, encompassing 85.7% of the global rice area and contributing 89.8% of global rice production. Africa ranks second, covering 10.3% of the global rice area but only contributing 4.7% to global production [13]. America ranks third, accounting for 4.9% of the global rice area and 3.6% of global production [13]. Although Africa is host to a higher number of rice-growing countries (≈41) than Asia (≈31), its yield level remains significantly lower (1.4 t/ha) compared to Asia (3.2 t/ha) and the global average of 3.1 t/ha [13]. Rice production occurs under different agricultural systems, often determined by surface-water regimes. These include dryland systems (upland or slash-and-burn) and wetland systems (freshwater swamps, deepwater/mangrove swamps, and irrigated wetland) [7,14]. Mangrove swamp rice, a unique system found in West Africa, covers approximately 200,000 ha, primarily in Guinea-Bissau, Guinea Conakry, Senegal, Gambia, and Sierra Leone [15,16]. This farming system plays a crucial role in food security along the West African coast, contributing to more than 30% of the population’s caloric intake [17].
All over the world, pests and diseases are major constraints to rice production [18]. From production to consumption, approximately 800 insect species can attack rice [15], but this information is often scattered and lacks systematic compilation. This study aims to address this gap by compiling and analyzing the existing published data on rice insect pests and diseases. While the biotic network naturally controls most rice pests, a small number of insect species consistently pose a significant threat. According to Heinrichs and Muniappan [5], only 20 species in tropical Asia, 15 in Africa, and 20 in the Americas are of major economic importance, categorized as root and stem feeders, stem borers, gall midges, hoppers, foliage feeders, and panicle feeders. These pests, along with various diseases, can cause severe yield losses worldwide [19].
Rice production is particularly affected by stem borers, hoppers, and defoliators. A meta-analysis on yield impacts caused by a complex of stem borers in Asia found that a 1% increase in whiteheads corresponds to a 4% reduction in yield [20]. In Asia, major stem borers include Scirpophaga incertulas, which can cause yield losses ranging from 3 to 95% in India [21], and Chilo suppressalis [22]. In West Africa, key species such as Diopsis macrophthalma, Maliarpha separatella, Chilo zacconius, and Sesamia calamistis pose significant threats [5]. Additionally, rice gall midges (Orseolia spp.) affect rice production in both Asia and West Africa [19]. Hoppers, including leafhoppers (Cicadellidae) and planthoppers (Delphacidae), not only cause direct feeding damage, but also serve as a vector for viral diseases such as rice tungro, Rice Transitory Yellowing, ‘hoja blanca’ virus, and Rice Yellow Mottle Virus (RYMV) [23,24,25]. Defoliators such as rice leaf folders (Cnaphalocrocis spp.), armyworms (Mythimna separata, Spodoptera frugiperda), and swarming caterpillars (Spodoptera mauritia) significantly reduce photosynthesis and damage panicles [5]. Additionally, coleopteran pests, including African hispids and flea beetles, serve as vectors of RYMV [5]. Although numerous insect species attack rice, only a subset inflicts severe economic losses, highlighting the need for effective pest management strategies.
Rice diseases, caused by fungi, bacteria, viruses, and nematodes, affect various plant parts and significantly impact yields [26]. Among fungal pathogens, rice Blast (Pyricularia oryzae) is the most destructive globally, affecting all growth stages and manifesting as leaf Blast, neck rot, and panicle Blast [5,27,28]. Bacterial Blight (Xanthomonas oryzae pv. oryzae) is the most severe bacterial disease, widespread except in Europe [29]. While viral diseases generally cause minor losses, outbreaks can be devastating. In Asia, rice tungro spherical virus (RTSV) and Rice Grassy Stunt virus are major threats [30,31], ‘hoja blanca’ dominates in the Americas [23], and RYMV is the primary rice virus in Africa [32]. Nematodes also pose serious risks. The rice stem nematode (Ditylenchus angustus) causes Ufra disease, leading to 20–90% yield losses in Asia. The root-knot nematode (Meloidogyne graminicola), widespread in flooded conditions, can result in up to 80% losses in upland rice and 73% in irrigated systems. Other damaging species include the white tip nematode (Aphelenchoides besseyi) and rice cyst nematodes (Heterodera spp.) [33]. Managing these pests and diseases is critical for ensuring stable rice production and food security.
Notwithstanding all the studies performed, there is no solid compilation of the records and distribution of rice pests and diseases around the world, with significant gaps in knowledge regarding key African rice-producing regions, particularly in West Africa. This work, a literature-based assessment, follows PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and aims to create a solid foundation of information on the current body of knowledge on pests and diseases that affect rice productivity, covering both Asian and African rice. It provides a comprehensive overview of the top pests and diseases affecting rice globally, assesses their current distribution, and evaluates their impact on rice productivity, offering key insights for their understanding and management, especially in the context of climate change. By mapping the distribution of pests and diseases, this study could contribute to the development of early warning systems, as well as evaluating where existing pest and disease control methods remain effective under changing climate conditions in specific countries and/or regions. Additionally, distribution trends can help predict future outbreaks and inform proactive management strategies.
2. Methods
2.1. Data Extraction
For the data extraction of available publications assessing rice pests and diseases worldwide, with emphasis on West Africa and mangrove production, three databases were used: PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 20 April 2024), Web of Science (WOS) (https://www.webofscience.com/, accessed on 20 April 2024), and CAB Abstract (https://www.cabdirect.org/, accessed on 20 April 2024). Following the PRISMA guidelines [34], a set of specific keywords combination were searched according to four search queries: (i) rice pests and diseases characterization in the world; (ii) rice pests and diseases characterization in Africa; (iii) rice pests and diseases characterization in West Africa; (iv) characterization of mangrove rice pests and diseases—from world to West Africa (the main rice-producing region within the mangrove agroecosystem and the pivotal region of African rice—O. glaberrima—production). Different combinations of keywords using ‘AND’ included ‘Oryza sativa’, ‘Oryza glaberrima’, ‘rice’, ‘disease’, ‘pest’, ‘africa’, ‘west africa’, and ‘mangrove rice’. The complete list of keyword combinations, as well as the Boolean operators applied to each bibliographic database, is detailed at Table S1. To cover the largest number of studies in the data search, all publications were considered, regardless of written language, availability of the full text, or type of publication (e.g., review). Whenever articles in languages other than English were analyzed, a standard translation tool (Google Translator) was used to extract the most relevant information.
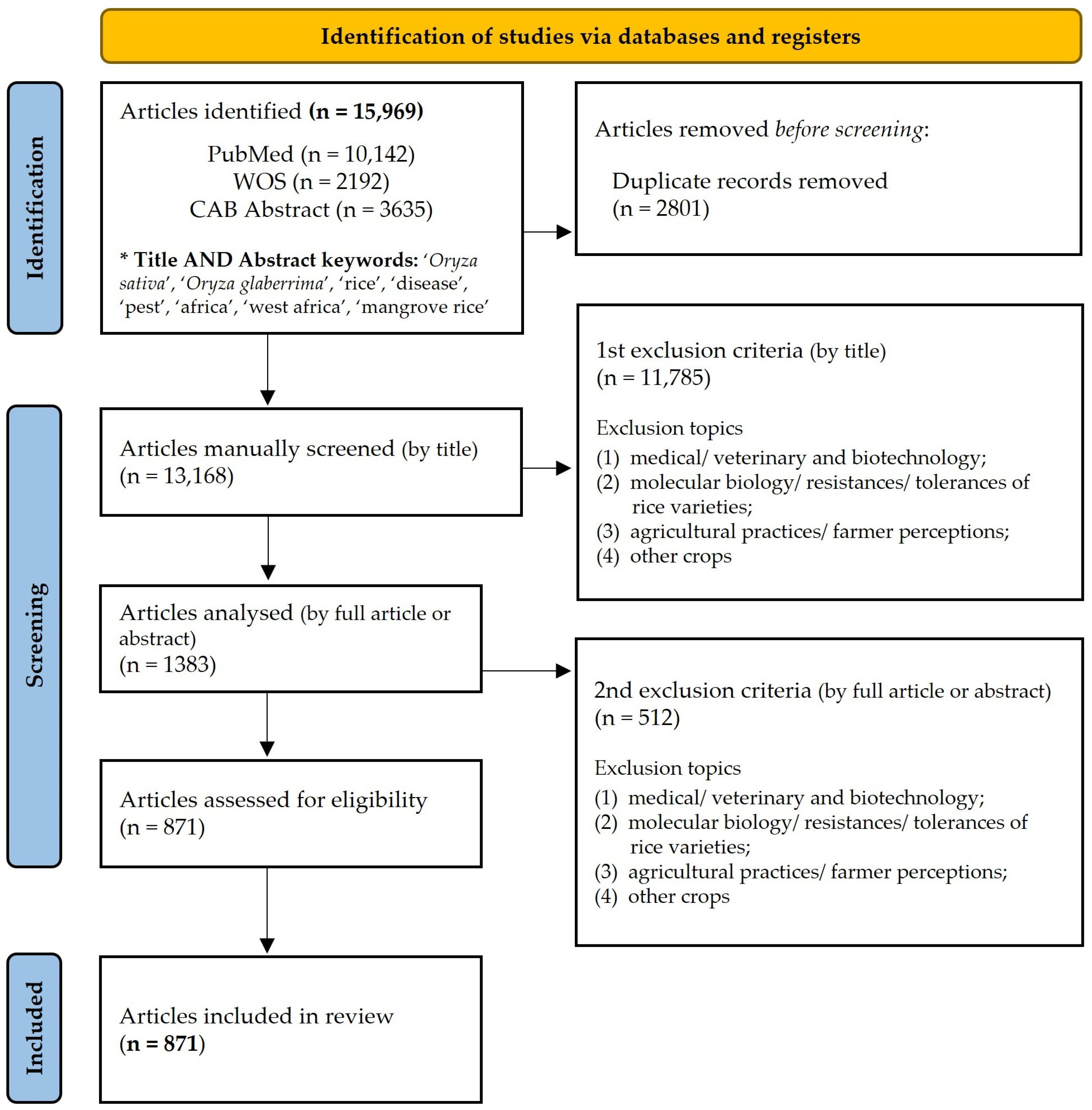
For the three databases (PubMed, WOS, CAB Abstract), searches were restricted to the title and abstract. After record assembly from the three databases, duplicates were removed by automatic comparison of titles using the excel tool. The articles were then excluded by applying the 1st exclusion criterion, which was manual curation (by title) of records not directly related to the subject, including at least one of the four disciplines: medical/veterinary and biotechnology; molecular biology/resistance/tolerance of rice varieties; agricultural practices/farmer perceptions; and other crops. To the remaining records, we applied the 2nd criterion: manual curation (by full article or abstract, whenever the full article was not available) of records not directly related to the subject, including the same disciples as the 1st criterion. Manually deleting articles can, naturally, lead to some errors of human perception. The entire process was carried out by the same person to always maintain the same observation criteria and to avoid deviation. The PRISMA flow diagram shows the number of records in each step of the selection process (Figure 1). The final set of records fitted the following inclusion criteria, representing information on pests and diseases of rice all over the world.
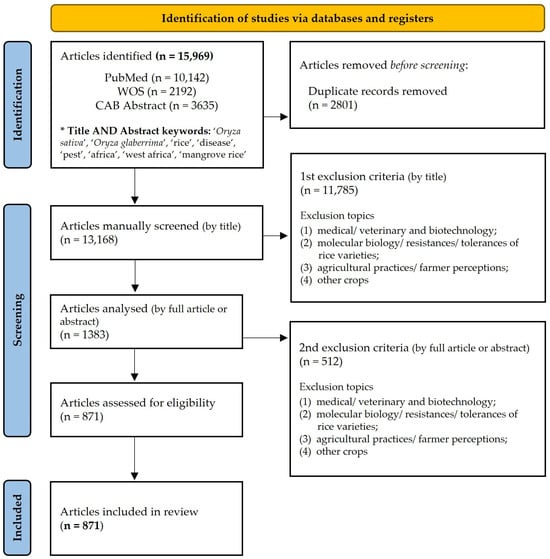
Figure 1.
PRISMA 2020 flow diagram. * For more details on keyword combination and Boolean operators applied to each bibliographic database, please check Table S1.
2.2. Data Curation Workflow
The first search was carried out based on article titles, resulting in a primary grouping into four main subjects: pests, diseases, pests and diseases, and predators. All the articles were searched to access the full article, and if not available, the respective abstract was collected. Each article was then carefully reviewed, one by one, to extract relevant data whenever available. The division criteria and all the extraction data were made by the same person to avoid bias. Once all the data were extracted, the scientific names of pests and diseases were revised to standardize them according to their accepted names by consulting GBIF, the Global Biodiversity Information Facility (https://www.gbif.org/, accessed on 6 July 2024). Data on insect pest taxonomy, synonyms, common names, host plants, and their respective distribution by continent, according to the literature screened, is compiled in Table S2.
2.3. Data Analysis
To create global maps presenting the number of records of pests and diseases per country, QGIS software v.3.28.4 [35] was employed. The shapefile map containing the world country borders was downloaded from ArcGIS Hub (https://hub.arcgis.com/datasets/esri::world-countries-generalized/explore (accessed on 12 July 2024). Additionally, chord diagrams depicting the relations between the genera of pests, fungi, bacteria, and viruses, and their distribution in African and Asian countries were generated using the ‘circlize’ package v. 0.4.15 [36] and the ‘chordDiagram’ function in R software v. 4.2.3 [37]. To analyze the estimated yield losses for each disease, the maximum percentage values reported in the studies for the top disease agents (bacteria, fungi, and viruses) were considered. Despite being aware of the potential bias of yield losses reported by each article, related to both methodologies and analyses, the reported values were represented as yield loss intervals using box plot graphs. These graphs were generated using the ‘graphics’ package and the ‘boxplot’ function in R software v. 4.2.3 [37]. To illustrate the rice plant part affected by each disease, a Sankey diagram was constructed using the RAWGraphs tool [38]. In these diagrams, the thickness of each chord is proportional to the number of pests or diseases found in each country/region or affecting each part of the plant.
3. Results
3.1. Databases Assessment on Rice Pests and Diseases
The search on PubMed, WOS, and CAB Abstract retrieved 15,969 articles (RAW data available at Figshare [39]. Among these, 10,142 were from PubMed (64%), 2192 were from WOS (14%), and 3635 were from CAB Abstract (23%). Repeated articles were removed, leaving 13,168 articles. Afterward, articles were excluded by applying the first exclusion criterion, which involved including at least one of the four disciplines: medical/veterinary and biotechnology; molecular biology/resistances/tolerances; agricultural practices/farmer perceptions; and other crops. This manual curation resulted in a selection of 1383 articles. From those, 512 were removed according to the second exclusion criterion, based on the analysis of the respective full article (or abstract when the full article was not available). Finally, 871 articles fitted the inclusion criteria and were carefully analyzed for data extraction and analysis (Figure 1). From these 871 articles, a total of 1598 records were identified. For better clarity, the term ‘article’ is applied to the article publication and the term ‘record’ to the data entry inside each article (e.g., one article can mention two diseases, which is the same as one article with two records). The majority of the studies are in English (81%), with Chinese as the second most abundant language (7%). Of the 871 articles, 58% presented the full publication available, while in the remaining (42%) articles, only the abstract was available, and these mostly belonged to the subject of disease (32%), with 6% on the subject of pests.
By assessing the distribution of studies according to the year of publication (Figure S1), the first increasing trend is detected in the decade of the 1960s, followed by a less steep but greater increase in the 1990s, with a fast increase trend in the following two decades.
3.2. Geographical Distribution of the Records
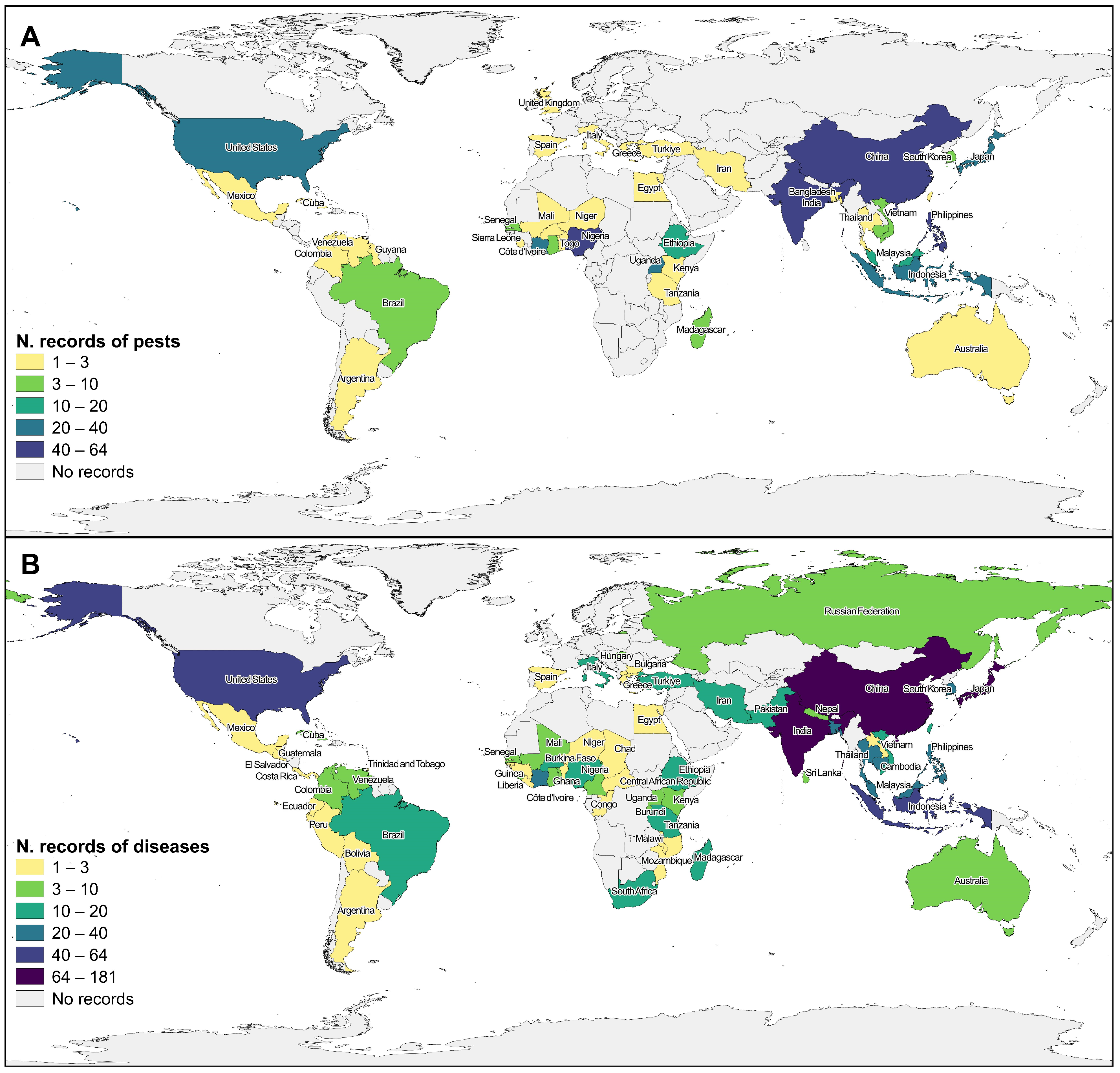
Research studies were conducted in 78 countries for pests (Figure 2A) and diseases (Figure 2B), covering all continents except for Antarctica. The majority of the records were reported in Asia (998), mainly India (26%, 258), China (22%, 220), and the Philippines (9%, 88), and the rest were distributed by 20 countries. Africa (359) was the second most studied continent, with the majority of the records conducted in Nigeria (16%, 59), Ivory Coast (11%, 39), and Uganda (8%, 30), and the rest distributed by 28 countries. In third place stands America (150), with the biggest number of records in the USA (57%, 85), followed by Brazil (13%, 20), and Colombia (7%, 10) (Figure 2). Thirty-four percent of rice-producing countries are not represented in the collection of articles analyzed in this work, and cross-referencing our data with FAOSTAT [40] shows that two major producers (>1,000,000 t), Myanmar, and North Korea, are not represented.
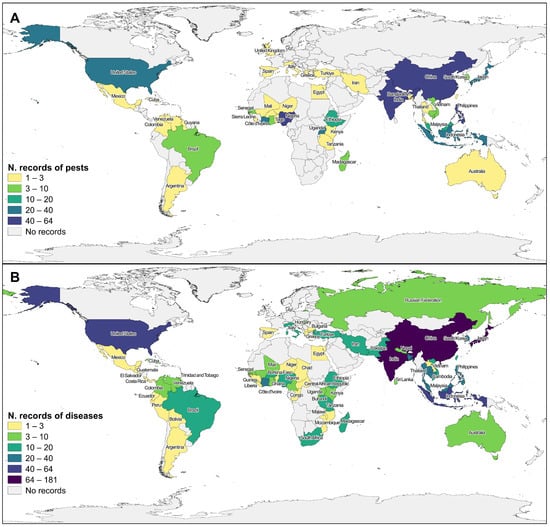
Figure 2.
Geographical distribution of total records included in the review for pests (A) and diseases (B). Countries with no data available are represented in gray. Color differences represent different record value classes according to records screened.
Regarding reported rice species, studies on O. glaberrima are almost limited to Africa, with the exception of one article (with two records) in Asia [41], which was conducted under laboratory conditions. Among the articles providing information about the studied rice species, only one article on pests refers to O. glaberrima, mentioning the whitefly Aleurocybotus indicus (synonymous with Vasdavidius indicus), Hemiptera, in West Africa, but not considering it a major pest [42]. Nevertheless, 15 articles on O. glaberrima diseases are reported for Africa, resulting in 40 records. Wild relative rice species are mostly associated with disease records, mainly represented by Rice Yellow Mottle Virus (RYMV).
3.3. Pests Affecting Rice Production
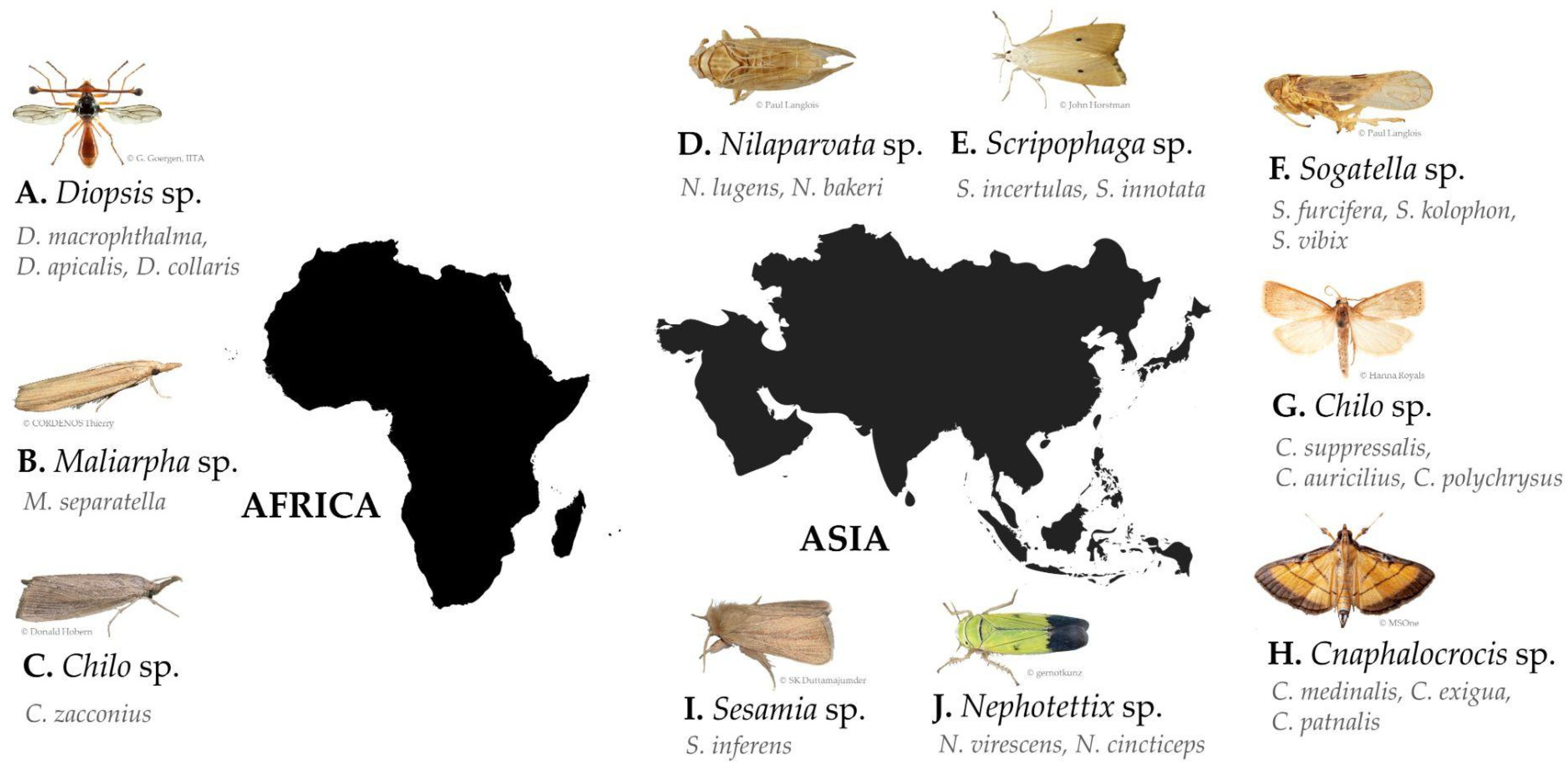
A total of 343 records of pests associated with rice damage were identified from the 140 final publications grouped in the pest subject. These records are represented by three phyla: Arthropoda (335), Chordata (6, with 4 from the Mammal class and 2 from the Bird class), and Annelida (2). Arthropoda records, with the highest number, are dominated by the class Insecta, with 329 records. Insecta branches into seven orders, mostly represented by Lepidoptera and Hemiptera species. Among these, 84 genera have been recorded, representing 118 species. The 329 insect records were characterized by the type of study applied: pest status (260), identification (44), and pest risk (25). Of the total records of the class Insecta, 44 provided information on being a major pest. Given the global distribution of records and the low number of resulting records, a minimum of six records per genus was classified as an important top pest for Africa and Asia. Having defined this, it is possible to observe that, in Africa, references predominate in three genera: Diopsis (17%), Maliarpha (8%), and Chilo (6%). Of these genera, the following species are represented: D. macrophthalma, D. apicalis, D. collaris, M. separatella, and C. zacconius (Figure 3A–C). In Asia, there are seven genera most referred to: Nilaparvata (10%), Scirpophaga (9%), Sogatella (7%), Chilo (6%), Cnaphalocrocis (6%), Sesamia (4%), and Nephotettix (3%). Of these genera, the following species are represented: (a) Nilaparvata lugens, and only with one record, N. bakeri; (b) Scirpophaga incertulas, S. innotata; (c) Sogatella furcifera, and with only one record each, S. kolophon and S. vibix; (d) Chilo suppressalis, and only with one record each, C. auricilius and C. polychrysus; (e) Cnaphalocrocis medinalis, and with two records, C. exigua, and with only one record C. patnalis; (f) Sesamia inferens; and (g) Nephotettix spp. (including N. virescens and N. cincticeps) (Figure 3D–J). Please check Table S2 for insect pest synonyms and other relevant data.
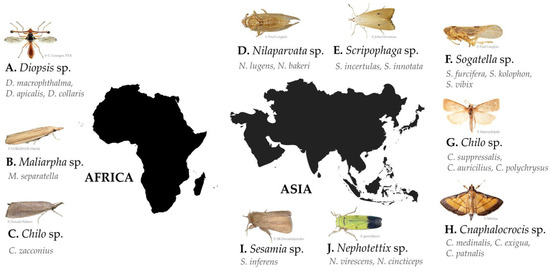
Figure 3.
Top pests identified in Africa (A–C) and in Asia (D–J).
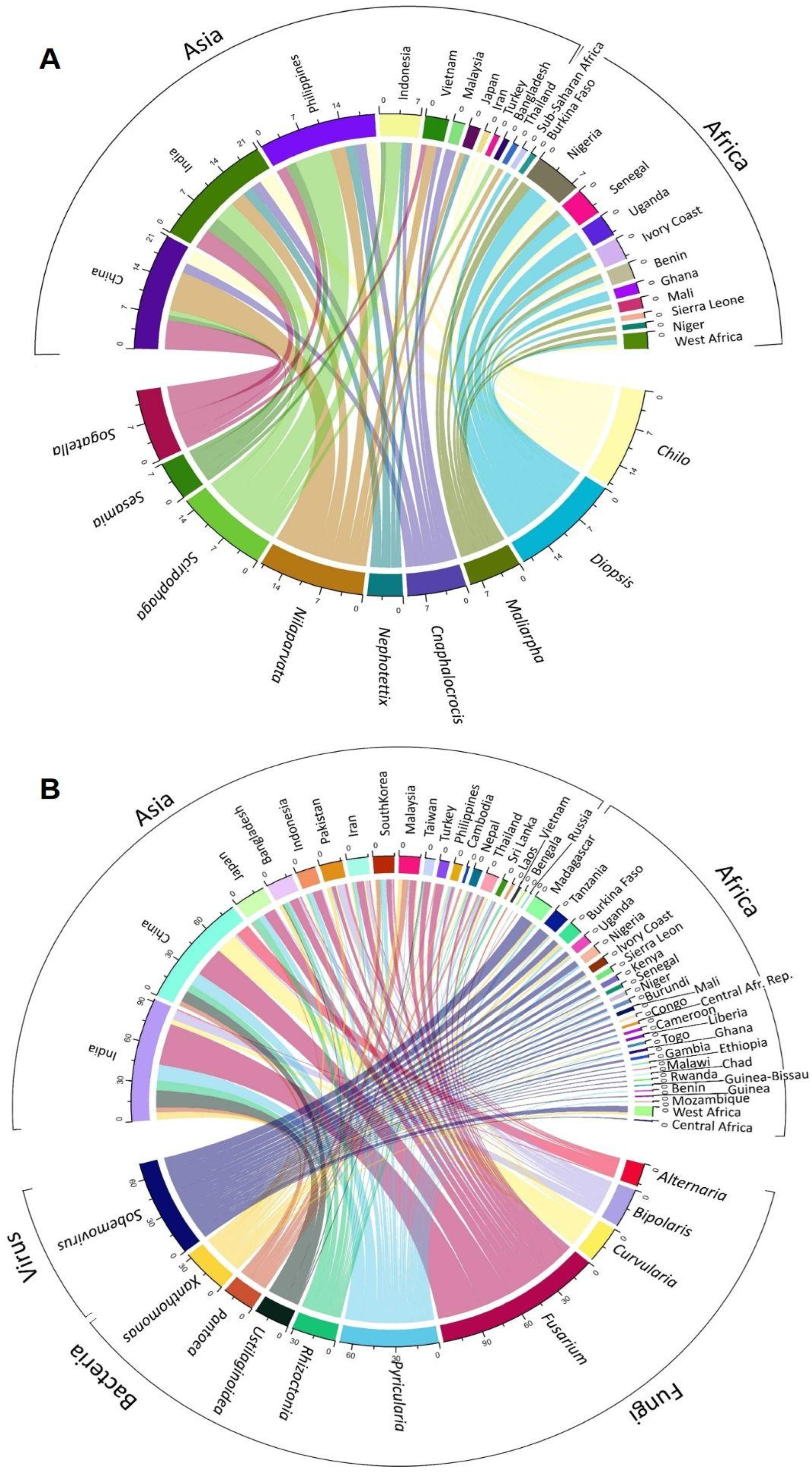
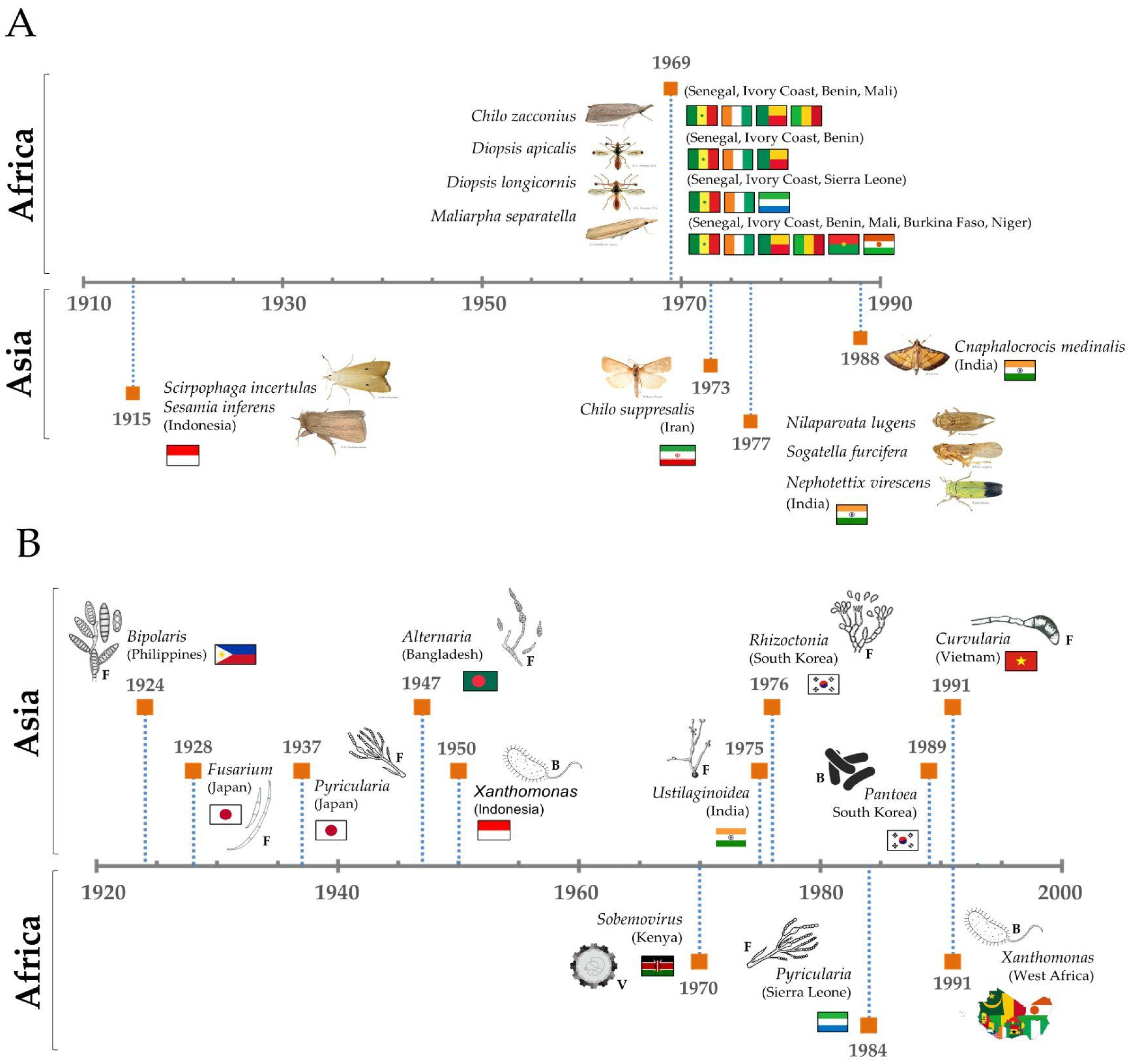
A chord diagram illustrates the distribution of each top pest genus known by country in the studies analyzed (Figure 4A), and a detailed analysis allows us to rebuild a timeline of each top pest (Figure 5A), where it is possible to observe that genus. Chilo was first mentioned in Africa in 1969 (C. zacconius, in Senegal, Ivory Coast, Benin, Mali), and in 1970 in Asia (C. suppresalis, in Iran).
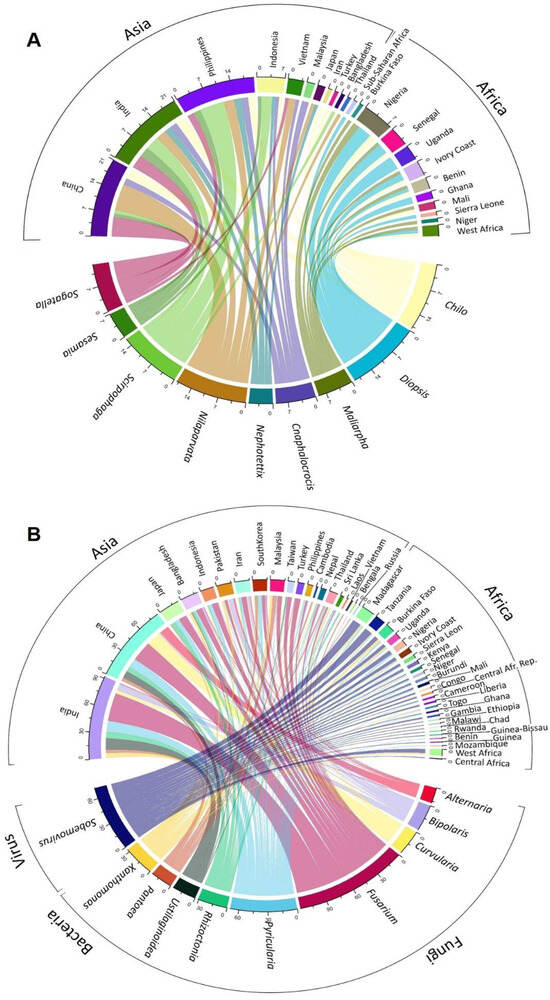
Figure 4.
Chord diagram shows the number of top pest genera (A) and diseases (B) recorded in each country or region (e.g., West Africa, sub-Sahara). Diseases grouped by microorganism type: fungi, bacteria, and virus.
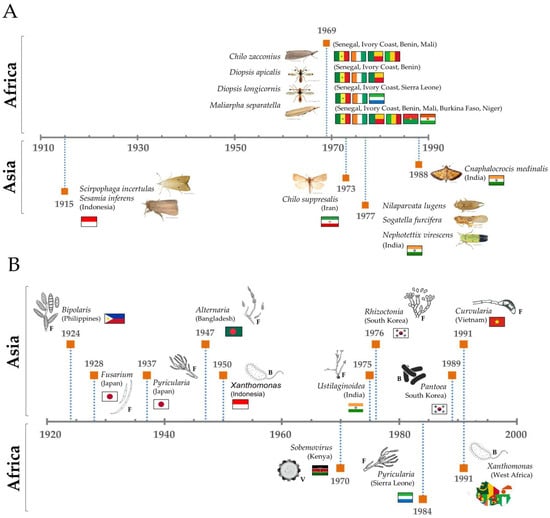
Figure 5.
Timeline scheme with the first record compiled of each top pest (A) and disease (B) and its respective country of study. Letters correspond to the type of pathogen: F = fungi, B = bacteria and V = virus.
With limited representation, on the American continent (40 records from 29 articles), rice damage is primarily associated with the stink bug genus Oebalus (Hemiptera). In Europe (six records from five articles), the most common species is the rice water weevil, Lissorhoptrus oryzophilus (Coleoptera). Data from Oceania is represented by only two records from a single article. Given this limited representation, any conclusions derived from these continents should be interpreted with caution.
3.4. Diseases Affecting Rice Productivity
A total of 1033 records of diseases associated with rice were identified from the 648 final publications grouped on the disease subject. These records are represented by seven types of pathogens (fungi, virus, bacteria, nematode, phytoplasma, mycoplasma, and oomycete), but mostly represented by fungi. Eight records were classified as missing data due to lack of available information in the articles (e.g., an absence of microorganism group identification). Of the 1033 records, only 371 disease records presented field methods by accessing incidence and/or severity; 304 reported pathogenicity tests; 433 mentioned having an impact on the yield, and only 238 mentioned a percentage or range of yield loss.
Agents were grouped in genera and ranked according to the number of records found and classified as a top disease causal agent when presenting equal or over fifteen records per genus (Figure 4B). A detailed analysis allowed us to rebuild a timeline of each top disease causal agent (Figure 5B). To provide some examples, Pyricularia was first mentioned in Asia in 1937 for Japan, while in Africa it was only mentioned in 1984, for Sierra Leone [43]. Similarly, Xanthomonas was first mentioned in 1950 in Indonesia (Asia), and only in 1991 in West Africa [44]. An example of the application of this type of observation could be the study of the temporal expansion of causal agents between continents.
The top fungal disease causal agent in Africa is the genus Pyricularia (34%), with P. oryzae (95%) being the most abundant species, and always associated with Blast diseases. Top bacterial diseases belong to the genus Xanthomonas (68%), with X. oryzae pv. oryzicola (60%) being the dominant species associated with two diseases, Bacterial Leaf Streak (89%) and Bacterial Leaf Blight (11%). Top virus disease belongs to the genus Sobemovirus (99%), represented only in the African continent and always associated with Rice Yellow Mottle Virus. In Asia, the top fungal diseases causal agents belong to the genera Fusarium (31%), Pyricularia (13%), Rhizoctonia (9%), Bipolaris (7%), Curvularia (7%), Ustilaginoidea (6%), and Alternaria (4%). Of these genera, the following species are represented: F. fujikuroi (60%), of which 96% is associated with Bakanae disease; P. oryzae (76%), with 95% associated with Blast diseases; R. solani (79%), with 88% associated with Sheath Blight and 12% with Rice Sheath disease; B. oryzae (79%), associated with Brown Spot diseases (65%) and rice grain discoloration (13%); C. lunata (59%), mainly associated with Rice Spikelet Rot disease (38%), Leaf Spot of Rice (19%), and rice grain discoloration (13%); U. virens (100%), always associated with False Smut; A. alternata (47%), associated with Rice Spikelet Rot disease (75%); and A. padwickii (29%), associated with Stackburn rice disease (40%), rice drain discoloration (20%), Rice Spikelet Rot disease (20%), and seed-borne fungal diseases (20%). The top bacterial diseases are represented by the genera Pantoea (30%) and Xanthomonas (26%), with the following main species: P. ananatis (61%), mostly associated with Bacterial Blight diseases (64%), rice grain discoloration (14%), and 7% to Bacterial Palea Browning; and Xanthomonas oryzae pv. oryzae (65%), always associated with Bacterial Blight diseases.
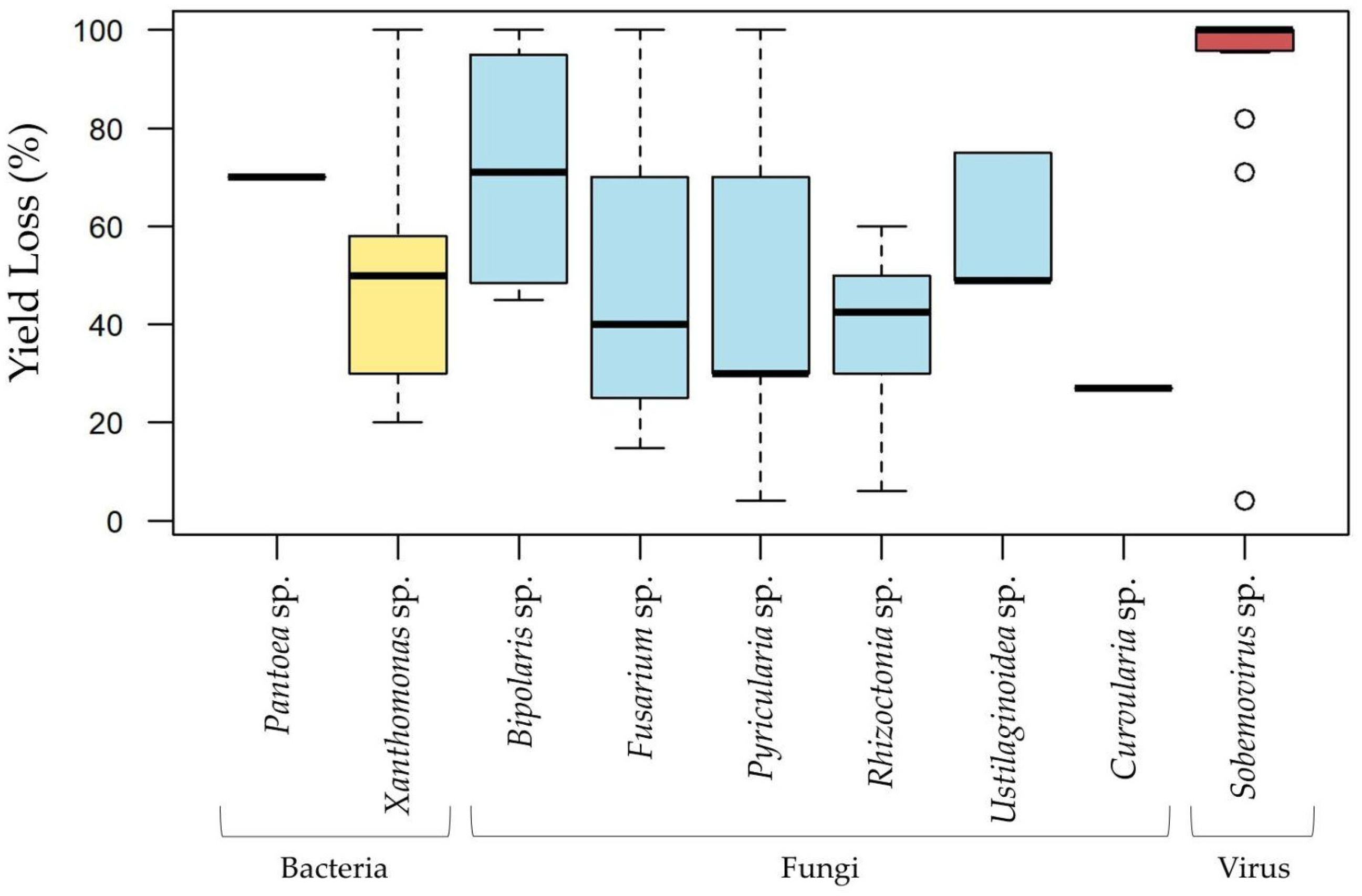
Maximum yield loss percentages at the disease level retrieved from the records screened show that one bacteria genus (Xanthomonas), three fungal genera (Bipolaris, Fusarium, and Pyricularia), and one viral genus (Sobemovirus) were reported to cause total yield loss (Figure 6). The bacterial genus Pantoea was associated with losses of 70%, while the fungal genera Rhizoctonia, Ustilaginoidea, and Curvularia were reported with maximum losses of 60%, 75%, and 27%, respectively (Figure 6).
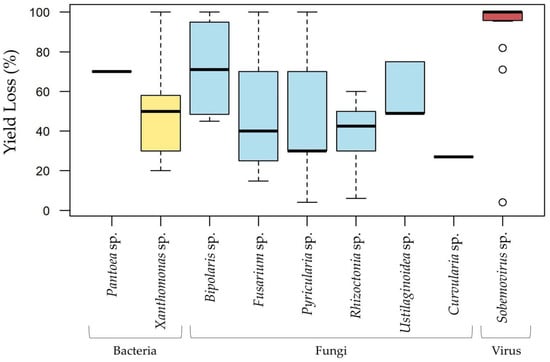
Figure 6.
Maximum yield loss percentages recorded for each causal agent corresponding to the top disease pathogens (bacteria, fungi, virus), based on 104 articles and 183 records from Africa and Asia. The most represented species include Pantoea ananatis, Xanthomonas oryzae pv. oryzicola, X.s oryzae pv. oryzae, Bipolaris oryzae, Fusarium fujikuroi, Pyricularia oryzae, Rhizoctonia solani, Ustilaginoidea virens, and Curvularia lunata.
The yield loss data were primarily based on bibliographic references (96%), with only 4% derived from field experiments. These experiments focused on three rice diseases and their causal agents. Two studies examined Brown Spot disease (Bipolaris oryzae): one conducted in Indonesia in 2019 [45] used a randomized block design with two sets (diseased and control plots), measuring grain yield at harvest. The other, from Nigeria in the early 1970s [46], applied the paired plant technique to calculate yield loss based on disease incidence. A third study on Sheath Blight (Rhizoctonia solani) in India (2013–2015) [47] tracked disease occurrence, incidence, severity, and fungal population in the soil, with yield loss correlated with disease incidence. Lastly, a study on Rice Yellow Mottle Virus (RYMV) in Niger in the late 2000s [48] surveyed irrigated rice fields, recording incidence and disease symptoms, with yield loss calculated from incidence levels. Considering these data, there is a clear gap in the evaluation of yield losses associated with major rice diseases, which is crucial for understanding their impact on rice productivity. Disease-causing agents may exhibit varying levels of severity in different regions of the world, even when affecting the same crop species, due to several factors (e.g., being better adapted to specific ecological conditions or being capable of overcoming the defenses of certain rice varieties). Such information is essential for assessing the severity of pathogens and designing region-specific disease management strategies to mitigate the impact of pathogens on rice production.
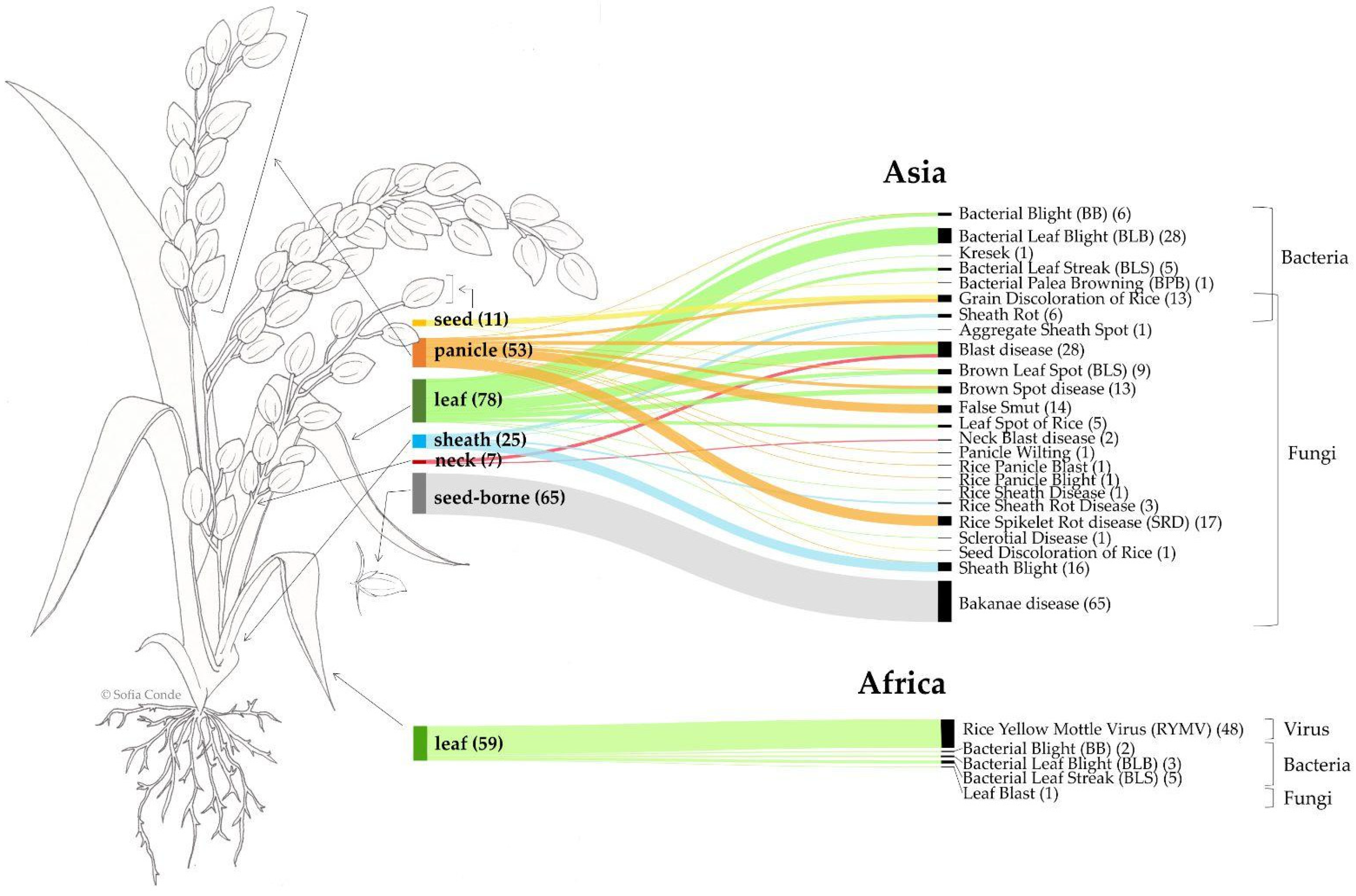
The results indicate that, in Asia, most recorded diseases affect the leaf, seed (seed-borne), or panicle (Figure 7). Bakanae disease (Fusarium fujikuroi) is exclusively seed-borne, while Bacterial Leaf Blight (Xanthomonas) affects only the leaf. Blast disease (Pyricularia oryzae) is documented to impact the leaf, panicle, and neck. Rice Spikelet Rot disease (Curvularia lunata) is confined to the panicle, whereas Sheath Blight (Rhizoctonia solani), primarily affecting the sheath, is also recorded on the panicle. In Africa, five different diseases were reported, all affecting the leaf.
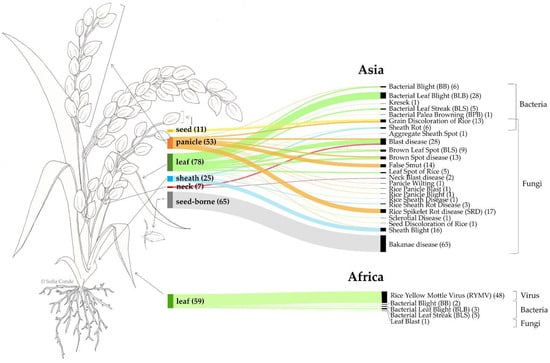
Figure 7.
Schematic representation of rice plant parts affected by each disease across continents (Africa vs. Asia) based on available data. Link widths are proportional to the number of records.
4. Discussion
A comprehensive keyword search strategy facilitated the coverage of an extensive body of literature on rice pests and diseases, yielding approximately 16,000 articles for initial screening. Subsequent analysis focused on the two primary rice-growing regions, Africa and Asia, documenting the most significant pests and diseases alongside their distribution patterns. The review revealed notable gaps in the literature, particularly regarding African rice-producing countries, and limited research applied to O. glaberrima. The lack of rice-producing countries in the explored databases may be due to relevant research being published in non-indexed sources like technical reports or government documents. This highlights the need for more peer-reviewed scientific articles to enhance accessibility and understanding of pest and disease dynamics in these regions. The scarcity of information on O. glaberrima is evident in the data available from EPPO (European and Mediterranean Plant Protection Organization) [49], which lists only two pests (Nilaparvata lugens and Orseolia oryzae) and three diseases (Xanthomonas oryzae pv. oryzae, X. oryzae pv. oryzicola, and Rice Yellow Mottle Virus). This limited dataset reflects the fact that O. glaberrima production is largely confined to West Africa, a region where research efforts remain sparse [50,51]. In contrast, O. sativa benefits from significantly more extensive documentation. The EPPO database identifies approximately 70 pests (including two snail species) and 44 diseases (including 10 nematodes) affecting this species. This disparity underscores the pressing need for increased research and data collection on O. glaberrima, particularly in West Africa, to better understand and address the challenges faced by this region’s rice cultivation.
4.1. Overall Assessment of Rice Pests
Top pest genera in Africa include Diopsis (Diptera), Maliarpha, and Chilo (Lepidoptera), consistent with findings reported in earlier studies [52,53,54]. Species such as Diopsis macrophthalma, D. apicalis, Maliarpha separatella, and Chilo zacconius are primarily distributed in West Africa, where they hold significant agricultural importance [5]. The Diopsis genus, commonly referred to as stalked-eye flies, is categorized into species groups, including the D. apicalis group, an Afrotropical cluster encompassing D. apicalis Dalman and D. macrophthalma Dalman [55]. Among these, D. apicalis is dominant in West Africa, while D. macrophthalma is widespread across sub-Saharan Africa, where it is considered the most common rice diopsid [55]. Both species were first reported in 1969 and are frequently found in West African countries. During the vegetative phase, the larvae of Diopsis bore galleries in rice stems, destroying the plant’s growing parts and causing a characteristic symptom known as ‘deadheart’ [56]. At later stages, Maliarpha separatella reduces plant height and grain yield, while Chilo zacconius, restricted to West Africa [57,58], similarly impacts grain weight and plant growth in susceptible rice varieties [59]. As climate change reshapes agricultural ecosystems, Diopsis is emerging as a more significant threat to rice production, namely due to the rising temperatures that accelerate the life cycle of Diopsis, leading to increased population growth and more frequent infestations, particularly in Africa [60].
In Asia, the top pest genera include the hemipterans Nilaparvata, Sogatella, and Nephotettix, and the lepidopterans Scirpophaga, Chilo, Cnaphalocrocis, and Sesamia. Among these, the brown planthopper (Nilaparvata lugens), native to Asia [61], is one of the most severe rice pests. In high populations, it causes leaf discoloration, progressing to ‘hopperburn’, which ultimately kills the plant. Additionally, N. lugens transmits plant viruses [61]. The yellow stem borer (Scirpophaga incertulas) is another major pest, particularly abundant throughout Asia [62,63], which attacks rice throughout its lifecycle. During the vegetative phase, it causes ‘deadheart’ symptoms, and at the reproductive stage, it leads to ‘whiteheads’, severely affecting yields [62,63]. Similarly, the striped stem borer (Chilo suppressalis), common in temperate and subtropical Asia [64], damages plants by causing ‘deadhearts’ and ‘whiteheads’ through larval feeding. The white-backed planthopper (Sogatella furcifera), a significant phloem-feeding pest, damages rice by sucking sap and transmitting viruses such as Southern Rice Black-Streaked Dwarf Disease and Rice Black-Streaked Dwarf Virus [65,66]. Another notable pest, the rice leaf folder (Cnaphalocrocis medinalis), limits photosynthesis by folding and feeding on rice leaves. Meanwhile, the pink stem borer (Sesamia inferens) causes symptoms like ‘deadhearts’ and ‘earheads’, resulting in yield losses [67]. Finally, the genus Nephotettix, including species such as N. virescens and N. nigropictus, is a significant vector of viral diseases. Nephotettix virescens transmits diseases like rice tungro and Rice Yellow Dwarf, while N. nigropictus is associated with rice tungro, Rice Yellow Dwarf, and Rice Gall Dwarf diseases. Climate change is intensifying the threat posed by key rice pests in Asia, leading to greater crop losses and food security concerns. Rising temperatures are increasing pest activity and disease transmission, particularly for Nephotettix spp. (green leafhoppers), which transmit rice tungro virus, and Nilaparvata lugens (brown planthoppers), which spread Rice Grassy Stunt and Ragged Stunt viruses. To mitigate these impacts, a combination of adaptive pest management, climate-resilient rice varieties, and improved monitoring systems adapted to region-specific climate conditions is essential.
These findings highlight the diverse threats posed by pest species in Africa and Asia, emphasizing the critical need for targeted pest management strategies.
4.2. Overall Assessment of Rice Diseases
In Africa, the top causal agents of rice diseases are viruses, mainly from the genera Sobemovirus, and also the genera Benyvirus. Sobemovirus, which causes Rice Yellow Mottle Virus (RYMV), is transmitted by pest vectors from 14 different genera, primarily belonging to the family Chrysomelidae [68]. In contrast, Benyvirus, responsible for Rice Stripe Necrosis, is associated with a single recorded vector: the protist Polymyxa graminis (order Plasmodiophorida, phylum Cercozoa).
In Asia, fungal and viral pathogens play a dominant role in rice diseases, often involving specific pest vectors. For instance, Sarocladium oryzae, the causative agent of sheath rot, is linked to vectors such as Leptocorisa acuta and Brevennia rehi, with additional references to mites from the family Tarsonemidae (Acari). Another fungal pathogen, Nakataea oryzae, which causes False Smut, is vectored by Nilaparvata lugens. Phytoplasma diseases, such as Rice Orange Leaf, are transmitted by leafhoppers like Nephotettix cincticeps and Inazuma dorsalis. Among viral diseases, rice tungro is prominently vectored by leafhoppers of the Nephotettix genus, particularly Nephotettix virescens, while Rice Stripe Virus relies on Laodelphax striatellus for its transmission.
Considering the most recorded diseases worldwide in the review, the five most representative diseases are Bakanae disease, Blast disease, Rice Yellow Mottle Virus, rice tungro disease, and Rice Spikelet Rot disease.
Climate change is increasing the spread and severity of rice diseases by altering environmental conditions. Rising temperatures and shifting rainfall patterns favor fungal pathogens like rice Blast and expand the range of insect vectors such as Nephotettix species, which transmit rice tungro virus disease. Extreme weather events further weaken plant defenses, increasing the frequency of disease outbreaks. Additionally, climate change disrupts biological pest control, intensifying disease pressure. To mitigate these risks, Climate Smart Agriculture Practices (CSAP) can help protect rice yields and enhance ecosystem resilience [69].
Top Diseases
Rice cultivation faces significant challenges from various diseases that threaten crop yields and food security worldwide. Among these, Bakanae disease stands out as a historically significant issue, caused predominantly by Fusarium fujikuroi [70]. First documented in Japan in 1828 [71], this seed-borne disease disrupts crop growth at all stages, leading to symptoms such as poor germination and abnormal seedling elongation due to the production of gibberellin, a mycotoxin [70]. While Bakanae disease is most prevalent in Asia, with substantial records from China, Indonesia, Malaysia, and India [72,73,74,75], it has also been reported, although less frequently, on other continents. The Fusarium genus, known for its taxonomic complexity and adaptability, not only causes crop diseases, but also poses risks to human health through mycotoxin contamination in rice, highlighting the importance of vigilant management [76,77,78]. Equally formidable is Blast disease, caused by Pyricularia oryzae (and occasionally P. grisea) [79], which is recognized as one of the most destructive diseases affecting rice worldwide [27,79]. Since its first identification in Japan in 1937 [80], Blast disease has spread to more than 85 rice-producing countries [27], including major agricultural regions in Asia and Africa. This pathogen targets critical plant structures, including grains, leaves, and panicles, significantly reducing yields and jeopardizing food supply chains [81].
On the African continent, the Rice Yellow Mottle Virus (RYMV) is a major viral pathogen [68], first detected in Kenya in 1966 [82]. Unlike other rice diseases with global distributions, RYMV remains confined to sub-Saharan Africa [83], where it is a major constraint on rice production [84]. Transmitted by coleopteran insects, the virus causes yellow mottling on leaves and incomplete panicle development, resulting in substantial yield losses [68]. Despite its localized presence, the severity of its impact underscores the need for focused research and management strategies. In contrast, Rice tungro disease, another viral disease, poses a severe risk in Asia [85,86,87], where it affects the growth and productivity of rice crops through its dual-viral infection mechanism: rice tungro bacilliform virus (RTBV) and rice tungro spherical virus (RTSV) [87]. Transmitted by leafhoppers, these viruses lead to stunted growth, yellow discoloration, and sterile panicles [87]. First reported in India and Indonesia in 1968 [88,89], rice tungro disease continues to plague Asian rice cultivation, making it a significant area of concern for food security in the region.
Fungal diseases like Rice Spikelet Rot further complicate rice production, caused by multiple pathogens, including F. proliferatum, Curvularia lunata, and Alternaria alternata [90]. Predominantly reported in China [91], this disease affects the rice panicle, causing grain discoloration and malformation as symptoms progress from reddish hues during flowering to dark brown and black at maturity [90]. Although less widespread than other diseases, its impact on grain quality and yield is no less critical.
Together, these diseases illustrate the complex and interconnected challenges faced by rice growers worldwide. From fungal infections to viral threats, these pathogens not only reduce crop yields, but also compromise grain quality and food safety. Addressing these issues requires a comprehensive approach, integrating research, monitoring, and effective management practices to safeguard global rice production.
4.3. Linking Diseases Agents as Major Constraints to Rice Productivity
Yield loss percentages provide valuable insights into the severity of concern associated with each rice disease [92]. Nevertheless, it is important to note that while many yield losses studies are conducted in controlled environments, field study conditions are far more complex, with various abiotic, chemical, geological, and biotic factors interacting, making yield loss highly variable and potentially influenced by multiple, less evident organisms, or other factors. Figure 6 highlights the evaluation of yield losses caused by bacterial (two genera), fungal (six genera), and viral pathogens (Sobemovirus), offering a comprehensive overview of their impact on rice productivity. Among bacterial genera, Pantoea has been reported to reduce rice yields by up to 70%, while Xanthomonas exhibits a broader impact, with losses ranging from 20% to 100%, underscoring its role as a key agent of cosmopolitan rice diseases. Fungal pathogens, including Pyricularia, Bipolaris, and Fusarium, are particularly destructive. These genera are associated with Blast disease, Brown Spot disease, and Bakanae disease, respectively, each capable of causing yield losses as high as 100%. These diseases are prevalent across rice-growing regions and are devastating to productivity across diverse ecological zones. The Sobemovirus genus, responsible for Rice Yellow Mottle Virus (RYMV), represents one of the most harmful rice diseases, with yield losses also reaching 100%. However, rice yield losses to RYMV have been reported to fluctuate greatly between 10% and 100%, depending on plant age prior to infection, susceptibility of rice variety, and environmental factors, both in experimental and field assays. Specific to the African continent, RYMV poses a significant threat to rice productivity, raising serious concerns among African rice-producing countries.
4.4. Implications for Rice Production
The native regions of O. sativa growth (India, Myanmar, Thailand, North Vietnam, and China) have documented major pests, such as Sogatella furcifera [93] and Nilaparvata lugens [3], as well as significant diseases, including Bakanae disease [71] and False Smut [94]. Similarly, in the native region of O. glaberrima, located in the floodplains of the Niger River in Africa and encompassing countries such as Guinea Conakry, Ivory Coast, Mali, Burkina Faso, Benin, Niger, Chad, Cameroon, and Nigeria [95,96], records highlight Rice Yellow Mottle Virus (RYMV) as a major disease affecting O. sativa (e.g., in Burkina Faso [97,98] and Ivory Coast [99]) and there is a single record of a major pest reported in Nigeria [59].
This review provides a global perspective on the distribution of pests and diseases that impact rice productivity. In Africa, the top pest genera include Diopsis, Maliarpha, and Chilo, whereas in Asia, the key genera are Nilaparvata, Scirpophaga, Sogatella, Chilo, Cnaphalocrocis, Sesamia, and Nephotettix. The top disease-causing agents in Africa include the fungal genus Pyricularia, the bacterial genus Xanthomonas, and the viral genus Sobemovirus. In contrast, in Asia, the major disease-causing agents include fungi such as Fusarium, Pyricularia, Rhizoctonia, Bipolaris, Curvularia, Ustilaginoidea, and Alternaria, alongside bacteria like Xanthomonas and Pantoea. Figure 7 underscores the patterns of plant tissue affected by these pathogens. In Africa, leaf damage predominates, with fungi, bacteria, and viruses collectively targeting rice leaves. Asia presents a more comprehensive dataset, reflecting extensive research in the region. The most frequently affected tissues are the leaf and seed, with seed-borne diseases like Bakanae (caused by Fusarium) and foliar diseases like Blast (caused by Pyricularia) being particularly prominent. Panicle, sheath, seed, and neck tissues also show vulnerability to various diseases. Among the diseases causing the most significant yield losses, fungi like Fusarium, Pyricularia, and Bipolaris are notable for their severe impact, with losses reported to reach 100%. Fusarium and Bipolaris, primarily recorded in Asia, are linked to Bakanae disease and Brown Spot disease, respectively. Bakanae primarily affects seed-borne plants, while Brown Spot disease targets leaves and panicles. Blast disease, caused by Pyricularia, is a widespread concern in both Africa and Asia, affecting multiple plant parts. In Africa, a particular threat is posed by the viral genus Sobemovirus, responsible for RYMV, which can cause yield losses of up to 100% and predominantly damages rice leaves. The geographic specificity of RYMV to Africa highlights the pressing need for targeted research and management strategies in African rice-producing regions.
Effective rice disease management maximizes yields, ensures food security, and supports sustainable farming. Key strategies include genetic resistance breeding, biological control, cultural practices, and responsible chemical use. Advances in molecular breeding have led to the development of resistant rice varieties against major diseases like rice Blast [100] and Bacterial Leaf Blight [101]. Biological control using microorganisms like Pseudomonas fluorescens, which demonstrate antagonistic effects against pathogens such as Bipolaris oryzae, reduces reliance on chemical pesticides [102]. Cultural practices such as integrated pest and disease management (IPM and IDM, respectively), water regulation, and crop rotation have been shown to reduce disease incidence [103,104]. Additionally, farmer education plays a key role in strengthening these efforts. Technology-driven methods, like predictive modeling, aid early detection [105], reducing yield losses. A holistic approach integrating these strategies will strengthen rice production and sustainability.
As a final consideration, in rice ecosystems, pests and diseases often interact synergistically, resulting in greater damage than their individual effects [106]. This interaction accelerates crop loss, reduces yields, and complicates management strategies. Primary pests directly reduce productivity, while secondary pests, such as stem borers [57], weaken plants and increase their susceptibility to disease. Stem borers create entry wounds that allow fungal pathogens to infect rice, weakening stems and attracting more pests. For instance, sheath rot incidence rises with plant density and insect damage, particularly at the panicle initiation stage, leading to upper leaf sheath rot and stunted panicle emergence [107]. Additionally, rice–fish polyculture [108,109] may help reduce insect pests and diseases in rice fields [110], potentially mitigating these synergistic effects.
5. Conclusions
This literature-based assessment consolidates global knowledge on rice pests and diseases, highlighting key threats in Africa and Asia. In Africa, the most significant pests are stem borers such as Diopsis spp., Maliarpha separatella, and Chilo zacconius, which damage rice stems, leading to white panicles and reduced grain quality. In Asia, significant pests include stem borers like Scirpophaga incertulas, Chilo suppressalis, and Sesamia inferens, alongside sap-sucking pests such as plant- and leafhoppers (e.g., Nilaparvata lugens). These pests not only cause direct damage to plants, but also serve as vectors for viruses, such as Rice Grassy Stunt virus and Rice Ragged Stunt virus, exacerbating their impact on rice productivity. This review reveals that leaves are the most commonly affected plant tissue by both pest and disease, often leading to secondary damage in other parts of the plant. While considerable research has been conducted in Asia, particularly in India, China, and the Philippines, there remains a significant gap in knowledge regarding African rice production systems. This disparity is especially pronounced for O. glaberrima, an indigenous African rice species, where understanding of pest and disease interactions remains limited. In addition, there is an urgent need to expand our understanding of field pests and diseases in both African and Asian rice systems that contribute to post-harvest losses or pose risks to human health, such as those caused by storage fungi and pests (e.g., [111,112]). Future studies should prioritize under-researched regions and species, exploring interdisciplinary approaches to examine pest and disease dynamics comprehensively. While this study focused only on the two rice species, O. sativa and O. glaberrima, future research could broaden this scope by investigating the interactions between rice subspecies and varieties with pests and diseases, providing valuable insights into their susceptibility and resistance. This could help refine breeding programs, improve pest management strategies, and enhance global food security in the face of evolving climate conditions. By addressing these knowledge gaps, research can support the development of targeted pest and disease management strategies, particularly for vulnerable regions like Africa. Ultimately, enhancing our understanding of biotic stresses on rice will be critical to securing global food security and improving the livelihoods of rice producers worldwide.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15070667/s1: Figure S1. Number of all publications, grouped by decades from 1910 to the present, regarding diseases and pests; Table S1. Search strategy in CAB Abstract [https://www.cabdirect.org/, accessed on 20 April 2024], Web of Science [https://www.webofscience.com/, accessed on 20 April 2024], and PubMed [https://pubmed.ncbi.nlm.nih.gov/, accessed on 20 April 2024] databases/search engines for each query; Table S2. List of insect pest species mentioned in the manuscript and their main synonyms. The list of synonyms was compiled according to the GBIF database [https://www.gbif.org/, accessed on 11 March 2025] (≡Homotypic synonym). Common names (in English), host plants, and continental distribution were provided based on the EPPO database [https://gd.eppo.int/, accessed on 11 March 2025]. A host plant species listed in bold represents a Major Host. * Synonyms consulted in NCBI taxonomy database [https://www.ncbi.nlm.nih.gov/taxonomy, accessed on 11 March 2025] or EPPO database when not available in GBIF; ** Distribution information was consulted in GBIF when not present in EPPO.
Author Contributions
Conceptualization, S.C. (Sofia Conde) and F.M.; methodology, S.C. (Sofia Conde) and F.M.; formal analysis, S.C. (Sofia Conde), S.C. (Sílvia Catarino) and F.M.; investigation, S.C. (Sofia Conde), S.C. (Sílvia Catarino), S.F. and F.M.; resources, S.C. (Sofia Conde), S.C. (Sílvia Catarino) and F.M.; data curation, S.C. (Sofia Conde), S.C. (Sílvia Catarino) and F.M.; writing—original draft preparation, S.C. (Sofia Conde), S.C. (Sílvia Catarino) and F.M.; writing—review and editing, S.C. (Sofia Conde), S.C. (Sílvia Catarino), S.F., M.P.T. and F.M.; visualization, S.C. (Sofia Conde), S.C. (Sílvia Catarino) and F.M.; supervision, F.M.; funding acquisition, M.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU-funded project “MALMON—Mangroves, mangrove rice, and mangrove people: sustainably improving rice production, ecosystems and livelihoods”, under grant contract FOOD/2019/412-700. S. Conde benefited from funds from the MALMON project through a PhD fellowship; S. Catarino was funded by GenoCash Project (PTDC/ASP-AGR/0760/2020, https://doi.org/10.54499/PTDC/ASP-AGR/0760/2020); F.M. and S.F. were funded by the Scientific Employment Stimulus—Individual Call (CEEC Individual) by National Funds FCT/MCTES—2022.00392.CEECIND/CP1738/CT0002, (https://doi.org/10.54499/2022.00392.CEECIND/CP1738/CT0002); and 2020.03526.CEECIND (https://doi.org/10.54499/2020.03526.CEECIND/CP1601/CP1649/CT0007), respectively. This study was also funded by National funds to the CEF (UIDB/00239/2020; https://doi.org/10.54499/UIDB/00239/2020), LEAF (UID/AGR/04129/2020; https://doi.org/10.54499/UIDB/04129/2020), and cE3c (UIDB/00329/2020; https://doi.org/10.54499/UIDB/00329/2020) research units funded by Fundação para a Ciência e a Tecnologia I.P. (FCT), Portugal, and by the Associate Laboratory TERRA (LA/P/0092/2020, https://doi.org/10.54499/LA/P/0092/2020).
Data Availability Statement
All of the data are available in the article sections and/or the Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ewete, F.K.; Olagbaju, R.A. The development of Aspavia armigera Fabricius (Hemiptera: Pentatomidae) and its status as a pest of cowpea and rice. Int. J. Trop. Insect Sci. 1990, 11, 171–177. [Google Scholar] [CrossRef]
- Alves, T.M.; Maia, A.H.N.; Barrigossi, J.A.F. Spatial distribution and coexisting patterns of adults and nymphs of Tibraca limbativentris (Hemiptera: Pentatomidae) in paddy rice fields. Environ. Entomol. 2016, 45, 1505–1514. [Google Scholar] [CrossRef]
- Hereward, J.P.; Cai, X.; Matias, A.M.A.; Walter, G.H.; Xu, C.; Wang, Y. Migration dynamics of an important rice pest: The brown planthopper (Nilaparvata lugens) across Asia—Insights from population genomics. Evol. Appl. 2020, 13, 2449–2459. [Google Scholar] [CrossRef]
- Statista. Worldwide Production of Grain in 2023/24, by Type (in Million Metric Tons). Available online: https://www.statista.com/statistics/263977/world-grain-production-by-type/ (accessed on 13 July 2024).
- Heinrichs, E.A.; Muniappan, R. IPM for Tropical Crops: Rice. CAB Rev. 2017, 12, 1–31. [Google Scholar] [CrossRef]
- Bernaola, L.; Cosme, M.; Schneider, R.W.; Stout, M. Belowground inoculation with arbuscular mycorrhizal fungi increases local and systemic susceptibility of rice plants to different pest organisms. Front. Plant Sci. 2018, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Teeken, B.; Nuijten, E.; Temudo, M.P.; Okry, F.; Mokuwa, A.; Struik, P.C.; Richards, P. Maintaining or abandoning African rice: Lessons for understanding processes of seed innovation. Hum. Ecol. 2012, 40, 879–892. [Google Scholar] [CrossRef]
- Linares, O.F. African rice (Oryza glaberrima): History and future potential. Proc. Natl. Acad. Sci. USA 2002, 99, 16360–16365. [Google Scholar] [CrossRef]
- GRiSP. Rice Almanac: Source Book for One of the Most Economic Activities on Earth, 4th ed.; International Rice Research Institute: Los Baños, Philippines, 2013. [Google Scholar]
- van Andel, T. African rice (Oryza glaberrima Steud.): Lost crop of the enslaved Africans discovered in Suriname. Econ. Bot. 2010, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van Andel, T.R.; Meyer, R.S.; Aflitos, S.A.; Carney, J.A.; Veltman, M.A.; Copetti, D.; Flowers, J.M.; Havinga, R.M.; Maat, H.; Purugganan, M.D.; et al. Tracing ancestor rice of Suriname Maroons back to its African origin. Nat. Plants 2016, 2, 16149. [Google Scholar] [CrossRef]
- Bagirov, V.; Treshkin, S.; Korobka, A.; Dereka, F.; Garkusha, S.; Kovalev, V.; Esaulova, L.; Kizinek, S. Scientific support of the rice growing industry of the agroindustrial complex of the Russian Federation in solving the problems of food security. E3S Web Conf. 2020, 210, 05006. [Google Scholar] [CrossRef]
- Samal, P.; Babu, S.C.; Mondal, B.; Mishra, S.N. The global rice agriculture towards 2050: An inter-continental perspective. Outlook Agric. 2022, 51, 164–172. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Sie, M.; Hijmans, R.J.; Otsuka, K. Increasing Rice Production in Sub-Saharan Africa: Challenges and Opportunities. Adv. Agron. 2007, 94, 55–133. [Google Scholar] [CrossRef]
- Agyen-Sampong, M. Mangrove swamp rice production in West Africa. In Dynamique et Usages de la Mangrove Dans les Pays des Rivières du Sud (du Sénégal à la Sierra Leone); Cormier-Salem, M.C., Ed.; ORSTOM Éditions: Paris, France, 1994; pp. 185–188. [Google Scholar]
- Adefurin, O.; Zwart, S.J. A Detailed Map of Rice Production Areas in Mangrove Ecosystems in West-Africa in 2013—Mapping of Mangrove Rice Systems Using Landsat 8 Satellite Imagery and Secondary Data; Technical Report; Africa Rice Center: Cotonou, Benin, 2017. [Google Scholar] [CrossRef]
- Pandey, S.; Byerlee, D.; Dawe, D.; Dobermann, A.; Mohanty, S.; Rozelle, S.; Hardy, B. Rice in the Global Economy: Strategic Research and Policy Issues for Food Security; International Rice Research Institute: Manila, Philippines, 2010. [Google Scholar]
- Mukherjee, A.K.; Bag, M.K.; Annamalai, M.; Adak, T.; Lenka, S.; Basana Gowda, G.; Prasanthi, G.; Raghu, S.; Baite, M.S.; Prabhukarthikeyan, S.R.; et al. Bio-intensive Management of Pest and Diseases of Rice. In Rice Research for Enhancing Productivity, Profitability and Climate Resilience; Pathak, H., Nayak, A.K., Jena, M., Singh, O.N., Samal, P., Sharma, S.G., Eds.; ICAR-National Rice Research Institute: Cuttack, India, 2018; pp. 404–418. [Google Scholar]
- Pathak, M.D.; Khan, Z.R. Insect Pests of Rice; IRRI: Manila, Philippines, 1994; p. 89. [Google Scholar]
- Wilson, B.E.; Villegas, J.M.; Stout, M.J.; Landry, K.J. Relative yield loss from stem borers (Lepidoptera: Crambidae) and rice water weevil (Coleoptera: Curculionidae) in rice. J. Econ. Entomol. 2021, 11, 1159–1165. [Google Scholar] [CrossRef]
- Sirvi, M.K.; Seervi, S.; Kumar, P. Pest management of yellow stem borer Scirpophaga incertulas in Rice. Just Agric. 2021, 1, 1–4. [Google Scholar]
- Mohapatra, S.D.; Raghu, S.; Prasanthi, G.; Baite, M.S.; Prabhukarthikeyan, S.R.; Yadav, M.K.; Basana Gowda, G.; Pandi, G.G.P.; Banerjee, A.; Aravindan, S.; et al. Bio-ecology of rice insect pests and diseases: Paving the way to climate-smart rice protection technologies. In Rice Research for Enhancing Productivity, Profitability and Climate Resilience; Pathak, H., Nayak, A.K., Jena, M., Singh, O.N., Samal, P., Sharma, S.G., Eds.; ICAR-National Rice Research Institute: Odisha, India, 2018; pp. 384–403. [Google Scholar]
- Morales, F.J.; Jennings, P.R. Rice hoja blanca: A complex plant-virus-vector pathosystem. CABI Rev. 2010, 5, 1–16. [Google Scholar] [CrossRef]
- Martin, J.E.; Jimenez, E.K.B.; Cruz, M.G.; Zhu-Salzman, K.; Way, M.O.; Badillo-Vargas, I.E. Assessing the potential infection of Tagosodes orizicolus (Hemiptera: Delphacidae) by Rice Hoja Blanca virus in Texas. J. Econ. Entomol. 2020, 113, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, Y.; Chen, H.; Liang, Z.; Chen, J.; Wu, R.; Zhang, T.; Zhou, G.; Yang, X. Identification, characterization and prevalence in southern China of a new iflavirus in the leafhopper Recilia dorsalis (Hemiptera: Cicadellidae). Virus Res. 2023, 323, 199005. [Google Scholar] [CrossRef]
- Narayanasamy, P. Detection of Fungal Pathogens in Plants. In Microbial Plant Pathogens-Detection and Disease Diagnosis; Springer: Dordrecht, The Netherlands, 2011; Volume 1, pp. 5–199. [Google Scholar] [CrossRef]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Sahebi, M.; Hashemi, F.S.G.; Yusuff, O.; Usman, M.G. Blast disease intimidation towards rice cultivation: A review of pathogen and strategies to control. J. Anim. Plant Sci. 2017, 27, 1058–1066. [Google Scholar]
- Kato, H. Rice blast disease. Pest. Outlook 2001, 12, 23–25. [Google Scholar] [CrossRef]
- Naqvi, S.A.H. Bacterial Leaf Blight of Rice: And overview of epidemiology and management with special reference to Indian sub-continent. Pak. J. Agric. Res. 2019, 32, 359. [Google Scholar] [CrossRef]
- Tennant, P.; Gubba, A.; Roye, M.; Fermin, G. Viruses as Pathogens: Plant Viruses. In Viruses; Tennant, P., Fermin, G., Foster, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 6; pp. 135–156. [Google Scholar] [CrossRef]
- Listihani, L.; Selangga, D.G.W.; Yuliadhi, K.A.; Yuniti, I.G.A.D.; Ariati, P.E.P. Molecular characterization of Rice ragged stunt virus and Rice grassy stunt virus on Rice in Gianyar, Bali, Indonesia. J. Trop. Plant Pest Dis. 2024, 24, 48–57. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Lyu, Y.; Huang, Z.; Zhang, X.; Sun, B.; Li, P.; Jing, X.; Li, H.; Zhang, C. A Review of Vector-Borne Rice Viruses. Viruses 2022, 14, 2258. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.; Plowright, A.; Peng, D. Nematode Parasites of Rice. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI Publishing: Cambridge, MA, USA, 2005; pp. 87–130. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 12 July 2024).
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 12 July 2024).
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017. [Google Scholar] [CrossRef]
- Conde, S.; Catarino, S.; Monteiro, F. RAW Data. In Figshare 2024. Available online: https://figshare.com/articles/dataset/RAW_Data_PRISMA_/26332849 (accessed on 20 August 2024).
- FAOSTAT. Statistical Database. Food and Agriculture Organization of the United Nations, Rome. Available online: https://www.fao.org/faostat/en/#home (accessed on 28 June 2024).
- Muniyappa, V.; Ramakrishnan, K. Mycoplasma and allied pathogens of plants, animals and human beings. In Proceedings of the Workshop held at University of Agricultural Sciences, Bangalore, India, 14–16 February 1979. [Google Scholar]
- Alam, M.S. Whitefly (Hemiptera: Aleyrodidae): A potential pest of rice in West Africa. Int. Rice Res. Newsl. 1989, 14, 38–39. [Google Scholar]
- Fomba, S.N. Rice disease situation in mangrove and associated swamps in Sierra Leone. Trop. Pest Manag. 1984, 30, 73–81. [Google Scholar] [CrossRef]
- Awoderv, V.A.; Bangura, N.; John, V.T. Incidence, distribution and severity of bacterial diseases on rice in West Africa. Trop. Pest Manag. 1991, 37, 113–117. [Google Scholar] [CrossRef]
- Mau, Y.S.; Ndiwa, A.S.S.; Oematan, S.S. Brown spot disease severity, yield and yield loss relationships in pigmented upland rice cultivars from East Nusa Tenggara, Indonesia. Biodiversitas 2020, 21, 1625–1634. [Google Scholar] [CrossRef]
- Aluko, M.O. Crop losses caused by the brown leaf spot disease of rice in Nigeria. Agric. Food Sci. 1975, 59, 609–613. [Google Scholar]
- Ahmad, F.; Khan, M.R. Incidence of sheath blight in irrigated rice and associated yield losses in northern India. Plant Dis. 2023, 107, 2907–2915. [Google Scholar] [CrossRef]
- Issaka, S.; Basso, A.; Sorho, F.; Onasanya, A.; Haougui, A.; Sido, A.Y.; Aké, S.; Fargette, D.; Séré, Y. Diagnosis and importance of rice yellow mottle disease epidemics in Niger republic. J. Appl. Biosci. 2012, 50, 3501–3511. [Google Scholar]
- EPPO. EPPO Global Database. Available online: https://gd.eppo.int (accessed on 9 July 2024).
- Eniayejuni, A. Scientific research in West Africa and the impact of international collaboration: An analysis in Scopus database, 1997–2017. Afr. J. Libr. 2020, 30, 1–5. [Google Scholar]
- Olufadewa, I.I.; Adesina, M.A.; Ayorinde, T. From Africa to the world: Reimagining Africa’s research capacity and culture in the global knowledge economy. J. Glob. Health 2020, 10, 010321. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, H.; Hill, S.B. Dormancy in the stalk-eyed fly, Diopsis longicornis MACQ, a pest of rice in Sub-Saharan Africa. Int. J. Trop. Insect Sci. 1995, 16, 303–310. [Google Scholar] [CrossRef]
- Akinsola, E.A.; Agyen-Sampong, M. The ecology, bionomics and control of rice stem-borers in West Africa. Int. J. Trop. Insect Sci. 1984, 5, 69–77. [Google Scholar] [CrossRef]
- Brenière, J. The importance of entomological problems in the development of rice-growing in West Africa. Agron. Trop. 1969, 24, 906–927, (In French with English Abstract). [Google Scholar]
- Feijen, H.R.; Feijen, C. Diopsis mayae sp. N. (Diptera, Diopsidae), a dominant stalk-eyed fly, occurring from South Africa to Saudi Arabia. Tijdschrift Voor Entomol. 2017, 160, 61–74. [Google Scholar] [CrossRef]
- Ba, N.M.; Dakouo, D.; Nacro, S.; Karamage, F. Seasonal abundance of lepidopteran stemborers and diopsid flies in irrigated fields of cultivated (Oryza sativa) and wild rice (Oryza longistaminata) in western Burkina Faso. Int. J. Trop. Insect Sci. 2008, 28, 30–36. [Google Scholar] [CrossRef]
- Khan, Z.R.; Litsinger, J.A.; Barrion, A.T.; Villanueva, F.F.D.; Fernandez, N.J.; Taylo, L.D. World Bibliography of Rice Stem Borers, 1794–1990; International Rice Research Institute: Manila, Philippines, 1991. [Google Scholar]
- De Prins, J.; De Prins, W. Afromoths, Online Database of Afrotropical Moth Species (Lepidoptera). World Wide Web Electronic Publication. Available online: http://www.afromoths.net (accessed on 10 July 2024).
- Umeh, E.D.N.; Joshi, R.C.; Ukwungwu, M.N. Biology, status and management of rice insect pests in Nigeria. Crop Prot. 1992, 11, 408–413. [Google Scholar] [CrossRef]
- Roland, B.; Bernard, G.C.; Elie, D.A.; Séraphin, Z.A. Diopsis (Diopsis thoracica and D. apicalis) damaging rice production in Africa: A review. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 33–41. [Google Scholar] [CrossRef]
- Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest categorisation of Nilaparvata lugens. EFSA J. 2023, 21, 7999. [Google Scholar] [CrossRef]
- Chen, Y.H.; Romena, A. Feeding Patterns of Scirpophaga incertulas (Lepidoptera: Crambidae) on Wild and Cultivated Rice During the Booting Stage. Environ. Entomol. 2006, 35, 1094–1102. [Google Scholar]
- Ali, M.P.; Bari, M.N.; Haque, S.S.; Kabir, M.M.M.; Nowrin, F.; Choudhury, T.R.; Mankin, R.W.; Ahmed, N. Response of a rice insect pest, Scirpophaga incertulas (Lepidoptera: Pyralidae) in warmer world. BMC Zool. 2020, 5, 6. [Google Scholar] [CrossRef]
- Jiang, M.X.; Cheng, J.A. Interactions between the striped stem borer Chilo suppressalis (Walk.) (Lep., Pyralidae) larvae and rice plants in response to nitrogen fertilization. J. Pest Sci. 2003, 76, 124–128. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, J.; Jia, H.; Miao, Y.; Hou, M. Sogatella furcifera saliva mucin-like protein is required for feeding and induces rice defences. Int. J. Mol. Sci. 2022, 23, 8239. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef] [PubMed]
- Baladhiya, H.C.; Sisodiya, D.B.; Pathan, N.P. A review on pink stem borer, Sesamia inferens Walker: A threat to cereals. J. Entomol. Zool. Stud. 2018, 6, 1235–1239. [Google Scholar]
- Kouassi, N.K.; N’Guessan, P.; Albar, L.; Fauquet, C.M.; Brugidou, C. Distribution and characterization of Rice yellow mottle virus: A threat to African farmers. Plant Dis. 2005, 89, 124–133. [Google Scholar] [CrossRef]
- Rajamani, K.; Chintagunta, L.; Raj, G.B. Climate Change Impact on Pests and Disease Intensity of Rice. Theor. Biol. Forum 2022, 11, 80–83. [Google Scholar] [CrossRef]
- Shakeel, Q.; Mubeen, M.; Sohail, M.A.; Ali, S.; Iftikhar, Y.; Tahir Bajwa, R.; Aqueel, M.A.; Upadhyay, S.K.; Divvela, P.K.; Zhou, L. An explanation of the mystifying bakanae disease narrative for tomorrow’s rice. Front. Microbiol. 2023, 14, 1153437. [Google Scholar] [CrossRef]
- Bashyal, B.M. Etiology of an emerging disease: Bakanae of rice. Indian Phytopathol. 2018, 71, 485–494. [Google Scholar] [CrossRef]
- Wang, G.C.; Chen, H.K.; Xu, P.S.; Bao, J.Y. Studies on the pathogens of the rice bakanae disease in Zhejiang. Acta Phytop. Sin. 1990, 20, 93–97, (In Chinese with English abstract). [Google Scholar]
- Bashyal, B.M.; Aggarwal, R. Molecular identification of Fusarium species associated with bakanae disease of rice (Oryza sativa) in India. Indian J. Agric. Sci. 2013, 83, 71–76. [Google Scholar]
- Zainudin, N.A.I.M.; Razak, A.A.; Salleh, B. Bakanae disease of rice in Malaysia and Indonesia: Etiology of the causal agent based on morphological, physiological and pathogenicity characteristics. J. Plant Prot. Res. 2008, 48, 475–485. [Google Scholar] [CrossRef]
- Zainudin, N.A.I.M.; Sidique, S.N.M.; Salleh, B. Genetic Diversity of Fusarium fujikuroi Isolated from Bakanae Disease of Rice on the Basis of Vegetative Compatibility. Pertanika J. Trop. Agric. Sci. 2008, 31, 271–278. [Google Scholar]
- Reddy, K.R.N.; Reddy, C.S.; Abbas, H.K.; Abel, C.A.; Muralidharan, K. Mycotoxigenic fungi, mycotoxins, and management of rice grains. Toxin Ver. 2008, 27, 287–317. [Google Scholar] [CrossRef]
- El-Shanshoury, A.E.-R.R.; El-Sabbagh, S.M.; Emara, H.A.; Saba, H.E. Occurrence of moulds, toxicogenic capability of Aspergillus flavus and levels of aflatoxins in maize, wheat, rice and peanut from markets in central delta provinces, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 852–865. [Google Scholar]
- Dong, F.; Xing, Y.J.; Lee, Y.W.; Mokoena, M.P.; Olaniran, A.O.; Xu, J.H.; Shi, J.R. Occurrence of Fusarium mycotoxins and toxigenic Fusarium species in freshly harvested rice in Jiangsu, China. World Mycotoxin J. 2020, 13, 201–212. [Google Scholar] [CrossRef]
- Xiang, Z.; Okada, D.; Asuke, S.; Nakayashiki, H.; Ikeda, K. Novel insights into host specificity of Pyricularia oryzae and Pyricularia grisea in the infection of gramineous plant roots. Mol. Plant Pathol. 2022, 23, 1658–1670. [Google Scholar] [CrossRef]
- Suzuki, H. Studies on the relations between the anatomical characters of the rice plant and its susceptibility to blast disease. J. Coll. Agric. 1937, 24, 181–264, (In Japanese with English Abstract). [Google Scholar]
- Muimba-Kankolongo, A. Cereal Production. In Food Crop Production by Smallholder Farmers in Southern Africa; Muimba-Kankolongo, A., Ed.; Academic Press: Ottawa, Canada, 2018; pp. 73–121. [Google Scholar] [CrossRef]
- Bakker, W. Rice yellow mottle, a mechanically transmissible virus disease of rice in Kenya. Neth. J. Plant Pathol. 1970, 76, 53–63. [Google Scholar] [CrossRef]
- Ochola, D.; Issaka, S.; Rakotomalala, M.; Pinel-Galzi, A.; Ndikumana, I.; Hubert, J.; Hébrard, E.; Séré, Y.; Tusiime, G.; Fargette, D. Emergence of rice yellow mottle virus in eastern Uganda: Recent and singular interplay between strains in East Africa and in Madagascar. Virus Res. 2015, 195, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Luk’yanchikov, V.P.; Sorokina, Z.A. Virus disease of rice. Zash. Rast. 1979, 12, 35–36, (In Russian with English Abstract). [Google Scholar]
- Azzam, O.; Chancellor, T.C.B. The biology, epidemiology, and management of rice tungro disease in Asia. Plant Dis. 2002, 86, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roy, S.; Tarafdar, J.; Senapat, B.K. Present status of rice tungro disease in West Bengal: Occurrence and characterization of viruses. J. Crop Weed 2009, 5, 229–232. [Google Scholar]
- Bunawan, H.; Dusik, L.; Bunawan, S.N.; Amin, N.M. Rice Tungro disease: From identification to disease control. World Appl. Sci. J. 2014, 31, 1221–1226. [Google Scholar]
- John, V.T. Identification and characterization of tungro, a virus disease of Rice in India. Plant Dis. Rep. 1968, 52, 871–875. [Google Scholar]
- Rivera, C.T.; Ou, S.H.; Tantere, D.M. Tungro disease of rice in Indonesia. Plant Dis. Rep. 1968, 52, 122–124. [Google Scholar]
- Huang, S.; Wang, L.; Liu, L.; Tang, S.; Zhu, D.; Savary, S. Rice spikelet rot disease in China—1. Characterization of fungi associated with the disease. Crop Prot. 2011, 30, 1–9. [Google Scholar] [CrossRef]
- Hu, S.; Yu, J.; Wei, K.; Lan, B.; Li, M.; Xie, B.; Zhu, Y.; Yang, Y.; Zhu, J.; Zhang, L.; et al. Studies on the biological characteristics and pathogenic identification of rice spikelet rot disease in Jiangxi province. Acta Agric. Univ. Jiangxiensis 2019, 41, 234–242. [Google Scholar]
- Cerda, R.; Avelino, J.; Gary, C.; Tixier, P.; Lechevallier, E.; Allinne, C. Primary and secondary yield losses caused by pests and diseases: Assessment and modeling in coffee. PLoS ONE 2017, 12, e0169133. [Google Scholar] [CrossRef]
- Ambikadevi, D. White backed plant hopper, Sogatella furcifera (Horvath) (Homoptera: Delphacidae) a major pest of rice in Kuttanad, Kerala. Insect Environ. 1998, 4, 36. [Google Scholar]
- Saha, M.; Chakraborty, A.; Bhattacharya, K. Aerobiology, epidemiology and disease forecasting of false smut disease of rice in West Bengal, India. Aerobiologia 2020, 36, 299–304. [Google Scholar] [CrossRef]
- FAO. Irrigation Potential in Africa: A Basin Approach; FAO: Rome, Italy, 1997; p. 117. Available online: https://openknowledge.fao.org/handle/20.500.14283/w4347e (accessed on 26 June 2024).
- Al-Gamal, S.A.; Sokona, Y.; Dodo, A.K. Climatic changes and groundwater resources in Africa. Int. J. Clim. Chang. Strateg. Manag. 2009, 1, 133–145. [Google Scholar] [CrossRef]
- Pinel, A.; N’Guessan, P.; Bousalem, M.; Fargette, D. Molecular variability of geographically distinct isolates of Rice yellow mottle virus in Africa. Arch. Virol. 2000, 145, 1621–1638. [Google Scholar] [CrossRef]
- Tollenaere, C.; Lacombe, S.; Wonni, I.; Barro, M.; Ndougonna, C.; Gnacko, F.; Sérémé, D.; Jacobs, J.M.; Hebrard, E.; Cunnac, S.; et al. Virus-bacteria rice co-infection in Africa: Field estimation, reciprocal effects, molecular mechanisms, and evolutionary implications. Front. Plant Sci. 2017, 8, 645. [Google Scholar] [CrossRef]
- Bertrand, G.N.; Fatogoma, S.; Brahima, K. Development of rice yellow mottle disease depending on topographic positions of the lowland in Côte d’Ivore. Plant Pathol. J. 2018, 17, 87–94. [Google Scholar] [CrossRef]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Asfaliza, R.; Latif, M.A. Blast resistance in rice: A review of conventional breeding to molecular approaches. Mol. Biol. Rep. 2012, 40, 2369–2388. [Google Scholar] [CrossRef]
- Chukwu, S.; Rafii, M.; Ramlee, S.; Ismail, S.; Hasan, M.; Oladosu, Y.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Karan, R.; Kalaimathi, D.; Renganathan, P.; Balabaskar, P. Isolation and characterization of phylloplane associated bacteria and its in vitro antagonistic activity against Bipolaris oryzae. Agric. Sci. Dig. 2022, 1–4. [Google Scholar] [CrossRef]
- Agbowuro, G.; Afolabi, M.; Olamiriki, E.; Awoyemi, S. Rice blast disease (Magnaporthe oryzae): A menace to rice production and humanity. Int. J. Pathog. Res. 2020, 4, 32–39. [Google Scholar] [CrossRef]
- Khatun, M.; Nessa, B.; Salam, M.; Kabir, M. Strategy for rice disease management in bangladesh. Bangladesh Rice J. 2021, 25, 23–36. [Google Scholar] [CrossRef]
- Kim, Y.; Roh, J.; Kim, H. Early forecasting of rice blast disease using long short-term memory recurrent neural networks. Sustainability 2017, 10, 34. [Google Scholar] [CrossRef]
- Wraight, S.P. Synergism between insect pathogens and entomophagous insects, and its potential to enhance biological control efficacy. In Predators and Parasitoids; Koul, O., Dhaliwal, G.S., Eds.; Taylor & Francis: London, UK; New York, NY, USA, 2003; p. 146. [Google Scholar]
- Sparks, A.; Castilla, N.P.; Cruz, C.M.V. Sheath Root. Rice Knowledge Bank. IRRI. Available online: http://www.knowledgebank.irri.org/training/fact-sheets/pest-management/diseases/item/sheath-rot (accessed on 14 March 2025).
- Xiuzhen, F. Rice-Fish Culture in China. Aquac. Asia 2003, 8, 44–46. [Google Scholar]
- Keleman, P.-J.; Durand, J.-D.; Simier, M.; Camará, A.; Sá, R.M.; Panfili, J. eDNA-based seasonal monitoring reveals fish diversity patterns in mangrove habitats of Guinea-Bissau, West Africa. Reg. Stud. Mar. Sci. 2025, 82, 104013. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Liu, L.; Fu, Q.; Zhu, D. Nonchemical pest control in China rice: A review. Agron. Sustain. Dev. 2014, 34, 275–291. [Google Scholar] [CrossRef]
- Conde, S.; Barai, A.; Catarino, S.; Costa, G.J.; Ferreira, S.; Tavares, I.; Ferreira, M.R.; Temudo, M.P.; Monteiro, F. Hidden secrets of mangrove swamp rice stored seeds in Guinea-Bissau: Assessment of fungal communities and implications for food security. Agronomy 2024, 14, 1870. [Google Scholar] [CrossRef]
- Conde, S.; Monteiro, F.; Catarino, S.; Ferreira, M.R.; Ferreira, S. Uninvited guests: New stored mangrove rice insect pests in Guinea-Bissau. J. Stored Prod. Res. 2025, 111, 102567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).