Physiological and Ecological Responses of Anoectochilus formosanus to Different Light Intensities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Treatments
2.2. Plant and Stomatal Morphology
2.3. Chlorophyll Content
2.4. Chlorophyll Fluorescence Parameters
2.5. Measurement of Polysaccharide and Flavonoid Contents
2.6. Statistical Analysis
3. Results
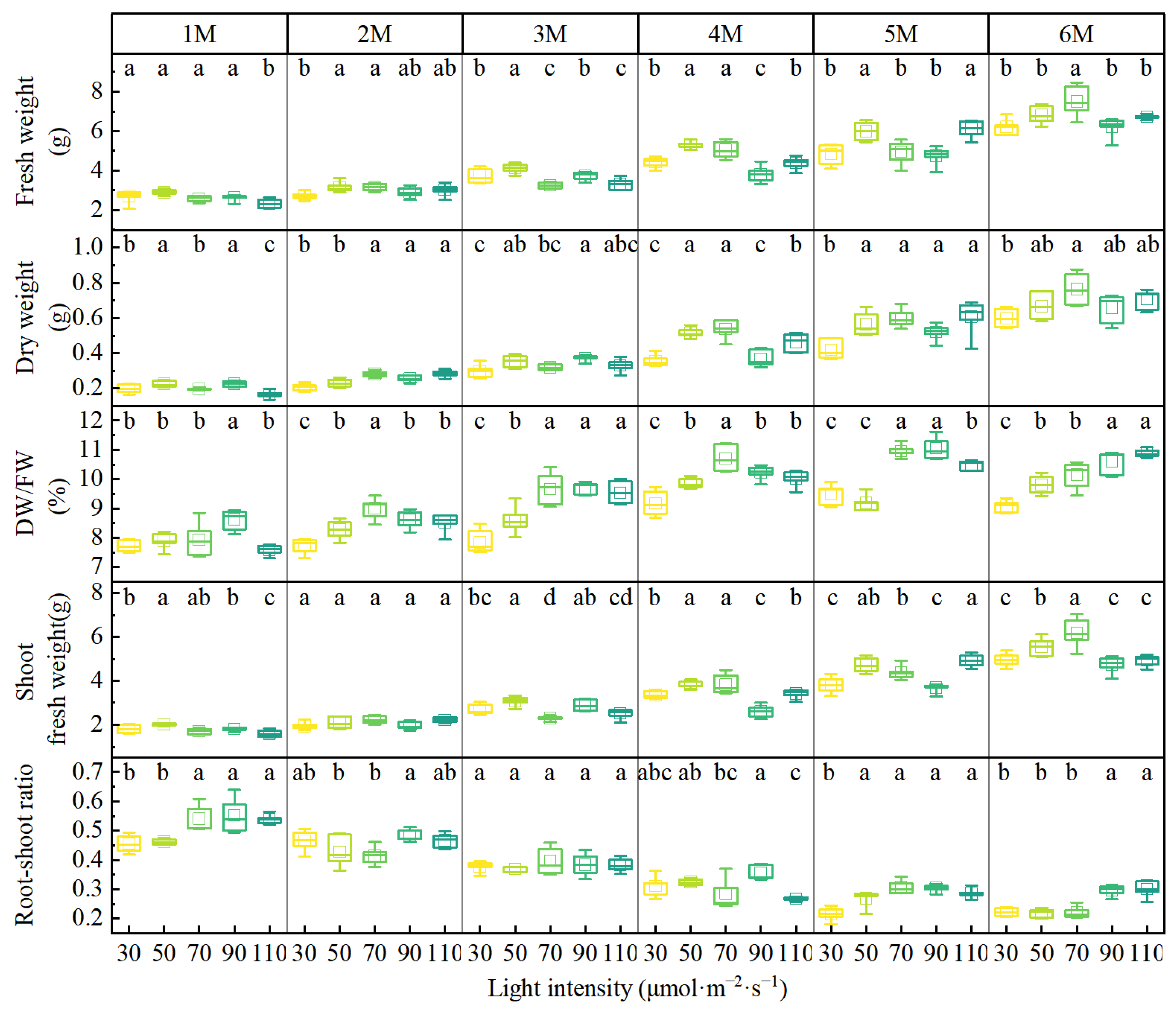
3.1. The Effects of Different Light Intensities on the Morphological Characteristics and Biomass Accumulation of A. formosanus
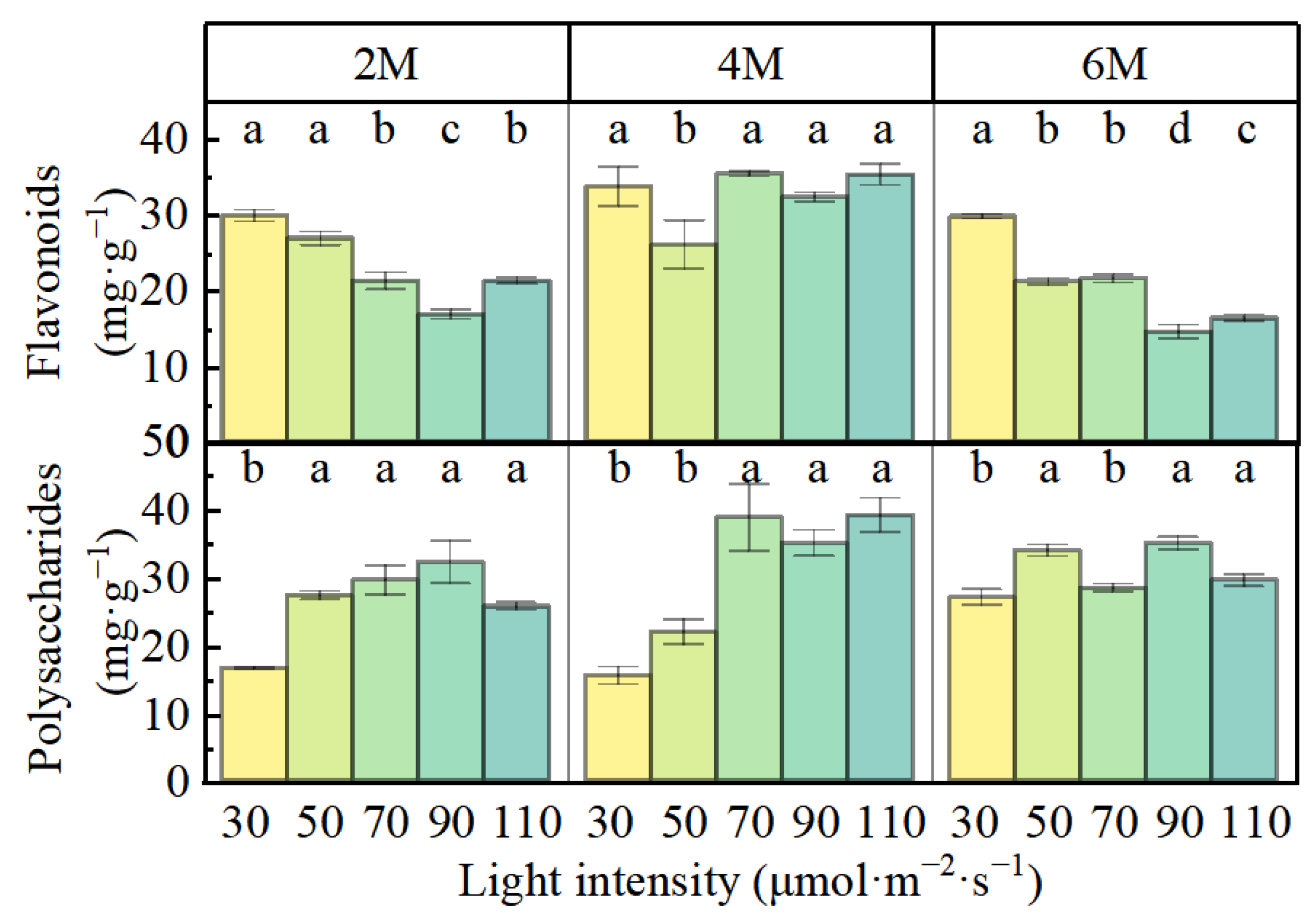
3.2. Flavonoid and Polysaccharide Contents
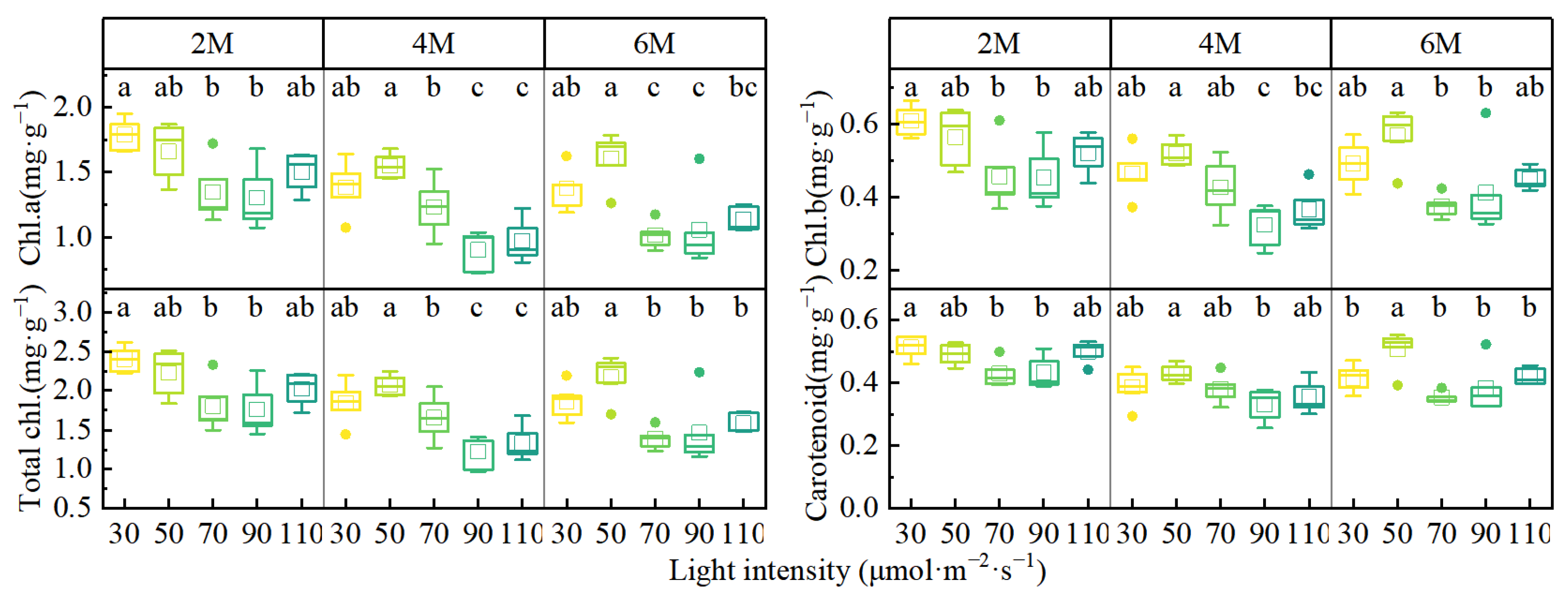
3.3. Chlorophyll Content
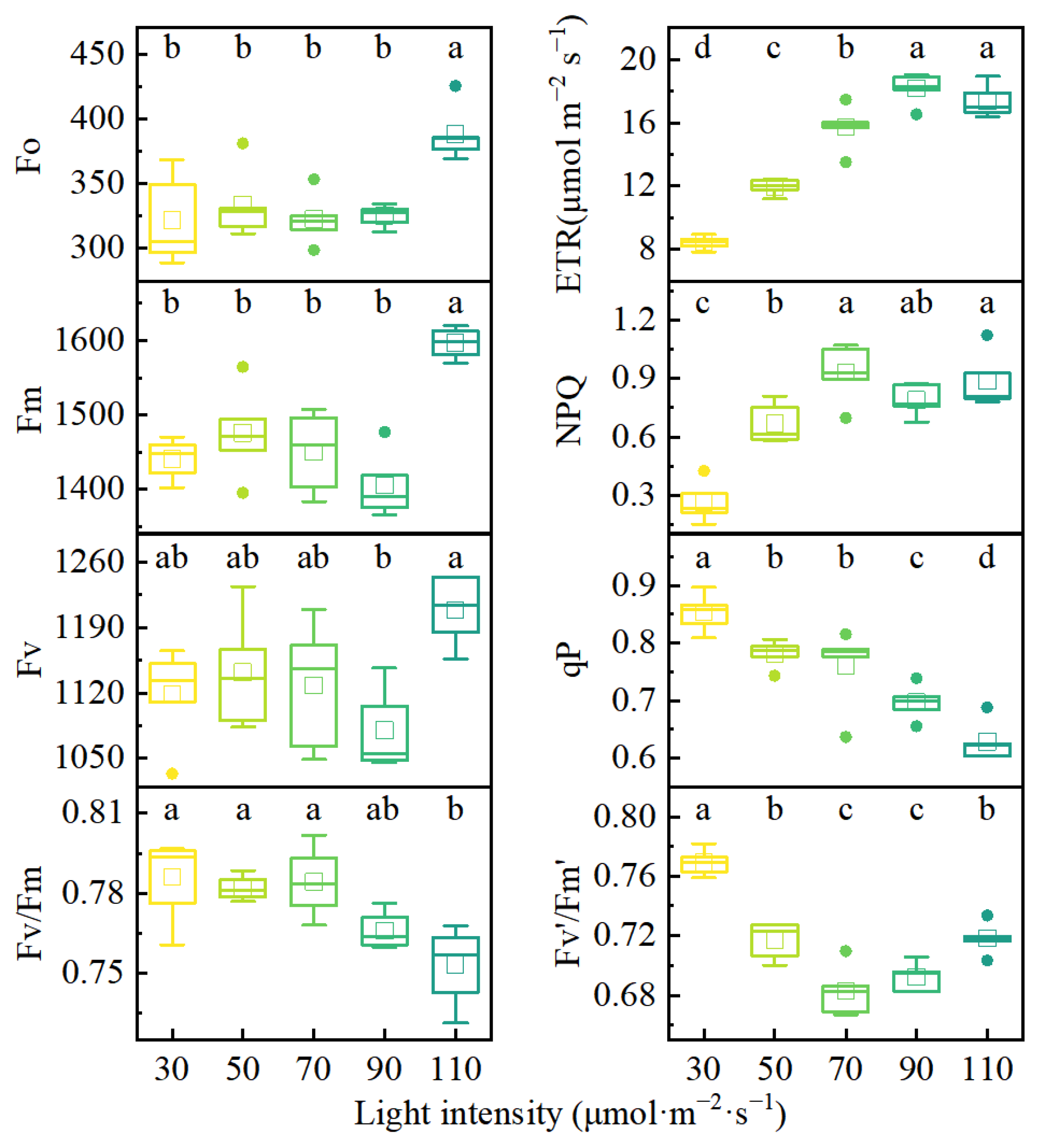
3.4. Changes in Fluorescence Parameters
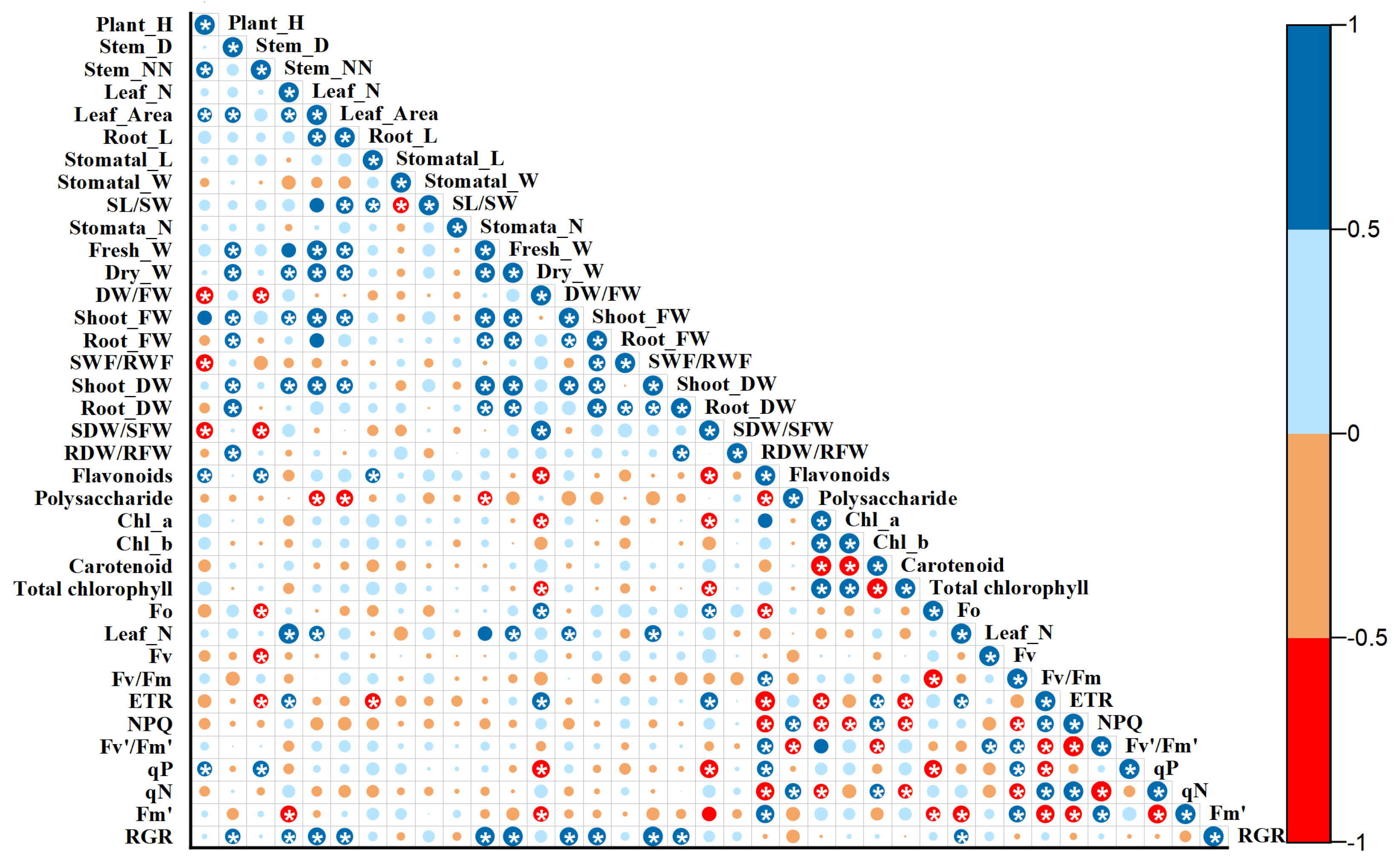
3.5. Correlation Analysis
3.6. Principal Component Analysis
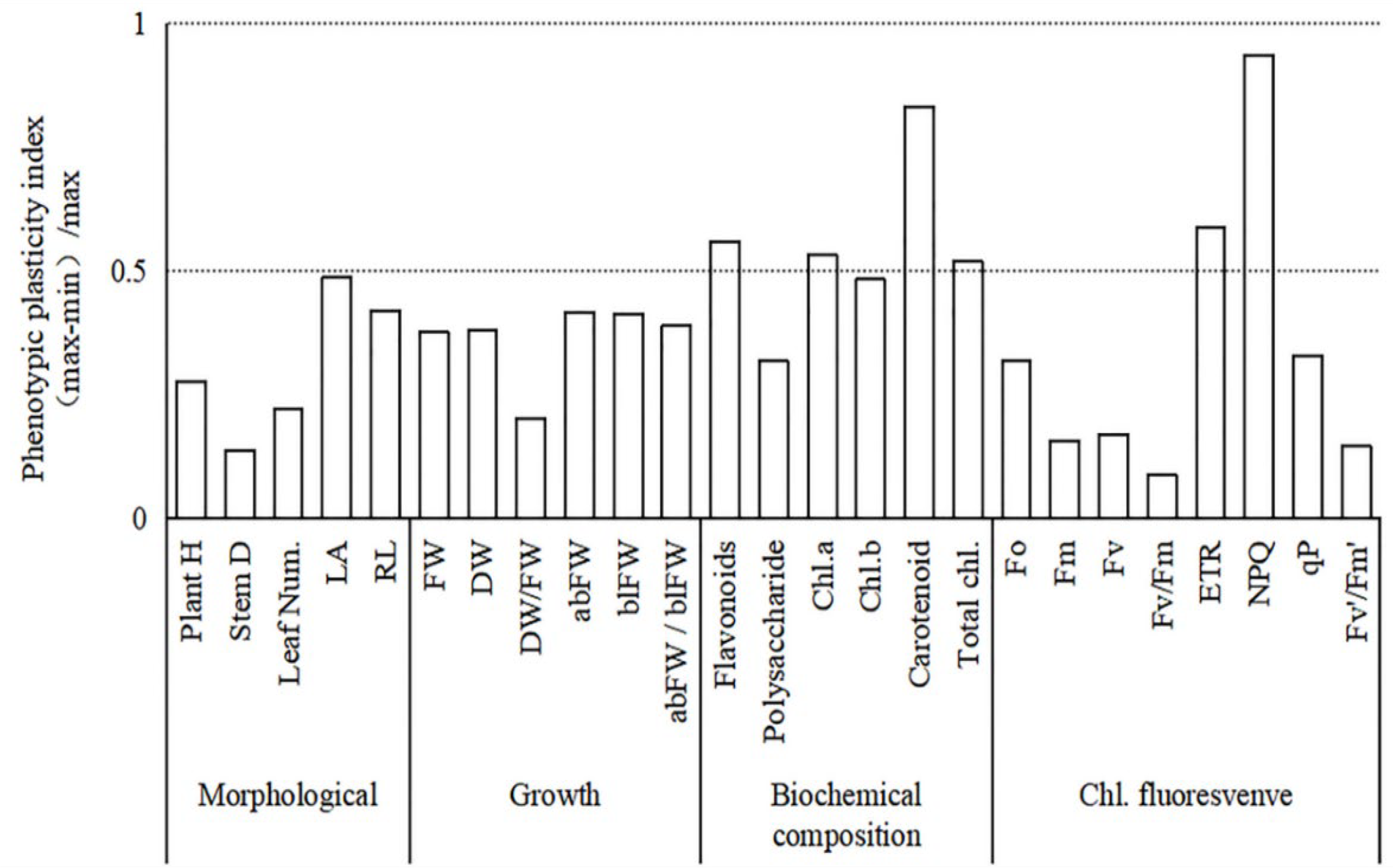
3.7. Plasticity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-López, N.F.; Cavatte, P.C.; Silva, P.E.; Martins, S.C.; Morais, L.E.; Medina, E.F.; DaMatta, F.M. Physiological and biochemical abilities of robusta coffee leaves for acclimation to cope with temporal changes in light availability. Physiol. Plant. 2013, 149, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar]
- Zhang, R.; Kong, Z.; Chen, S.; Ran, Z.; Ye, M.; Xu, J.; Zhou, C.; Liao, K.; Cao, J.; Yan, X. The comparative study for physiological and biochemical mechanisms of Thalassiosira pseudonana and Chaetoceros calcitrans in response to different light intensities. Algal Res. 2017, 27, 89–98. [Google Scholar]
- Pearcy, R.W. Functional Plant Ecology, 2nd ed.; Responses of Plants to Heterogeneous Light Environments; Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 213–258. ISBN 9780429122477. [Google Scholar]
- Jiang, H.; Tian, X.; Dong, S.; Bao, J. Effects of temperature and light intensity on the growth and biochemical composition of Sargassum thunbergii. Yingyong Shengtai Xuebao 2009, 20, 185. [Google Scholar] [PubMed]
- Zhao, N.; Xu, Q.; Wang, X. Ecological and physiological characteristics of Pinellia ternata under different shade conditions. Pak. J. Agric. Sci. 2022, 59, 925. [Google Scholar]
- Schumann, T.; Paul, S.; Melzer, M.; Dörmann, P.; Jahns, P. Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Front. Plant Sci. 2017, 8, 681. [Google Scholar]
- Campbell, W.; Black, C. The relationship of CO2 assimilation pathways and photorespiration to the physiological quantum requirement of green plant photosynthesis. BioSystems 1978, 10, 253–264. [Google Scholar]
- Gao, Z.; Khalid, M.; Jan, F.; Jiang, X.; Yu, X. Effects of light-regulation and intensity on the growth, physiological and biochemical properties of Aralia elata (miq.) seedlings. South Afr. J. Bot. 2019, 121, 456–462. [Google Scholar]
- Chen, C.; Luo, X.; Jin, G.; Cheng, Z.; Pan, X.; Zhu, G.; Li, S.; Zhu, Y.; Tang, N. Shading effect on survival, growth, and contents of secondary metabolites in micropropagated Anoectochilus plantlets. Braz. J. Bot. 2017, 40, 599–607. [Google Scholar]
- Chen, M.; Zeng, X.; Liu, Y.; Zhang, H.; Hu, Q. An orthogonal design of light factors to optimize growth, photosynthetic capability and metabolite accumulation of Anoectochilus roxburghii (Wall.) Lindl. Sci. Hortic. 2021, 288, 110272. [Google Scholar] [CrossRef]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.K.; Hung, N.K.; Anh, P.T.L.; Thu, N.T.; Hien, N.T.T.; Khanh, T.D.; Ha, C.H. LED lights promote growth and flavonoid accumulation of Anoectochilus roxburghii and are linked to the enhanced expression of several related genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, M.; Li, H.; Zeng, B.; Chang, Q.; Lai, Z. Effects of supplemental lighting with different light qualities on growth and secondary metabolite content of Anoectochilus roxburghii. PeerJ 2018, 6, e5274. [Google Scholar]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar]
- Heumann, H.G. Microwave-Stimulated Glutaraldehyde and Osmium Tetroxide Fixation of Plant Tissue: Ultrastructural Preservation in Seconds. Histochemistry 1992, 97, 341–347. [Google Scholar] [PubMed]
- Porra, R.J. The Chequered History of the Development and Use of Simultaneous Equations for the Accurate Determination of Chlorophylls a and b. Photosynthesis Research 2002, 73, 149–156. [Google Scholar]
- Huang, Y.; Chen, J. Comparison of polysaccharide and flavonoid contents in Anoictochilus roxburghii (wall.) lindl under different cultivation forms and harvest time. J. Trop. Org. 2012, 3, 174–176. [Google Scholar]
- Li, Y.; Kong, D.; Liang, H.-L.; Wu, H. Alkaloid content and essential oil composition of Mahonia breviracema cultivated under different light environments. J. Appl. Bot. Food Qual. 2018, 91, 171–179. [Google Scholar]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar]
- Nishimura, E.; Suzaki, E.; Irie, M.; Nagashima, H.; Hirose, T. Architecture and growth of an annual plant Chenopodium album in different light climates. Ecol. Res. 2010, 25, 383–393. [Google Scholar]
- Allahverdiyeva, Y.; Aro, E.-M. Photosynthetic responses of plants to excess light: Mechanisms and conditions for photoinhibition, excess energy dissipation and repair. In Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 275–297. [Google Scholar]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism underlying the shading-induced chlorophyll accumulation in tea leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Dumlao, M.R.; Darehshouri, A.; Cohu, C.M.; Muller, O.; Mathias, J.; Adams, W.W.; Demmig-Adams, B. Low temperature acclimation of photosynthetic capacity and leaf morphology in the context of phloem loading type. Photosynth. Res. 2012, 113, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological limits to plant phenotypic plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef]
- Verhoeven, A.; Adams Iii, W.; Demmig-Adams, B. The xanthophyll cycle and acclimation of Pinus ponderosa and Malva neglecta to winter stress. Oecologia 1999, 118, 277–287. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- You, T.; Barnett, S.M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Duan, B.; Lu, Y.; Yin, C.; Li, C. Morphological and physiological plasticity of woody plant in response to high light and low light. Chin. J. Appl. Environ. Biol. 2005, 11, 238–245. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Cen, C.; Chu, X.; Wei, H.; Mu, Y.; Gu, S.; Liu, H.; Ma, Z. Physiological and Ecological Responses of Anoectochilus formosanus to Different Light Intensities. Agriculture 2025, 15, 705. https://doi.org/10.3390/agriculture15070705
Li R, Cen C, Chu X, Wei H, Mu Y, Gu S, Liu H, Ma Z. Physiological and Ecological Responses of Anoectochilus formosanus to Different Light Intensities. Agriculture. 2025; 15(7):705. https://doi.org/10.3390/agriculture15070705
Chicago/Turabian StyleLi, Rui, Caihui Cen, Xuan Chu, Hongyu Wei, Yinghui Mu, Song Gu, Hongli Liu, and Zhiyu Ma. 2025. "Physiological and Ecological Responses of Anoectochilus formosanus to Different Light Intensities" Agriculture 15, no. 7: 705. https://doi.org/10.3390/agriculture15070705
APA StyleLi, R., Cen, C., Chu, X., Wei, H., Mu, Y., Gu, S., Liu, H., & Ma, Z. (2025). Physiological and Ecological Responses of Anoectochilus formosanus to Different Light Intensities. Agriculture, 15(7), 705. https://doi.org/10.3390/agriculture15070705






