Effect of Synthetic Fungicides Used in Conventional Strawberry Growing System on Hirsutella sp., an Entomopathogenic Fungus of Cyclamen Mite
Abstract
:1. Introduction
2. Materials and Methods
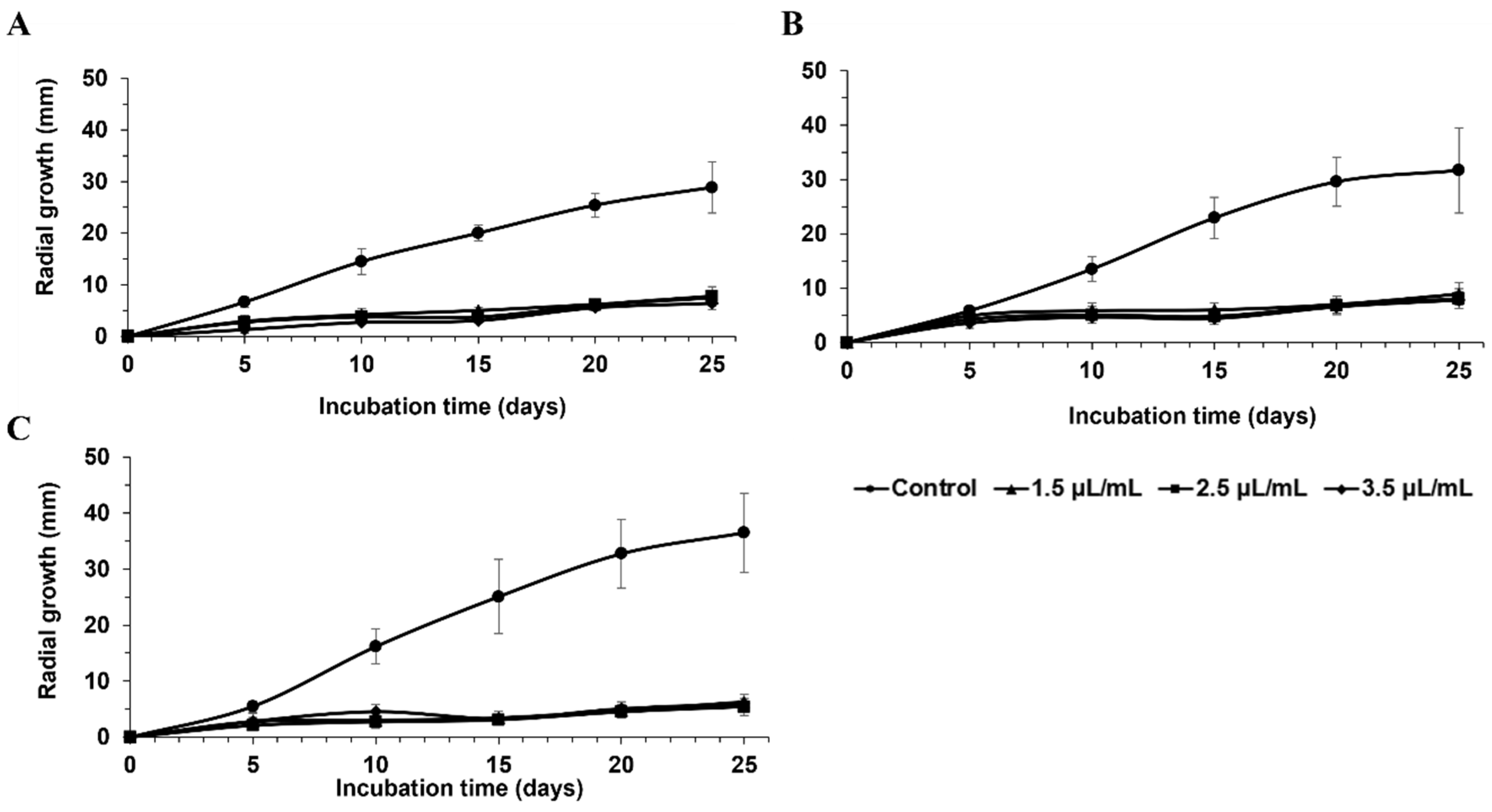
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdisc. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Koul, B.; Chopra, M.; Lamba, S. Microorganisms as biocontrol agents for sustainable agriculture. In Relationship Between Microbes and the Environment for Sustainable Ecosystem Services; Samuel, J., Kumar, A., Singh, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–68. [Google Scholar] [CrossRef]
- Ikiz, B.; Dasgan, H.Y.; Gruda, N.S. Utilizing the power of plant growth promoting rhizobacteria on reducing mineral fertilizer, improved yield, and nutritional quality of Batavia lettuce in a floating culture. Sci. Rep. 2024, 14, 1616. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Kitsiou, F.; Natsiopoulos, D.; Eliopoulos, P.A. Entomopathogenic fungi: Interactions and applications. Encyclopedia 2022, 2, 646–656. [Google Scholar] [CrossRef]
- Litwin, A.; Nowak, M.; Różalska, S. Entomopathogenic fungi: Unconventional applications. Rev. Environ. Sci. Biotechnol. 2020, 19, 23–42. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Yadav, P.K. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent. Food Agric. 2023, 9, 2180857. [Google Scholar] [CrossRef]
- Inglis, C.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycetous fungi for managing insect pests. In Fungi as Biocontrol Agents; CABI Books: Wallingford, UK, 2001; pp. 23–69. ISBN 9780851993560. [Google Scholar]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal. Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Dara, S.K. Managing strawberry pests with chemical pesticides and non-chemical alternatives. Int. J. Fruit Sci. 2016, 16, 129–141. [Google Scholar] [CrossRef]
- Government of Canada. Pesticides and Pest Management Reports and Publications; Government of Canada: Ottawa, ON, Canada, 2024; Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management.html (accessed on 23 February 2024).
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Schaefers, G.A. Seasonal densities and control of the cyclamen mite, Steneotarsonemus pallidus (Acarina: Tarsonemidae) on strawberry in New York. J. Econ. Entomol. 1963, 56, 565–571. [Google Scholar] [CrossRef]
- Stenseth, C.; Nordby, A.L.F. Damage, and control of the strawberry mite Steneotarsonemus pallidus (Acarina: Tarsonemidae), on strawberries. J. Hortic. Sci. 1976, 51, 24–49. [Google Scholar] [CrossRef]
- Harnois, M.; Lacroix, C. Tarsonème du Fraisier; Bulletin d’information No 17—Petits fruits, Réseau d’avertissements phytosanitaires; MAPAQ: Québec, QC, Canada, 2013. [Google Scholar]
- Bernier, V.; Lefebvre, N.; Khelifi, M.; Renkema, J.; Fournier, V. Control of Phytonemus pallidus (Acari: Tarsonemidae) from strawberry transplants using controlled atmosphere temperature treatment. J. Econ. Entomol. 2023, 116, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Alford, D.V. The effects of Tarsonemus fragariae Zimmermann (Acarina: Tarsonemidae) on strawberry yields. Ann. Appl. Biol. 1972, 70, 13–18. [Google Scholar] [CrossRef]
- Ajila, H.E.V.; Lemos, F.; Colares, F.; Ferreira, J.A.M.; Lofego, A.C.; Pallini, A. A new record of a pest mite on strwberry Phy-tonemus pallidus (Banks) (Acari: Tarsonemidae) arrives in Minas Gerais, Brazil. Fla. Entomol. 2018, 101, 529–532. [Google Scholar] [CrossRef]
- Łabanowska, B.H.; Piotrowski, W.; Korzeniowski, M.; Cuthbertson, A.G.S. Control of the strawberry mite, Phytonemus pallidus (Banks) in strawberry plantations by alternative acaricides. Crop Prot. 2015, 78, 5–14. [Google Scholar] [CrossRef]
- Fountain, M.T.; Harris, A.L.; Cross, J.V. The use of surfactants to enhance acaricide control of Phytonemus pallidus (Acari: Tarsonemidae) in strawberry. Crop Prot. 2010, 29, 1286–1292. [Google Scholar] [CrossRef]
- Johansen, N.S.; Trandem, N.; Le, V.H.; Stensvand, A. The potential for using aerated steam to eradicate strawberry mite and two-spotted spider mite on strawberry transplants. Exp. Appl. Acarol. 2022, 88, 243–262. [Google Scholar] [CrossRef]
- Renkema, J.M.; Takeda, F.; Janisiewicz, W. Ultraviolet-C irradiation has no short-term, direct effects on cyclamen mite (Phytonemus pallidus (Banks)) in strawberry. Can. J. Plant Sci. 2023, 103, 589–594. [Google Scholar] [CrossRef]
- Patenaude, S.; Tellier, S.; Fournier, V. Cyclamen mite (Acari: Tarsonemidae) monitoring in eastern Canada strawberry (Rosaceae) fields and its potential control by the predatory mite Neoseiulus cucumeris (Acari: Phytoseiidae). Can. Entomol. 2020, 152, 249–260. [Google Scholar] [CrossRef]
- Snowden, A.L. Soft fruits and berry fruits. In Post-Harvest Diseases and Disorders of Fruits and Vegetables; CRC Press: London, UK, 1990; pp. 238–269. [Google Scholar]
- Swett, C.L.; Butler, B.B.; Peres, N.A.; Koivunen, E.E.; Hellman, E.M.; Beaulieu, J.R. Using model-based fungicide programing to effectively control Botrytis and Anthracnose fruit rots in Mid-Atlantic strawberry fields and co-manage strawberry sap beetle (Stelidota geminate). Crop Prot. 2020, 134, 105175. [Google Scholar] [CrossRef]
- Vischetti, C.; Feliziani, E.; Landi, L.; De Bernardi, A.; Marini, E.; Romanazzi, G. Effectiveness of four synthetic fungicides in the control of post-harvest gray mold of strawberry and analyses of residues on fruit. Agronomy 2024, 14, 65. [Google Scholar] [CrossRef]
- Lagnaoui, A.; Radcliffe, E.B. Potato fungicides interfere with entomopathogenic fungi impacting population dynamics of green peach aphid. Am. J. Potato Res. 1998, 75, 19–25. [Google Scholar] [CrossRef]
- Latteur, G.; Jansen, J.-P. Effects of 20 fungicides on the infectivity of conidia of the aphid entomopathogenic fungus Erynia neoaphidis. BioControl 2002, 47, 435–444. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Wright, D.J.; Leather, S.R. The effects of farming system and fertilisers on pests and natural enemies: A synthesis of current research. Agric. Ecosyst. Environ. 2011, 141, 261–270. [Google Scholar] [CrossRef]
- Zaller, J.G.; Brühl, C.A. Non-target effects of pesticides on organisms inhabiting agroecosystems. Front. Environ. Sci. 2019, 7, 75. [Google Scholar] [CrossRef]
- Srinivasulu, M.; Ortiz, D.R. Effect of pesticides on bacterial and fungal populations in Ecuadorian tomato cultivated soils. Environ. Process. 2017, 4, 93–105. [Google Scholar] [CrossRef]
- Celar, F.A.; Kos, K. Effects of selected herbicides and fungicides on growth, sporulation and conidial germination of entomopathogenic fungus Beauveria bassiana. Pest Manag. Sci. 2016, 72, 2110–2117. [Google Scholar] [CrossRef]
- Fiedler, Ż.; Sosnowska, D. Side effects of fungicides and insecticides on entomopathogenic fungi in vitro. J. Plant Prot. Res. 2018, 57, 355–360. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model application of entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef]
- Afandhi, A.; Choliq, F.A.; Fernando, I.; Marpaung, Y.M.A.N.; Setiawan, Y. Occurrence of soil-inhabiting entomopathogenic fungi within a conventional and organic farm and their virulence against Spodoptera litura. Biodiversitas. J. Biol. Divers. 2022, 23, 1172–1180. [Google Scholar] [CrossRef]
- Samal, I.; Bhoi, T.K.; Vyas, V.; Majhi, P.K.; Mahanta, D.K.; Komal, J.; Singh, S.; Kumar, P.V.D.; Acharya, L.K. Resistance to fungicides in entomopathogenic fungi: Underlying mechanisms, consequences, and opportunities for progress. Trop. Plant Pathol. 2024, 49, 5–17. [Google Scholar] [CrossRef]
- Bałazy, S.; Wrzosek, M.; Sosnowska, D.; Tkaczuk, C.; Muszewska, A. Laboratory trials to infect insects and nematodes by some acaropathogenic Hirsutella strains (Mycota: Clavicipitaceous anamorphs). J. Invertebr. Pathol. 2008, 97, 103–113. [Google Scholar] [CrossRef]
- Simmons, D.R.; Kepler, R.M.; Rehner, S.A.; Groden, E. Phylogeny of Hirsutella species (Ophiocordycipitaceae) from the USA: Remedying the paucity of Hirsutella sequence data. IMA Fungus 2015, 6, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zou, X.; Cao, W.; Xu, Z.; Liang, Z. Two new species of Hirsutella (Ophiocordycipitaceae, Sorariomycetes) that are parasitic on lepidopteran insects from China. MycoKeys 2021, 82, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Dara, S.K. Compatibility of the entomopathogenic fungus Beauveria bassiana with some fungicides used in California strawberry. Open Plant Sci. J. 2017, 10, 29–34. [Google Scholar] [CrossRef]
- Roberti, R.; Righini, H.; Masetti, A.; Maini, S. Compatibility of Beauveria bassiana with fungicides in vitro and on zucchini plants infested with Trialeurodes vaporariorum. Biol. Control 2017, 113, 39–44. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Majchrowska-Safaryan, A. Effect of selected fungicides on the growth of acaropathogenic fungi from the genus Hirsutella. Appl. Ecol. Env. Res. 2020, 18, 3897–3905. [Google Scholar] [CrossRef]
- Yáñez, M.; France, A. Effects of fungicides on the development of the entomopathogenic fungus Metarhizium anisopliae var. anisopliae. Chil. J. Agr. Res. 2010, 70, 390–398. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Mietkiewski, R. Effect of selected pesticides on the growth of fungi from Hirsutella genus isolated from phytophagous mites. J. Plant Prot. Res. 2005, 45, 171–179. [Google Scholar]
- Tkaczuk, C.; Łabanowska, B.H.; Miętkiewski, R. The influence of pesticides on the growth of fungus Hirsutella nodulosa (Petch) — entomopathogen of strawberry mite (Phytonemus pallidus ssp. fragariae Zimm.). J. Fruit Ornam. Plant Res. 2004, 12, 119–126. [Google Scholar]
- Ishii, H.; Watanabe, H.; Yamaoka, Y.; Schnabel, G. Sensitivity to fungicides in isolates of Colletotrichum gloeosporioides and C. acutatum species complexes and efficacy against anthracnose diseases. Pestic. Biochem. Physiol. 2022, 182, 105049. [Google Scholar] [CrossRef]
- Bruck, D.J. Impact of fungicides on Metarhizium anisopliae in the rhizosphere, bulk soil and in vitro. BioControl 2009, 54, 597–606. [Google Scholar] [CrossRef]
- Erdoğan, O.; Sağlan, Z. In vitro compatibility of entomopathogenic fungi Beauveria bassiana (BALS.) Vuill. with different fungicides. Black Sea J. Agric. 2023, 6, 416–421. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Reilly, C.C.; Hotchkiss, M.W.; Wood, B.W. The potential for enhanced fungicide resistance in Beauveria bassiana through strain discovery and artificial selection. J. Invertebr. Pathol. 2002, 81, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A functional overview of conservation biological control. Crop Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Van Emden, H.F. Conservation biological control of insect pests. CABI Rev. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Tixier, M.-S. Predatory mites (Acari: Phytoseiidae) in agro-ecosystems and conservation biological control: A review and explorative approach for forecasting plant-predatory mite interactions and mite dispersal. Front. Ecol. Evol. 2018, 6, 192. [Google Scholar] [CrossRef]
- Díaz-Lucas, M.F.; Maza, N.; Kirschbaum, D.S.; Rocca, M.; Greco, N.M. The potential of the hoverfly Allograpta exotica as a biological control agent of the strawberry aphid Chaetosiphon fragaefolii. J. Appl. Èntomol. 2024, 148, 191–198. [Google Scholar] [CrossRef]
- Ottaviano, M.F.G.; Cédola, C.V.; Sánchez, N.E.; Greco, N.M. Conservation biological control in strawberry: Effect of different pollen on development, survival, and reproduction of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2015, 67, 507–521. [Google Scholar] [CrossRef]
- Sigsgaard, L.; Betzer, C.; Naulin, C.; Eilenberg, J.; Enkegaard, A.; Kristensen, K. The effect of floral resources on parasitoid and host longevity: Prospects for conservation biological control in strawberries. J. Insect Sci. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
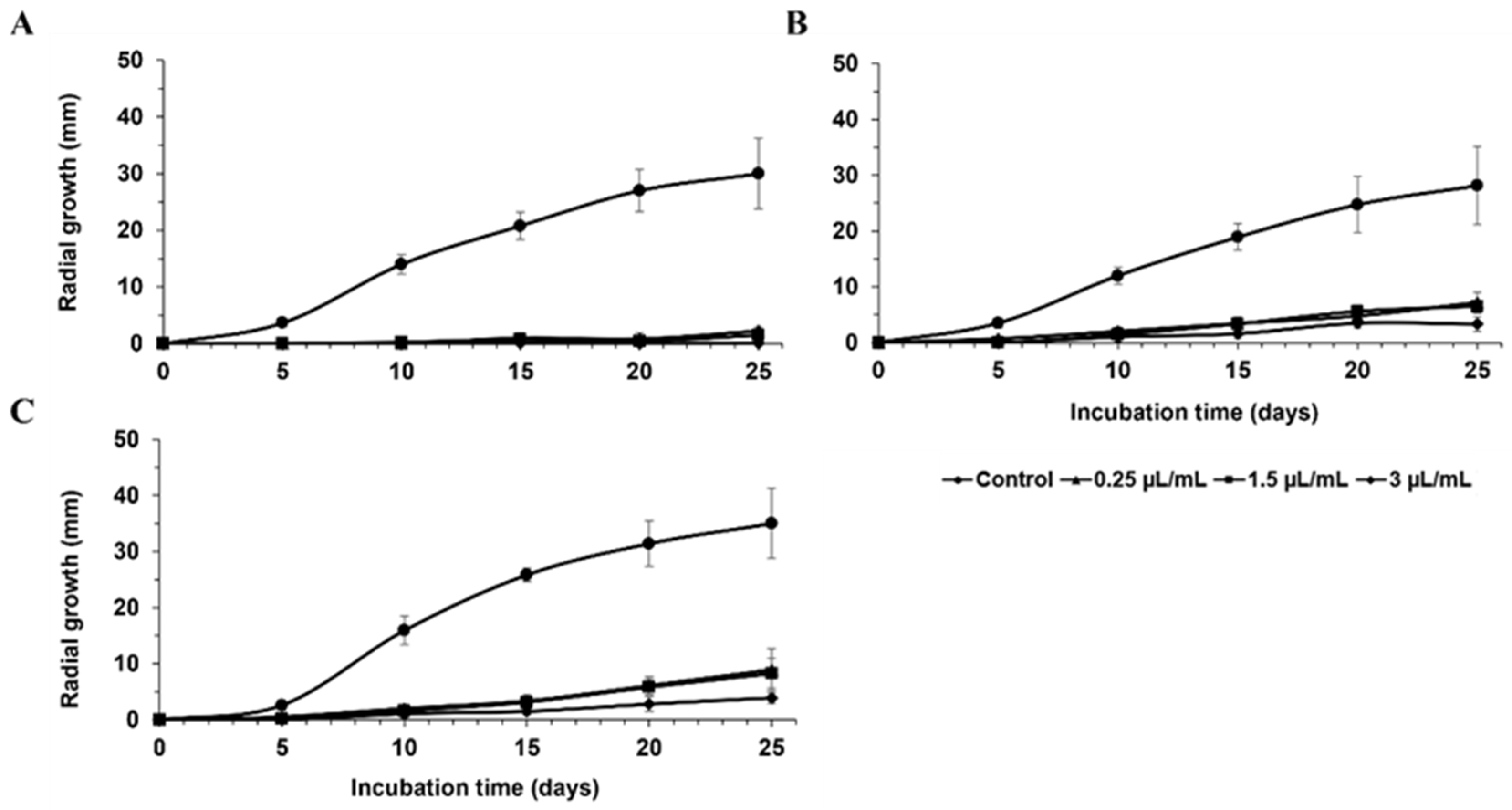
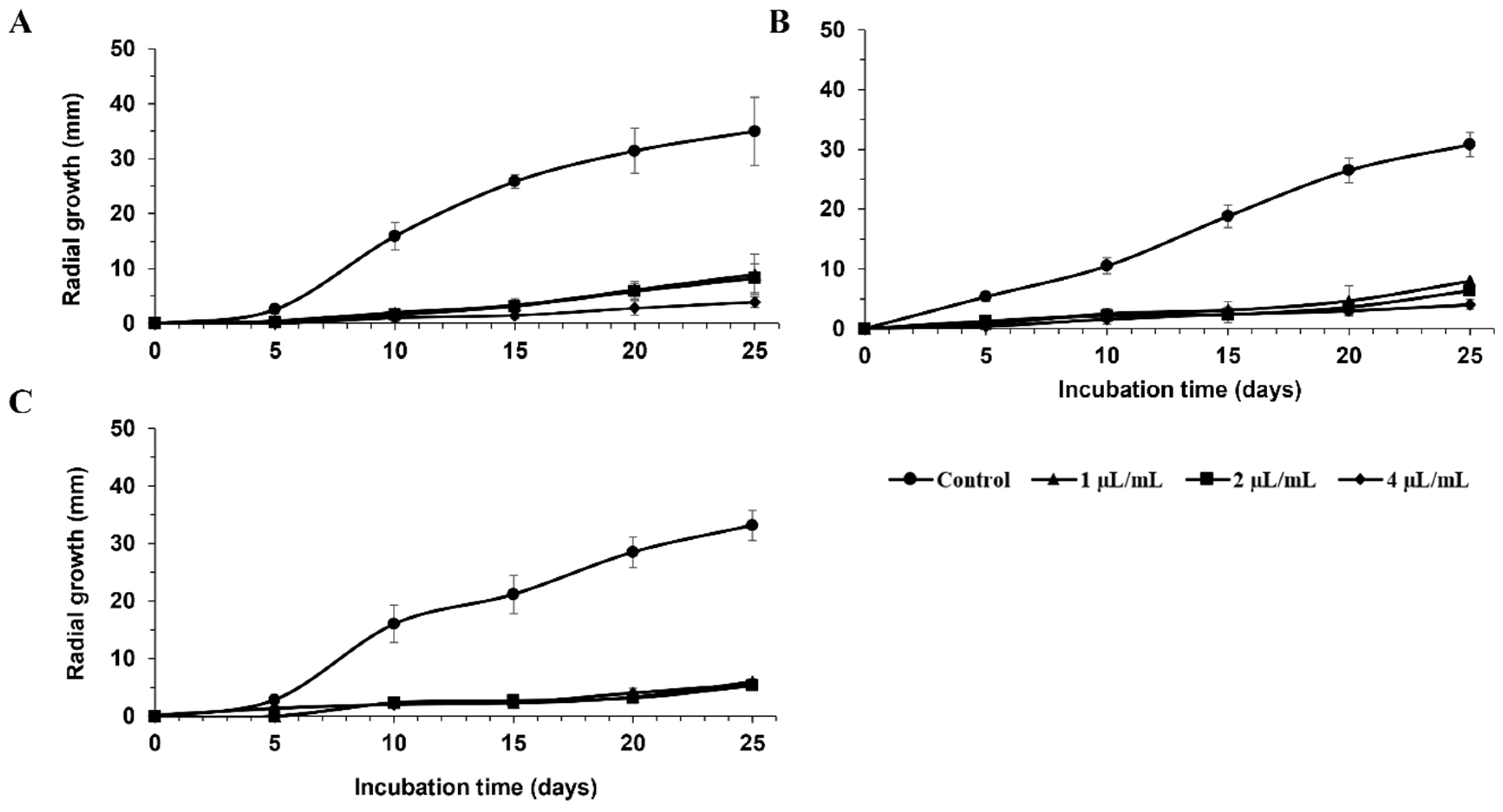
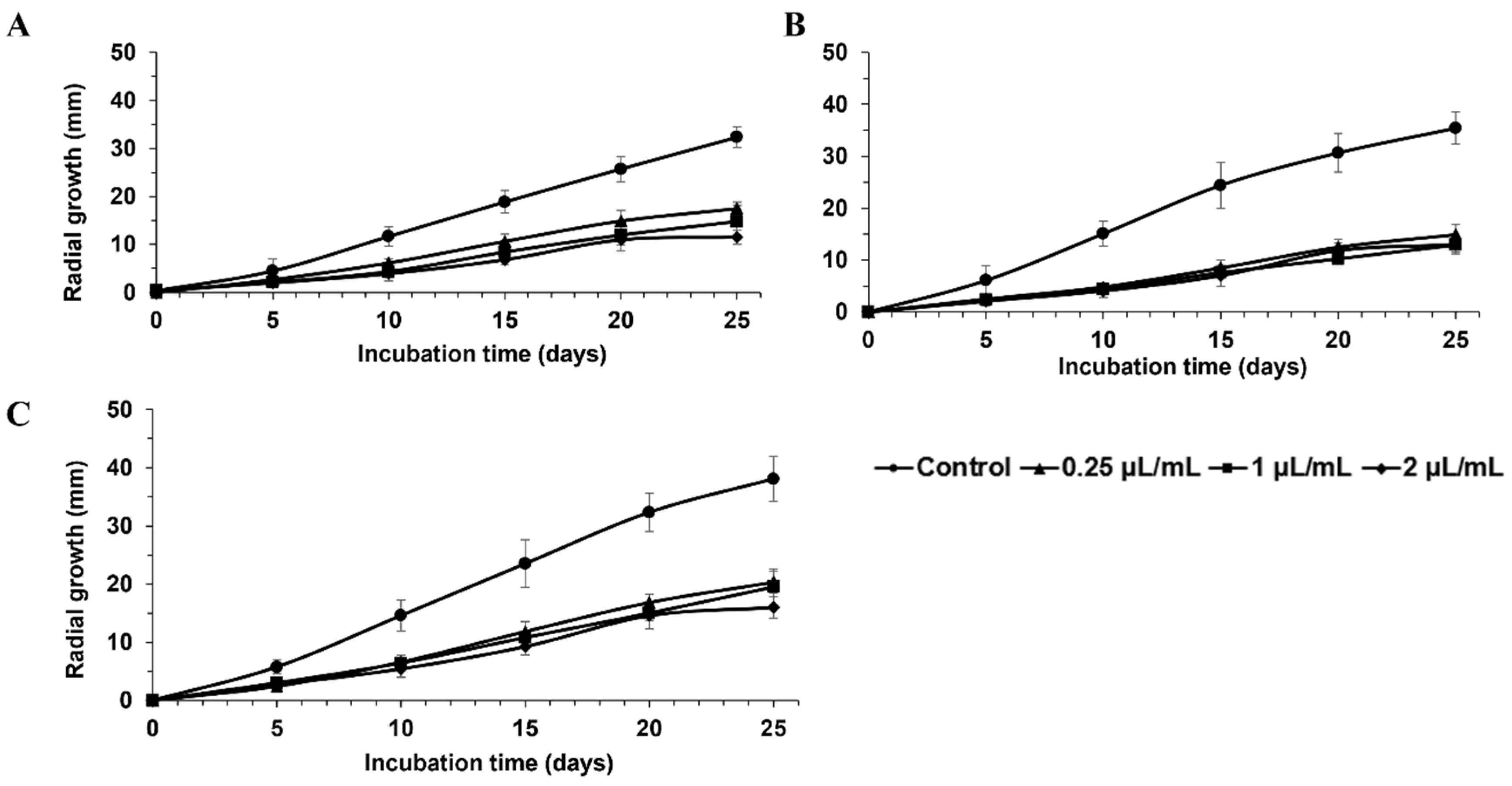
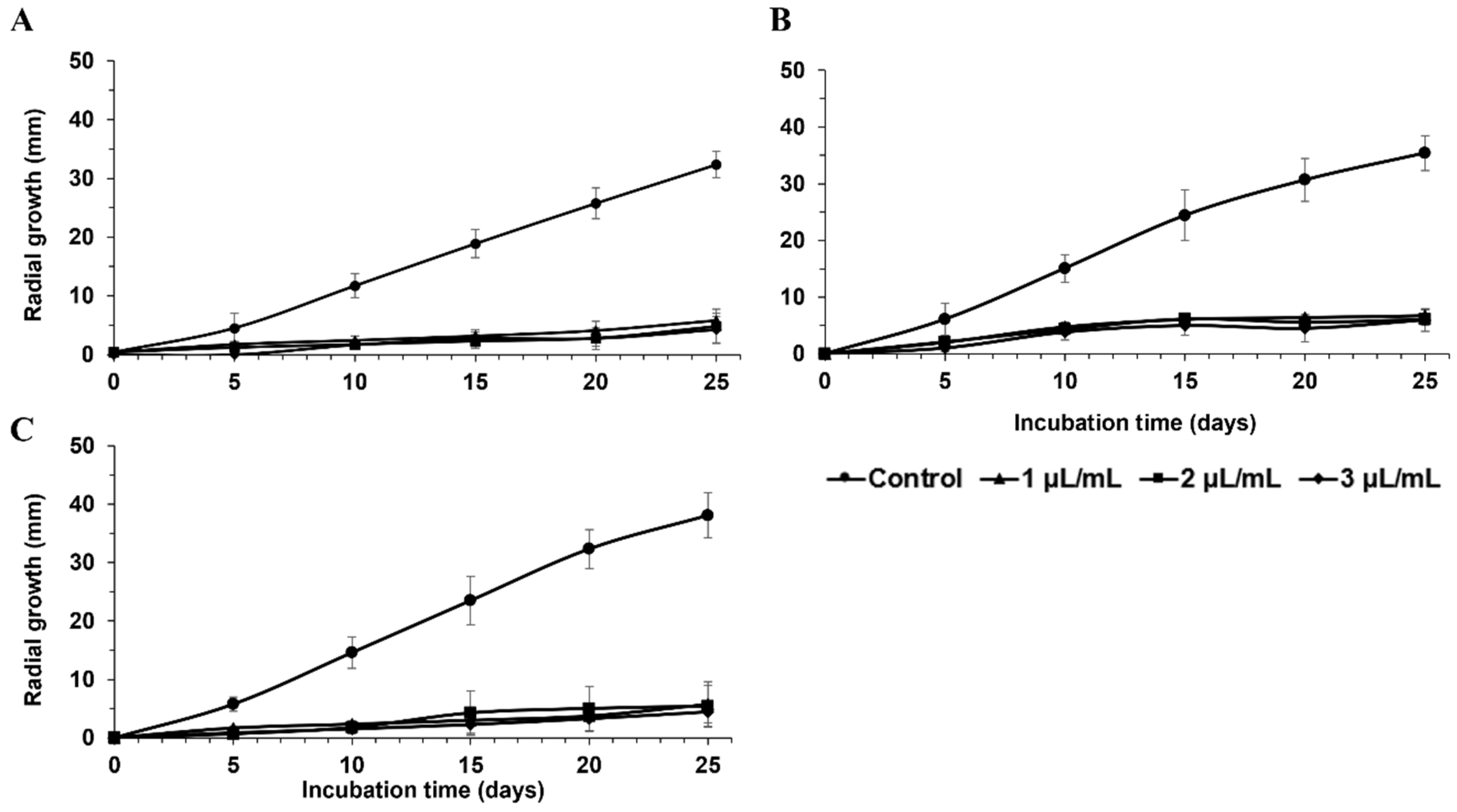
| FRAC Code a | Product | Manufacturer | Active Ingredient(s) | Concentrations Tested |
|---|---|---|---|---|
| 3 | Fullback® 125 SC | FMC of Canada Limited (Calgary, AB, Canada) | Flutriafol | 0.5 and 2 µL/mL |
| 3 | Mettle ® 125 ME | Gowan Company LLC (Yuma, AZ, USA) | Tetraconazole | 0.25 and 2 µL/mL |
| 3 | NovaTM | Corteva Agriscience Canada Company (Calgary, AB, Canada) | Myclobutanil | 0.25 and 1 mg/mL |
| 3/11 | Quadris top® | Syngenta Canada Inc. (Guelph, ON, Canada) | Difenoconazole/Azoxystrobin | 1 and 7 µL/mL |
| 7 | FontelisTM | Corteva Agriscience Canada Company | Penthiopyrad | 2 and 16 µL/mL |
| 7 | Kenja® 400SC | ISK Bioscience Corporation (Painesville, OH, USA) | Isofetamid | 1, 2, and 3 µL/mL |
| 9 | ScalaMD SC | Bayer CropScience (Calgary, AB, Canada) | Pyrimethanil | 1, 2, and 4 µL/mL |
| 11 | Evito® 480 SC | UPL AgroSolutions Canada Inc. (Guelph, ON, Canada) | Fluoxastrobin | 0.25, 1.25, and 3 µL/mL |
| 11 | Flint | Bayer CropScience | Trifloxystrobin | 0.25 and 1.5 mg/mL |
| 11 | Intuity® | Valent Canada, Inc. (Guelph, ON, Canada) | Mandestrobin | 0.5 and 9 µL/mL |
| 7/11 | MerivonMD | BASF Canada Inc. (Mississauga, ON, Canada) | Fluxapyroxad/Pyraclostrobin | 0.5 and 9 µL/mL |
| 7/11 | PristineMD WG | BASF Canada Inc. | Boscalid/ Pyraclostrobin | 1 and 4 mg/mL |
| 7/12 | Miravis® Prime | Syngenta Canada Inc. | Pydiflumetofen/Fludioxonil | 1 and 5 µL/mL |
| 9/12 | Switch® 62.5 WG | Syngenta Canada Inc. | Cyprodinil/ Fludioxonil | 1 and 5 mg/mL |
| 17 | Elevate® 50 WDG | UPL AgroSolutions Canada Inc. | Fenhexamid | 1.5, 2.5, and 3.5 mg/mL |
| 50 | Property® 300SC | ISK Bioscience Corporation | Pyriofenone | 0.25, 1, and 2 µL/mL |
| M 04 | Maestro® 80 WSP | UPL AgroSolutions Canada Inc. | Captan | 1.5 and 3.5 mg/mL |
| H. nodulosa H0 | Hirsutella sp. H94 | H. nodulosa H98 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Capitale-Nationale | Montérégie | Chaudière-Appalaches | ||||||
| Growing system | Organic | Conventional | Conventional | ||||||
| Sequence comparison | GenBank accession no. | Query cover | Per. identity | GenBank accession no. | Query cover | Per. identity | GenBank accession no. | Query cover | Per. identity |
| ITS | KJ524680.1 | 100% | 100% | KJ524690.1 | 100% | 99.64% | KM652172.1 | 100% | 99.83% |
| nrLSU | KM652117.1 | 100% | 99.9% | – | – | – | KM652117.1 | 100% | 99.9% |
| KJ524711.1 | 100% | 100% | – | – | – | KJ524711.1 | 100% | 100% | |
| tef1 | OQ979221.1 | 100% | 100% | – | – | – | KM652000.1 | 100% | 100% |
| KM652008.1 | 100% | 100% | – | – | – | KM652008.1 | 100% | 100% | |
| Percent Inhibition of Radial Growth (%) | ||||||
|---|---|---|---|---|---|---|
| Fungicide | H. nodulosa H0 | Hirsutella sp. H94 | H. nodulosa H98 | |||
| Min | Max | Min | Max | Min | Max | |
| Fullback® 125 SC | 100 ± 0 a a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| Mettle® 125 ME | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| NovaTM | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| Quadris top® | 100 ± 0 a | 100 ± 0 a | 97 ± 3 ab | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| Intuity® | 95 ± 3 ab | 99 ± 3 ab | 84 ± 5 cdef | 85 ± 3 cd | 89 ± 3 bcde | 90 ± 3 bcd |
| Miravis® Prime | 95 ± 3 ab | 98 ± 3 ab | 94 ± 6 abc | 94 ± 7 abc | 97 ± 2 ab | 100 ± 0 a |
| Evito® 480 SC | 92 ± 2 abc | 100 ± 1 a | 73 ± 11 fg | 88 ± 3 bcd | 74 ± 14 g | 89 ± 3 cde |
| Switch® 62.5 WG | 89 ± 4 bcd | 96 ± 5 abcd | 91 ± 3 abcd | 97 ± 3 ab | 95 ± 5 abc | 99 ± 2 a |
| Flint | 87 ± 2 bcd | 88 ± 7 bcdef | 73 ± 9 fg | 84 ± 1 d | 79 ± 5 efg | 91 ± 3 bcd |
| Maestro® 80 WSP | 82 ± 8 de | 86 ± 3 cdef | 83 ± 6 cdef | 85 ± 5 cd | 85 ± 3 cdef | 91 ± 3 bcd |
| PristineMD WG | 83 ± 4 de | 90 ± 6 abcde | 88 ± 1 abcd | 91 ± 5 abcd | 90 ± 1 abcd | 97 ± 3 ab |
| ScalaMD SC | 81 ± 7 de | 89 ± 3 abcde | 75 ± 8 efg | 88 ± 2 bcd | 76 ± 7 fg | 82 ± 2 e |
| MerivonMD | 81 ± 9 de | 84 ± 14 ef | 87 ± 7 bcde | 89 ± 8 bcd | 90 ± 5 abcd | 96 ± 5 abc |
| FontelisTM | 83 ± 3 cde | 84 ± 4 def | 79 ± 4 defg | 85 ± 4 cd | 84 ± 5 defg | 90 ± 2 bcd |
| Kenja® 400SC | 82 ± 4 de | 81 ± 7 ef | 81 ± 4 defg | 83 ± 3 de | 85 ± 9 cdef | 86 ± 9 de |
| Elevate® 50 WDG | 74 ± 4 e | 77 ± 7 f | 70 ± 10 g | 73 ± 11 e | 82 ± 5 defg | 83 ± 4 e |
| Property® 300SC | 47 ± 3 f | 65 ± 6 g | 58 ± 7 h | 63 ± 6 f | 46 ± 7 h | 58 ± 4 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duclos, A.; Delisle-Houde, M.; Moisan-De Serres, J.; Tellier, S.; Fournier, V.; Tweddell, R.J. Effect of Synthetic Fungicides Used in Conventional Strawberry Growing System on Hirsutella sp., an Entomopathogenic Fungus of Cyclamen Mite. Agriculture 2025, 15, 715. https://doi.org/10.3390/agriculture15070715
Duclos A, Delisle-Houde M, Moisan-De Serres J, Tellier S, Fournier V, Tweddell RJ. Effect of Synthetic Fungicides Used in Conventional Strawberry Growing System on Hirsutella sp., an Entomopathogenic Fungus of Cyclamen Mite. Agriculture. 2025; 15(7):715. https://doi.org/10.3390/agriculture15070715
Chicago/Turabian StyleDuclos, Andréa, Maxime Delisle-Houde, Joseph Moisan-De Serres, Stéphanie Tellier, Valérie Fournier, and Russell J. Tweddell. 2025. "Effect of Synthetic Fungicides Used in Conventional Strawberry Growing System on Hirsutella sp., an Entomopathogenic Fungus of Cyclamen Mite" Agriculture 15, no. 7: 715. https://doi.org/10.3390/agriculture15070715
APA StyleDuclos, A., Delisle-Houde, M., Moisan-De Serres, J., Tellier, S., Fournier, V., & Tweddell, R. J. (2025). Effect of Synthetic Fungicides Used in Conventional Strawberry Growing System on Hirsutella sp., an Entomopathogenic Fungus of Cyclamen Mite. Agriculture, 15(7), 715. https://doi.org/10.3390/agriculture15070715





