Sustainability Indicators of the Banana and Lemongrass Intercropping System in Different Harvest Seasons: Growth, Yield, Seasonality and Essential Oil Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
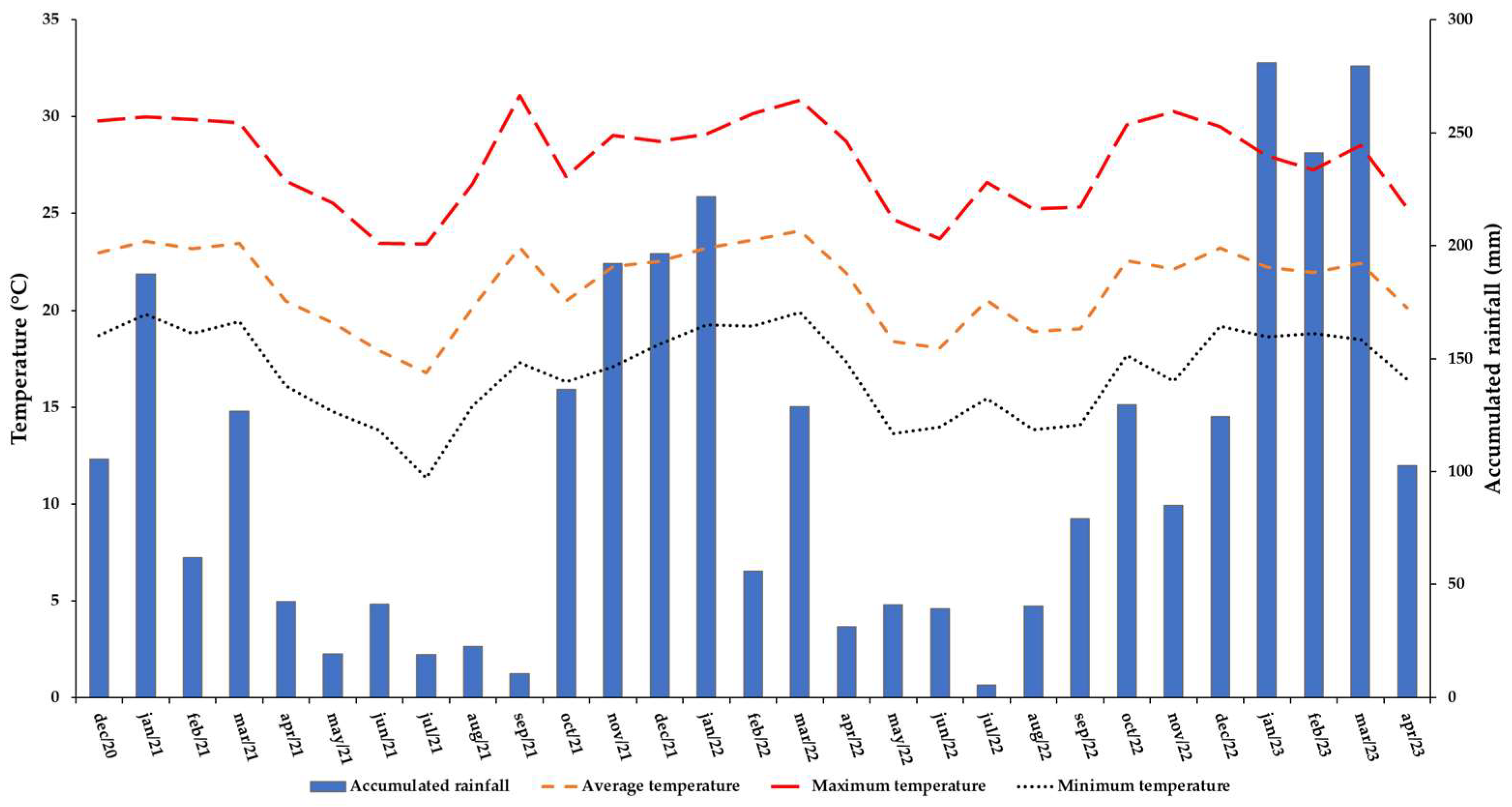
2.2. Experimental Site Management
2.2.1. Banana Growing
2.2.2. Lemongrass Growing
2.3. Cropping Systems and Harvesting Seasons
2.4. Experimental Design
2.5. Harvesting
2.6. Growth and Yield Assessments
2.7. Determination of Land Equivalent Ratio
2.8. Lemongrass Essential Oil Extraction
2.9. Lemongrass Essential Oil Content and Yield
2.10. Essential Oil Analysis by Gas Chromatography Coupled with Mass Spectrometry (GC and GC-MS)
2.11. Statistical Analysis
3. Results
3.1. Banana Growth and Yield
3.2. First Growing Season of Lemongrass
3.3. Second Growing Season of Lemongrass
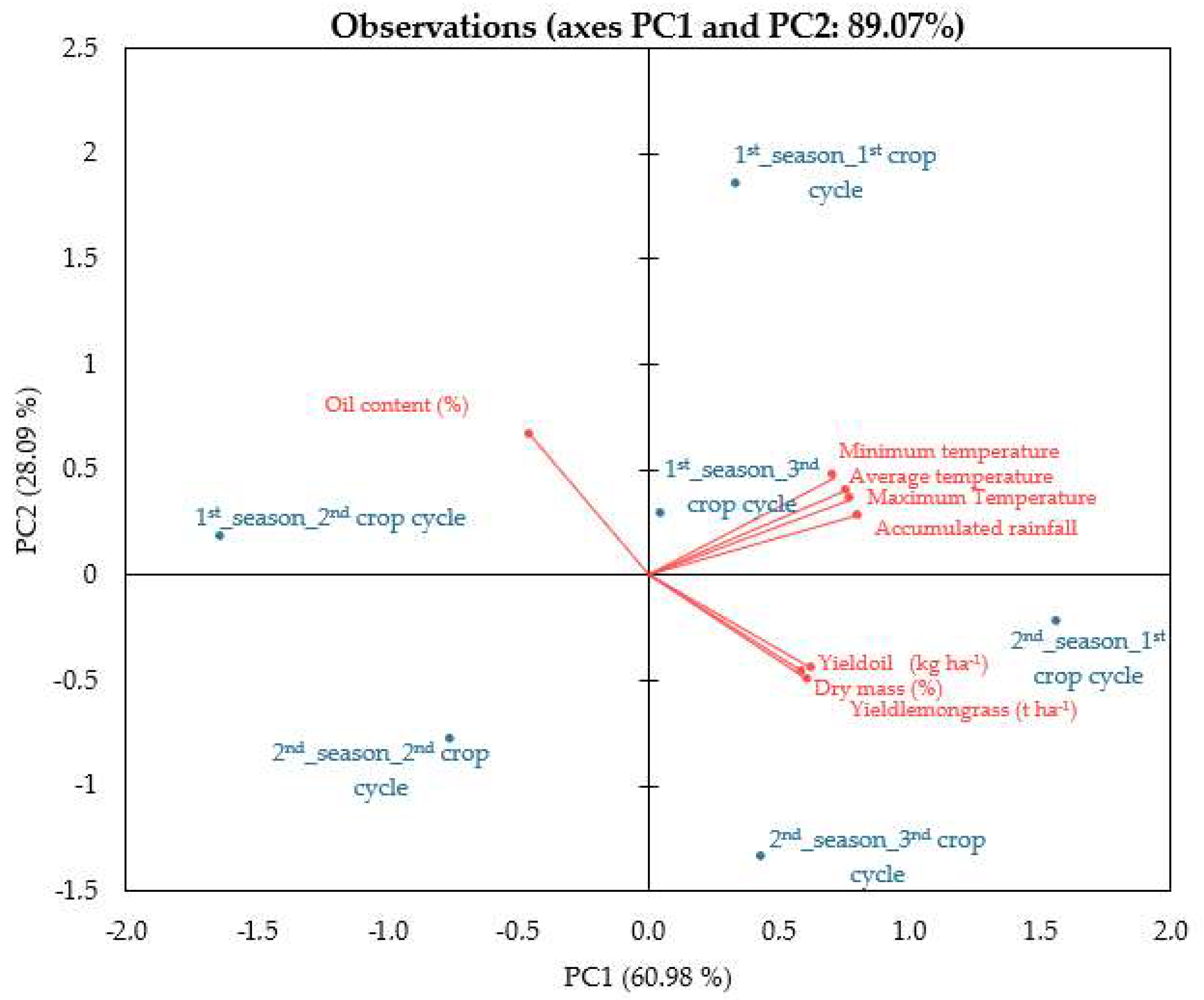
3.4. Principal Component Analysis
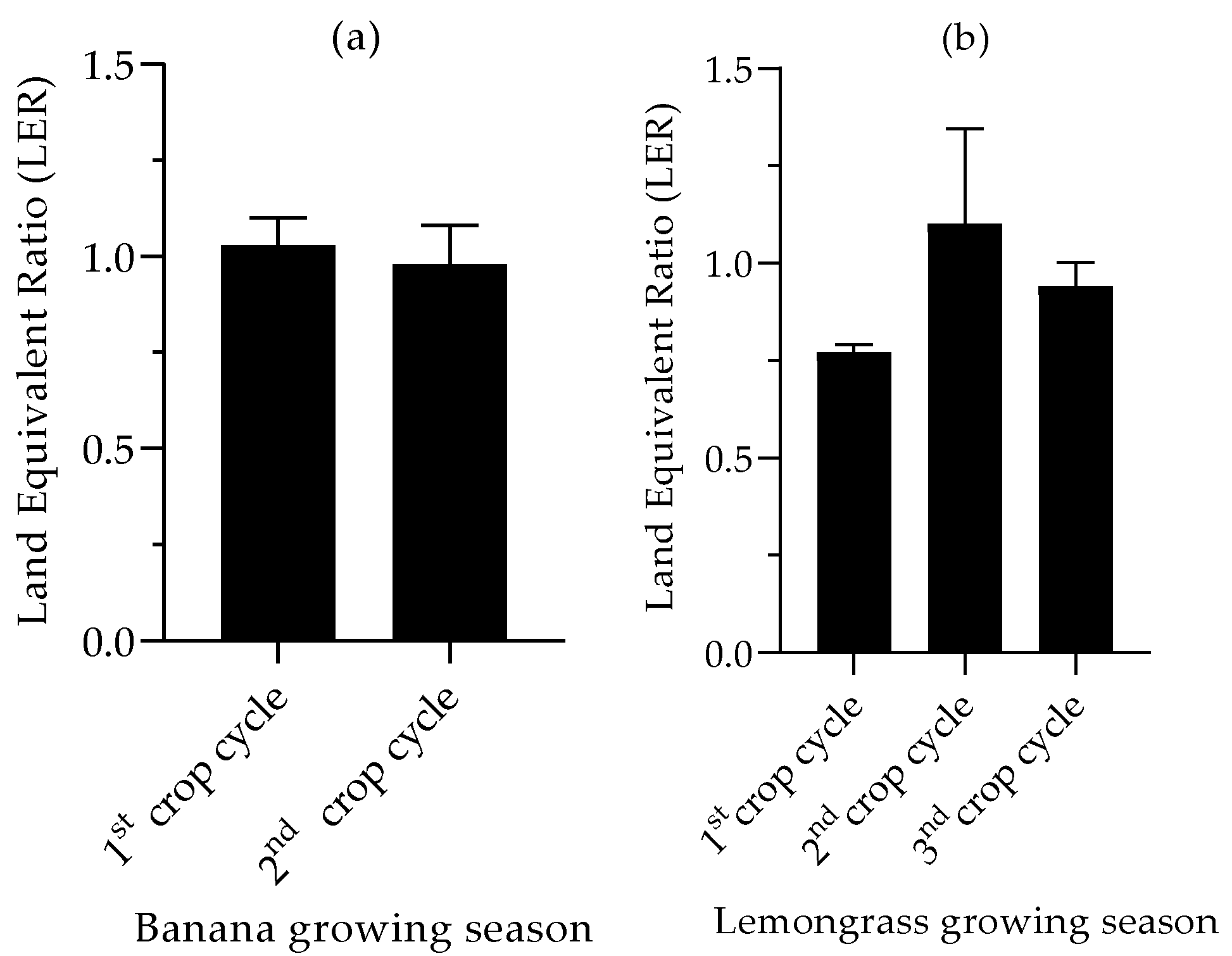
3.5. Land Equivalent Ratio
3.6. Chemical Composition of Essential Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yogendra, N.D.; Keerhi, P.E.; Nazeer, M.; Jnanesha, A.C.; Verma, R.S.; Sundaresan, V. Livelihood Enhancement and Resource Use Efficiency under Lemongrass with Food Crops. Acta Ecol. Sin. 2023, 44, 266–274. [Google Scholar] [CrossRef]
- Singh, M.; Shivaraj, B. Intercropping studies in lemongrass (Cymbopogon flexuosus) (Steud. Wats.). Agron. Crop Sci. 1998, 190, 23–26. [Google Scholar] [CrossRef]
- Carvalho Neta, R.N.F.; Sousa, D.B.P.; Barros, M.F.S.; Nunes, K.B.; Torres, H.S.; Assis, E.B.V.; Farias, L.F.; Turri, R.J.G. Usos potenciais de óleos essenciais em remediação ambiental: Uma revisão. Res. Soc. Dev. 2021, 10, e3210716146. [Google Scholar] [CrossRef]
- Majewska, E.; Kozowska, M.; Skakowska, E.G.; Kowalska, D.; Tarnowski, K. Lemongrass (Cymbopogon citratus) Essential Oil: Extraction, Composition, Bioactivity and Uses for Food Preservation—A Review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Oliveira, C.C.A.; Santos, J.S. Active compounds of lemon grass (Cymbopogon citratus): A review. Res. Soc. Dev. 2021, 10, e263101220281. [Google Scholar] [CrossRef]
- Kamaruddin, Z.H.; Jumaidin, R.; Selamat, M.Z.; Ilya, R.A. Characteristics and Properties of Lemongrass (Cymbopogon Citratus): A Comprehensive Review. J. Nat. Fibers 2021, 19, 8101–8118. [Google Scholar] [CrossRef]
- Pinto, D.A.; Mantovani, E.C.; Melo, E.C.; Sediyama, G.C.; Vieira, G.H.S. Produtividade e qualidade do óleo essencial de capim limão, Cymbopogon citratus, DC., submetido a diferentes lâminas de irrigação. Rev. Bras. Plantas Med. 2014, 16, 54–61. [Google Scholar] [CrossRef]
- Bakshi, P.; Bhushan, A.; Bali, K.; Kour, K. Intercropping in Fruit Orchards: A Way Forward for Doubling the Farmer’s Income. Int. J. Agric. Sci. 2019, 11, 9274–9276. [Google Scholar]
- Almeida, U.O.; Andrade Neto, R.C.; Lunz, A.M.P.; Cades, M.; Costa, D.A.; Araújo, J.M.; Teixeira Júnior, D.L.; Rodrigues, M.J.S. Production of Banana, Cultivar D’Angola, Intercropped with Açai Single in Different Planting Arrangements. RBAS 2019, 9, 80–89. [Google Scholar]
- Ntamwira, J.; Ocimati, W.; Kearsley, E.; Safari, N.; Bahati, L.; Amini, D.; Lubobo, A.K.; Waswa, B.; Blomme, G. The Integration of Shade-Sensitive Annual Crops in Musa spp. Plantations in South Kivu, Democratic Republic of Congo. Agronomy 2021, 11, 368. [Google Scholar] [CrossRef]
- Alves, E.P.; Silva, M.L.; Oliveira Neto, S.N.; Barrela, T.P.; Santos, R.H.S. Economic Analysis of a Coffee-Banana System of a Family-Based Agriculture at the Atlantic Forest Zone, Brazil. Cienc. Agrotec. 2015, 39, 232–239. [Google Scholar] [CrossRef]
- Rodrigues de Jesus, P.R.; Leonel, S.; Leonel, M.; Cândido, H.T.; Tecchio, M.A. Variability, sustainability and productivity of banana and lemongrass intercropping based on yield components and competitive indices. Scientia Hortic. 2025, 340, 113946. [Google Scholar] [CrossRef]
- Ferreira, T.M.C.; Vasconcelos, M.; Cantão, B.P.; Silva, J.L.; Aguiar, W.K. Land Use Based on Agroforestry System: A Study at São Domingos do Capim County, Pará. Rev. Ciênc. Agroamb. 2016, 14, 93–99. [Google Scholar]
- Almeida, G.M.; Rodrigues, J.G.L. Desenvolvimento de plantas através da interferência de auxinas, citocininas, etileno e giberelinas. Braz. J. Appl. Technol. Agric. Sci. 2016, 9, 111–117. [Google Scholar] [CrossRef]
- Napoleão, G.M.; Rodrigues de Jesus, P.R.; Leonel, S. Cultivar Diversification of Banana Production in Brazil, 2021. Agron. Sci. Biotechnol. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Damatto Júnior, E.R.; Campos, A.J.; Manoel, L.; Moreira, G.C.; Leonel, S.; Evangelista, R.M. Produção e caracterização de frutos de bananeiras ‘Prata-Anã’ e ‘Prata-Zulu’. Rev. Bras. Frutic. 2005, 127, 440–443. [Google Scholar] [CrossRef]
- Oliveira, S.G.; Bonfim, F.P.G.; Alves, L.F.; Marques, I.B.; Araújo, E.O. Alelopatia de capim-cidreira na germinação, vigor de sementes e no desenvolvimento inicial do tomate-cereja. Cad. Ciênc. Agrar. 2018, 10, 7–12. [Google Scholar]
- Ashishi, K.; Jnasnesha, R.K.; Lal, D.; Dubey, B.K. Intercropping and mixed herb distillation for high-quality oil yield using lemon-scented basil (Ocimum africanum Lur.) cv. CIM-Jyoti and lemongrass (Cymbopogon flexuosus) (Nees ex Steud.) cv. Krishna. Acta Ecol. Sin. 2022, 42, 269–273. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Zhang, S.; Miao, Y.; Zhang, Y.; Li, Z.; Zhao, J.; Zhang, J.; Qin, X.; Yao, Y. Plant Interaction Patterns Shape of Microbial Community and Nutrient Cycling in Different Intercropping Scenarios of Aromatic Plant Species. Front. Microbiol. 2022, 13, 888789. [Google Scholar] [CrossRef]
- Leonel, S.; Leonel, M.; Jesus, P.R.R.d.; Tecchio, M.A.; Silva, M.d.S.; Cândido, H.T.; Molha, N.Z.; Ouros, L.F.d. Achievements of Banana (Musa sp.)-Based Intercropping Systems in Improving Crop Sustainability. Horticulturae 2024, 10, 956. [Google Scholar] [CrossRef]
- Rodrigues de Jesus, P.R.; Leonel, S.; Leonel, M.; Cândido, H.T.; Molha, N.Z.; Domiciano, V.M.; Ouros, L.F.; Tecchio, M.A. Performance and Leaf Nutritional Content of Banana Cultivars Intercropped with Lemongrass. Rev. Caatinga 2024, 37, e12448. [Google Scholar] [CrossRef]
- Cunha, A.R.; Martins, D. Classificação climática para os municípios de Botucatu e São Manuel, SP. Irriga 2009, 14, 1–11. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.d.; Oliveira, V.A.d.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.d.; Araújo Filho, J.C.d.; Oliveira, J.B.d.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa: Brasília, Brazil, 2013; 353p. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Nomura, E.S.; Damatto Júnior, E.R.; Kobori, R.T.; Penteado, L.A.C. Tratos culturais. In Cultivo de Bananeira, 1st ed.; Nomura, E.S., Damatto Júnior, E.R., Eds.; Manual Técnico CDRS; Graça D’Auria: Campinas, Brazil, 2020; Volume 1, pp. 49–71. [Google Scholar]
- Oliveira, H.S.; Lemos, O.F.; Miranda, V.S.; Moura, H.C.P.; Campelo, M.F.; Santos, L.R.R. Estabelecimento e multiplicação in vitro de brotos no processo de micropropagação de cultivares de bananeira (Musa spp.). Acta Amazon. 2011, 41, 369–376. [Google Scholar] [CrossRef]
- Nomura, E.S.; Damatto Júnior, E.R.; Mendonça, J.C.; Kobori, R.T.; Penteado, L.A.C. Calagem e adubação. In Cultivo de Bananeira, 1st ed.; Nomura, E.S., Damatto Júnior, E.R., Eds.; Manual Técnico CDRS; Graça D’Auria: Campinas, Brazil, 2020; Volume 1, pp. 73–88. [Google Scholar]
- Carvalho, L.M.d. Orientações Técnicas Para o Cultivo de Plantas Medicinais, Aromáticas e Condimentares; Circular Técnica 70; Embrapa Tabuleiros Costeiros: Aracaju, Brazil, 2015; 11p. [Google Scholar]
- May, A.; Bovi, O.A.; Maia, N.B.; Moraes, A.R.A.; Pinheiro, M.Q.; Mario, M. Influência do intervalo entre cortes sobre produção de biomassa de duas espécies de capim limão. Hortic. Bras. 2008, 26, 379–382. [Google Scholar] [CrossRef]
- Teixeira, L.A.J.; Nomura, E.S.; Damatto-Junior, E.R.; Fuzitani, E.J. Banana. In Instruções Agrícolas Para as Principais Culturas Econômicas; Aguiar, A.T.E., Gonçalves, C., Paterniani, M.E.A.G., Tucci, M.G.S., Castro, C.E.F., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 2014; pp. 46–51. [Google Scholar]
- Bolfarini, A.C.; Putti, F.; Azevedo, J.M.; Silva, M.; Ferreira, R.B.; Leonel, M.; Tecchio, M.A.; Leonel, S. Application of phosphate fertilization on banana hybrid ‘FHIA 18’ and its impact on production performance. Aust. J. Crop Sci. 2020, 14, 744–750. [Google Scholar] [CrossRef]
- Facanali, R.; Marques, M.O.M.; Hantao, L.W. Metabolic Profiling of Varronia curassavica Jacq. Terpenoids by Flow Modulated Two-Dimensional Gas Chromatography Coupled to Mass Spectrometry. Separations 2020, 7, 18. [Google Scholar] [CrossRef]
- Gimenes, L.; Silva, J.C.R.L.; Facanali, R.; Hantao, L.W.; Siqueira, W.J.; Marques, M.O.M. Essential Oils of New Lippia alba Genotypes Analyzed by Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography (GC × GC) and Chemometric Analysis. Molecules 2021, 26, 2332. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; 809p, Available online: https://diabloanalytical.com/ms/essential-oil-components-by-gcms/essential_oil_components_ebook.pdf (accessed on 10 July 2023).
- Van Den Dool, H.; Kratz, D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 10 July 2023).
- Van Asten, P.J.A.; Wairegi, L.W.I.; Musaka, D.; Uringi, N.O. Agronomic and Economic Benefits of Coffee-Banana Intercropping in Uganda’s Smallholder Farming Systems. Agric. Syst. 2011, 104, 326–334. [Google Scholar] [CrossRef]
- Huss, C.; Holmes, K.D.; Blubaugh, C. Benefits and Risks of Intercropping for Crop Resilience and Pest Management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Mazzafera, P.; Favarin, J.L.; Andrade, S.A.L. Editorial: Intercropping Systems in Sustainable Agriculture. Front. Sustain. Food Syst. 2021, 5, 634361. [Google Scholar] [CrossRef]
- Maitra, A.; Hossain, A.; Brestic, M.; Skalicky, P.; Gitari, H. Intercropping: A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Eskandari, H. A General Overview on Intercropping and Its Advantage in Sustainable Agriculture. J. Appl. Environ. Biol. Sci. 2011, 1, 482–486. [Google Scholar]
- Cassidy, E.S.; West, P.C.; Gerber, J.S.; Foley, J.A. Redefining Agricultural Yields: From Tonnes to People Nourished per Hectare. Environ. Res. Lett. 2013, 8, 034015. [Google Scholar] [CrossRef]
- Kaliz, B.; Zuk-Golaszewska, K.; Radawiec, W.; Golaszewski, J. Land Use Indicators in the Context of Land Use Efficiency. Sustainability 2023, 15, 1106. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Research of United Nations. FAOSTAT: Food and Agriculture Data. 2023. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 December 2023).
- Thakur, N.S.; Mohanty, S.; Gunaga, R.P.; Gajbhiye, N.A. Melia dubia Cav. Spatial Geometries Influence the Growth, Yield and Essential Oil Principles Content of Cymbopogon flexuosus (Nees Ex Steud.) W.Watson. Agroforest Syst. 2020, 94, 985–995. [Google Scholar] [CrossRef]
- Mwithiga, G.; Maina, S.; Mutuvi, P.; Gitari, J. Lemongrass (Cymbopogon flexuosus) Growth Rate, Essential Oil Yield and Composition as Influenced by Different Soil Conditioners Under Two Watering Regimes. Heliyon 2024, 10, e25540. [Google Scholar] [CrossRef]
- Yeshitila, W.S. Herbage and Essential Oil Yield of Two Lemongrass (Cymbopogon citratus) Varieties as Affected by Harvesting Cycle and Number of Tillers per Planting Slips at Wondo Genet, Southern Ethiopia. Int. J. Res. Stud. Agric. Sci. 2019, 5, 18–22. [Google Scholar] [CrossRef]
- Kishore, K.; Rupa, T.R.; Samant, D. Influence of Shade Intensity on Growth, Biomass Allocation, Yield and Quality of Pineapple in Mango-Based Intercropping System. Sci. Hortic. 2021, 278, 109868. [Google Scholar] [CrossRef]
- Ocimati, W.; Groot, J.C.J.; Blomme, G.; Timler, C.J.; Remans, R.; Taulya, G.; Ntamwira, J.; Tittonell, P. A Multi-Objective Model Exploration of Banana-Canopy Management and Nutrient Input Scenarios for Optimal Banana-Legume Intercrop Performance. Agronomy 2021, 11, 311. [Google Scholar] [CrossRef]
- Kumar, M.; Krishna, A.K.; Kumar, S.; Lal, R.K. Influence of Lemongrass (Cymbopogon flexuosus (Nees ex Steud.)) Essential Oil Yield Under Intercropping with Pomegranate (Punica granatum L.) with Special Reference to the Plant-Soil Relationship. J. Med. Plants Stud. 2021, 9, 219–225. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Bonilla, O.H.; Lucena, E.M.P. Influência da Sazonalidade e do Ciclo Circadiano no Rendimento e Composição Química dos Óleos Essenciais de Croton spp. da Caatinga. Iheringia Série Botânica 2018, 73, 31–38. [Google Scholar] [CrossRef]
- Madi, Y.F.; Choucry, M.A.; Meselhy, M.R.; El-Kashoury, E.A. Essential Oil of Cymbopogon citratus Cultivated in Egypt: Seasonal Variation in Chemical Composition and Anticholinesterase Activity. Nat. Prod. Res. 2020, 35, 4063–4067. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.K.N.; Nguyen, D.C.; Phu, T.N.N.; Ho, V.T.T.; Vo Nguyen, D.V.; Bach, L.G.; Nguyen, T.D. Research on Lemongrass Oil Extraction Technology (Hydrodistillation, Microwave-Assisted Hydrodistillation). Indones. J. Chem. 2019, 19, 1000–1007. [Google Scholar] [CrossRef]
- Silva, T.L.M.; Rosa, G.I.; Santos, M.A.L.; Graf, S.L.; Maia, B.H.L.N.; Beltrame, F.L.; Ferrari, P.C. Lemongrass Essential Oil (Cymbopogon citratus (DC) Stapf.) Seasonal Evaluation and Microencapsulation by Spray-Drying. Braz. Arch. Biol. Technol. Curitiba 2023, 66, e23230016. [Google Scholar] [CrossRef]
- Brasil. Farmacopeia Brasileira, 6th ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2019. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/farmacopeia-brasileira/6a-edicao-volume-2 (accessed on 10 December 2023).
- Bayala, B.; Bassole, I.H.N.; Maqdasy, S.; Baron, S.; Simpore, J.; Lobaccaro, J.M.A. Cymbopogon citratus and Cymbopogon giganteus Essential Oils Have Cytotoxic Effects on Tumor Cell Cultures. Identification of Citral as a New Putative Antiproliferative Molecule. Biochimie 2018, 153, 162–170. [Google Scholar] [CrossRef]
- Zielińska, A.; Martins-Gomes, C.; Ferreira, N.R.; Silva, A.M.; Nowak, I.; Souto, E.B. Anti-inflammatory and Anti-cancer Activity of Citral: Optimization of Citral-loaded Solid Lipid Nanoparticles (SLN) Using Experimental Factorial Design and LUMiSizer®. Int. J. Pharm. 2018, 553, 428–440. [Google Scholar] [CrossRef]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical Properties and Therapeutic Potential of Citral, a Monoterpene Isolated from Lemongrass. Med. Chem. 2021, 17, 2–12. [Google Scholar] [CrossRef]
- Radünz, A.L.; Radünz, M.; Bizollo, A.R.; Tramontin, M.A.; Radünz, L.L.; Mariot, M.P.; Tempel-Stumpf, E.R.; Calisto, J.F.F.; Zaniol, F.; Albeny-Simões, D.; et al. Insecticidal and Repellent Activity of Native and Exotic Lemongrass on Maize Weevil. Braz. J. Biol. 2022, 84, e252990. [Google Scholar]

| Chemical Soil Properties | Planting | Sole Crop | Intercropping |
|---|---|---|---|
| pH (1:2.5 soil/CaCl2 suspension 0.01 mol L−1) | 5.6 | 4.9 | 5.3 |
| Soil organic matter (g kg−1) | 11.8 | 13.9 | 16.1 |
| Presin (mg kg−1) | 9.2 | 15.0 | 13.2 |
| S (mg kg−1) | 5.4 | 9.3 | 8.9 |
| H + Al (mmolc dm−3) | 15.1 | 17.2 | 17.3 |
| K (mmolc dm−3) | 1.3 | 1.6 | 1.8 |
| Ca (mmolc dm−3) | 16.4 | 19.7 | 21.6 |
| Mg (mmolc dm−3) | 6.0 | 6.9 | 7.1 |
| Sun of bases (mmolc dm−3) | 23.7 | 28.2 | 30.5 |
| Cation exchange capacity (mmolc dm−3) | 38.0 | 42.0 | 49.0 |
| Base saturation (%) | 61.0 | 65.0 | 69.0 |
| Fe (mg kg−1) | 32.0 | 20.0 | 25.0 |
| Cu (mg lg−1) | 2.4 | 2.2 | 2.8 |
| B (mg kg−1) | 0.3 | 0.7 | 0.9 |
| Mn (mg kg−1) | 8.6 | 8.4 | 9.3 |
| Zn (mg kg−1) | 2.3 | 4.1 | 4.7 |
| Cropping System | Plant Height (m) | Pseudostem Diameter (cm) | Number of Leaves | Yield (kg−1 Plant−1 Year−1) | Cumulative Yield (kg Plant−1) |
|---|---|---|---|---|---|
| Intercropping | 2.1 ± 0.30 a | 17.0 ± 1.36 a | 7.7 ± 1.25 a | 17.0 ± 5.71 a | 34.1 ± 1.10 a |
| Sole crop | 2.0 ± 0.18 b | 16.9 ± 1.12 a | 7.8 ± 0.96 a | 17.2 ± 6.27 a | 34.4 ± 1.30 a |
| Overall mean | 2.1 | 17.0 | 7.8 | 17.1 | 34.3 |
| CV (%) | 3.3 | 5.3 | 6.2 | 6.8 | 4.8 |
| 1st crop cycle | 1.9 ± 0.13 b | 16.0 ± 0.60 b | 7.0 ± 0.54 b | 11.6 ± 0.64 b | - |
| 2nd crop cycle | 2.3 ± 0.21 a | 17.9 ± 0.75 a | 8.6 ± 0.83 a | 22.7 ± 1.18 a | - |
| Overall mean | 2.1 | 16.9 | 7.8 | 17.2 | - |
| CV (%) | 10.7 | 3.0 | 7.3 | 6.5 | - |
| Crop Cycles | Yieldlemongrass (t ha−1) | Dry Mass (%) | Oil Content (%) | Yieldoil (kg ha−1) |
|---|---|---|---|---|
| 1st crop cycle | 0.66 ± 0.05 b | 32.00 ± 0.54 a | 1.37 ± 0.18 a | 3.08 ± 0.54 c |
| 2nd crop cycle | 1.27 ± 0.31 b | 27.00 ± 0.91 b | 1.47 ± 0.14 a | 5.18 ± 1.47 b |
| 3rd crop cycle | 8.08 ± 0.99 a | 31.00 ± 1.45 a | 1.10 ± 0.02 b | 15.2 ± 2.48 a |
| Source of variation | p-value | |||
| Cropping system (CS) | 0.856 ns | 0.055 ns | 0.562 ns | 0.606 ns |
| Crop cycle (CC) | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| CS X CC | 0.951 ns | 0.085 ns | 0.152 ns | 0.257 ns |
| CVCS (%) | 15.5 | 5.5 | 11.1 | 5.81 |
| CVCC (%) | 18.9 | 8.2 | 9.3 | 9.2 |
| Productive Indicators | Cropping System | Crop Cycles | Average Cropping System | ||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||
| Yieldlemongrass (t ha−1) | Intercropped | 10.0 ± 0.36 b | 1.79 ± 0.24 a | 12.7 ± 0.98 b | 8.13 b |
| Sole crop | 15.8 ± 2.13 a | 1.71 ± 0.30 a | 14.9 ± 1.81 a | 10.80 a | |
| Average crop cycles | 12.90 b | 1.75 c | 13.80 a | * | |
| Yieldoil (kg ha−1) | Intercropped | 23.0 ± 1.08 b | 3.25 ± 0.98 a | 24.0 ± 0.55 b | 16.75 b |
| Sole crop | 30.5 ± 0.91 a | 2.16 ± 0.65 a | 27.2 ± 0.99 a | 19.95 a | |
| Average crop cycles | 26.75 a | 2.71 b | 25.60 a | ** | |
| Essential oil content (%) | Intercropped | 0.62 ± 0.02 a | 0.70 ± 0.04 a | 0.58 ± 0.04 a | 0.63 a |
| Sole crop | 0.56 ± 0.03 a | 0.52 ± 0.09 b | 0.63 ± 0.04 a | 0.57 b | |
| Average crop cycles | 0.59 a | 0.61 a | 0.61 a | * | |
| N° | Substances | 1st Crop Cycle | 2nd Crop Cycle | 3rd Crop Cycle | |||||
|---|---|---|---|---|---|---|---|---|---|
| IRLCal. | IRLlit. | Mon. | Interc. | Mon. | Interc. | Mon. | Interc. | ||
| 1 | 6-metil-5-hepten-2-ona | 993 | 981 | 0.25 | 0.25 | 0.23 | 0.28 | 0.46 | 0.41 |
| 2 | n-Octanal | 1011 | 998 | 0.05 | 0.05 | 0.06 | 0.08 | 0.10 | 0.08 |
| 2 | o-Cymene | 1031 | 1022 | 0.10 | 0.15 | tr | tr | tr | Tr |
| 3 | Limonene | 1034 | 1024 | 0.24 | 0.28 | 0.35 | 0.40 | 0.49 | 0.49 |
| 4 | (Z)-β-Ocimene | 1038 | 1032 | 0.05 | tr | tr | tr | tr | tr |
| 5 | n.i. | 1058 | tr | tr | 0.03 | 0.07 | 0.07 | 0.08 | |
| 6 | 4-Nonanone | 1077 | 1078 * | 0.27 | 0.26 | 0.30 | 0.39 | 0.34 | 0.36 |
| 7 | Linalool | 1106 | 1095 | 0.41 | 0.39 | 0.45 | 0.49 | 0.58 | 0.54 |
| 8 | n.i. | 1117 | 0.09 | tr | 0.03 | 0.07 | 0.06 | 0.06 | |
| 9 | exo-Isocitral | 1151 | 1140 | 0.10 | 0.10 | 0.16 | 0.16 | 0.16 | 0.16 |
| 10 | n.i. | 1159 | 0.16 | 0.13 | 0.13 | 0.16 | 0.15 | 0.13 | |
| 11 | n.i. | 1169 | 0.16 | 0.16 | 0.62 | 0.65 | 0.66 | 0.64 | |
| 12 | Rosuferan epoxide | 1178 | 1173 | 0.16 | 0.13 | 0.10 | 0.16 | 0.30 | 0.29 |
| 13 | (E)-Isocitral (=Isogeranial) | 1188 | 1177 | 1.09 | 1.09 | 1.17 | 1.27 | 1.31 | 1.26 |
| 14 | (4Z)-Decennial | 1200 | 1193 | 0.07 | 0.05 | 0.10 | 0.08 | 0.08 | 0.06 |
| 15 | n.i. | 1206 | 0.22 | 0.23 | 0.15 | 0.17 | 0.31 | 0.28 | |
| 16 | n-Decanal | 1214 | 1201 | 0.09 | 0.07 | 0.09 | 0.09 | 0.13 | 0.10 |
| 17 | n.i. | 1230 | 0.06 | 0.06 | 0.05 | 0.09 | 0.06 | 0.07 | |
| 18 | n.i. | 1241 | 0.10 | 0.10 | 0.11 | 0.14 | 0.10 | 0.11 | |
| 19 | Neral (=Z-citral) | 1249 | 1235 | 34.25 | 34.12 | 35.75 | 36.35 | 37.05 | 37.32 |
| 20 | Geraniol | 1259 | 1249 | 0.60 | 0.56 | tr | 0.07 | 0.58 | 0.36 |
| 21 | Geranial (=E-citral) | 1280 | 1264 | 60.06 | 60.64 | 59.47 | 58.59 | 56.36 | 56.69 |
| 22 | n.i. | 1350 | 0.08 | 0.08 | 0.18 | 0.00 | 0.08 | 0.04 | |
| 23 | Geranyl acetate | 1384 | 1379 | 1.01 | 0.95 | 0.20 | 0.15 | 0.25 | 0.20 |
| 24 | γ-Cadinene | 1520 | 1513 | 0.21 | 0.14 | 0.15 | 0.12 | 0.07 | 0.08 |
| 25 | Cariophyllene oxide | 1592 | 1582 | 0.30 | 0.22 | 0.21 | 0.18 | 0.25 | 0.25 |
| Total number of substances identified | 99.41 | 99.47 | 98.78 | 99.47 | 98.49 | 98.65 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, P.R.R.d.; Leonel, S.; Silva, M.d.S.; Bonfim, F.P.G.; Leonel, M.; Cândido, H.T.; Tecchio, M.A.; Molha, N.Z.; Domiciano, V.M. Sustainability Indicators of the Banana and Lemongrass Intercropping System in Different Harvest Seasons: Growth, Yield, Seasonality and Essential Oil Properties. Agriculture 2025, 15, 758. https://doi.org/10.3390/agriculture15070758
Jesus PRRd, Leonel S, Silva MdS, Bonfim FPG, Leonel M, Cândido HT, Tecchio MA, Molha NZ, Domiciano VM. Sustainability Indicators of the Banana and Lemongrass Intercropping System in Different Harvest Seasons: Growth, Yield, Seasonality and Essential Oil Properties. Agriculture. 2025; 15(7):758. https://doi.org/10.3390/agriculture15070758
Chicago/Turabian StyleJesus, Paulo Ricardo Rodrigues de, Sarita Leonel, Marcelo de Souza Silva, Filipe Pereira Giardini Bonfim, Magali Leonel, Hebert Teixeira Cândido, Marco Antonio Tecchio, Nicholas Zanette Molha, and Vinicius Martins Domiciano. 2025. "Sustainability Indicators of the Banana and Lemongrass Intercropping System in Different Harvest Seasons: Growth, Yield, Seasonality and Essential Oil Properties" Agriculture 15, no. 7: 758. https://doi.org/10.3390/agriculture15070758
APA StyleJesus, P. R. R. d., Leonel, S., Silva, M. d. S., Bonfim, F. P. G., Leonel, M., Cândido, H. T., Tecchio, M. A., Molha, N. Z., & Domiciano, V. M. (2025). Sustainability Indicators of the Banana and Lemongrass Intercropping System in Different Harvest Seasons: Growth, Yield, Seasonality and Essential Oil Properties. Agriculture, 15(7), 758. https://doi.org/10.3390/agriculture15070758






