Influence of Biochar Organic Carbon Composition and Thermal Stability on Nitrate Retention and Tomato Yield on Soilless Biochar Amended Growth Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Method
2.1.1. Media
2.1.2. Tomato Production
2.1.3. Sample Collection and Laboratory Processing
2.2. Physicochemical Properties
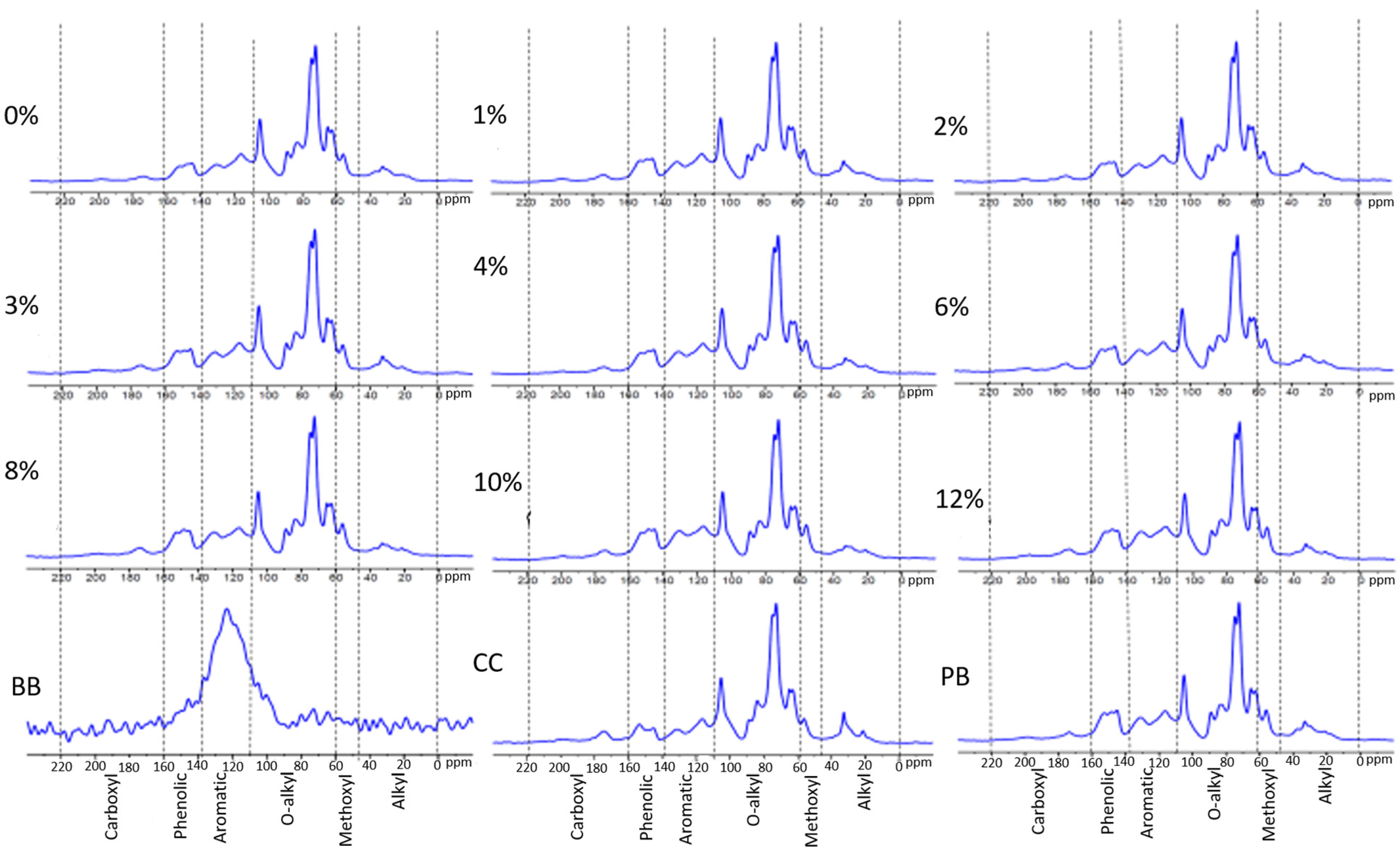
2.3. 13C Solid—State Nuclear Magnetic Resonance Analysis
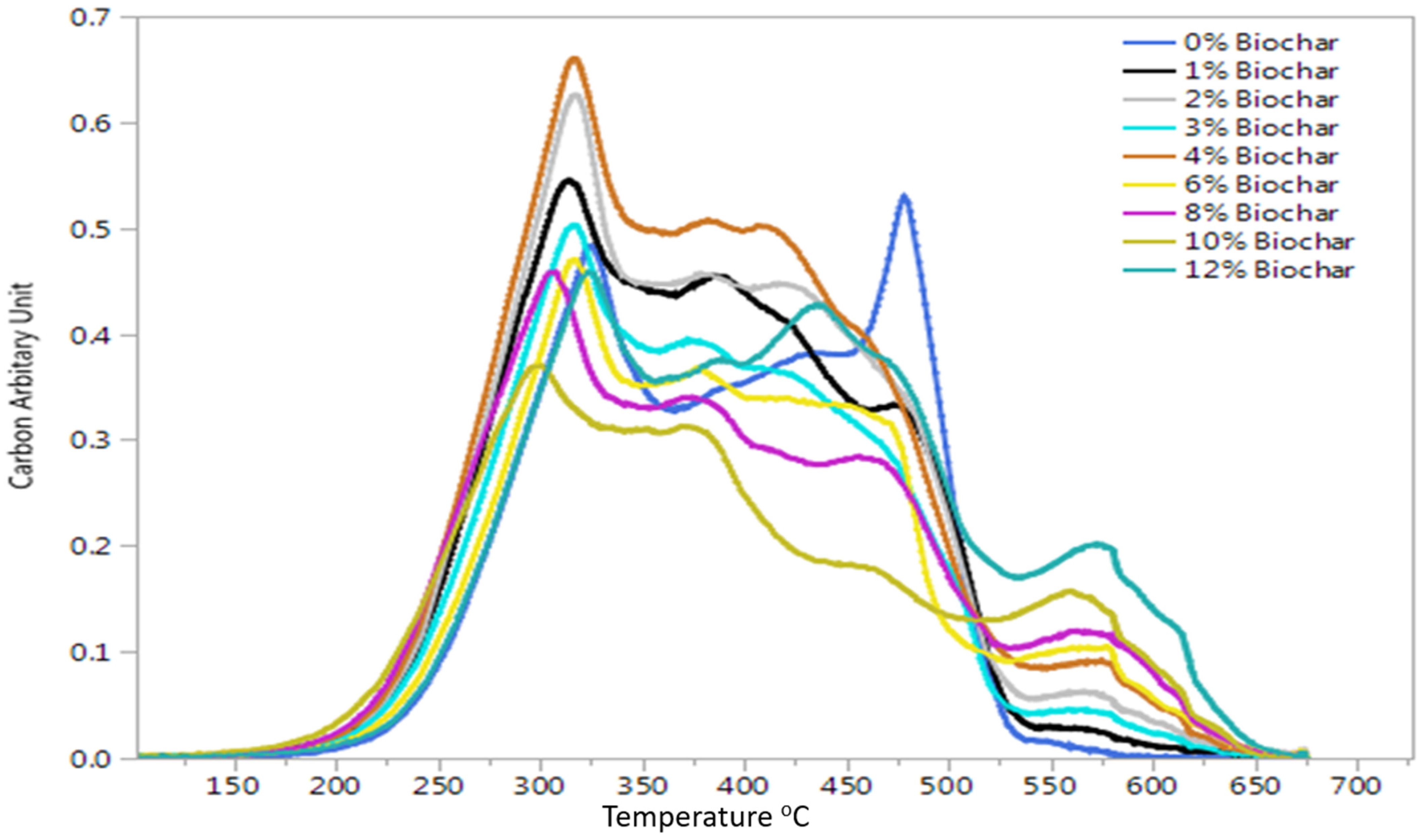
2.4. Thermal Stability Analysis Using Multi-Elemental Scanning Thermal Analysis
2.5. Statistical Analysis
3. Results
3.1. Biochar Amended Soilless Media Physicochemical Properties
3.2. C Solid State Nuclear Magnetic Resonance
3.3. Multi-Elemental Scanning Thermal Analysis Results
3.4. Nitrate Retention
3.5. Tomatoes Yield
4. Discussion
4.1. Biochar Amendment Altered the Soilless Media’s Physical-Chemical Properties
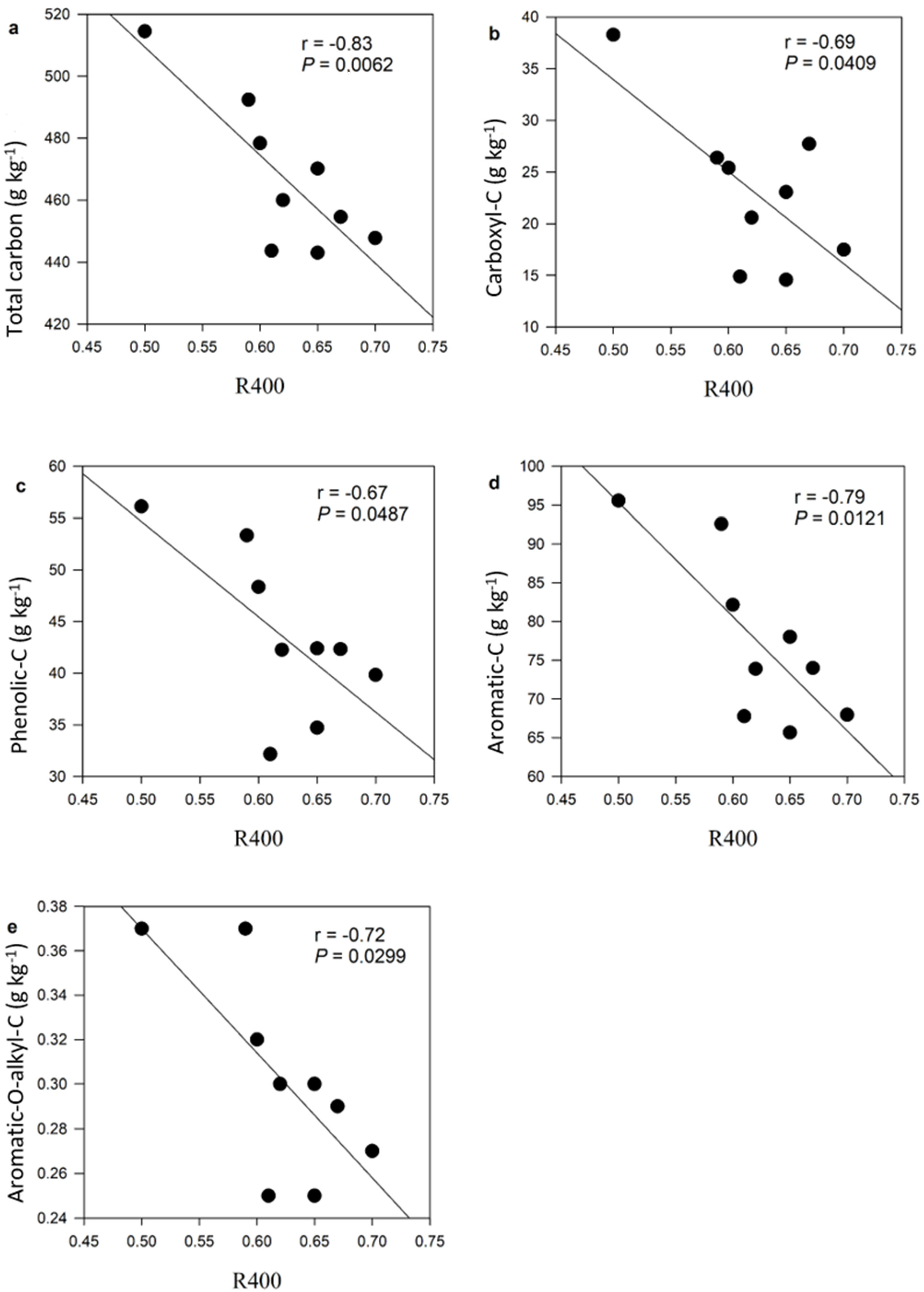
4.2. Amendment of Soilless Media with Biochar Influences Organic Carbon Composition and Thermal Stability
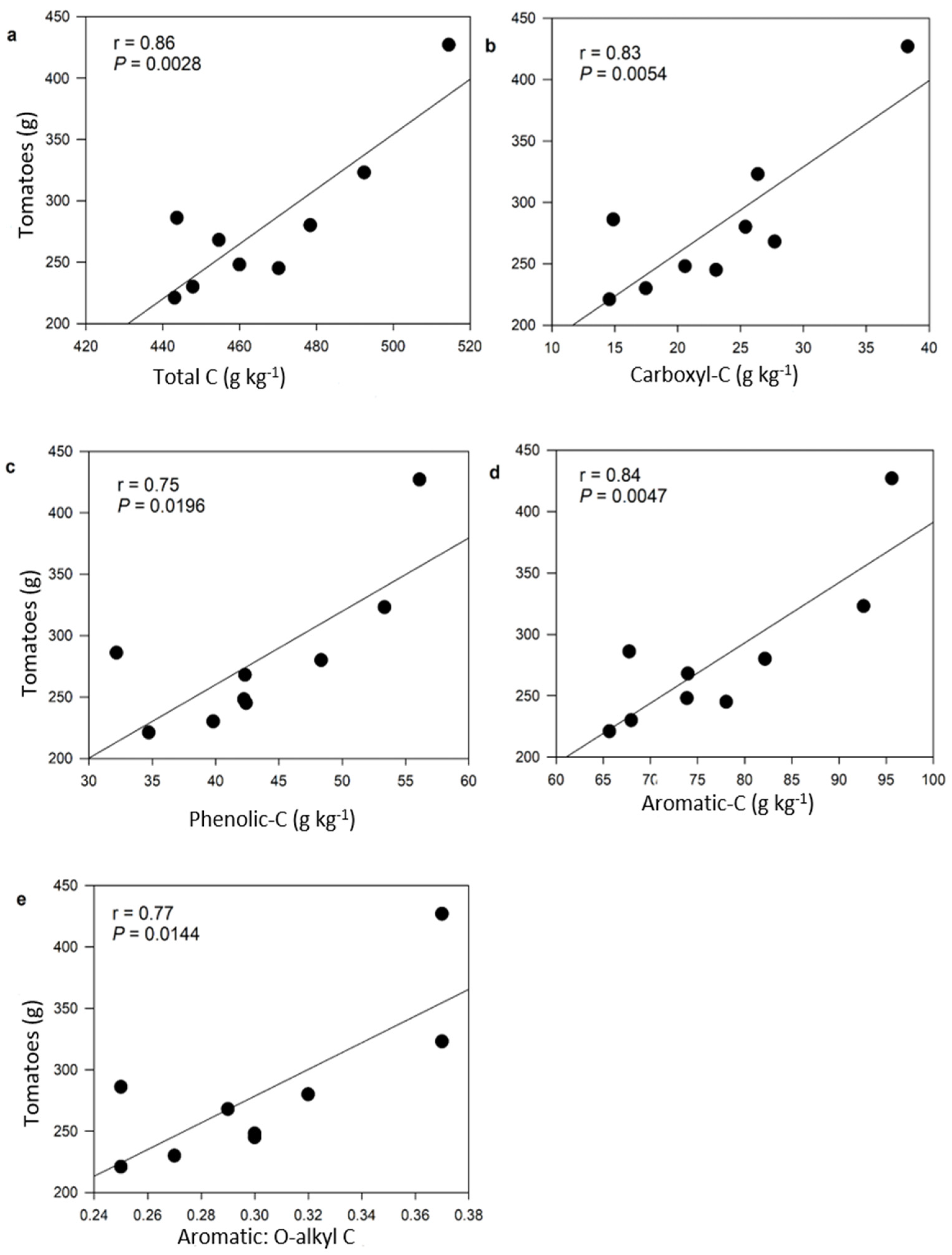
4.3. Organic Carbon Composition and Thermal Stability Influence Nitrate Retention
4.4. Enhanced Nitrate Retention Improves Tomatoes Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Zhang, A.; Bian, R.; Hussain, Q.; Li, L.; Pan, G.; Zheng, J.; Zheng, J. Change in net global warming potential of a rice–wheat cropping system with biochar soil amendment in a rice paddy from China. Agric. Ecosyst. Environ. 2013, 173, 37–45. [Google Scholar] [CrossRef]
- Cao, P.; Lu, C.C.; Yu, Z. Historical nitrogen fertilizer used in agricultural ecosystems of the contiguous United States during 1850–2015: Application rate, timing, and fertilizer types. Earth Syst. Sci. Data Discuss. 2018, 10, 969. [Google Scholar] [CrossRef]
- Follett, J.R.; Follett, R.F.; Herz, W.C. Environmental and human impacts of reactive nitrogen. In Advances in Nitrogen Management for Water Quality; Soil and Water Conservation Society: Ankeny, IA, USA, 2010; pp. 1–37. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636. [Google Scholar] [CrossRef]
- Osei, G.K. Influence of Biochar Carbon Composition and Thermal Stability on Nitrogen Retention, Microbial Diversity and Radionuclides in Soilless Growth Media. Ph.D. Thesis, Agricultural and Mechanical University, Tallahassee, FL, USA, 2021. [Google Scholar]
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. Nitrogen. Assim. Plants 2021, 37, 2–17. [Google Scholar]
- Benbi, D.K.; Richter, J. A critical review of some approaches to modelling nitrogen mineralization. Biol. Fertil. Soils 2002, 35, 168–183. [Google Scholar] [CrossRef]
- Aczel, M.R. What is the nitrogen cycle, and why is it key to life? Front. Young Minds 2019, 7, 14. [Google Scholar] [CrossRef]
- Ribaudo, M. Reducing agriculture’s nitrogen footprint: Are new policy approaches needed? Amber Waves Econ. Food Farming Nat. Resour. Rural. Am. 2011, 9, 34–39. [Google Scholar]
- Basso, B.; Shuai, G.; Zhang, J.; Robertson, G.P. Yield stability analysis reveals sources of large-scale nitrogen loss from the US Midwest. Sci. Rep. 2019, 9, 5774. [Google Scholar] [CrossRef]
- De Notaris, C.; Rasmussen, J.; Sørensen, P.; Olesen, J.E. Nitrogen leaching: A crop rotation perspective on the effect of N surplus, field management and use of catch crops. Agric. Ecosyst. Environ. 2018, 255, 1–11. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Withers, P.J.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of Biochar on Container Substrate Properties and Growth of Plants—A Review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems–A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless culture. In Good Agricultural Practices for Greenhouse Vegetable Crops. Principles for Mediterranean Climate Areas; FAO: Rome, Italy, 2013; pp. 303–354. [Google Scholar]
- Rezaei-Nejad, A.; Ismaili, A. Changes in growth, essential oil yield and composition of geranium (Pelargonium graveolens L.) as affected by growing media. J. Sci. Food Agric. 2014, 94, 905–910. [Google Scholar] [CrossRef]
- Ngatia, L.W.; Hsieh, Y.P.; Nemours, D.; Fu, R.; Taylor, R.W. Potential phosphorus eutrophication mitigation strategy: Biochar carbon composition, thermal stability and pH influence phosphorus sorption. Chemosphere 2017, 180, 201–211. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Oxfordshire, UK, 2015. [Google Scholar]
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Sun, L.; Li, L.; Chen, Z.; Wang, J.; Xiong, Z. Combined effects of nitrogen deposition and biochar application on emissions of N2O, CO2 and NH3 from agricultural and forest soils. Soil. Sci. Plant Nutr. 2014, 60, 254–265. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Gcb Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; Volume 105, pp. 47–82. [Google Scholar]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil biochar amendment as a climate change mitigation tool: Key parameters and mechanisms involved. J. Environ. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef]
- Konare, H.; Yost, R.S.; Doumbia, M.; McCarty, G.W.; Jarju, A.; Kablan, R. Loss on ignition: Measuring soil organic carbon in soils of the Sahel, West Africa. Afr. J. Agric. Res. 2010, 5, 3088–3095. [Google Scholar]
- Beck, D.A.; Johnson, G.R.; Spolek, G.A. Amending green roof soil with biochar to affect runoff water quantity and quality. Environ. Pollut. 2011, 159, 2111–2118. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Hatfield, G.R.; Maciel, G.E.; Erbatur, O.; Erbatur, G. Qualitative and quantitative analysis of solid lignin samples by carbon-13 nuclear magnetic resonance spectrometry. Anal. Chem. 1987, 59, 172–179. [Google Scholar] [CrossRef]
- Espina, R.; Yu, L.; Wang, J.; Tong, Z.; Vashishtha, S.; Talaat, R.; Scatina, J.; Mutlib, A. Nuclear magnetic resonance spectroscopy as a quantitative tool to determine the concentrations of biologically produced metabolites: Implications in metabolites in safety testing. Chem. Res. Toxicol. 2009, 22, 299–310. [Google Scholar] [CrossRef]
- Cody, G.D.; Alexander, C.O.D.; Tera, F. Solid-state (1H and 13C) nuclear magnetic resonance spectroscopy of insoluble organic residue in the Murchison meteorite: A self-consistent quantitative analysis. Geochim. Cosmochim. Acta 2002, 66, 1851–1865. [Google Scholar] [CrossRef]
- Hsieh, Y.P. A novel multi elemental scanning thermal analysis (MESTA) method for the identification and characterization of solid substances. J. AOAC Int. 2007, 90, 54–59. [Google Scholar]
- Disnar, J.R.; Jacob, J.; Morched-Issa, M.; Lottier, N.; Arnaud, F. Assessment of peat quality by molecular and bulk geochemical analysis: Application to the Holocene record of the Chautagne marsh (Haute Savoie, France). Chem. Geol. 2008, 254, 101–112. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Knicker, H. Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org. Geochem. 2011, 42, 867–890. [Google Scholar] [CrossRef]
- Disnar, J.R.; Guillet, B.; Kéravis, D.; Di-Giovanni, C.; Sebag, D. Soil organic matter (SOM) characterization by Rock-Eval pyrolysis: Scope and limitations. Org. Geochem. 2003, 34, 327–343. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice–Maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Pasian, C.; Lal, R.; Lopez, R.; Dıaz, M.J.; Fernandez, M. Morpho-physiological plant quality when biochar and vermicompost are used as growing media replacement in urban horticulture. Urban For. Urban Green. 2018, 34, 175–180. [Google Scholar] [CrossRef]
- Blok, C.; Van der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for Horticultural Rooting Media Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef]
- Dong, D.; Feng, Q.; Mcgrouther, K.; Yang, M.; Wang, H.; Wu, W. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J. Soils Sediments 2015, 15, 153–162. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Schomberg, H. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Spielvogel, S.; Prietzel, J.; Kögel-Knabner, I. Soil organic matter stabilization in acidic forest soils is preferential and soil type-specific. Eur. J. Soil Sci. 2008, 59, 674–692. [Google Scholar] [CrossRef]
- Haumaier, L.; Zech, W. Black carbon—Possible source of highly aromatic components of soil humic acids. Org. Geochem. 1995, 23, 191–196. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Jocteur Monrozier, L.; Robin, A.M. Action de la faune du sol sur une litière de feuillu: Application de techniques pyrolytiques à l’étude des modifications subies par les feuilles de charme (Carpinus betulus) ingérées par Glomeris marginata. Rev. D’ecologie Biol. Sol. 1988, 25, 203–214. [Google Scholar]
- Schulz, H.; Dunst, G.; Glaser, B. No effect level of co-composted biochar on plant growth and soil properties in a greenhouse experiment. Agronomy 2014, 4, 34–51. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Cheng, Y.; Cai, Z.; Müller, C.; Zhang, J. Composition of soil recalcitrant C regulates nitrification rates in acidic soils. Geoderma 2019, 337, 965–972. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar as plant growth promoter: Better off alone or mixed with organic amendments? Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Hao, X.; Zhou, D. Transport of biochar particles in saturated granular media: Effects of pyrolysis temperature and particle size. Environ. Sci. Technol. 2013, 47, 821–828. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R. Soil water repellency: Its causes, characteristics and hydro-geomorphological significance. Earth-Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Archanjo, B.S.; Mendoza, M.E.; Albu, M.; Mitchell, D.R.; Hagemann, N.; Mayrhofer, C.; Anh Mai, T.L.; Weng, Z.; Kappler, A.; Behrens, S.; et al. Nanoscale analyses of the surface structure and composition of biochars extracted from field trials or after co-composting using advanced analytical electron microscopy. Geoderma 2017, 294, 70–79. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Hagemann, N.; Kammann, C.I.; Schmidt, H.P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Joseph, S. Erratum: Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 12378. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil. Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Sombroek, W.I.M.; Ruivo, M.D.L.; Fearnside, P.M.; Glaser, B.; Lehmann, J. Amazonian dark earths as carbon stores and sinks. In Amazonian Dark Earths; Springer: Dordrecht, The Netherlands, 2003; pp. 125–139. [Google Scholar]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil. 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. The effects of biochar, compost and their mixture and nitrogen fertilizer on yield and nitrogen use efficiency of barley grown on a Nitisol in the highlands of Ethiopia. Sci. Total Environ. 2016, 569, 869–879. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, G.; Wu, G.; Kibue, G.W.; Li, L.; Zhang, X.; Zheng, J.; Zheng, J.; Cheng, K.; Joseph, S.; et al. Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere 2016, 142, 106–113. [Google Scholar] [CrossRef] [PubMed]


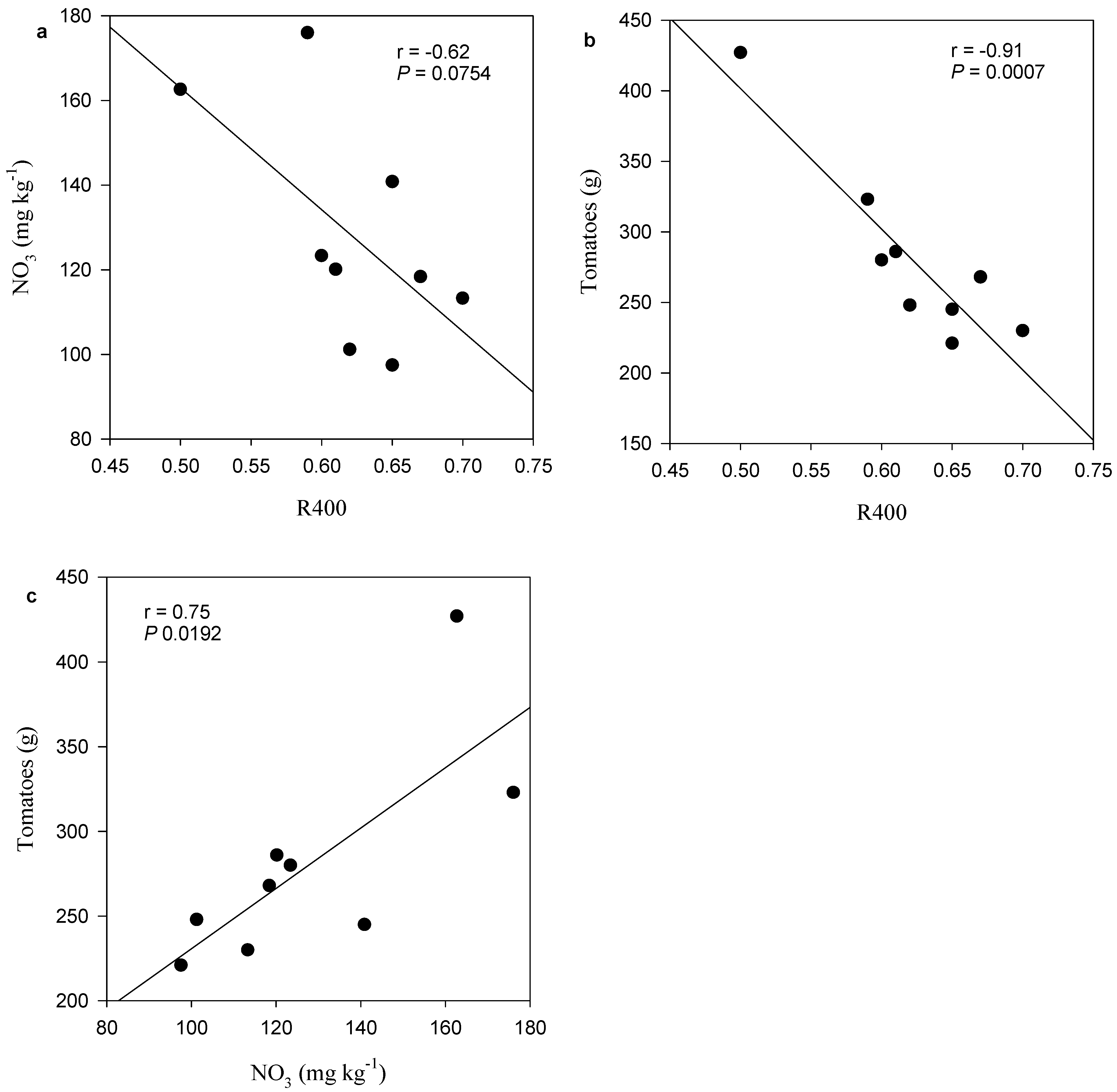
| Biochar | pH | LOI (%) | NH4+ (mg kg−1) | NO3− (mg kg−1) | N (g kg−1) | C (g kg−1) | C:N |
|---|---|---|---|---|---|---|---|
| 0% | 6.15 ± 0.03 d | 90.55 ± 0.90 a | 14.73 ± 2.73 a | 120.16 ± 16.57 bc | 4.17 ± 0.07 a | 443.67 ± 3.03 c | 106.51 ± 0.77 b |
| 1% | 6.02 ± 0.01 d | 90.58 ± 0.95 a | 16.87 ± 1.35 a | 113.30 ± 10.01 c | 3.60 ± 0.10 ab | 447.80 ± 7.61 bc | 124.53 ± 1.35 ab |
| 2% | 6.03 ± 0.06 d | 91.27 ± 0.41 a | 17.33 ± 1.12 a | 97.51 ± 25.24 c | 3.33 ± 0.09 b | 443.03 ± 4.08 c | 133.11 ± 1.49 a |
| 3% | 6.23 ± 0.09 d | 90.47 ± 0.16 a | 21.12 ± 1.30 a | 118.39 ± 12.98 bc | 3.30 ± 0.00 b | 454.60 ± 6.55 bc | 137.76 ± 1.07 a |
| 4% | 6.47 ± 0.05 bc | 89.30 ± 1.20 a | 19.71 ± 3.23 a | 140.86 ± 11.01 abc | 3.63 ± 0.03 ab | 470.17 ± 4.83 abc | 129.45 ± 1.20 ab |
| 6% | 6.46 ± 0.02 c | 90.21 ± 0.44 a | 16.16 ± 1.20 a | 101.23 ± 17.95 c | 3.30 ± 0.15 b | 459.97 ± 22.71 bc | 139.39 ± 1.21 a |
| 8% | 6.68 ± 0.02 ab | 90.18 ± 0.40 a | 17.20 ± 2.19 a | 123.36 ± 17.55 bc | 3.53 ± 0.26 ab | 478.40 ± 6.48 abc | 136.68 ± 2.27 a |
| 10% | 6.76 ± 0.01 a | 88.69 ± 1.09 a | 14.30 ± 1.46 a | 176.01 ± 2.37 a | 3.60 ± 0.17 ab | 492.43 ± 8.69 ab | 137.31 ± 1.85 a |
| 12% | 6.84 ± 0.02 a | 90.85 ± 0.91 a | 12.71 ± 0.43 a | 162.65 ± 21.20 ab | 3.80 ± 0.11 ab | 514.47 ± 8.07 a | 135.76 ± 1.89 a |
| p value | <0.0001 | 0.4618 | 0.1078 | 0.0386 | 0.0044 | 0.0006 | 0.0024 |
| Biochar | Carboxyl | Phenolic | Aromatic | O-Alkyl | Methoxyl | Alkyl | Alkyl:O-Alkyl | Aromatic: O-Alkyl |
|---|---|---|---|---|---|---|---|---|
| 0% | 14.89 | 32.17 | 67.76 | 267.87 | 27.12 | 33.86 | 0.13 | 0.25 |
| 1% | 17.48 | 39.82 | 67.96 | 250.51 | 30.81 | 41.22 | 0.16 | 0.27 |
| 2% | 14.58 | 34.72 | 65.66 | 258.72 | 30.32 | 39.04 | 0.15 | 0.25 |
| 3% | 27.72 | 42.32 | 73.98 | 256.09 | 24.38 | 30.11 | 0.12 | 0.29 |
| 4% | 23.06 | 42.40 | 78.04 | 260.22 | 30.10 | 36.35 | 0.14 | 0.30 |
| 6% | 20.59 | 42.24 | 73.88 | 247.96 | 31.93 | 43.38 | 0.17 | 0.30 |
| 8% | 25.40 | 48.34 | 82.16 | 256.75 | 30.97 | 34.79 | 0.14 | 0.32 |
| 10% | 26.38 | 53.34 | 92.60 | 253.08 | 30.68 | 36.36 | 0.14 | 0.37 |
| 12% | 38.29 | 56.11 | 95.59 | 261.63 | 28.47 | 34.38 | 0.13 | 0.37 |
| Biochar | LTSC (g kg−1) | HTSC (g kg−1) | R400 |
|---|---|---|---|
| 0% | 268.65 | 175.02 | 0.61 |
| 1% | 311.72 | 136.08 | 0.70 |
| 2% | 290.09 | 152.94 | 0.65 |
| 3% | 306.68 | 147.92 | 0.67 |
| 4% | 303.81 | 166.36 | 0.65 |
| 6% | 283.71 | 176.26 | 0.62 |
| 8% | 285.47 | 192.93 | 0.60 |
| 10% | 291.06 | 201.37 | 0.59 |
| 12% | 255.74 | 258.73 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osei, G.K.; Ngatia, L.W.; Abazinge, M.D.; Bolques, A.; Jagoe, C.; Owens, M.A.; Mwashote, B.; Fu, R. Influence of Biochar Organic Carbon Composition and Thermal Stability on Nitrate Retention and Tomato Yield on Soilless Biochar Amended Growth Media. Agriculture 2025, 15, 865. https://doi.org/10.3390/agriculture15080865
Osei GK, Ngatia LW, Abazinge MD, Bolques A, Jagoe C, Owens MA, Mwashote B, Fu R. Influence of Biochar Organic Carbon Composition and Thermal Stability on Nitrate Retention and Tomato Yield on Soilless Biochar Amended Growth Media. Agriculture. 2025; 15(8):865. https://doi.org/10.3390/agriculture15080865
Chicago/Turabian StyleOsei, George K., Lucy W. Ngatia, Michael D. Abazinge, Alejandro Bolques, Charles Jagoe, Marcia A. Owens, Benjamin Mwashote, and Riqiang Fu. 2025. "Influence of Biochar Organic Carbon Composition and Thermal Stability on Nitrate Retention and Tomato Yield on Soilless Biochar Amended Growth Media" Agriculture 15, no. 8: 865. https://doi.org/10.3390/agriculture15080865
APA StyleOsei, G. K., Ngatia, L. W., Abazinge, M. D., Bolques, A., Jagoe, C., Owens, M. A., Mwashote, B., & Fu, R. (2025). Influence of Biochar Organic Carbon Composition and Thermal Stability on Nitrate Retention and Tomato Yield on Soilless Biochar Amended Growth Media. Agriculture, 15(8), 865. https://doi.org/10.3390/agriculture15080865






