The Potential of Pequi Oil as a Modulator of Chaperone Expression to Minimize Heat Stress in Laying Hens
Abstract
1. Introduction
2. Materials and Methods
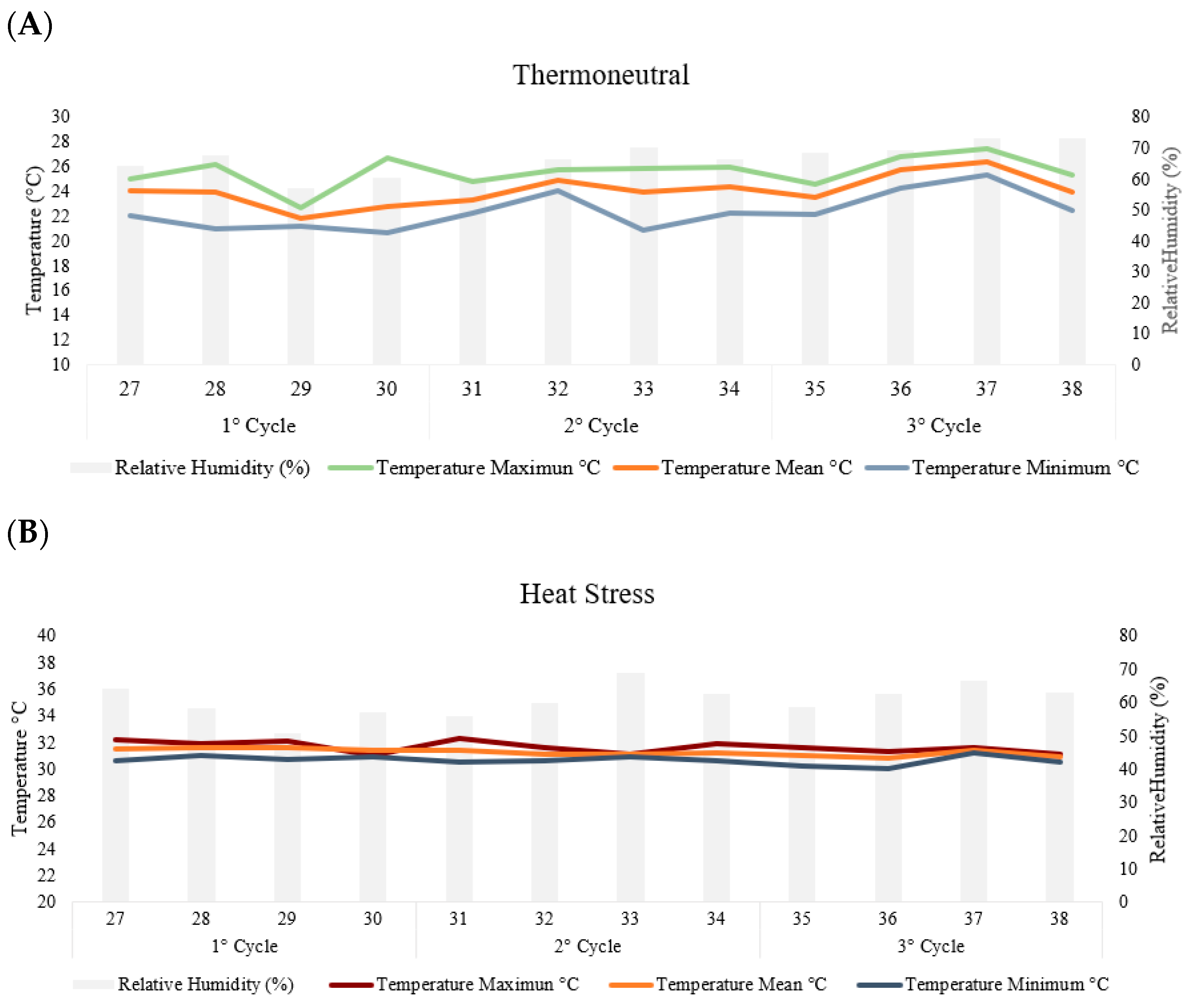
2.1. Experimental Design, Diets, and Treatments
2.2. Protein Relative Abundance Variance Analysis by Shotgun LC–MS/MS
2.2.1. Liver Tissue Analysis
2.2.2. Liver Tissue Protein Preparation for Shotgun LC–MS/MS Analysis
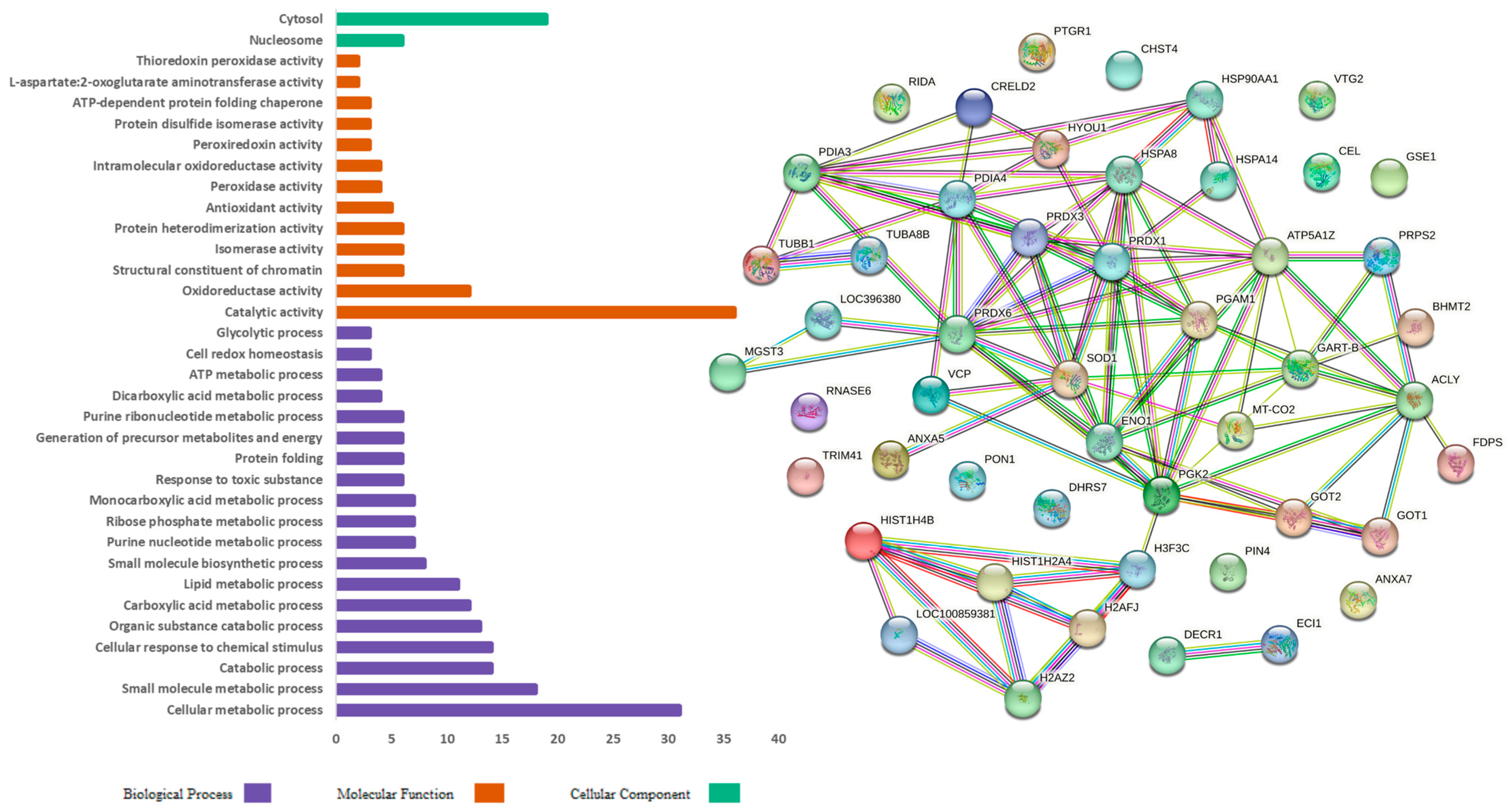
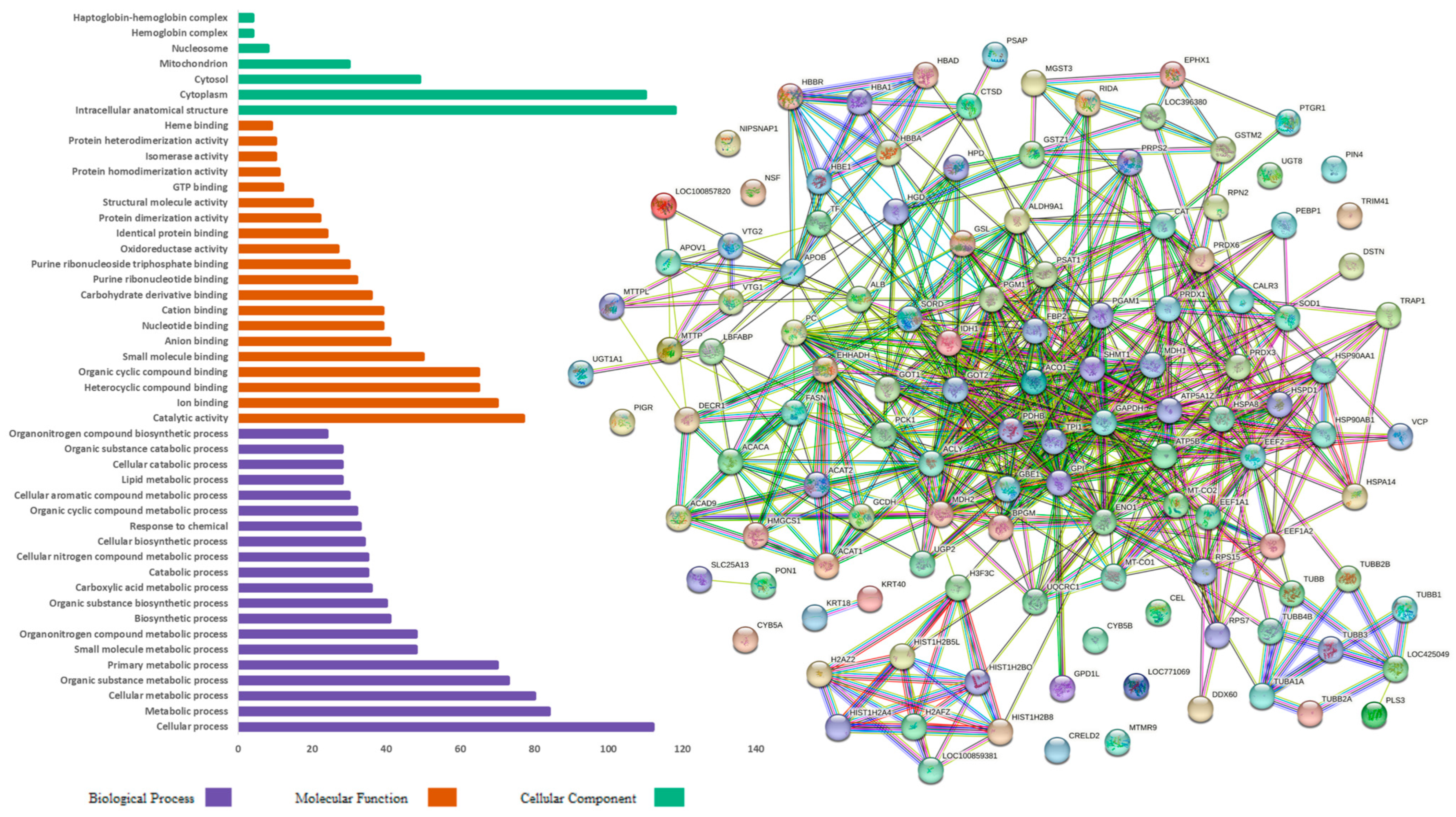
3. Results
4. Discussion
4.1. Heat Shock Proteins
4.2. Antioxidant System
4.3. Lipid Metabolism
4.4. Immune System
4.5. Unique Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCG1 | Binding cassette subfamily E member 1 |
| AMBIC | Bicarbonate |
| BD | Basal diet |
| BiP | Binding immunoglobulin protein |
| BP | Biological process |
| CAT | Catalase |
| CC | Cellular component |
| CEUA | Ethics Committee on the Use of Animals |
| CO | Control diet |
| DTT | Dithiothreitol |
| FMVZ | School of Veterinary Medicine and Animal Science |
| FOXO | Forkhead box O |
| HO | 1- Heme oxygenase-1 |
| HS | Heat stress |
| HSPs | Heat shock proteins |
| IBB | Department of Chemistry and Biochemistry |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-6 | Interleukin-6 |
| LBM | Bioanalytical and Metalloproteomics Laboratory |
| LC-MS/MS- | Liquid chromatography–tandem mass spectrometry |
| MF | Molecular function |
| NF-kB | Nuclear factor kappa B |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| PDI | Protein disulfide-isomerase |
| PO | Pequi oil |
| PPIase | Peptidyl-prolyl cis/trans isomerase |
| PRXs | Peroxiredoxins |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor-alpha |
| UNESP | São Paulo State University |
References
- Surai, P.F. Vitagenes in Avian Biology and Poultry Health; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020. [Google Scholar]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Belal, S.; Kang, D.; Cho, E.; Park, G.; Shim, K. Taurine Reduces Heat Stress by Regulating the Expression of Heat Shock Proteins in Broilers Exposed to Chronic Heat. Braz. J. Poult. Sci. 2018, 20, 479–486. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Chen, M.; Sun, Y.; Safdar, A.; Pasha, R.H.; Munir, M.; Ding, C. Oxidative Stress in Poultry: Lessons from the Viral Infections. Oxid. Med. Cell. Longev. 2018, 14, 5123147. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Freitas, C.; da Vera, E.; Chiea, A. Effect of the application of an enzymatic pretreatment on bioactive compounds of Caryocar brasiliense Camb pulp oil. J. Food Process. Preserv. 2018, 42, 13828. [Google Scholar] [CrossRef]
- Batista, F.O.; Sousa, R.S. Compostos bioativos em frutos pequi (Caryocar brasiliense Camb.) E baru (Dipteryx alata vogel) e seus usos potenciais: Uma revisão. Braz. J. Dev. 2019, 5, 9259–9270. [Google Scholar] [CrossRef]
- Guglielmetti, C.; Manfredi, M.; Brusadore, S.; Sciuto, S.; Esposito, G.P.G.U.; Magnani, L.; Gili, S.; Marengo, E.; Acutis, P.L.; Mazza, M. Two-dimensional gel and shotgun proteomics approaches to distinguish fresh and frozen-thawed curled octopus (Eledone cirrhosa). J. Proteom. 2018, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kang, D.A.; Shim, K.S. Proteomic changes in broiler liver by body weight differences under chronic heat stress. Poult. Sci. 2022, 101, 101794. [Google Scholar] [CrossRef]
- Rostagno, H.S.; Albino, L.F.T.; Hannas, M.I.; Donzele, J.L.; Sakomura, N.K.; Perazzo, F.G.; Saraiva, A.; Teixeira, M.L.; Rodrigues, P.B.; Oliveira, R.F.; et al. Tabelas brasileiras para aves e suínos: Composição de Alimentos e Exigências Nutricionais. Univ. Fed. Viçosa Dep. Zootec. 2017, 4, 451–488. [Google Scholar]
- Da Silva, J.; Andrade, L.G.; Rodrigues, P.A.D.; Cordeiro, L.G.; Lima, G.A.; Lopes, J.L.; Castillo, E.O.F.; Martins, R.A.; Assunção, A.S.A.; Vieira, J.C.S.; et al. Plasma Proteome Alterations of Laying Hens Subjected to Heat Stress and Fed a Diet Supplemented with Pequi Oil (Caryocar brasiliense Camb.): New Insights in the Identification of Heat Stress Biomarkers. Biomolecules 2024, 14, 1424. [Google Scholar] [CrossRef]
- Cruvinel, J.M.; Urayama, P.M.G.; Oura, C.Y.; Kaiser, F.K.L.; Santos, T.S.; Alves, B.A.; Kadri, S.M.; Corrêa, C.R.; Sartori, J.R.; Pezzato, A.C. Pequi Oil (Caryocar brasiliense Camb.) Attenuates the Adverse Effects of Cyclical Heat Stress and Modulates the Oxidative Stress-Related Genes in Broiler Chickens. Animals 2023, 13, 1896. [Google Scholar] [CrossRef]
- Almeida, E.C.; Faria, V.D.; Cirinêu, F.D.; Santiago, M.G.A.; Miotto, B.; Vieira, J.C.S.; Braga, C.P.; Adamec, J.; Fernandes, A.A.H.; Buzalaf, M.A.R.; et al. Metalloproteomic Investigation of Hg-Binding Proteins in Renal Tissue of Rats Exposed to Mercury Chloride. Int. J. Mol. Sci. 2024, 25, 164. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.O.; Chemelo, V.S.; Aragão, W.A.B.; Puty, B.; Dionizio, A.; Teixeira, F.B.; Fernandes, M.S.; Silva, M.C.F.; Fernandes, L.M.P.; de Oliveira, E.H.C.; et al. From Molecules to Behavior in Long-Term Inorganic Mercury Intoxication: Unraveling Proteomic Features in Cerebellar Neurodegeneration of Rats. Int. J. Mol. Sci. 2021, 23, 111. [Google Scholar] [CrossRef]
- Bollengier-lee, S.; Mitchell, M.A.; Utomo, D.B.; Williams, P.E.; Whitehead, C.C. Influence of high dietary vitamin E supplementation on egg production and plasma characteristics in hens subjected to heat stress. Br. Poult. Sci. 1998, 39, 106–112. [Google Scholar] [CrossRef]
- Pasri, P.; Pukkung, C.; Mermillod, P.; Gérard, N.; Jantasaeng, O.; Sirisopapong, M.; Okrathok, S.; Thiabching, T.; Rakngam, S.; Kamkajon, K.; et al. Alleviating heat stress on broiler breeder hens: Effect of dietary antioxidant supplementation on reproductive performance, egg quality, offspring growth, and antioxidant capacity. J. Appl. Poult. Res. 2025, 34. [Google Scholar] [CrossRef]
- Gouda, A.; El-Monairy, M.M.A.; Hassan, H.M.A.; Hamouda, Y.M.A.; Youssef, A.M.A. Lycopene Supplementation Enhances Growth Performance, Antioxidant Enzymes, Heat Shock Protein 70, and Some Biochemical and Immune Parameters in Broiler Chickens Exposed to Heat Stress. Poult. Sci. J. 2025, 13, 127–134. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Miao, J.; Zhang, X.; Xiong, Y.; Ma, F.; Ding, J.; He, S. Bamboo leaf flavonoids ameliorate cyclic heat stress-induced oxidative damage in broiler liver through activation of Keap1-Nrf2 signaling pathway. Poul. Sci. 2025, 104, 104952. [Google Scholar] [CrossRef]
- Fathi, M.; Zarrinkavyani, K.; Biranvand, Z.; Mustafa, Y. The Effect of Silymarin on Antioxidant, Performance, Immunoglobulin Protein Levels, Cecal Microbiota, and Hemobiochemical Indicators in Heat Stressd Broilers. Poult. Sci. J. 2025, 13, 115–126. [Google Scholar] [CrossRef]
- Xu, L.; Gao, P.; Wu, H.; Gao, Y.; Ji, H.; Huang, X.; Zhang, S.; Fan, W.; Song, S. Lactobacillus plantarum 4-2 alleviates cyclic heat stress-induced oxidative stress and damage in the ileum of laying hens via Keap1-Nrf2 pathway. J. Therm. Biol. 2025, 127, 104072. [Google Scholar] [CrossRef]
- Hashemitabar, S.H.; Hosseinian, S.A. The comparative effects of probiotics on growth, antioxidant indices and intestinal histomorphology of broilers under heat stress condition. Sci. Rep. 2024, 14, 23471. [Google Scholar] [CrossRef]
- Yilmaz, E.; Gul, M. Effects of essential oils on heat-stressed poultry: A review. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Herring, G.; Gawlik, D.E. The Role of Stress Proteins in the Study of Allostatic Overload in Birds: Use and Applicability to Current Studies in Avian Ecology. Sci. World J. 2007, 7, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Nielsen, M.; Carcione, T.; Li, S.; Shi, J. Apolipoprotein E regulates mitochondrial function through the PGC-1α-sirtuin 3 pathway. Aging 2019, 11, 11148–11156. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, C.; Chen, C.; Chan, H.; Wang, S.; Tang, P.; Chen, C.; Lee, Y.; Chen, S.; Huang, S. Annotation of differential protein expression in the hypothalami of layer-type Taiwan country chickens in response to acute heat stress. J. Therm. Biol 2018, 77, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Cara, J.; Aluru, N.; Moyano, F.; Vijayan, M. Food-deprivation induces HSP70 and HSP90 protein expression in larval gilthead sea bream and rainbow trout. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 142, 426–431. [Google Scholar] [CrossRef]
- Yousefi, M.; Adineh, H.; Taheri Mirghaed, A.; Hoseini, S.M. Co-Supplementation of Diet with Saccharomyces cerevisiae and Thymol: Effects on Growth Performance, Antioxidant and Immunological Responses of Rainbow Trout, Oncorhynchus mykiss. Animals 2025, 15, 302. [Google Scholar] [CrossRef]
- Ashraf, E.; Ahmed, M.F.; Shahin, S.A.; Omar, A.A.; Zayed, M.M.; Abdel-Rahim, M.M. Dietary rosemary oil with/without zymogen forte improves water quality, growth hormones, immune-physiological response, stress resilience, and health status of Chelon ramada grown in groundwater. BMC Vet. Res. 2025, 21, 27. [Google Scholar] [CrossRef]
- Büyükdeveci, M.E.; Cengizler, İ.; Balcázar, J.L.; Demirkale, İ. Effects of two host-associated probiotics Bacillus mojavensis B191 and Bacillus subtilis MRS11 on growth performance, intestinal morphology, expression of immune-related genes and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus iniae. Dev. Comp. Immunol. 2023, 138, 104553. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Singh, R.; Teicher, B.A.; Wright, J.E.; Trites, D.H.; Shklar, G. Induction of a 70kD protein associated with the selective cytotoxicity of beta-carotene in human epidermal carcinoma. Biochem. Biophys. Res. Commun. 1990, 169, 941–946. [Google Scholar] [CrossRef]
- Toba, T.; Shidoji, Y.; Fujii, J.; Moriwaki, H.; Muto, Y.; Suzuki, T.; Ohishi, N.; Yagi, K. Growth suppression and induction of heat-shock protein-70 by 9-cis β-carotene in cervical dysplasia-derived cells. Life Sci. 1997, 61, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Sun, M.; Hoxie, R.; Kotler, J.L.M.; Friedman, L.J.; Gelles, J.; Street, T.O. The endoplasmic reticulum chaperone BiP is a closure-accelerating cochaperone of Grp94. Proc. Natl. Acad. Sci. USA 2022, 119, e2118793119. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, C. Protein disulfide–isomerase, a folding catalyst and a redox-regulated chaperone. Free Radic. Biol. Med. 2015, 83, 305–313. [Google Scholar] [CrossRef]
- Gavini, N.; Tungtur, S.; Pulakat, L. Peptidyl-Prolyl cis/trans Isomerase-Independent Functional NifH Mutant of Azotobacter vinelandii. J. Bacteriol. 2006, 188, 6020–6025. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Rattan, S.I.S. Primary Stress Response Pathways for Preconditioning and Physiological Hormesis. In The Science of Hormesis in Health and Longevity; Rattan, S.I.S., Kyriazi, M., Eds.; Academic Press: London, UK, 2019; Volume 39, pp. 35–51. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis After Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transplant. 2018, 27, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Stud-ies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [CrossRef]
- Brito, R.M.; Barcia, M.T.; Almeida Farias, C.A.; Zambiazi, R.C.; Fujimori, M.; Honorio-França, A.C.; França, E.L.; Pertuzatti, P.B. Bioactive compounds of pequi pulp and oil extracts modulate antioxidant activity and antiproliferative activity in cocultured blood mononuclear cells and breast cancer cells. Food Nutr. Res. 2022, 66, 10-29219. [Google Scholar] [CrossRef]
- Han, D.S.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar]
- Ray, S.D.; Krmic, M.; Hussain, A.; Marvilli, C.; Fabian, R.; Niha, A.; Danai, M.; Smith, Z.; Jalshgari, A.; Malick Alhariri, A.; et al. Toxicity of Natural Products, 4th ed.; Academic Press: Oxford, UK, 2023; Volume 4. [Google Scholar] [CrossRef]
- Pinheiro, D.S.; Jesuíno, R.S.A. O Gene da Paraoxonase 1 (PON1) no Contexto Doença Arterial Coronariana. Arq. Bras. Cardiol. 2022, 119, 602–603. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A. Peroxiredoxin Evolution and the Regulation of Hydrogen Peroxide Signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Sobotta, M.C.; Liou, W.; Stöcker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.D.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- Burley, R.W.; Evans, A.J.; Pearson, J.A. Molecular Aspects of the Synthesis and Deposition of Hens’ Egg Yolk with Special Reference to Low Density Lipoprotein. Poul. Sci. 1993, 72, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G.; von Hunolstein, C.; Visca, P.; Orsi, N.; Antonini, E. Studies of the antimicrobial activity of ovotransferrin. PubMed 1983, 5, 97–105. [Google Scholar]
- Ibrahim, H.R.; Hoq, M.d.I.; Aoki, T. Ovotransferrin possesses SOD-like superox-ide anion scavenging activity that is promoted by copper and manganese binding. Int. J. Biol. Macromol. 2007, 41, 631–640. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021, 30, 619–663. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.; Song, Q.Q.; Guo, Y.Y.; Jiao, H.C.; Song, Z.G.; Lin, H. Corticosterone regulation of ovarian follicular development is dependent on the energy status of laying hens. J. Lipid Res. 2013, 54, 1860–1876. [Google Scholar] [CrossRef]
- Kumari, K.N.R.; Nath, X.J. Ameliorative measures to counter heat stress in poultry. Worlds Poult. Sci. J. 2018, 74, 117–130. [Google Scholar] [CrossRef]
- Lu, Z.T.; He, X.T.; Ma, B.; Zhang, L.; Li, J.C.; Jiang, Y.; Zhou, G.J.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poul. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, G.; Liu, H. Enoyl-CoA hydratase. reaction, mechanism, and inhibition. Bioorg. Med. Chem. 2003, 11, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, R.; Wei, H.; Wang, Y.; Chen, Y.; Zhang, H.; Li, X.; Liu, H.; Bao, J. Enriched environment housing improved the laying hen’s resistance to transport stress via modulating the heat shock protective response and inflammation. Poul. Sci. 2021, 100, 100939. [Google Scholar] [CrossRef]
- Abare, M.Y.; Rahayu, S.; Tugiyanti, E. Review: The role of heat shock proteins in chicken: Insights into stress adaptation and health. Res. Vet. Sci. 2023, 165, 105057. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, C.; Enríquez, S.L.; la Paz, S.M.; Geniz, I.; Quiroz, M.E.R.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef]
- Monda, J.K.; Ge, X.; Hunkeler, M.; Donovan, K.A.; Ma, M.W.; Jin, C.Y.; Leonard, M.; Fischer, E.S.; Bennett, E.J. HAPSTR1 localizes HUWE1 to the nucleus to limit stress signaling pathways. Cell Rep. 2023, 42, 112496. [Google Scholar] [CrossRef]
- Yano, M.; Nakamuta, S.; Wu, X.; Okumura, Y.; Kido, H. A Novel Function of 14-3-3 Protein: 14-3-3ζ Is a Heat-Shock–related Molecular Chaperone That Dissolves Thermal-aggregated Proteins. Mol. Biol. Cell. 2006, 17, 4769–4779. [Google Scholar] [CrossRef]
- Pennington, K.; Chan, T.; Torres, M.; Andersen, J. The dynamic and stress-adaptive signaling hub of 14-3-3: Emerging mechanisms of regulation and context-dependent protein–protein interactions. Oncogene 2018, 37, 5587–5604. [Google Scholar] [CrossRef]
- Xue, J.; Fan, J.; Li, Y.; Wu, W.; Yan, Q.; Zheng, Q. ABCG1 Attenuates Oxidative Stress Induced by H2O2 through the Inhibition of NADPH Oxidase and the Upregulation of Nrf2-Mediated Antioxidant Defense in Endothelial Cells. Anal. Cell. Pathol. 2020, 2020, 2095645. [Google Scholar] [CrossRef] [PubMed]
- Kardassis, D.; Gafencu, A.; Zannis, V.I.; Davalos, A. Regulation of HDL Genes: Transcriptional, Posttranscriptional, and Posttranslational. Handb. Exp. Pharmacol. 2014, 224, 113–179. [Google Scholar] [CrossRef]
- Roll, A.A.P.; Forgiarini, J.; Xavier, E.G.; Lopes, D.C.N.; Roll, V.F.B.; Rutz, F. Replacing soybean oil with increasing levels of soybean acid oil in diets of coturnix quails (Coturnix coturnix coturnix) and the effects on egg quality. An. Acad. Bras. Cienc. 2017, 90, 529–539. [Google Scholar] [CrossRef] [PubMed]

| Protein Access | Protein | Control ¹ | 0.6% PO 2 | |||
|---|---|---|---|---|---|---|
| Heat shock proteins | ||||||
| O73885 | Heat shock cognate 71 kDa protein | 1.00 | ||||
| A0A8V0ZAR1 | Heat shock protein 90 alpha family class B member 1 | 0.04 | ||||
| A0A8V0YRK7 | Heat shock protein 90 alpha family class A member 1 | 0.99 | 1.00 | |||
| P11501 | Heat shock protein HSP 90-alpha | 0.02 | 1.00 | |||
| A0A8V1A5Z3 | Heat shock 70 kDa protein 8 | 0.01 | 1.00 | |||
| A0A5H3A935 | Heat shock 70 kDa protein | 0.03 | 1.00 | |||
| A0A8V0Z8E9 | Heat shock 70 kDa protein 2 | 1.00 | ||||
| A0A4P8G8N8 | Heat shock protein 70 | 0.03 | 1.00 | Unexpressed | ||
| Q90593 | Endoplasmic reticulum chaperone BiP | 0.98 | ||||
| Q5ZL72 | 60 kDa heat shock protein_ mitochondrial | 0.03 | ||||
| Q2XQE5 | Heat shock protein 90 | 1.00 | ||||
| Q5ZMA5 | Histidine kinase/HSP90-like ATPase domain-containing protein | 1.00 | ||||
| A0A8V0Z0N7 | Peptidyl-prolyl cis/trans isomerase | 0.03 | 1.00 | |||
| Antioxidant proteins | 0.01 | |||||
| A0A8V0WZA6 | Protein disulfide-isomerase | 1.00 | 1.00 | |||
| Q8JG64 | Protein disulfide-isomerase A3 | 1.00 | ||||
| A0A8V0XTG7 | Protein disulfide-isomerase A4 | 1.00 | ||||
| A0A8V0X7N5 | Paraoxonase | 0.05 | ||||
| P26697 | Glutathione S-transferase 3 | 0.98 | ||||
| A0A8V1A317 | Catalase | 1.00 | ||||
| Q5ZL24 | Catalase core domain-containing protein | 1.00 | ||||
| F1NQS2 | Glutathione transferase | 0.01 | 1.00 | |||
| A0A8V0XMJ1 | Glutathione S-transferase zeta 1 | 0.99 | ||||
| A0A8V0YAW2 | Superoxide dismutase [Cu-Zn] | 0.03 | 1.00 | |||
| A0A8V0YJA6 | Superoxide dismutase 1_ soluble | 1.00 | ||||
| A0A8V1AHJ8 | Glutathione S-transferase | 0.01 | ||||
| A0A8V1A5G1 | Peroxiredoxin 1 | 0.03 | ||||
| A0A8V0X8L4 | Peroxiredoxin 3 | 0.03 | ||||
| Q5ZJF4 | Peroxiredoxin-6 | 0.04 | ||||
| P19121 | Albumin | 1.00 | ||||
| A0A8V0Y7H9 | Microsomal glutathione S-transferase 3 | 1.00 | ||||
| Lipid metabolism | ||||||
| A0A8V0XX39 | Apolipoprotein B | 0.99 | ||||
| P87498 | Vitellogenin-1 | 0.98 | ||||
| P02845 | Vitellogenin-2 | 0.01 | ||||
| P02789 | Ovotransferrin | 1.00 | ||||
| A0A8V1AKX1 | Fatty acid synthase | 0.96 | ||||
| P11029 | Acetyl-CoA carboxylase | 1.00 | ||||
| A0A8V0Y492 | Enoyl-CoA hydratase | 0.04 | ||||
| A0A8V0YYX3 | Enoyl-CoA delta isomerase 1 | 0.05 | 0.98 | |||
| P11029 | Acetyl-CoA carboxylase | 1.00 | ||||
| A0A8V0ZEH5 | Diazepam binding inhibitor_ acyl-CoA binding protein | 0.02 | 0.99 | |||
| Q9PRL8 | Acyl-CoA-binding protein | 1.00 | ||||
| A0A8V0Z420 | Hydroxysteroid 17-beta dehydrogenase 12 | 0.95 | ||||
| Immune system | ||||||
| A0A8V0ZVD7 | Immunoglobulin lambda-like polypeptide 1 | 0.03 | ||||
| P20763 | Ig lambda chain C region | 0.02 | ||||
| A0A8V0XHB9 | Ig-like domain-containing protein | 1.00 |
| Protein Access | Protein | 0.6% Pequi Oil |
| P79781 | Ubiquitin-40S ribosomal protein S27a | Uniquely |
| P0CG62 | Polyubiquitin-B | Uniquely |
| A0A8V0ZMQ2 | Ubiquitin B | Uniquely |
| Q91021 | Ubiquitin-like domain-containing protein | Uniquely |
| Q9PST8 | Polyubiquitin | Uniquely |
| A0A8V1A7E5 | Cullin 3 | Uniquely |
| Q5ZKJ2 | 14-3-3 domain-containing protein | Uniquely |
| Q5ZLQ6 | 14-3-3 protein beta/alpha | Uniquely |
| Q5ZMT0 | 14-3-3 protein epsilon | Uniquely |
| Q5F3W6 | 14-3-3 protein gamma | Uniquely |
| Q5ZMD1 | 14-3-3 protein theta | Uniquely |
| Q5ZKC9 | 14-3-3 protein zeta | Uniquely |
| A0A8V0YM11 | Stratifin | Uniquely |
| A0A8V0ZD14 | HUWE1-associated protein modifying stress responses | Uniquely |
| Q5ZJX6 | ATP binding cassette subfamily E member 1 | Uniquely |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.A.D.; da Silva, J.A.; Vieira, J.C.S.; de Lima, G.A.; Cordeiro, L.G.; Castillo, E.O.F.; de Lima Lopes, J.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; Sartori, J.R. The Potential of Pequi Oil as a Modulator of Chaperone Expression to Minimize Heat Stress in Laying Hens. Agriculture 2025, 15, 867. https://doi.org/10.3390/agriculture15080867
Rodrigues PAD, da Silva JA, Vieira JCS, de Lima GA, Cordeiro LG, Castillo EOF, de Lima Lopes J, Buzalaf MAR, de Magalhães Padilha P, Sartori JR. The Potential of Pequi Oil as a Modulator of Chaperone Expression to Minimize Heat Stress in Laying Hens. Agriculture. 2025; 15(8):867. https://doi.org/10.3390/agriculture15080867
Chicago/Turabian StyleRodrigues, Paola Aparecida Damázio, Joyce Andrade da Silva, José Cavalcante Souza Vieira, Gabrieli Andressa de Lima, Laís Garcia Cordeiro, Elis Omar Figueroa Castillo, Júlia de Lima Lopes, Marília Afonso Rabelo Buzalaf, Pedro de Magalhães Padilha, and José Roberto Sartori. 2025. "The Potential of Pequi Oil as a Modulator of Chaperone Expression to Minimize Heat Stress in Laying Hens" Agriculture 15, no. 8: 867. https://doi.org/10.3390/agriculture15080867
APA StyleRodrigues, P. A. D., da Silva, J. A., Vieira, J. C. S., de Lima, G. A., Cordeiro, L. G., Castillo, E. O. F., de Lima Lopes, J., Buzalaf, M. A. R., de Magalhães Padilha, P., & Sartori, J. R. (2025). The Potential of Pequi Oil as a Modulator of Chaperone Expression to Minimize Heat Stress in Laying Hens. Agriculture, 15(8), 867. https://doi.org/10.3390/agriculture15080867









