Description, Identification, and Growth of Ectomycorrhizae in Tuber sinense-Mycorrhized Castanea mollissima Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Seedlings
2.3. Inoculum Production and Seedling Inoculation
2.4. Molecular Identification of Truffle Ectomycorrhizae
2.5. Statistical Analysis of Ectomycorrhizal Colonization Rates
2.6. Morphological Observations of Truffle Ectomycorrhizae
2.6.1. Macroscopic Morphology Under Stereomicroscopy
2.6.2. Preparation and Light Microscopic Examination of Resin-Embedded Sections
2.6.3. Preparation and Laser Confocal Microscopy Examination of Vibratome Sections
2.7. Morphological Statistics and Statistical Analysis
2.8. Mycorrhizal Model Construction Based on Microstructure
3. Results and Discussion
3.1. ITS Identification of Truffle Ectomycorrhizae
3.2. Tuber Ectomycorrhizal Root Colonization Rates
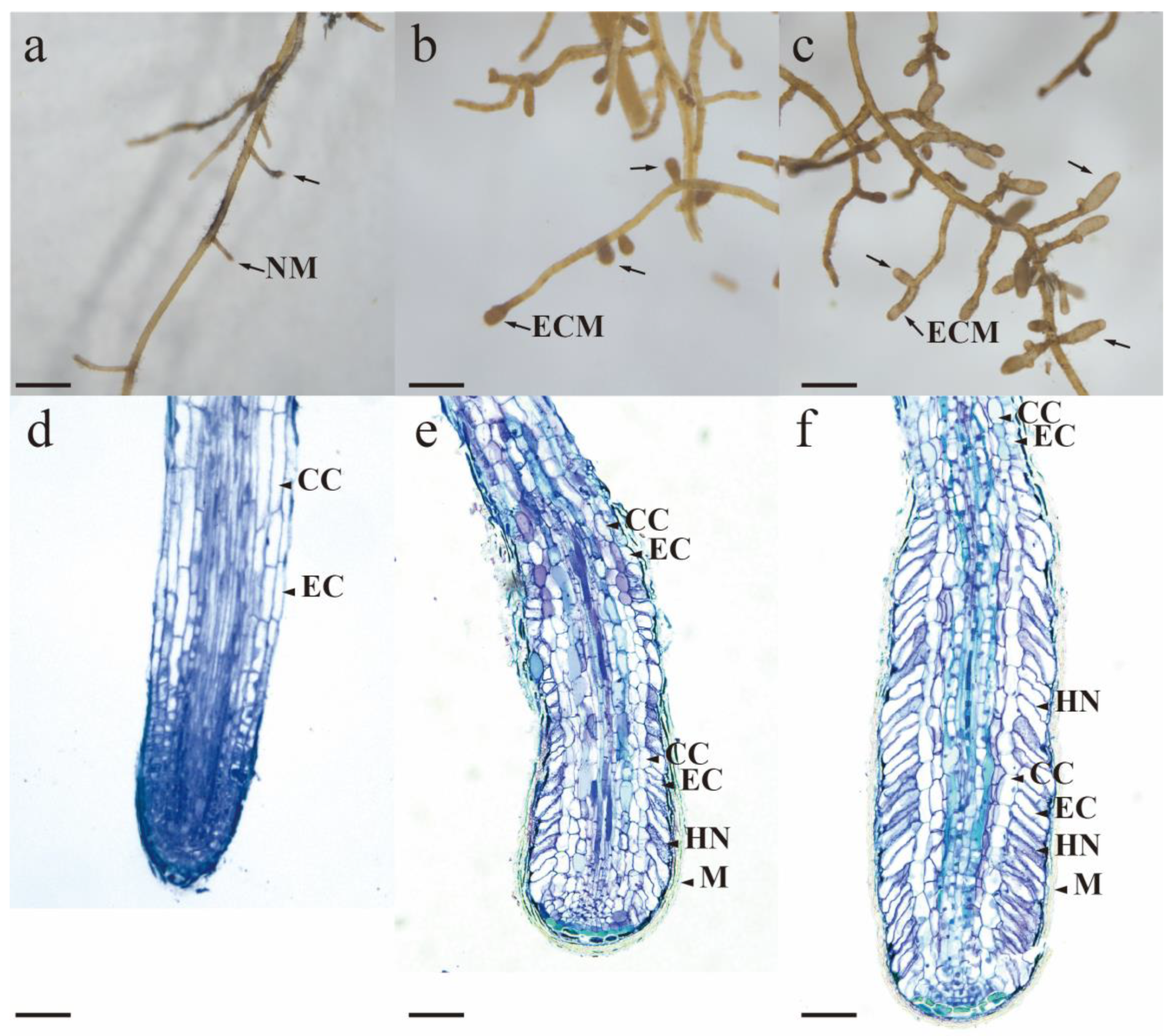
3.3. Macroscopic Morphology Under Stereomicroscopy
3.4. Characterization of Mycorrhizal Colonization Morphology by Light Microscopy
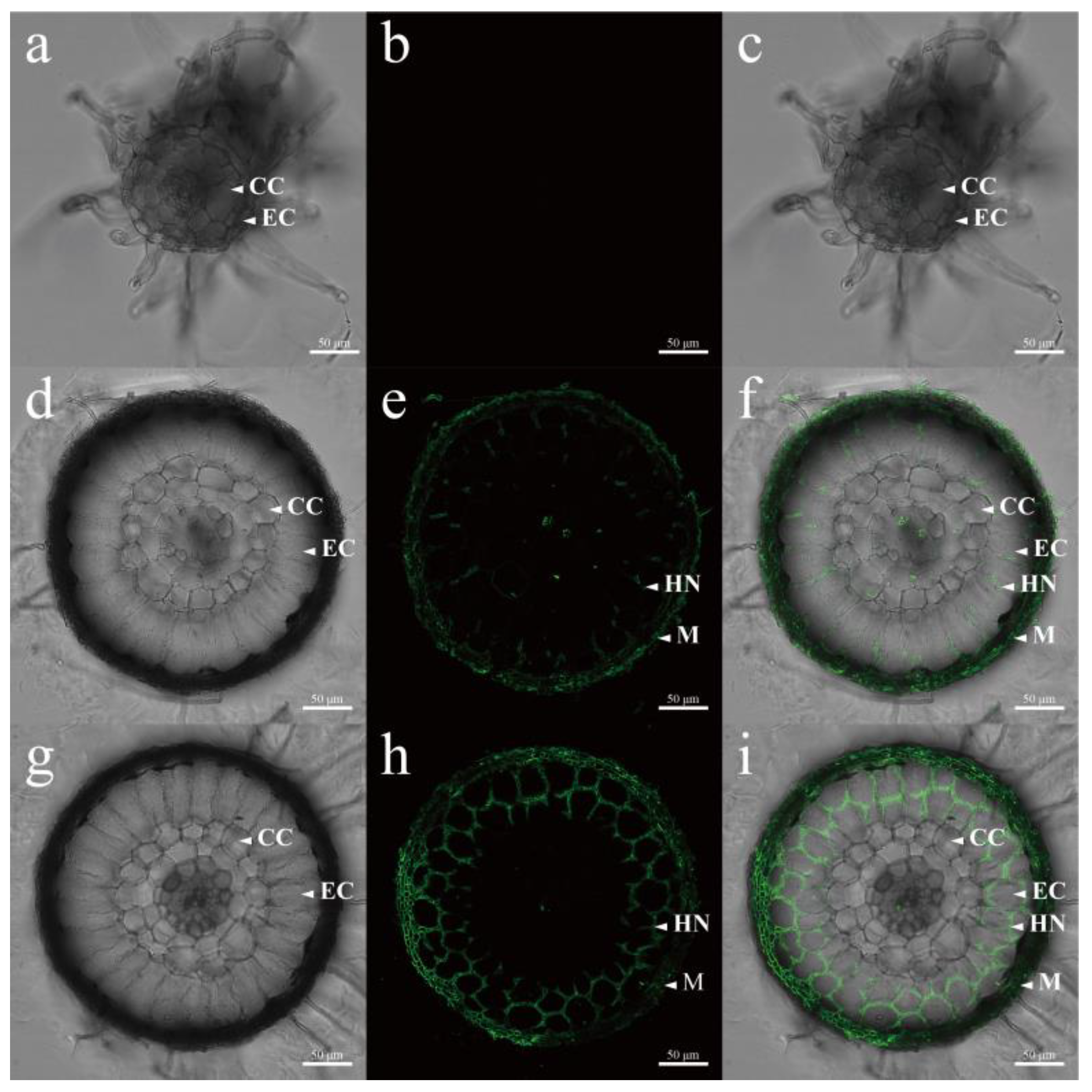
3.5. Laser Confocal Microscopy Analysis of Hyphal Growth
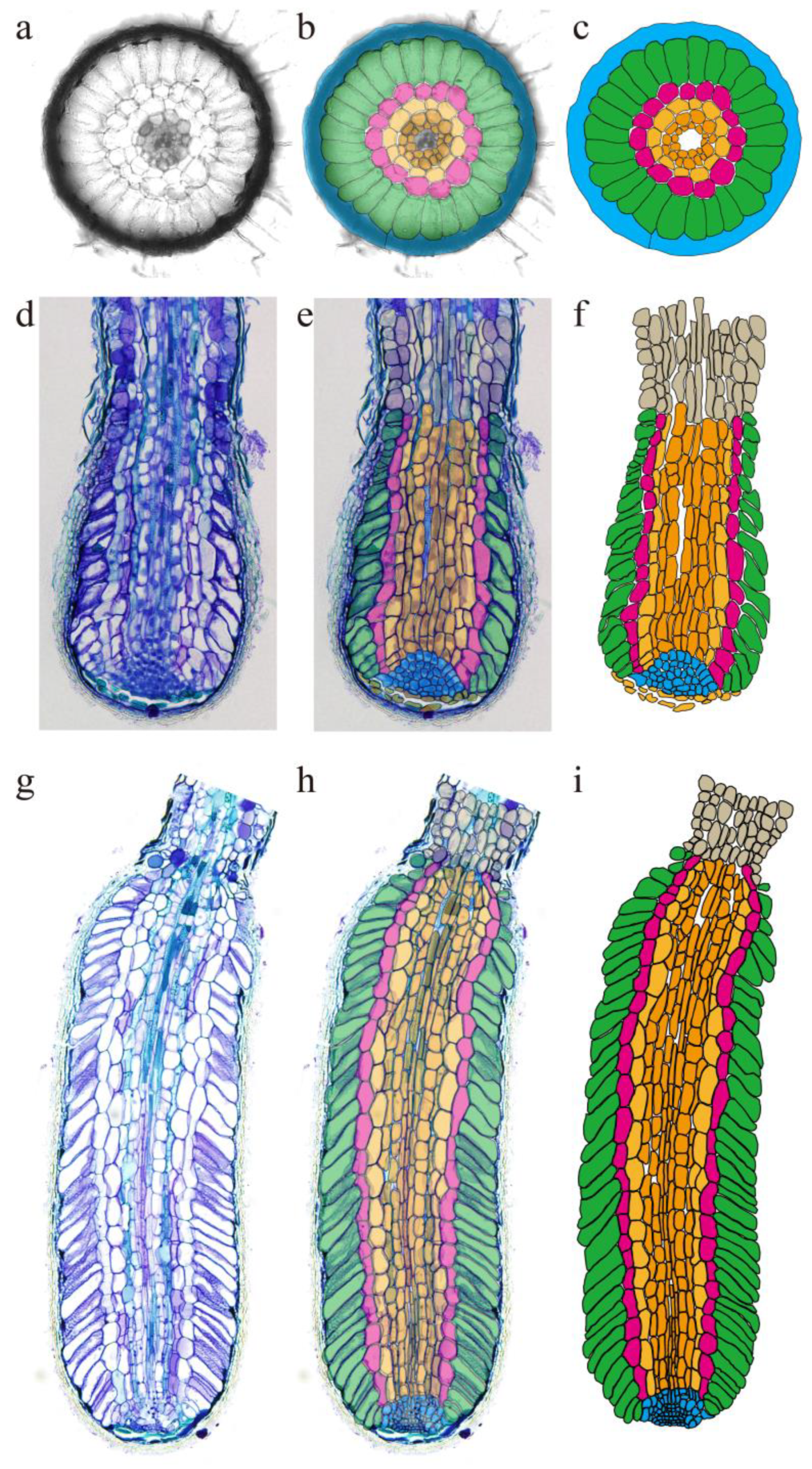
3.6. Mycorrhizal Model Construction Based on Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.P.; Li, X.L.; Ye, L.; Huang, Y.; Kang, Z.J.; Zhang, B.; Zhang, X.P. Colonization by Tuber melanosporum and Tuber indicum affects the growth of Pinus armandii and phoD alkaline phosphatase encoding bacterial community in the rhizosphere. Microbiol. Res. 2020, 239, 126520. [Google Scholar] [CrossRef] [PubMed]
- Zambonelli, A.; Iotti, M.; Murat, C. (Eds.) True Truffle (Tuber spp.) in the World: Soil Ecology, Systematics and Biochemistry; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31436-5. [Google Scholar]
- Huang, L.; Li, Y.; Yuan, J.; Wan, S.; Colinas, C.; He, X.; Shi, X.; Wang, Y.; Yu, F. Tuber indicum and T. lijiangense colonization differentially regulates plant physiological responses and mycorrhizosphere bacterial community of Castanopsis rockii seedlings. Front. Plant Sci. 2023, 14, 1134446. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Wang, Y.L.; Guerin-Laguette, A.; Wang, R.; Zhang, P.; Li, Y.M.; Yu, F.Q. Ectomycorrhizal synthesis between two Tuber species and six tree species: Are different hostfungus combinations having dissimilar impacts on host plant growth? Mycorrhiza 2022, 32, 341–351. [Google Scholar] [CrossRef]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, F.Q.; Zhang, C.; Liu, C.; Yang, M.; Li, S. Edible ectomycorrhizal fungi and their cultivation in China. In Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences; Pérez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F.Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 31–60. ISBN 9783030373788. [Google Scholar]
- Tschaplinski, T.J.; Plett, J.M.; Engle, N.L.; Deveau, A.; Cushman, K.C.; Martin, M.Z.; Doktycz, M.J.; Tuskan, G.A.; Brun, A.; Kohler, A.; et al. Populus trichocarpa and Populus deltoides exhibit different metabolomic responses to colonization by the symbiotic fungus Laccaria bicolor. Mol. Plant-Microbe Interact. 2014, 27, 546–556. [Google Scholar] [CrossRef]
- Daguerre, Y.; Levati, E.; Ruytinx, J.; Tisserant, E.; Morin, E.; Kohler, A.; Montanini, B.; Ottonello, S.; Brun, A.; Veneault-Fourrey, C.; et al. Regulatory networks underlying mycorrhizal development delineated by genome-wide expression profiling and functional analysis of the transcription factor repertoire of the plant symbiotic fungus Laccaria bicolor. BMC Genom. 2017, 18, 737. [Google Scholar] [CrossRef]
- Tang, N.; Lebreton, A.; Xu, W.; Dai, Y.; Yu, F.; Martin, F.M. Transcriptome profiling reveals differential gene expression of secreted proteases and highly specific gene repertoires involved in Lactarius-Pinus symbioses. Front. Plant Sci. 2021, 12, 714393. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Huang, L.L.; Guerin-Laguette, A.; Wang, R.; Yu, F.Q. Characterization of Tuber indicum (Pezizales, Tuberaceae) mycorrhizae synthesized with four host trees exotic to China. Symbiosis 2020, 82, 215–224. [Google Scholar] [CrossRef]
- Nahberger, T.U.; Benucci, G.M.N.; Kraigher, H.; Grebenc, T. Effect of earthworms on mycorrhization, root morphology and biomass of silver fir seedlings inoculated with black summer truffle (Tuber aestivum Vittad.). Sci. Rep. 2021, 11, 6167. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guerin Laguette, A.; Butler, R.; Huang, L.; Yu, F. The European delicacy Tuber melanosporum forms mycorrhizae with some indigenous Chinese Quercus species and promotes growth of the oak seedlings. Mycorrhiza 2019, 29, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Kong, Z. Cellular basis of legume-rhizobium symbiosis. Plant Commun. 2024, 5, 101045. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal symbiosis in plant growth and stress adaptation: From genes to ecosystems. Annu. Rev. Plant Biol. 2022, 74, 569–607. [Google Scholar] [CrossRef]
- Wan, S.P.; Yu, F.Q.; Tang, L.; Wang, R.; Wang, Y.; Liu, P.G.; Wang, X.H.; Zheng, Y. Ectomycorrhizae of Tuber huidongense and T. liyuanum with Castanea mollissima and Pinus armandii. Mycorrhiza 2016, 26, 249–256. [Google Scholar] [CrossRef]
- Li, X.; Ye, L.; Zhang, X.; Tan, H.; Li, Q. Root-tip cutting and uniconazole treatment improve the colonization rate of Tuber indicum on Pinus armandii seedlings in the greenhouse. Microb. Biotechnol. 2020, 13, 535–547. [Google Scholar] [CrossRef]
- Kinoshita, A.; Obase, K.; Yamanaka, T. Ectomycorrhizae formed by three Japanese truffle species (Tuber japonicum, T. longispinosum, and T. himalayense) on indigenous oak and pine species. Mycorrhiza 2018, 28, 679–690. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Murat, C. Forty years of inoculating seedlings with truffle fungi: Past and future perspectives. Mycorrhiza 2015, 25, 77–81. [Google Scholar] [CrossRef]
- Donnini, D.; Benucci, G.M.N.; Bencivenga, M.; Baciarelli-Falini, L. Quality assessment of truffle-inoculated seedlings in Italy: Proposing revised parameters for certification. For. Syst. 2014, 23, 385–393. [Google Scholar] [CrossRef]
- Sillo, F.; Brunetti, C.; Marroni, F.; Vita, F.; Santos Nascimento, L.B.; Vizzini, A.; Mello, A.; Balestrini, R.; dos Santos Nascimento, L.B. Systemic effects of Tuber melanosporum inoculation in two Corylus avellana genotypes. Tree Physiol. 2022, 42, 1463–1480. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate map of Medicago truncatula root nodules. Development 2014, 141, 3517–3528. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Wei, X.; Jin, X.; Wang, L.; Fan, K.; Zou, K.; Zheng, Z.; Saridis, G.; Zhao, N.; Zhou, D.; et al. ARBUSCULAR MYCORRHIZA-INDUCED KINASES AMK8 and AMK24 associate with the receptor-like kinase KINASE3 to regulate arbuscular mycorrhizal symbiosis in Lotus japonicus. Plant Cell 2023, 35, 2006–2026. [Google Scholar] [CrossRef]
- Hill, R.A.; Wong-Bajracharya, J.; Anwar, S.; Coles, D.; Wang, M.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Martin, F.; Anderson, I.C.; et al. Abscisic acid supports colonization of Eucalyptus grandis roots by the mutualistic ectomycorrhizal fungus Pisolithus microcarpus. New Phytol. 2021, 233, 966–982. [Google Scholar] [CrossRef]
- de Chaumont, F.; Dallongeville, S.; Chenouard, N.; Hervé, N.; Pop, S.; Provoost, T.; Meas-Yedid, V.; Pankajakshan, P.; Lecomte, T.; Le Montagner, Y.; et al. Icy: An open bioimage informatics platform for extended reproducible research. Nat. Methods 2012, 9, 690–696. [Google Scholar] [CrossRef]
- Jo, L.; Kajala, K. ggPlantmap: An open-source R package for the creation of informative and quantitative ggplot maps derived from plant images. J. Exp. Bot. 2024, 75, 5366–5376. [Google Scholar] [CrossRef]
- Fan, L.; Li, T.; Xu, Y.Y.; Yan, X.Y. Species diversity, phylogeny, endemism and geography of the truffle genus Tuber in China based on morphological and molecular data. Persoonia-Mol. Phylogeny Evol. Fungi 2022, 48, 175–202. [Google Scholar] [CrossRef]
- Gwon, J.H.; Park, H.; Eom, A.H. Mycorrhization of Quercus spp. with Tuber huidongense and T. himalayense collected in Korea. Mycobiology 2022, 50, 104–109. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.L.; Li, T.; Sun, H.J.; Xiong, W.P.; Li, Y. Chinese black truffles: Tuber yigongense sp. nov., taxonomic reassessment of T. indicum s.l., and re-examination of the T. sinense isotype. Mycotaxon 2018, 133, 183–196. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.H.; Cheewangkoon, R.; Zhao, R.L. Uneven distribution of prokaryote-derived horizontal gene transfer in fungi: A lifestyle-dependent phenomenon. mBio 2025, 16, e0285524. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Calvo, E.; Marco, P.; Fischer, M.; Creydt, M. Mass spectrometry-based non-targeted lipidome analysis and extraction of markers for the authentication of white and black truffle species and their origin determination. Agriculture 2024, 14, 2350. [Google Scholar] [CrossRef]
- Geng, L.; Wang, X.; Yu, F.; Deng, X.; Tian, X.; Shi, X.; Xie, X.; Liu, P.; Shen, Y. Mycorrhizal synthesis of Tuber indicum with two indigenous hosts, Castanea mollissima and Pinus armandii. Mycorrhiza 2009, 19, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Mrak, T.; Grebenc, T.; Friedrich, S.; Münzenberger, B. Description, identification, and growth of Tuber borchii Vittad. mycorrhized Pinus sylvestris L. seedlings on different lime contents. Mycorrhiza 2024, 34, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barreda, S.; Molina-Grau, S.; Reyna, S. Fertilisation of Quercus seedlings inoculated with Tuber melanosporum: Effects on growth and mycorrhization of two host species and two inoculation methods. Iforest-Biogeosci. For. 2017, 10, 267–272. [Google Scholar] [CrossRef]
- Marozzi, G.; Sánchez, S.; Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Albertini, E.; Donnini, D. Mycorrhization of pecan (Carya illinoinensis) with black truffles: Tuber melanosporum and Tuber brumale. Mycorrhiza 2017, 27, 303–309. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sánchez-García, M.; Morin, E.; Andreopoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef]
- Liao, H.-L.; Chen, Y.; Vilgalys, R. Metatranscriptomic study of common and host-specific patterns of gene expression between pines and their symbiotic ectomycorrhizal fungi in the genus Suillus. PLoS Genet. 2016, 12, e1006348. [Google Scholar] [CrossRef]
- Basso, V.; Kohler, A.; Miyauchi, S.; Singan, V.; Guinet, F.; Šimura, J.; Novák, O.; Barry, K.W.; Amirebrahimi, M.; Block, J.; et al. An ectomycorrhizal fungus alters sensitivity to jasmonate, salicylate, gibberellin, and ethylene in host roots. Plant Cell Environ. 2020, 43, 1047–1068. [Google Scholar] [CrossRef]
- Plett, K.L.; Buckley, S.; Plett, J.M.; Anderson, I.C.; Lundberg-Felten, J.; Jämtgård, S. Novel microdialysis technique reveals a dramatic shift in metabolite secretion during the early stages of the Interaction between the ectomycorrhizal fungus Pisolithus microcarpus and its host Eucalyptus grandis. Microorganisms 2021, 9, 1817. [Google Scholar] [CrossRef]
- Agerer, R. Fungal relationships and structural identity of their ectomycorrhizae. Mycol Prog. 2006, 5, 67–107. [Google Scholar] [CrossRef]
- Rambold, G.; Agerer, R. DEEMY—The concept of a characterization and determination system for ectomycorrhizae. Mycorrhiza 1997, 7, 113–116. [Google Scholar] [CrossRef]
- Álvarez-Lafuente, A.; Benito-Matías, L.F.; Peñuelas-Rubira, J.L.; Suz, L.M. Multi-cropping edible truffles and sweet chestnuts: Production of high-quality Castanea sativa seedlings inoculated with Tuber aestivum, its ecotype T. uncinatum, T. brumale, and T. macrosporum. Mycorrhiza 2018, 28, 29–38. [Google Scholar] [CrossRef]
- Ori, F.; Leonardi, M.; Faccio, A.; Sillo, F.; Iotti, M.; Pacioni, G.; Balestrini, R. Synthesis and ultrastructural observation of arbutoid mycorrhizae of black truffles (Tuber melanosporum and T. aestivum). Mycorrhiza 2020, 30, 715–723. [Google Scholar] [CrossRef]
- Lancellotti, E.; Iotti, M.; Zambonelli, A.; Franceschini, A. Characterization of Tuber borchii and Arbutus unedo mycorrhizas. Mycorrhiza 2014, 24, 481–486. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal biogeography. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Martin, F.M.; van der Heijden, M.G.A. The mycorrhizal symbiosis: Research frontiers in genomics, ecology, and agricultural application. New Phytol. 2024, 242, 1486–1506. [Google Scholar] [CrossRef]
- Ruytinx, J.; Miyauchi, S.; Hartmann-Wittulsky, S.; de Freitas Pereira, M.; Guinet, F.; Churin, J.-L.; Put, C.; Le Tacon, F.; Veneault-Fourrey, C.; Martin, F.; et al. A transcriptomic atlas of the ectomycorrhizal fungus Laccaria bicolor. Microorganisms 2021, 9, 2612. [Google Scholar] [CrossRef]
- Peterson, R.L.; Massicotte, H.B. Exploring structural definitions of mycorrhizas, with emphasis on nutrient-exchange interfaces. Can. J. Bot. 2004, 82, 1074–1088. [Google Scholar] [CrossRef]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef]
- Larsen, P.E.; Sreedasyam, A.; Trivedi, G.; Desai, S.; Dai, Y.; Cseke, L.J.; Collart, F.R. Multi-omics approach identifies molecular mechanisms of plant-fungus mycorrhizal interaction. Front. Plant Sci. 2015, 6, 1061. [Google Scholar] [CrossRef] [PubMed]

| Sample | Colonization Rate (%) | Mantle Cell Layers | Mantle Thickness (μm) | Epidermal Cell Perimeter (μm) | Epidermal Cell Cross-Sectional Area (μm2) |
|---|---|---|---|---|---|
| NI | - | - | - | 152.70 ± 22.51 a | 2295.21 ± 336.81 b |
| T3 | 24.4 ± 5.3 b | 2–3 | 4.09 ± 0.62 b | 44.30 ± 7.12 c | 1551.88 ± 558.33 c |
| T8 | 45.2 ± 8.6 a | 3–5 | 5.23 ± 0.56 a | 79.70 ± 9.48 b | 2353.89 ± 653.93 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, W.; Cao, Q.; Yang, R.; Qin, Y.; Zhang, G. Description, Identification, and Growth of Ectomycorrhizae in Tuber sinense-Mycorrhized Castanea mollissima Seedlings. Agriculture 2025, 15, 868. https://doi.org/10.3390/agriculture15080868
Wang Y, Zhang W, Cao Q, Yang R, Qin Y, Zhang G. Description, Identification, and Growth of Ectomycorrhizae in Tuber sinense-Mycorrhized Castanea mollissima Seedlings. Agriculture. 2025; 15(8):868. https://doi.org/10.3390/agriculture15080868
Chicago/Turabian StyleWang, Yiyang, Weiwei Zhang, Qingqin Cao, Rui Yang, Yong Qin, and Guoqing Zhang. 2025. "Description, Identification, and Growth of Ectomycorrhizae in Tuber sinense-Mycorrhized Castanea mollissima Seedlings" Agriculture 15, no. 8: 868. https://doi.org/10.3390/agriculture15080868
APA StyleWang, Y., Zhang, W., Cao, Q., Yang, R., Qin, Y., & Zhang, G. (2025). Description, Identification, and Growth of Ectomycorrhizae in Tuber sinense-Mycorrhized Castanea mollissima Seedlings. Agriculture, 15(8), 868. https://doi.org/10.3390/agriculture15080868





