Impact of Peas (Pisum Sativum L.) as a Sustainable Source of Protein in Growing Pigs’ Diets on Production Efficiency, Nitrogen Metabolism and Gastrointestinal Tract Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Purchases and Chemical Analyses
2.2. Ethics, Animals, Housing, and Experimental Diets
2.3. Measurements, Sample Collection, and Procedures
2.3.1. Pig Performances
2.3.2. Nitrogen Metabolism
2.3.3. Blood Sample Collection for Plasma Protein Profile
2.3.4. Measurements and Intestinal Content Analysis
Intestinal Segments and Intestinal Content pH Measurements
Short-Chain Fatty Acid (SCFA) Analysis
Intestinal Microbiota Assessment
2.3.5. Intestinal Segments Collected for Histological Analysis
2.4. Statistical Analysis
3. Results
3.1. Pisum sativum L. Chemical Composition
3.2. Growth Performance, Nitrogen Balance, and Digestibility Coefficients
3.3. The Protein Profile in Blood Samples
3.4. Intestinal Content Assessment of Short Chain Fatty Acids
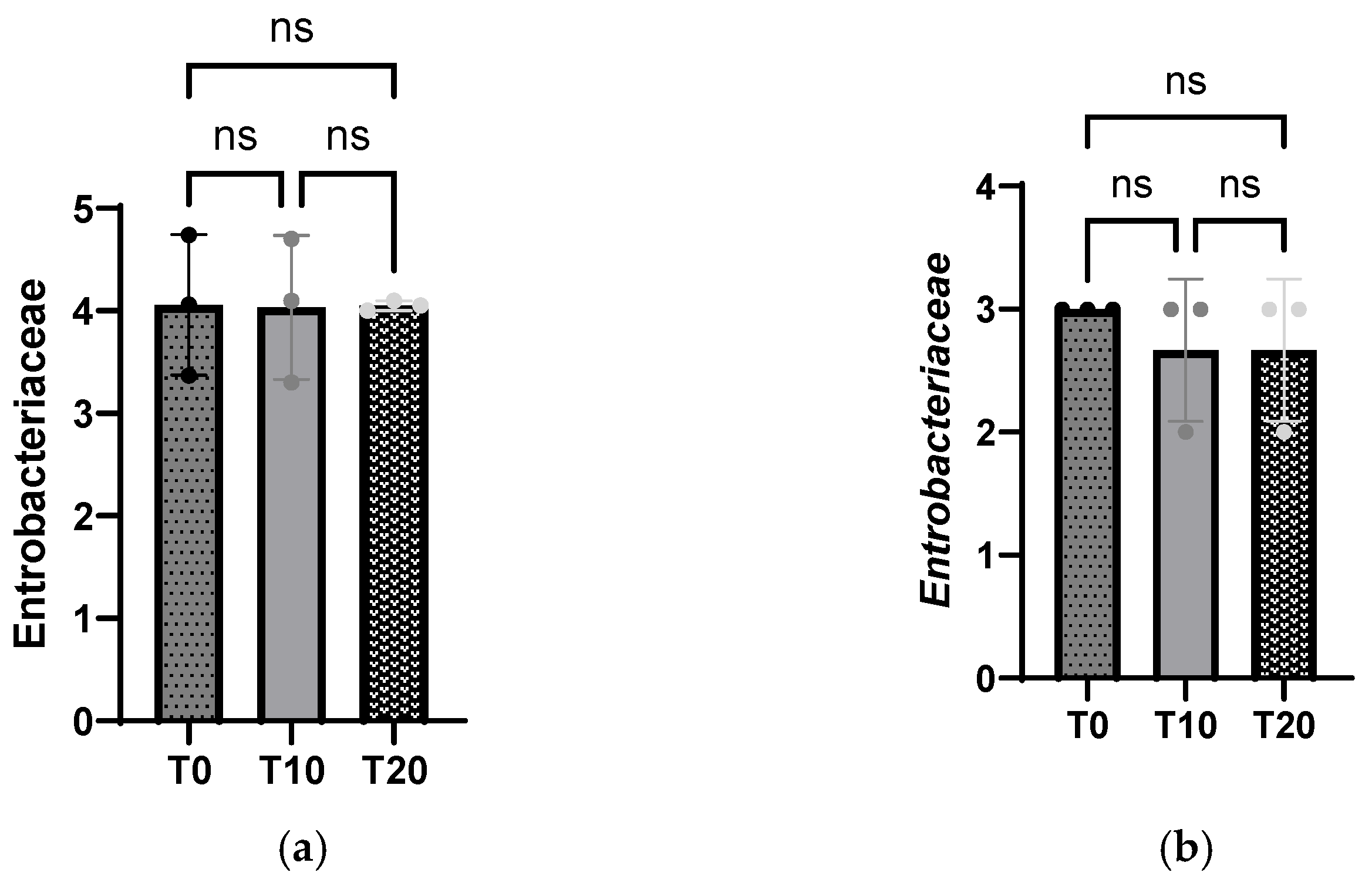
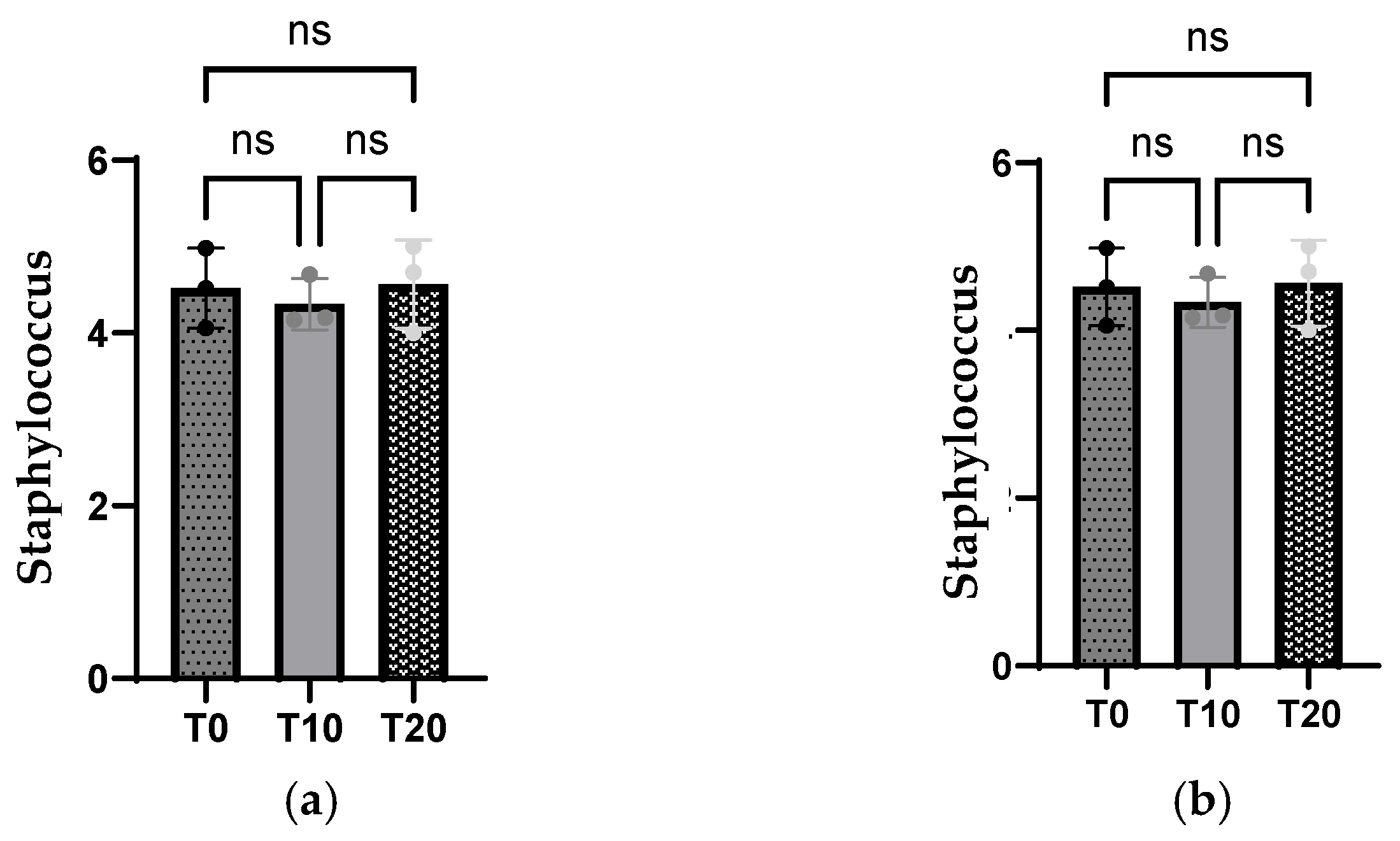
3.5. Intestinal Population Microbiota from Ileal and Cecal Segments
3.6. Intestinal Length and pH Measurements
3.7. Intestinal Morphology of Small Intestine
3.8. Correlation Between Performance Parameters, Nitrogen Metabolism, Short Chain Fatty Acids, Intestinal Microbiota, and Intestinal Villus/Crypt Ratio in Pigs’ Growing Phase
4. Discussion
4.1. Pisum sativum L. Chemical Composition
4.2. Growth Performance, Nitrogen Balance, and Digestibility Coefficients
4.3. The Protein Profile in Blood Samples
4.4. Short Chain Fatty Acid Intestinal Content
4.5. Microbial Status of Growing Pigs
4.6. Intestinal Length and pH Measurements
4.7. Intestinal Morphology
4.8. Correlation Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Colgrave, S.M. Allergenicity of novel and traditional protein sources in food production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef] [PubMed]
- Khanashyam, A.C.; Mundanat, A.S.; Sajith Babu, K.; Thorakkattu, P.; Krishnan, R.; Abdullah, S.; Bekhit, A.E.A.; McClements, D.J.; Santivarangkna, C.; Nirmal, N.P. Emerging alternative food protein sources: Production process, quality parameters, and safety point of view. Crit. Rev. Biotechnol. 2025, 45, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Krajewska, A.; Dziki, D.; Hassoon, W.H. Sustainability of alternatives to animal protein sources, a comprehensive review. Sustainability 2024, 16, 7701. [Google Scholar] [CrossRef]
- Cusworth, G.; Garnett, T.; Lorimer, J. Legume dreams: The contested futures of sustainable plant-based food systems in Europe. Glob. Environ. Change 2021, 69, 102321. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Qazanfarzadeh, Z.; Majzoobi, M.; Sheiband, S.; Oladzadabbasabad, N.; Esmaeili, Y.; Barrow, C.J.; Timms, W. Alternative proteins; A path to sustainable diets and environment. Curr. Res. Food Sci. 2024, 9, 100882. [Google Scholar] [CrossRef]
- Singh, N.; Jain, P.; Ujinwal, M.; Langyan, S. Escalate protein plates from legumes for sustainable human nutrition. Front. Nutr. 2022, 9, 977986. [Google Scholar] [CrossRef] [PubMed]
- Tuśnio, A.; Taciak, M.; Barszcz, M.; Święch, E.; Bachanek, I.; Skomiał, J. Effect of replacing soybean meal by raw or extruded pea seeds on growth performance and selected physiological parameters of the ileum and distal colon of pigs. PLoS ONE 2017, 12, e0169467. [Google Scholar] [CrossRef]
- Parrini, A. Soybean replacement by alternative protein sources in pig nutrition and its effect on meat quality. Animals 2023, 13, 494. [Google Scholar] [CrossRef]
- Zaworska, A.; Kasprowicz-Potocka, M.; Rutkowski, A.; Jamroz, D. The influence of dietary raw and extruded field peas (Pisum sativum L.) on nutrient digestibility and performance of weaned and fattening pigs. Anim. Feed Sci. Technol. 2018, 240, 25–34. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Witek-Krowiak, A.; Gersz, A.; Moustakas, K.; Iwaniuk, J.; Grzędzicki, M.; Korczyński, M. Innovative high digestibility protein feed materials reducing environmental impact through improved nitrogen-use efficiency in sustainable agriculture. J. Environ. Manag. 2021, 291, 112693. [Google Scholar] [CrossRef]
- White, G.A.; Smith, L.A.; Houdijk, J.G.M.; Homer, D.; Kyriazakis, I.; Wiseman, J. Replacement of soybean meal with peas and faba beans in growing/finishing pig diets: Effect on performance, carcass composition and nutrient excretion. Anim. Feed Sci. Technol. 2015, 209, 202–210. [Google Scholar] [CrossRef]
- Lestingi, A. Alternative and sustainable protein sources in pig diet: A review. Animals 2024, 14, 310. [Google Scholar] [CrossRef]
- Thavarajah, D.; Lawrence, T.; Boatwright, L.; Windsor, N.; Johnson, N.; Kay, J.; Shipe, E.; Kumar, S.; Thavarajah, P. Organic dry pea (Pisum sativum L.): A sustainable alternative pulse-based protein for human health. PLoS ONE 2023, 18, e0284380. [Google Scholar] [CrossRef] [PubMed]
- Pulse Canada. Available online: https://pulsecanada.com (accessed on 20 October 2024).
- Wu, D.T.; Li, W.X.; Wan, J.J.; Hu, Y.C.; Gan, R.Y.; Zou, L. A comprehensive review of pea (Pisum sativum L.): Chemical composition, processing, health benefits, and food applications. Foods 2023, 12, 2527. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.H. Field Peas in Diets Fed to Swine; SDSU Extension Special Series. 1; South Dakota State University: Brookings, SD, USA, 2006; Available online: https://openprairie.sdstate.edu/extension_circ/1/ (accessed on 17 February 2025).
- Bărbieru, A. Relation of quantitative traits in winter peas (Pisum sativum L.). Rom. Agric. Res. 2023, 40, 199–207. [Google Scholar] [CrossRef]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef]
- Shurson, G.C.; Kerr, B.J. Challenges and opportunities for improving nitrogen utilization efficiency for more sustainable pork production. Front. Anim. Sci. 2023, 4, 1204863. [Google Scholar] [CrossRef]
- Ball, M.E.E.; Smyth, S.; Beattie, V.E.; McCracken, K.J.; McCormack, U.; Muns, R.; Gordon, F.J.; Bradford, R.; Reid, L.A.; Magowan, E. The environmental impact of lowering dietary crude protein in finishing pig diets—The effect on ammonia, odour and slurry production. Sustainability 2022, 14, 12016. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, R.; Qiao, M.; Ma, Y.; Li, T.; Li, N.; Song, L. The pea oligosaccharides could stimulate the in vitro proliferation of beneficial bacteria and enhance anti-inflammatory effects via the NF-κB pathway. Foods 2024, 13, 626. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Burlacu, G.H.; Cavache, A.; Burlacu, R.; Surdu, I. Potentialul Productiv Al Nutrețurilor și Utilizarea Lor, 1st ed.; Editura Ceres: Bucharest, Romania, 2002; p. 1. [Google Scholar]
- Hlatini, V.A.; Ncobela, C.N.; Chimonyo, M. Nitrogen balance response to varying levels of dietary protein in slow-growing Windsnyer pigs. S. Afr. J. Anim. Sci. 2020, 50, 644–653. [Google Scholar] [CrossRef]
- Mihaila, G.; Habeanu, M.; Lefter, N.; Gheorghe, A.; Dumitru, M.; Marin, I.; Vidu, L.; Nicolae, G.C.; Popa, D.; Marin, M. Influence of Guar Meal from pig compound feed on productive performance, nitrogen metabolism, and greenhouse gas emissions. Agriculture 2023, 13, 2156. [Google Scholar] [CrossRef]
- Norsvin, T. Feeding Manual Norsvin Duroc Progeny; Topigs Norsvin: ’s-Hertogenbosch, The Netherlands, 2017. [Google Scholar]
- Commission Regulation EC 152/2009; Laying Down the Methods of Sampling and Analysis for the Official Control of Feed. Official Journal of the European Union: Luxembourg, 2009.
- ISO 5983-2:2009; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content. Part 2: Block Digestion and Steam Distillation Method. International Organization for Standardization: Geneva, Switzerland, 2009.
- Moreira, I.; Fraga, A.L.; Paiano, D.; Oliveira, G.C.; Scapinello, C.; Martins, E.N. Nitrogen balance of starting barrow pigs fed on increasing lysine levels. Braz. Arch. Biol. Technol. 2004, 47, 85–91. [Google Scholar] [CrossRef]
- Directive 2010/63/EU; The European Parliament and the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union: Luxembourg, 2010; L 276/33-79. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng (accessed on 18 April 2025).
- Sorescu, I.; Dumitru, M.; Ciurescu, G. Lactobacillus spp. and Enterococcus faecium strains isolation, identification, preservation and quantitative determinations from turkey gut content. Biotechnol. Lett. 2019, 24, 41–49. [Google Scholar] [CrossRef]
- Ciurescu, G.; Dumitru, M.; Gheorghe, A.; Untea, A.E.; Drăghici, R. Effect of Bacillus subtilis on growth performance, bone mineralization, and bacterial population of broilers fed with different protein sources. Poult. Sci. 2020, 99, 5960–5971. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Giger-Reverdin, S.; Noblet, J.; Renaudeau, D.; Lessire, M.; Lebas, F. Pea Seeds; Feedipedia, A Programme by INRAE, CIRAD, AFZ and FAO. 2017. Available online: https://feedipedia.org/node/264 (accessed on 20 October 2024).
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Stanojevic, S.; Pesic, M.; Pavlicevic, M.; Zlatkovic, B.; Jankovic, M. Functional properties of protein hydrolysates from pea (Pisum sativum L) seeds. Int. J. Food Sci. Technol. 2012, 47, 1457–1467. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.C.; Asavajaru, P.; Lam, A. Pea: A sustainable vegetable protein crop. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 145–164. [Google Scholar] [CrossRef]
- Jørgensen, H.; Zhao, X.Q.; Eggum, B.O. The influence of dietary fibre and environmental temperature on the development of the gastrointestinal tract, digestibility, degree of fermentation in the hind-gut and energy metabolism in pigs. Br. J. Nutr. 1996, 75, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, F.; Jondreville, C.; Williatte-Hazouard, I.; Skiba, F.; Carrouée, B.; Gatel, F. Ileal digestibility of protein and amino acids of feed peas with different trypsin inhibitor activity in pigs. Can. J. Anim. Sci. 2000, 80, 643–652. [Google Scholar] [CrossRef]
- de Almeida Costa, G.E.; da Silva Queiroz-Monici, K.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Gawalko, E.J. Effect of variety and processing on nutrients and certain anti-nutrients in field peas (Pisum sativum). Food Chem. 2008, 111, 132–138. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Li, Y. Pea protein composition, functionality, modification, and food applications: A review. Adv. Food Nutr. Res. 2022, 101, 71–127. [Google Scholar]
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A perspective on pea allergy and pea allergens. Trends Food Sci. Technol. 2021, 116, 186–198. [Google Scholar] [CrossRef]
- Mihailović, V.; Mikić, A.; Erić, P.; Vasiljević, S.; Ćupina, B.; Katić, S. Protein pea in animal feeding. Biotechnol. Anim. Husb. 2005, 21, 281–285. [Google Scholar] [CrossRef]
- ITCF; UNIP; PGRO. Feed Peas—A Quality Raw Material for Animal Feeding; Institut Technique des Céréales et des Fourrages (ITCF): Paris, France; Union Nationale Interprofessionnelle des Plantes Riches en Protéines (UNIP), Processors & Growers Research Organisation (PGRO): Peterborough, UK, 1996. [Google Scholar]
- UNIP; ITCF. Peas—Utilisation in Animal Feeding; Union Nationale Interprofessionelle des Plants Riches en Protéines (UNIP) and Institut Technique des Céréals et des Fourrages (ITCF): Paris, France, 1995; p. 102. [Google Scholar]
- Sońta, M.; Batorska, M.; Więcek, J.; Rekiel, A. Performance results and concentrations of biochemical indices and mineral elements in blood serum of fatteners fed diets containing mixtures of raw seeds of pea (Pisum sativum L.) or blue lupin (Lupinus angustifolius L.). Animals 2020, 10, 858. [Google Scholar] [CrossRef]
- Prandini, A.; Morlacchini, M.; Moschini, M.; Fusconi, G.; Masoero, F.; Piva, G. Raw and extruded pea (Pisum sativum) and lupin (Lupinus albus var. Multitalia) seeds as protein sources in weaned piglets’ diets: Effect on growth rate and blood parameters. Ital. J. Anim. Sci. 2005, 4, 385–394. [Google Scholar]
- Hanczakowska, E.; Księżak, J.; Świątkiewicz, M. Efficiency of pea seeds in sow, piglet and fattener feeding. Anim. Prod. Sci. 2019, 59, 304–313. [Google Scholar] [CrossRef]
- Purwin, C.; Stanek, M. Nutrient digestibility and nitrogen balance in growing-finishing pigs fed legume-based diets. Ann. UMCS Zootech. 2011, 29, 52–61. [Google Scholar] [CrossRef]
- Francine, Q.; Florence, G.L.; Aurélie, W.; Jean-Yves, D.; Etienne, L. Eco-friendly diet: Nutrient digestibility, nitrogen and energy balances and growth performance of growing pigs. J. Anim. Sci. 2024, 102, skae203. [Google Scholar] [CrossRef]
- Smith, L.A.; Houdijk, J.G.M.; Homer, D.; Kyriazakis, I. Effects of dietary inclusion of pea and faba bean as a replacement for soybean meal on grower and finisher pig performance and carcass quality. J. Anim. Sci. 2013, 91, 3733–3741. [Google Scholar] [CrossRef]
- Stein, H.H. Field peas can be included in the phase 2 diet for nursery pigs without adverse effects on pig performance. J. Anim. Sci. 2002, 80, 1000–1010. [Google Scholar]
- Stein, H.H.; Everts, A.K.R.; Sweeter, K.K.; Peters, D.N.; Maddock, R.J.; Wulf, D.M.; Pedersen, C. The influence of dietary field peas (Pisum sativum L.) on pig performance, carcass quality, and the palatability of pork. J. Anim. Sci. 2006, 84, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Loan, N.V.T.H.; van Kinh, L.; Riley, W.; Huyen, L.T.T.; Ngoan, L.D. The effect of replacing soybean meal by field pea (Pisum sativum) seed meal in diets on growth performance, carcass traits and meat quality of finishing pigs in the tropical climate conditions of Viet Nam. Asian-Australas. J. Anim. Sci. 2023, 35, 81. [Google Scholar]
- Degola, L.; Jonkus, D. The influence of dietary inclusion of peas, faba bean and lupin as a replacement for soybean meal on pig performance and carcass traits. Agron. Res. 2018, 16, 389–397. [Google Scholar] [CrossRef]
- Sirtori, F.; Crovetti, A.; Acciaioli, A.; Bonelli, A.; Pugliese, C.; Bozzi, R.; Campodoni, G.; Franci, O. Effect of replacing a soy diet with Vicia faba and Pisum sativum on performance, meat and fat traits of Cinta Senese pigs. Ital. J. Anim. Sci. 2015, 14, 3659. [Google Scholar] [CrossRef]
- Iowa State University. Available online: https://vetmed.iastate.edu (accessed on 12 February 2025).
- Kalakonda, A.; Jenkins, B.A.; John, S. Physiology, bilirubin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470290/ (accessed on 17 February 2025).
- Guerra Ruiz, A.R.; Crespo, J.; López Martínez, R.M.; Iruzubieta, P.; Casals Mercadal, G.; Lalana Garcés, M.; Lavin, B.; Morales Ruiz, M. Measurement and clinical usefulness of bilirubin in liver disease. Adv. Lab. Med. 2021, 2, 352–372. [Google Scholar] [CrossRef]
- Laboklin. Available online: https://laboklin.com (accessed on 10 December 2024).
- Wen, S.; Arakawa, H.; Tamai, I. Uric acid in health and disease: From physiological functions to pathogenic mechanisms. Pharmacol. Ther. 2024, 256, 108615. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, P.; Mosiichuk, N.; Woliński, J.; Yatsenko, T.; Grujic, D.; Lozinska, L.; Pieszka, M.; Święch, E.; Pierzynowski, S.G.; Goncharova, K. Oral uricase eliminates blood uric acid in the hyperuricemic pig model. PLoS ONE 2017, 12, e0179195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 61. [Google Scholar] [CrossRef]
- Che, L.; Chen, H.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Huang, Z.; Chen, D. Long-term intake of pea fibre affects colonic barrier function, bacterial and transcriptional profile in pig model. Nutr. Cancer 2014, 66, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, H.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Chen, D. Dietary pea fibre alters the microbial community and fermentation with increase in fibre degradation-associated bacterial groups in the colon of pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e254–e261. [Google Scholar] [CrossRef]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibres on fecal microbiota composition and short-chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Hooda, S.; Pieper, R.; Zijlstra, R.T.; van Kessel, A.G.; Mosenthin, R.; Gänzle, M.G. Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl. Environ. Microbiol. 2010, 76, 3692–3701. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fibre on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fibre and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fibre in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lærke, H.N.; Hedemann, M.S. The digestive system of the pig. In Nutritional Physiology of Pigs; Bach Knudsen, K.E., Kjeldsen, N.J., Poulsen, H.D., Jensen, B.B., Eds.; Videncenter for Svineproduktion: København, Danmarks, 2012. [Google Scholar]
- Prakatur, I.; Miskulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miskulin, I.; Domacinovic, M. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals 2019, 9, 301. [Google Scholar] [CrossRef]
- Kelly, D.D.; Smyth, J.A.; McCracken, K.J. Digestive development of the early-weaned pig: 2. Effect of level of food intake on digestive enzyme activity during the immediate post-weaning period. Br. J. Nutr. 1991, 65, 181–188. [Google Scholar] [CrossRef]
- Jin, L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Crenshaw, J.D. Effects of dietary fibre on intestinal growth, cell proliferation, and morphology in growing pigs. J. Anim. Sci. 1994, 72, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.E.; Pethick, D.W.; Mullan, B.P.; Hampson, D.J. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br. J. Nutr. 2001, 86, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.R. Characterization of the Effects of Intestinal Physiology Modified by Exogenous Enzymes and Direct-Fed Microbial on Intestinal Integrity, Energy Metabolism, Body Composition and Performance of Laying Hens and Broiler Chickens. PhD Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Parker, A.; Maclaren, O.J.; Fletcher, A.G.; Muraro, D.; Kreuzaler, P.A.; Byrne, H.M.; Maini, P.K.; Watson, A.J.; Pin, C. Cell proliferation within small intestinal crypts is the principal driving force for cell migration on villi. FASEB J. 2017, 31, 636–649. [Google Scholar] [CrossRef]
- Souza da Silva, M.; Perricone, V.; Vieira, S.L.; Lemme, A.; Hancock, J.D. Effects of different levels of dietary pea protein on broiler performance. Poult. Sci. 2021, 100, 101478. [Google Scholar]
- Quinteiro-Filho, W.M.; Gomes, A.V.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-de-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012, 41, 421–427. [Google Scholar] [CrossRef]
- Rehman, H.; Rosenkranz, C.; Böhm, J.; Zentek, J. Dietary inulin affects the morphology but not the sodium-dependent glucose and glutamine transport in the jejunum of broilers. Poult. Sci. 2007, 86, 118–122. [Google Scholar] [CrossRef]
- Landero, J.L.; Wang, L.F.; Beltranena, E.; Zijlstra, R.T. Diet nutrient digestibility and growth performance of weaned pigs fed field pea. Anim. Feed Sci. Technol. 2014, 198, 295–303. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.B.; Che, L.Q.; Yu, B.; He, J.; Yu, J.; Han, G.Q.; Huang, Z.Q.; Zheng, P.; Chen, D.W. Impact of fibre types on gut microbiota, gut environment and gut function in fattening pigs. Anim. Feed Sci. Technol. 2014, 195, 101–111. [Google Scholar] [CrossRef]
- Torrallardona, D.; Harris, C.I.; Fuller, M.F. Lysine synthesized by the gastrointestinal microflora of pigs is absorbed, mostly in the small intestine. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1177–E1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, J.; Song, X.; Yang, M.; Wang, H.; Wu, Y. Feeding dietary fermentable fibre improved fecal microbial composition and increased acetic acid production in a nursery pig model. J. Anim. Sci. 2023, 101, skad260. [Google Scholar] [CrossRef]
- Van Nevel, C.J.; Dierick, N.A.; Decuypere, J.A.; De Smet, S.M. In vitro fermentability and physicochemical properties of fibre substrates and their effect on bacteriological and morphological characteristics of the gastrointestinal tract of newly weaned piglets. Arch. Anim. Nutr. 2006, 60, 477–500. [Google Scholar] [CrossRef]

| Ingredients, % | Growing–Finishing Phase Diet | ||
|---|---|---|---|
| T0 | T10 | T20 | |
| Corn meal | 54.85 | 48.66 | 42.58 |
| Triticale | 18.00 | 18.00 | 17.89 |
| Soybean meal (46%) | 23.50 | 19.63 | 15.75 |
| Peas | - | 10.00 | 20.00 |
| Monocalcium phosphate | 0.75 | 0.73 | 0.70 |
| Calcium carbonate | 1.00 | 1.00 | 1.00 |
| Salt | 0.45 | 0.46 | 0.46 |
| DL-methionine (98%) | 0.07 | 0.07 | 0.07 |
| L-lysine HCl (99%) | 0.30 | 0.36 | 0.42 |
| L-threonine (99%) | 0.07 | 0.09 | 0.12 |
| Vitamin–mineral premix * | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 |
| Calculated nutrient content | |||
| ME, kcal/kg | 3255.82 | 3239.58 | 3223.77 |
| CP, % | 17.50 | 17.50 | 17.50 |
| DCP, % | 15.47 | 15.38 | 15.28 |
| EE, % | 2.52 | 2.36 | 2.20 |
| CF, % | 3.70 | 3.95 | 4.20 |
| Ca, % | 3.49 | 0.86 | 0.85 |
| DP, % | 0.25 | 0.25 | 0.25 |
| DLys, % | 0.96 | 0.96 | 0.95 |
| DMet, % | 0.31 | 0.31 | 0.31 |
| DThr, % | 0.55 | 0.55 | 0.55 |
| DTrp, % | 0.14 | 0.14 | 0.14 |
| DArg, % | 0.95 | 0.97 | 0.99 |
| Nutrients | Value 1 |
|---|---|
| ME, MJ/kg DM 2 | 15.4 ± 0.84 |
| Moisture, % | 9.46 ± 0.14 |
| DM, % | 90.54 ± 0.89 |
| dDM, % | 52.56 ± 0.78 |
| OM, % | 86.98 ± 1.31 |
| dOM, % | 54.05 ± 0.81 |
| CP 3, % | 23.90 ±0.36 |
| dCP, % | 75.09 ± 1.14 |
| EE, % | 1.20 ± 0.31 |
| CF, % | 6.00 ± 0.45 |
| Ash, % | 3.56 ± 0.35 |
| NES 4, % | 55.88 ± 1.89 |
| Parameters | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| T0 | T10 | T20 | |||
| Growth performance | |||||
| BW at 70 days (kg) | 25.00 | 26.50 | 25.83 | 1.23 | 0.703 |
| BW at 105 days (kg) | 69.50 | 69.50 | 67.00 | 1.77 | 0.549 |
| FCR (feed/gain) | 2.51 b | 2.72 a | 2.78 a | 0.05 | 0.017 |
| Feed intake | |||||
| ADFI (kg) | 2.46 b | 2.78 a | 2.61 a | 114 | 0.018 |
| DMI (kg) | 2.16 b | 2.44 a | 2.30 a | 99.9 | 0.018 |
| N intake (g) | 60.41 | 68.45 | 64.30 | 2.80 | 0.176 |
| Nitrogen output | |||||
| N fecal output (g) | 11.22 | 10.85 | 8.51 | 1.18 | 0.172 |
| N urinary output (g) | 14.57 | 17.75 | 17.80 | 2.31 | 0.571 |
| TNO | 25.79 | 28.60 | 26.31 | 2.46 | 0.684 |
| Coefficients of digestibility | |||||
| N digestibility, % | 81.26 | 83.41 | 86.47 | 2.07 | 0.242 |
| ATTND coefficient | 0.81 | 0.83 | 0.88 | 0.02 | 0.259 |
| AMN coefficient | 0.57 | 0.58 | 0.58 | 0.04 | 0.988 |
| N retained (g/day) | 34.61 b | 39.80 a | 37.66 ab | 3.90 | 0.042 |
| BVP, % | 70.69 | 69.27 | 67.70 | 3.79 | 0.865 |
| NPU, % | 57.40 | 57.85 | 58.70 | 3.72 | 0.970 |
| Parameters | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| T0 | T10 | T20 | |||
| Protein profile | |||||
| T-Bil mg/dL | 0.103 b | 0.400 ab | 0.800 a | 0.103 | 0.030 |
| T-Pro g/dL | 5.550 | 6.267 | 5.867 | 0.514 | 0.687 |
| Alb g/dL | 3.650 | 3.967 | 3.933 | 0.338 | 0.826 |
| BUN mg/dL | 21.50 | 24.00 | 20.00 | 1.330 | 0.196 |
| UA mg/dL | 0.450 b | 0.650 ab | 1.067 a | 0.131 | 0.046 |
| Cre mg/dL | 1.200 | 1.400 | 1.167 | 0.099 | 0.296 |
| Parameters | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| T0 | T10 | T20 | |||
| Cecum | |||||
| SCFA (µmol/g) | |||||
| Acetic acid C2 | 43.63 b | 58.01 a | 53.51 a | 1.340 | 0.017 |
| Propionic acid C3 | 19.70 | 25.06 | 23.71 | 1.610 | 0.426 |
| Isobutyric acid i-C4 | 0.22 | 0.30 | 0.30 | 0.036 | 0.603 |
| Butyric acid C4 | 8.60 b | 12.33 a | 12.84 a | 0.415 | 0.017 |
| Isovaleric acid i-C5 | 0.24 | 0.37 | 0.32 | 0.045 | 0.544 |
| Valeric acid C5 | 0.55 | 0.69 | 0.93 | 0.063 | 0.447 |
| Total SCFAs | 72.93 | 96.74 | 91.60 | - | - |
| SCFA (%) | |||||
| Acetic acid C2 | 59.82 | 59.96 | 58.42 | 1.500 | 0.739 |
| Propionic acid C3 | 27.01 | 25.90 | 25.88 | 1.810 | 0.904 |
| Isobutyric acid i-C4 | 0.30 | 0.30 | 0.33 | 0.040 | 0.876 |
| Butyric acid C4 | 11.80 b | 12.75 ab | 14.01 a | 0.488 | 0.048 |
| Isovaleric acid i-C5 | 0.33 | 0.38 | 0.35 | 0.050 | 0.823 |
| Valeric acid C5 | 0.75 | 0.71 | 1.01 | 0.107 | 0.166 |
| Ileum | |||||
| SCFA (µmol/g) | |||||
| Acetic acid C2 | 7.55 b | 27.09 a | 24.13 a | 1.090 | 0.001 |
| Propionic acid C3 | 0.37 | 0.32 | 0.26 | 0.028 | 0.316 |
| Isobutyric acid i-C4 | 0.11 b | 0.26 a | 0.23 a | 0.010 | 0.003 |
| Butyric acid C4 | 0.11 | 0.32 | 0.50 | 0.051 | 0.059 |
| Isovaleric acid i-C5 | 0.15 b | 0.39 a | 0.54 a | 0.043 | 0.018 |
| Valeric acid C5 | 0.06 | 0.04 | 0.05 | 0.004 | 0.159 |
| Total SCFAs | 8.34 | 28.42 | 25.70 | - | - |
| SCFA (%) | |||||
| Acetic acid C2 | 90.56 | 95.33 | 93.90 | 6.740 | 0.879 |
| Propionic acid C3 | 4.41 a | 1.11 b | 0.99 b | 0.396 | 0.001 |
| Isobutyric acid i-C4 | 1.26 a | 0.92 b | 0.90 b | 0.075 | 0.027 |
| Butyric acid C4 | 1.26 | 1.13 | 1.93 | 0.268 | 0.160 |
| Isovaleric acid i-C5 | 1.80 | 1.37 | 2.10 | 0.411 | 0.494 |
| Valeric acid C5 | 0.72 a | 0.13 b | 0.18 b | 0.060 | 0.001 |
| Parameters | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| T0 | T10 | T20 | |||
| pH cecum | 5.47 | 5.38 | 5.29 | 0.051 | 0.164 |
| pH jejunum | 6.21 | 6.23 | 6.26 | 0.049 | 0.830 |
| Duodenum length (cm/kg) | 0.30 | 0.31 | 0.29 | 0.023 | 0.841 |
| Ileum length (cm/kg) | 0.26 b | 0.25 b | 0.36 a | 0.030 | 0.049 |
| Cecum length (cm/kg) | 0.37 | 0.38 | 0.40 | 0.008 | 0.094 |
| Total digestive tract (cm/kg) | 37.62 b | 36.69 b | 43.67 a | 1.090 | 0.013 |
| Parameters | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| T0 | T10 | T20 | |||
| Villus (length) µm | |||||
| Duodenum | 615.7 a | 527.1 ab | 438.9 b | 31.3 | 0.002 |
| Jejunum | 511.0 | 457.3 | 541.6 | 43.5 | 0.391 |
| Ileum | 437.9 | 361.9 | 380.7 | 30.9 | 0.203 |
| Villus (width) µm | |||||
| Duodenum | 147.6 b | 213.3 a | 143.7 b | 12.2 | 0.012 |
| Jejunum | 110.0 b | 164.3 a | 178.9 a | 18.1 | 0.003 |
| Ileum | 155.4 | 173.4 | 185.6 | 16.2 | 0.399 |
| Villus area (length × width) | |||||
| Duodenum | 77,408 | 66,402 | 60,687 | 7382 | 0.259 |
| Jejunum | 37,180 b | 68,553 a | 88,413 a | 8238 | 0.001 |
| Ileum | 63,492 | 59,736 | 59,786 | 5224 | 0.876 |
| Crypts (depth) µm | |||||
| Duodenum | 616.6 | 676.5 | 602.1 | 31.7 | 0.219 |
| Jejunum | 445.8 b | 558.5 a | 589.9 a | 26.1 | 0.004 |
| Ileum | 451.1 b | 666.9 a | 587.5 a | 31.5 | 0.0001 |
| Villus/crypt ratio | |||||
| Duodenum | 1.03 a | 0.89 ab | 0.77 b | 0.066 | 0.035 |
| Jejunum | 1.08 | 1.03 | 0.93 | 0.126 | 0.651 |
| Ileum | 1.01 a | 0.54 b | 0.65 b | 0.085 | 0.001 |
| Pearson’s Correlation | Performances | Nitrogen Balance | Volatile Fatty Acids | Intestinal Microbiota | Intestinal Morphology | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cecum | Ileum | Lactobacilli | Villus/Crypt Ratio (V:C) | |||||||||||||||||
| BW | FCR | ADFI | N Intake | TNO | N Retained | dN | C2 | C3 | C4 | C2 | C3 | C4 | Ileum | Cecum | Duodenum | Jejunum | Ileum | |||
| Performance | BW | 1 | ||||||||||||||||||
| FCR | −0.672 | 1 | ||||||||||||||||||
| ADFI | 0.036 | 0.716 | 1 | |||||||||||||||||
| Nitrogen balance | N Intake | 0.019 | 0.728 | 1.000 ** | 1 | |||||||||||||||
| TNO | 0.342 | 0.466 | 0.951 | 0.946 | 1 | |||||||||||||||
| N Retained | −0.370 | 0.937 | 0.915 | 0.922 | 0.746 | 1 | ||||||||||||||
| dN | −0.912 | 0.917 | 0.377 | 0.393 | 0.074 | 0.719 | 1 | |||||||||||||
| Volatile fatty acids | Cecum | C2 | −0.211 | 0.866 | 0.969 | 0.973 | 0.846 | 0.986 | 0.594 | 1 | ||||||||||
| C3 | −0.275 | 0.897 | 0.951 | 0.956 | 0.809 | 0.995 * | 0.646 | 0.998 * | 1 | |||||||||||
| C4 | −0.592 | 0.995 * | 0.784 | 0.794 | 0.555 | 0.968 | 0.871 | 0.913 | 0.,938 | 1 | ||||||||||
| Ileum | C2 | −0.373 | 0.938 | 0.914 | 0.921 | 0.744 | 1.000 ** | 0.721 | 0.986 | 0.995 * | 0.969 | 1 | ||||||||
| C3 | 0.891 | −0.935 | −0.421 | −0.437 | −0.122 | −0.752 | −0.999 * | −0.632 | −0.682 | −0.894 | −0.754 | 1 | ||||||||
| C4 | −0.843 | 0.965 | 0.507 | 0.522 | 0.217 | 0.812 | 0.990 * | 0.704 | 0.749 | 0.933 | 0.814 | −0.995 * | 1 | |||||||
| Lactobacilli | Ileum | −0.444 | 0.962 | 0.879 | 0.888 | 0.690 | 0.997 * | 0.773 | 0.970 | 0.984 | 0.985 | 0.997 * | −0.802 | 0.856 | 1 | |||||
| Cecum | −0.529 | 0.984 | 0.829 | 0.839 | 0.617 | 0.984 | 0.831 | 0.941 | 0.962 | 0.997 * | 0.985 | −0.856 | 0.902 | 0.995 * | 1 | |||||
| Intestinal morphology | V:C ratio | Duodenum | 0.843 | −0.965 | −0.507 | −0.522 | −0.217 | −0.812 | −0.990 * | −0.704 | −0.749 | −0.933 | −0.814 | 0.995 * | −1.000 ** | −0.856 | −0.902 | 1 | ||
| Jejunum | 0.945 | −0.877 | −0.293 | −0.310 | 0.015 | −0.654 | −0.996 * | −0.519 | −0.575 | −0.823 | −0.656 | 0.991 * | −0.973 | −0.713 | −0.777 | 0.973 | 1 | |||
| Ileum | 0.294 | −0.905 | −0.945 | −0.950 | −0.798 | −0.997 * | −0.660 | −0.996 * | −1.000 ** | −0.944 | −0.996 * | 0.696 | −0.762 | −0.987 | −0.967 | 0.762 | 0.590 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaite, T.D.; Cornescu, G.M.; Gagniuc, E.; Cismileanu, A.E.; Gal, C.; Dumitru, M.; Toma, S.M. Impact of Peas (Pisum Sativum L.) as a Sustainable Source of Protein in Growing Pigs’ Diets on Production Efficiency, Nitrogen Metabolism and Gastrointestinal Tract Health. Agriculture 2025, 15, 897. https://doi.org/10.3390/agriculture15080897
Panaite TD, Cornescu GM, Gagniuc E, Cismileanu AE, Gal C, Dumitru M, Toma SM. Impact of Peas (Pisum Sativum L.) as a Sustainable Source of Protein in Growing Pigs’ Diets on Production Efficiency, Nitrogen Metabolism and Gastrointestinal Tract Health. Agriculture. 2025; 15(8):897. https://doi.org/10.3390/agriculture15080897
Chicago/Turabian StylePanaite, Tatiana Dumitra, Gabriela Maria Cornescu, Elvira Gagniuc, Ana Elena Cismileanu, Claudiu Gal, Mihaela Dumitru, and Smaranda Mariana Toma. 2025. "Impact of Peas (Pisum Sativum L.) as a Sustainable Source of Protein in Growing Pigs’ Diets on Production Efficiency, Nitrogen Metabolism and Gastrointestinal Tract Health" Agriculture 15, no. 8: 897. https://doi.org/10.3390/agriculture15080897
APA StylePanaite, T. D., Cornescu, G. M., Gagniuc, E., Cismileanu, A. E., Gal, C., Dumitru, M., & Toma, S. M. (2025). Impact of Peas (Pisum Sativum L.) as a Sustainable Source of Protein in Growing Pigs’ Diets on Production Efficiency, Nitrogen Metabolism and Gastrointestinal Tract Health. Agriculture, 15(8), 897. https://doi.org/10.3390/agriculture15080897








