Cover Crops for Carbon Mitigation and Biodiversity Enhancement: A Case Study of an Olive Grove in Messinia, Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Design
2.2. Carbon Sequestration Assessement
2.2.1. Soil Organic Carbon Stock
2.2.2. Carbon Accumulation in the Olive Tree
2.2.3. Carbon Accumulation in the Groundcover Flora
2.2.4. C Sequestration
2.3. Soil Arthropod Biodiversity
2.4. Data Analysis
3. Results
3.1. Carbon Sequestration
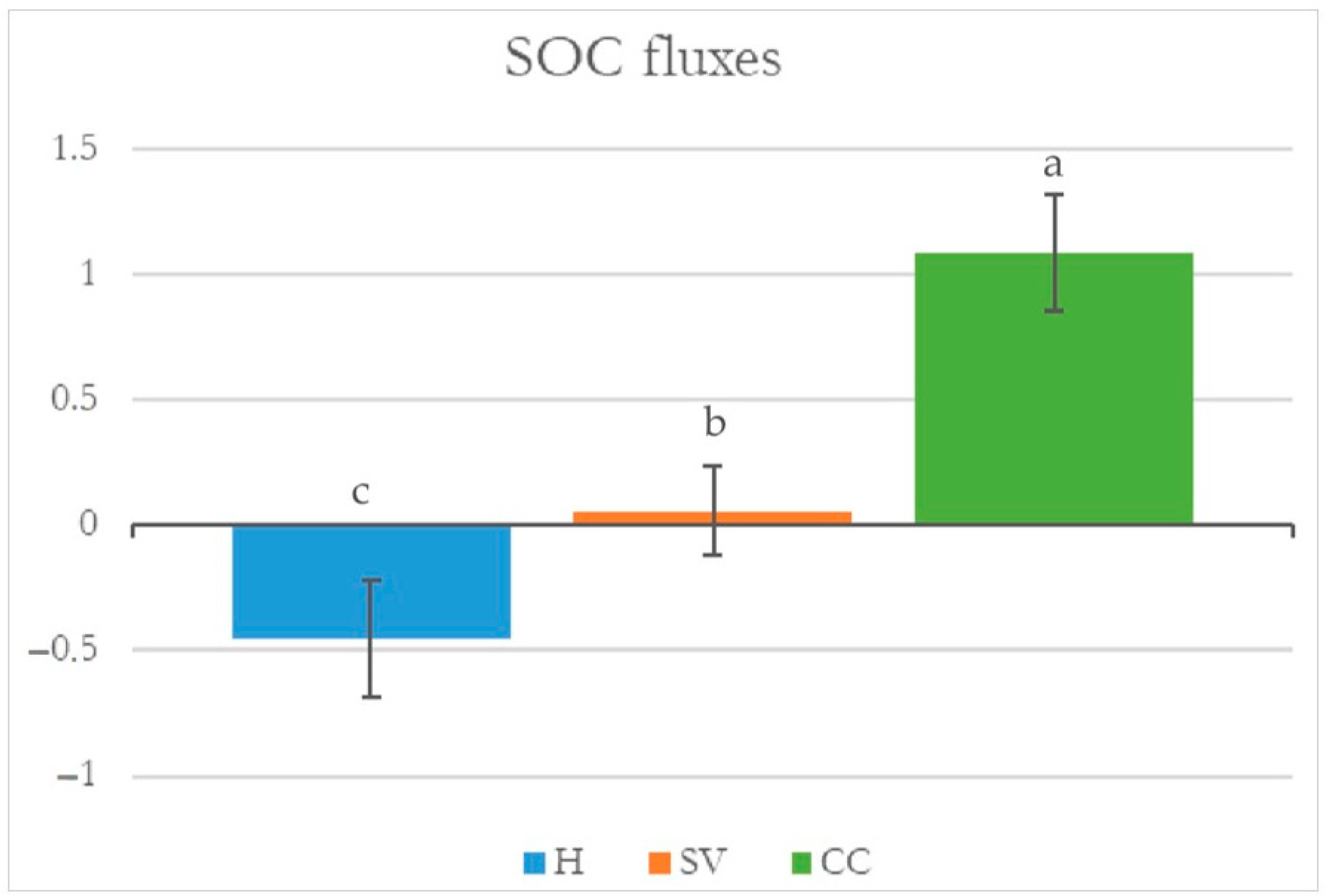
3.1.1. Soil Organic Carbon
3.1.2. Carbon Accumulated in Plant Material
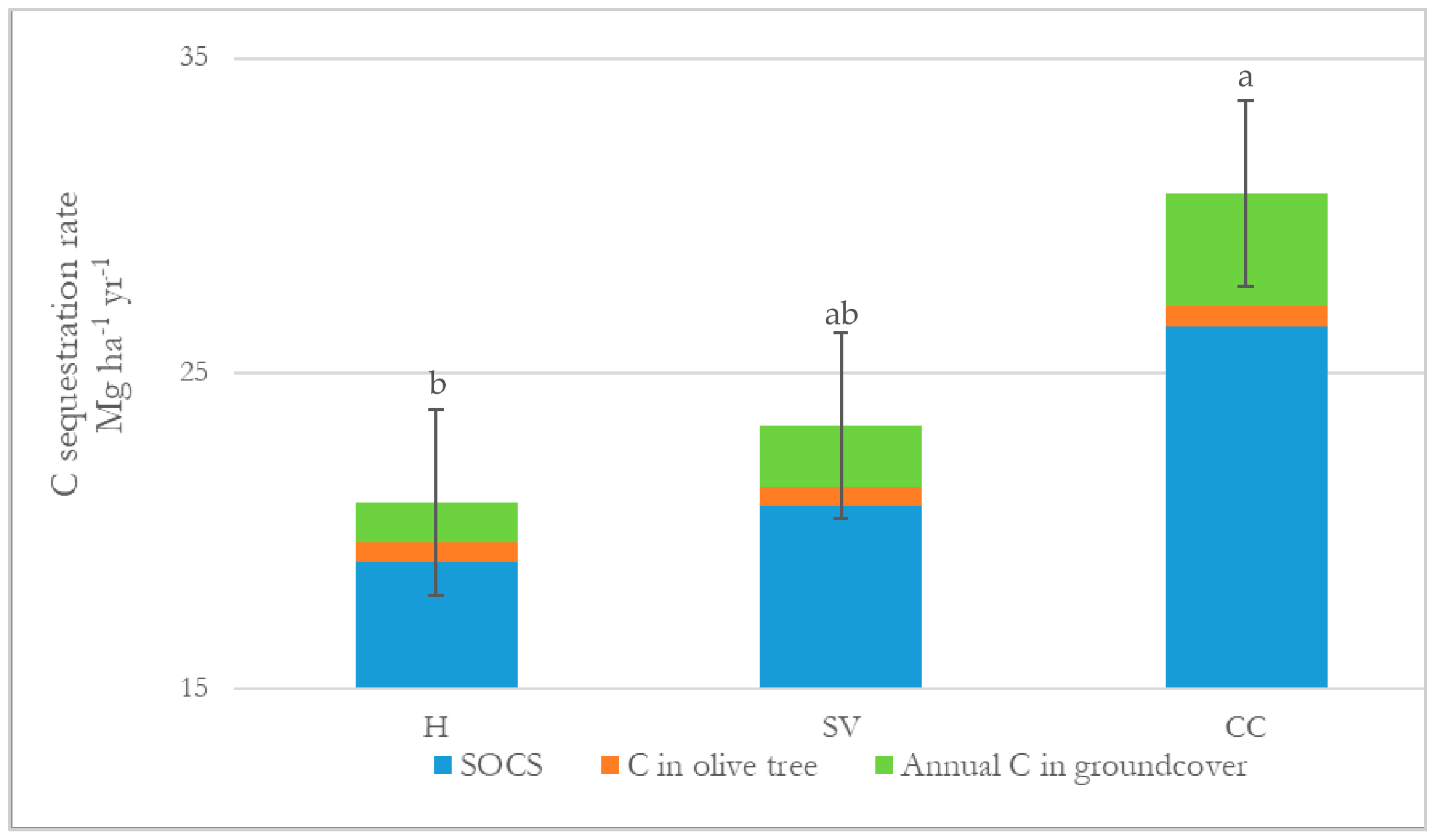
3.1.3. Annual C Sequestration Rate
3.2. Soil Arthropod Diversity
3.2.1. Total Soil Dueling Arthropod Abundance
3.2.2. Functionally Relevant Taxa Abundance
3.2.3. Specific Taxa
4. Discussion
4.1. C Sequestration
4.2. Soil Arthropod Biodiversity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | χ2 (p) |
| Arachnidae | 0.203 |
| Hymenoptera | 0.265 |
| Hemiptera | 0.294 |
| Isopoda | 1.68 |
| Chilopoda | 3.478 |
| Coleoptera | 0.563 |
| Orthoptera | 1.505 |
| Blattodea | 2.032 |
| Dermaptera | 0.566 |
| Diplopoda | 2.662 |
| Abundance/ha | 1.745 |
| Functional taxa | |
| BPC | 1.216 |
| NC | 0.474 |
| H′ | 1.377 |
| 1-D | 0.533 |
References
- IOC. Statistics. 2023. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/12/IOC-Olive-Oil-Dashboard-2.html (accessed on 7 February 2023).
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Change 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Terzi, M.; Barca, E.; Cazzato, E.; D’amico, F.S.; Lasorella, C.; Fracchiolla, M. Effects of weed control practices on plant diversity in a homogenous olive-dominated landscape (South-East of Italy). Plants 2021, 10, 1090. [Google Scholar] [CrossRef]
- Vahamidis, P.; Chachalis, D.; Akrivou, A.; Karanasios, E.; Ganopoulou, M.; Argiri, A.; Markellou, E. Weed Species’ Diversity and Composition as Shaped by the Interaction of Management, Site, and Soil Variables in Olive Groves of Southern Greece. Agronomy 2024, 14, 640. [Google Scholar] [CrossRef]
- Allen, H.D.; Randall, R.E.; Amable, G.S.; Devereux, B.J. The impact of changing olive cultivation practices on the ground flora of olive groves in the Messara and Psiloritis regions, Crete, Greece. Land Degrad. Dev. 2006, 17, 249–273. [Google Scholar] [CrossRef]
- Cavoski, A. An ambitious and climate-focused commissionagenda for post COVID-19 EU. Environ. Politics 2020, 29, 1112–1117. [Google Scholar] [CrossRef]
- Ellis, E.C. Land use and ecological change: A 12,000-year history. Annu. Rev. Environ. Resour. 2021, 46, 1–33. [Google Scholar] [CrossRef]
- Gross, C.D.; Harrison, R.B. The case for digging deeper: Soil organic carbon storage, dynamics, and controls in our changing world. Soil Syst. 2019, 3, 28. [Google Scholar] [CrossRef]
- Tautges, N.E.; Chiartas, J.L.; Gaudin, A.C.M.; O’Geen, A.T.; Herrera, I.; Scow, K.M. Deep soil inventories reveal that impacts of cover crops and compost on soil carbon sequestration differ in surface and subsurface soils. Glob. Change Biol. 2019, 25, 3753–3766. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- LaSalle, T.J.; Hepperly, P. Regenerative Organic Farming: A Solution to Global Warming; Rodale Institute: Allentown, PA, USA, 2008. [Google Scholar]
- Duval, M.E.; Galantini, J.A.; Capurro, J.E.; Martinez, J.M. Winter cover crops in soybean monoculture: Effects on soil organic carbon and its fractions. Soil Tillage Res. 2016, 161, 95–105. [Google Scholar] [CrossRef]
- Novara, A.; Cerda, A.; Barone, E.; Gristina, L. Cover crop management and water conservation in vineyard and olive orchards. Soil Tillage Res. 2021, 208, 104896. [Google Scholar] [CrossRef]
- Gómez-Muñoz, B.; Hatch, D.; Bol, R.; García-Ruiz, R. Nutrient dynamics during decomposition of the residues from a sown legume or ruderal plant cover in an olive oil orchard. Agric. Ecosyst. Environ. 2014, 184, 115–123. [Google Scholar] [CrossRef]
- Milgroom, J.; Soriano, M.A.; Garrido, J.M.; Gómez, J.A.; Fereres, E. The influence of a shift from conventional to organic olive farming on soil management and erosion risk in southern Spain. Renew. Agric. Food Syst. 2007, 22, 1–10. [Google Scholar] [CrossRef]
- Ramos, M.E.; Benítez, E.; García, P.A.; Robles, A.B. Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: Effects on soil quality. Appl. Soil. Ecol. 2010, 44, 6–14. [Google Scholar] [CrossRef]
- Haynes, R.J.; Beare, M.H. Aggregation and organic matter storage in meso-thermal, humid soils. In Structure and Organic Matter Storage in Agricultural Soils; CRC Press: Boca Raton, FL, USA, 2020; pp. 213–262. [Google Scholar]
- Moukanni, N.; Brewer, K.M.; Gaudin, A.C.M.; O’Geen, A.T. Optimizing carbon sequestration through cover cropping in Mediterranean agroecosystems: Synthesis of mechanisms and implications for management. Front. Agron. 2022, 4, 844166. [Google Scholar] [CrossRef]
- Gómez, J.A.; Campos, M.; Guzmán, G.; Castillo-Llanque, F.; Vanwalleghem, T.; Lora, A.; Giráldez, J.V. Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ. Sci. Pollut. Res. 2018, 25, 977–989. [Google Scholar] [CrossRef]
- Bugg, R.L.; Waddington, C. Using cover crops to manage arthropod pests of orchards: A review. Agric. Ecosyst. Environ. 1994, 50, 11–28. [Google Scholar] [CrossRef]
- Stavrianakis, G.; Sentas, E.; Stattegger, S.R.; Tscheulin, T.; Kizos, T. Effect of olive grove’s understorey management on arthropod diversity. Agroecol. Sustain. Food Syst. 2024, 48, 1115–1138. [Google Scholar] [CrossRef]
- Gkisakis, V.; Volakakis, N.; Kollaros, D.; Bàrberi, P.; Kabourakis, E.M. Soil arthropod community in the olive agroecosystem: Determined by environment and farming practices in different management systems and agroecological zones. Agric. Ecosyst. Environ. 2016, 218, 178–189. [Google Scholar] [CrossRef]
- Stiling, P.; Cornelissen, T. What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol. Control 2005, 34, 236–246. [Google Scholar] [CrossRef]
- Caon, L.; Vargas, R. Threats to soils: Global trends and perspectives. Global Soil Partnership Food and Agriculture Organization of the United Nations. In A Contribution from the Intergovernmental Technical Panel on Soils; Brajendra, Ed.; Global land outlook working paper; United Nations: Manhattan, NY, USA, 2017. [Google Scholar]
- de Paz, V.; Tobajas, E.; Rosas-Ramos, N.; Tormos, J.; Asís, J.D.; Baños-Picón, L. Effect of organic farming and agricultural abandonment on beneficial arthropod communities associated with olive groves in western Spain: Implications for Bactrocera oleae management. Insects 2022, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Tortosa, F.S.; Carpio, A.J. Structure of canopy and ground-dwelling arthropod communities in olive orchards is determined by the type of soil cover. Eur. J. Èntomol. 2021, 118, 159–170. [Google Scholar] [CrossRef]
- Walinga, I.; Kithome, M.; Novozamsky, I.; Houba, V.J.G.; van der Lee, J.J. Spectrophotometric Determination of Organic Carbon in Soil. Commun. Soil Sci. Plant Anal. 1992, 23, 1935–1944. [Google Scholar] [CrossRef]
- Lopez-Bellido, P.J.; Lopez-Bellido, L.; Fernandez-Garcia, P.; Muñoz-Romero, V.; Lopez-Bellido, F.J. Assessment of carbon sequestration and the carbon footprint in olive groves in Southern Spain. Carbon Manag. 2016, 7, 161–170. [Google Scholar] [CrossRef]
- Blake, G.R. Bulk density. Methods of Soil Analysis. Part 1; SSSA: Madison, WI, USA, 1986. [Google Scholar]
- Throop, H.; Archer, S.; Monger, H.; Waltman, S. When bulk density methods matter: Implications for estimating soil organic carbon pools in rocky soils. J. Arid. Environ. 2012, 77, 66–71. [Google Scholar] [CrossRef]
- Grossman, R.B.; Reinsch, T.G. Bulk density and linear extensibility. In Methods of Soil Analysis Part 4, SSSA Book Ser. 5; Dane, J.H., Topp, G.C., Eds.; SSSA: Madison, WI, USA, 2002; pp. 201–254. [Google Scholar]
- Bateni, C.; Ventura, M.; Tonon, G.; Pisanelli, A. Soil carbon stock in olive groves agroforestry systems under different management and soil characteristics. Agrofor. Syst. 2021, 95, 951–961. [Google Scholar] [CrossRef]
- Gómez, J.A.; Guzmán, G.; Vanwalleghem, T.; Vanderlinden, K. Spatial Variability of Soil Organic Carbon Stock in an Olive Orchard at Catchment Scale in Southern Spain. Int. Soil Water Conserv. Res. 2023, 11, 311–326. [Google Scholar] [CrossRef]
- Velázquez-Martí, B.; Sajdak, M.; López-Cortés, I.; Callejón-Ferre, A.J. Wood characterization for energy application proceeding from pruning Morus alba L., Platanus hispanica Münchh. and Sophora japonica L. in urban areas. Renew. Energy 2014, 62, 478–483. [Google Scholar] [CrossRef]
- Ilarioni, L.; Nasini, L.; Brunori, A.N.T.O.N.I.O.; Proietti, P. Experimental measurement of the biomass of Olea europaea L. Afr. J. Biotechnol. 2013, 12, 1216–1222. [Google Scholar]
- Poeplau, C.; Reiter, L.; Berti, A.; Kätterer, T. Qualitative and quantitative response of soil organic carbon to 40 years of crop residue incorporation under contrasting nitrogen fertilisation regimes. Soil Res. 2016, 55, 1–9. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Cotes, B.; Campos, M.; Pascual, F.; García, P.A.; Ruano, F. Comparing taxonomic levels of epigeal insects under different farming systems in Andalusian olive agroecosystems. Appl. Soil Ecol. 2010, 44, 228–236. [Google Scholar] [CrossRef]
- Timms, L.L.; Bowden, J.J.; Summerville, K.S.; Buddle, C.M. Does species-level resolution matter? Taxonomic sufficiency in terrestrial arthropod biodiversity studies. Insect Conserv. Divers. 2013, 6, 453–462. [Google Scholar] [CrossRef]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F.; et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef]
- Gkisakis, V.D.; Kollaros, D.; Kabourakis, E.M. Entomologia Hellenica; Hellenica Entomological Society: Athens, Greece, 2017. [Google Scholar]
- Gonçalves, M.F.; Pereira, J.A. Abundance and diversity of soil arthropods in the olive grove ecosystem. J. Insect Sci. 2012, 12, 20. [Google Scholar] [CrossRef]
- Gonçalves, F.M.; Rodrigues, M.C.; Pereira, J.A.; Thistlewood, H.; Torres, L.M. Natural mortality of immature stages of Bactrocera oleae (Diptera: Tephritidae) in traditional olive groves from north-eastern Portugal. Biocontrol Sci. Technol. 2012, 22, 837–854. [Google Scholar] [CrossRef]
- Urbaneja, A.; Marí, F.G.; Tortosa, D.; Navarro, C.; Vanaclocha, P.; Bargues, L.; Castañera, P. Influence of ground predators on the survival of the Mediterranean fruit fly pupae, Ceratitis capitata, in Spanish citrus orchards. BioControl 2006, 51, 611–626. [Google Scholar] [CrossRef]
- Stork, N.E.; Eggleton, P. Invertebrates as determinants and indicators of soil quality. Am. J. Altern. Agric. 1992, 7, 38–47. [Google Scholar] [CrossRef]
- Wurst, S. Plant-mediated links between detritivores and aboveground herbivores. Front. Plant Sci. 2013, 4, 380. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: www.R-project.org/ (accessed on 21 November 2024).
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Cover crops and carbon sequestration: Lessons from US studies. Soil Sci. Soc. Am. J. 2022, 86, 501–519. [Google Scholar] [CrossRef]
- Peregrina, F.; Pérez-Álvarez, E.P.; García-Escudero, E. The short term influence of aboveground biomass cover crops on C sequestration and β–glucosidase in a vineyard ground under semiarid conditions. Span. J. Agric. Res. 2014, 12, 1000–1007. [Google Scholar] [CrossRef]
- Repullo-Ruiberriz de Torres, M.A.; Moreno-García, M.; Ordóñez-Fernández, R.; Rodríguez-Lizana, A.; Rodríguez, B.C.; García-Tejero, I.F.; Zuazo, V.H.D.; Carbonell-Bojollo, R.M. Cover crop contributions to improve the soil nitrogen and carbon sequestration in almond orchards (SW Spain). Agronomy 2021, 11, 387. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Mezzapesa, G.N.; Stellacci, A.M.; Ferrara, G.; Occhiogrosso, G.; Petrelli, G.; Castellini, M.; Spagnuolo, M. Cover crop for a sustainable viticulture: Effects on soil properties and table grape production. Agronomy 2020, 10, 1334. [Google Scholar] [CrossRef]
- Carbonell-Bojollo, R.; González-Sánchez, E.J.; de Torres, M.R.R.; Ordóñez-Fernández, R.; Domínguez-Gimenez, J.; Basch, G. Soil organic carbon fractions under conventional and no-till management in a long-term study in southern Spain. Soil Res. 2015, 53, 113–124. [Google Scholar] [CrossRef]
- Rodríguez-Lizana, A.; de Torres, M.Á.R.-R.; Carbonell-Bojollo, R.; Moreno-García, M.; Ordóñez-Fernández, R. Study of C, N, P and K release from residues of newly proposed cover crops in a Spanish olive grove. Agronomy 2020, 10, 1041. [Google Scholar] [CrossRef]
- Bechara, E.; Papafilippaki, A.; Doupis, G.; Sofo, A.; Koubouris, G. Nutrient dynamics, soil properties and microbiological aspects in an irrigated olive orchard managed with five different management systems involving soil tillage, cover crops and compost. J. Water Clim. Change 2018, 9, 736–747. [Google Scholar] [CrossRef]
- Ghimire, R.; Lamichhane, S.; Acharya, B.S.; Bista, P.; Sainju, U.M. Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: A review. J. Integr. Agric. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Ordóñez-Fernández, R.; de Torres, M.A.R.-R.; Márquez-García, J.; Moreno-García, M.; Carbonell-Bojollo, R.M. Legumes used as cover crops to reduce fertilisation problems improving soil nitrate in an organic orchard. Eur. J. Agron. 2018, 95, 1–13. [Google Scholar] [CrossRef]
- Almagro, M.; López, J.; Boix-Fayos, C.; Albaladejo, J.; Martínez-Mena, M. Belowground carbon allocation patterns in a dry Mediterranean ecosystem: A comparison of two models. Soil Biol. Biochem. 2010, 42, 1549–1557. [Google Scholar] [CrossRef]
- Manivannan, S.; Balamurugan, M.; Parthasarathi, K.; Gunasekaran, G.; Ranganathan, L.S. Effect of vermicompost on soil fertility and crop productivity-beans (Phaseolus vulgaris). J. Environ. Biol. 2009, 30, 275–281. [Google Scholar]
- Gkisakis, V.D.; Kollaros, D.; Bàrberi, P.; Livieratos, I.C.; Kabourakis, E.M. Soil arthropod diversity in organic, integrated, and conventional olive orchards and different agroecological zones in Crete, Greece. Agroecol. Sustain. Food Syst. 2015, 39, 276–294. [Google Scholar] [CrossRef]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does organic farming benefit biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Ecological Methods; Methuen & Co.: London, UK, 1966. [Google Scholar]
- Iannotta, N.; Belfiore, T.; Noce, M.E.; Scalercio, S.; Vizzarri, V. The Impact of Some Compounds Utilised in Organic Olive Groves on the Non-Target Arthropod Fauna: Canopy and Soil Levels. In Proceedings of the Ecoliva 2007, VI Jornadas Internacionales de Olivar Ecologico, Puente de Génave (Jaén), Spain, 22–25 March 2007. [Google Scholar]
- Jerez-Valle, C.; García, P.A.; Campos, M.; Pascual, F. A simple bioindication method to discriminate olive orchard management types using the soil arthropod fauna. Appl. Soil Ecol. 2014, 76, 42–51. [Google Scholar] [CrossRef]
- Hanson, H.I.; Birkhofer, K.; Smith, H.G.; Palmu, E.; Hedlund, K. Agricultural land use affects abundance and dispersal tendency of predatory arthropods. Basic Appl. Ecol. 2017, 18, 40–49. [Google Scholar] [CrossRef]
- Holland, J.M.; Reynolds, C.J. The impact of soil cultivation on arthropod (Coleoptera and Araneae) emergence on arable land. Pedobiologia 2003, 47, 181–191. [Google Scholar] [CrossRef]
- Perner, J.; Malt, S. Assessment of changing agricultural land use: Response of vegetation, ground-dwelling spiders and beetles to the conversion of arable land into grassland. Agric. Ecosyst. Environ. 2003, 98, 169–181. [Google Scholar] [CrossRef]
- Briere, J.F.; Pracros, P.; Le Roux, A.Y.; Pierre, J.S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Danne, A.; Thomson, L.J.; Sharley, D.J.; Penfold, C.M.; Hoffmann, A.A. Effects of native grass cover crops on beneficial and pest invertebrates in Australian vineyards. Environ. Entomol. 2010, 39, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Franco, J.; Vasconcelos, T.; Branco, M. Effect of ground cover vegetation on the abundance and diversity of beneficial arthropods in citrus orchards. Bull. Entomol. Res. 2010, 100, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Fonte, S.J.; Shrestha, A.; Daane, K.M.; Mitchell, J.P. Winter cover crops and no-till promote soil macrofauna communities in irrigated, Mediterranean cropland in California, USA. Appl. Soil Ecol. 2021, 166, 104068. [Google Scholar] [CrossRef]
- Orsini, M. Mortality and Predation of Olive Fly (Bactrocera oleae) Pupae on the Soil in a Davis, California Olive Orchard; UC Berkeley: Berkeley, CA, USA, 2006. [Google Scholar]
- Volakakis, N.; Kabourakis, E.; Rempelos, L.; Kiritsakis, A.; Leifert, C. Effect of different cover crops, mass-trapping systems and environmental factors on invertebrate activity in table olive orchards—Results from field experiments in Crete, Greece. Agronomy 2022, 12, 2576. [Google Scholar] [CrossRef]
- Paredes, D.; Campos, M.; Cayuela, L. El control biológico de plagas de artrópodos por conservación: Técnicas y estado del arte. Ecosistemas 2013, 22, 56–61. [Google Scholar]




| Olive Agroecosystem Service | Taxa |
|---|---|
| Biological pest control (BPC) | Araneae Chilopoda Dermaptera Hymenoptera (Formicidae) Coleoptera |
| Nutrient cycling (NC) | Isopoda Diplopoda |
| Carbon Fluxes | SOC | Soil C Stock | Annual C in Olive Tree | Annual C Groundcover | C Sequestration | ||||
|---|---|---|---|---|---|---|---|---|---|
| Year | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| H | 1 ± 0.1 a | 0.9 ± 0.2 b | 21.7 ± 2.8 a | 16.4 ± 1.9 b | 0.616 | 1.2 ± 0.6 a | 0.7 ± 0.2 b | 24.2 ± 2 a | 17.7 ± 2 b |
| SV | 1.1 ± 0.03 a | 1.1 ± 0.2 b | 23.7 ± 2.4 a | 18 ± 3.4 b | 0.611 | 1.8 ± 1.1 a | 2 ± 1.1 b | 26 ± 1 a | 20.6 ± 4 ab |
| CC | 1.2 ± 0.1 a | 1.5 ± 0.4 a | 26 ± 4.7 a | 27 ± 6.1 a | 0.629 | 3.1 ± 1.3 a | 4 ± 1.4 a | 29.8 ± 4 a | 31.6 ± 5 a |
| ANOVA (F) | 5.87 | 5.108 | 9.258 | 37.704 | |||||
| Sig. (p) | 0.013 | 0.020 | 0.020 | <0.001 | |||||
| Season | Autumn | Spring | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | H | SV | CC | H | SV | CC | H | SV | CC |
| Arachnidae | 94 ± 2 | 84 ± 4 | 92 ± 1 | 108 ± 4 | 60 ± 1 | 74 ± 1 | 202 | 144 | 166 |
| Hymenoptera | 102 ± 10 | 64 ± 4 | 188 ± 9 | 356 ± 4 | 462 ± 5 | 354 ± 3 | 458 | 526 | 542 |
| Hemiptera | 4 ± 1 | 4 ± 0.4 | 2 ± 1 | 44 ± 1 | 18 ± 1 | 44 ± 1 | 48 | 22 | 46 |
| Isopoda | 54 ± 6 | 62 ± 3 | 102 ± 2 | 90 ± 1 | 54 ± 1 | 50 ± 1 | 144 | 116 | 152 |
| Chilopoda | 18 ± 1 | 22 ± 1 | 36 ± 6 | 8 ± 1 | 6 ± 0.2 | 16 ± 0.1 | 26 | 28 | 52 |
| Coleoptera | 24 ± 9 | 16 ± 3 | 24 ± 22 | 70 ± 1 | 78 ± 1 | 118 ± 2 | 94 | 94 | 142 |
| Orthoptera | 12 ± 2 | 0 | 4 ± 1 | 14 ± 1 | 22 ± 1 | 24 ± 0.4 | 26 | 22 | 28 |
| Blattodea | 84 ± 3 | 16 ± 4 | 40 ± 4 | 12 ± 1 | 8 ± 1 | 24 ± 1 | 96 | 24 | 64 |
| Dermaptera | 4 ± 0.3 | 12 ± 4 | 14 ± 1 | 52 ± 1 | 84 ± 2 | 118 ± 5 | 56 | 96 | 132 |
| Diplopoda | 8 ± 0.3 | 20 ± 1 | 126 ± 1 | 18 ± 1 | 36 ± 1 | 18 ± 1 | 26 | 56 | 144 |
| Abundance/ha | 404 | 300 | 628 | 772 | 828 | 840 | 1176 | 1128 | 1468 |
| BPC | 242 | 198 | 354 | 594 | 690 | 680 | 836 | 888 | 1034 |
| NC | 62 | 82 | 228 | 108 | 90 | 68 | 170 | 172 | 296 |
| S | 10 | 9 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| H′ | 1.86 | 1.88 | 1.81 | 1.72 | 1.54 | 1.84 | 1.79 | 1.75 | 1.87 |
| 1-D | 0.82 | 0.82 | 0.79 | 0.66 | 0.66 | 0.78 | 0.79 | 0.74 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michail, I.; Pantazis, C.; Solomos, S.; Michailidis, M.; Molassiotis, A.; Gkisakis, V. Cover Crops for Carbon Mitigation and Biodiversity Enhancement: A Case Study of an Olive Grove in Messinia, Greece. Agriculture 2025, 15, 898. https://doi.org/10.3390/agriculture15080898
Michail I, Pantazis C, Solomos S, Michailidis M, Molassiotis A, Gkisakis V. Cover Crops for Carbon Mitigation and Biodiversity Enhancement: A Case Study of an Olive Grove in Messinia, Greece. Agriculture. 2025; 15(8):898. https://doi.org/10.3390/agriculture15080898
Chicago/Turabian StyleMichail, Ioanna, Christos Pantazis, Stavros Solomos, Michail Michailidis, Athanassios Molassiotis, and Vasileios Gkisakis. 2025. "Cover Crops for Carbon Mitigation and Biodiversity Enhancement: A Case Study of an Olive Grove in Messinia, Greece" Agriculture 15, no. 8: 898. https://doi.org/10.3390/agriculture15080898
APA StyleMichail, I., Pantazis, C., Solomos, S., Michailidis, M., Molassiotis, A., & Gkisakis, V. (2025). Cover Crops for Carbon Mitigation and Biodiversity Enhancement: A Case Study of an Olive Grove in Messinia, Greece. Agriculture, 15(8), 898. https://doi.org/10.3390/agriculture15080898









