Brassinosteroids Alleviate Ethylene-Induced Copper Oxide Nanoparticle Toxicity and Ultrastructural and Stomatal Damage in Rice Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of CuO NPs
2.2. Plant Material and Growth Conditions
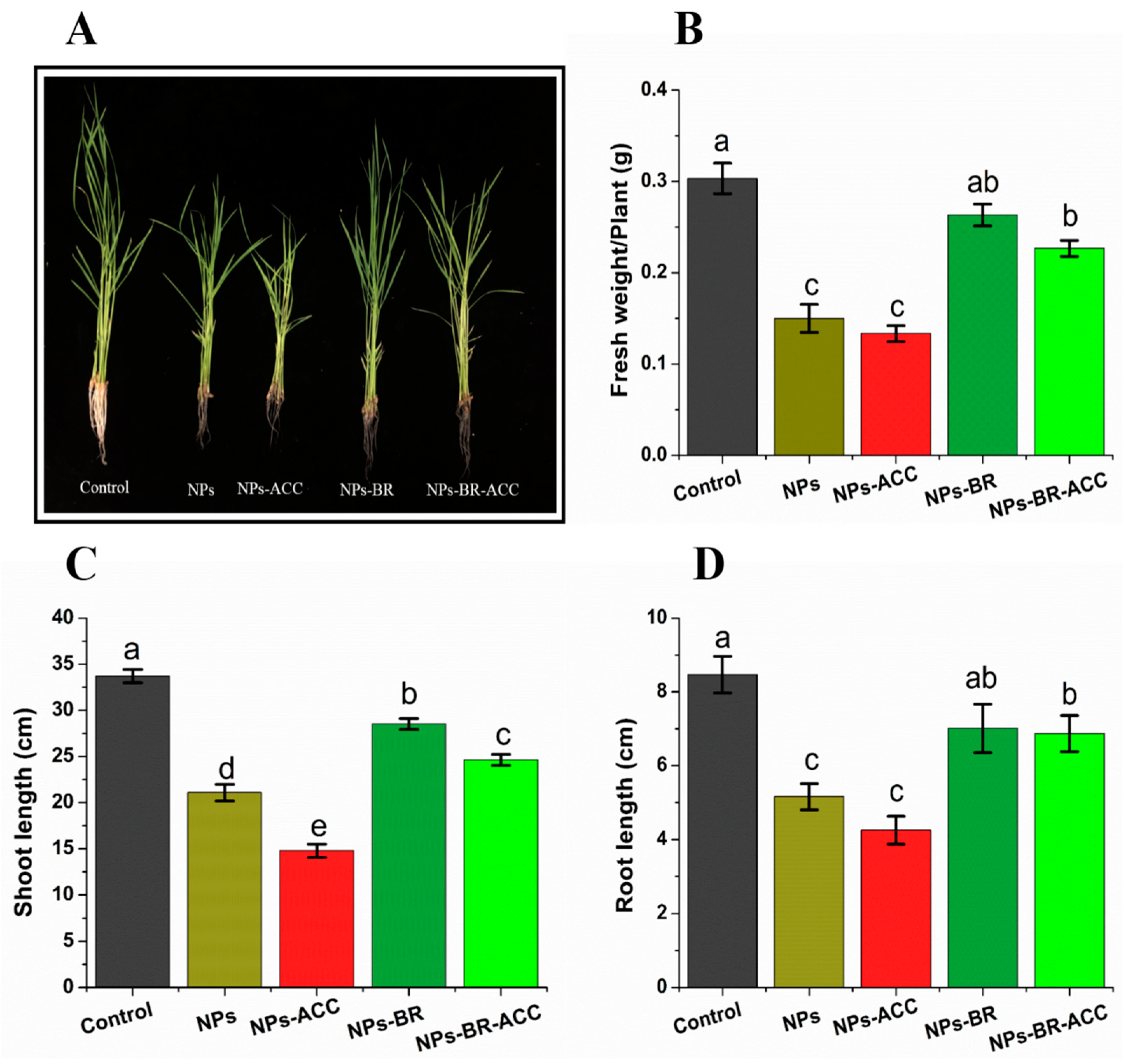
2.3. Determination of Biomass Accumulation
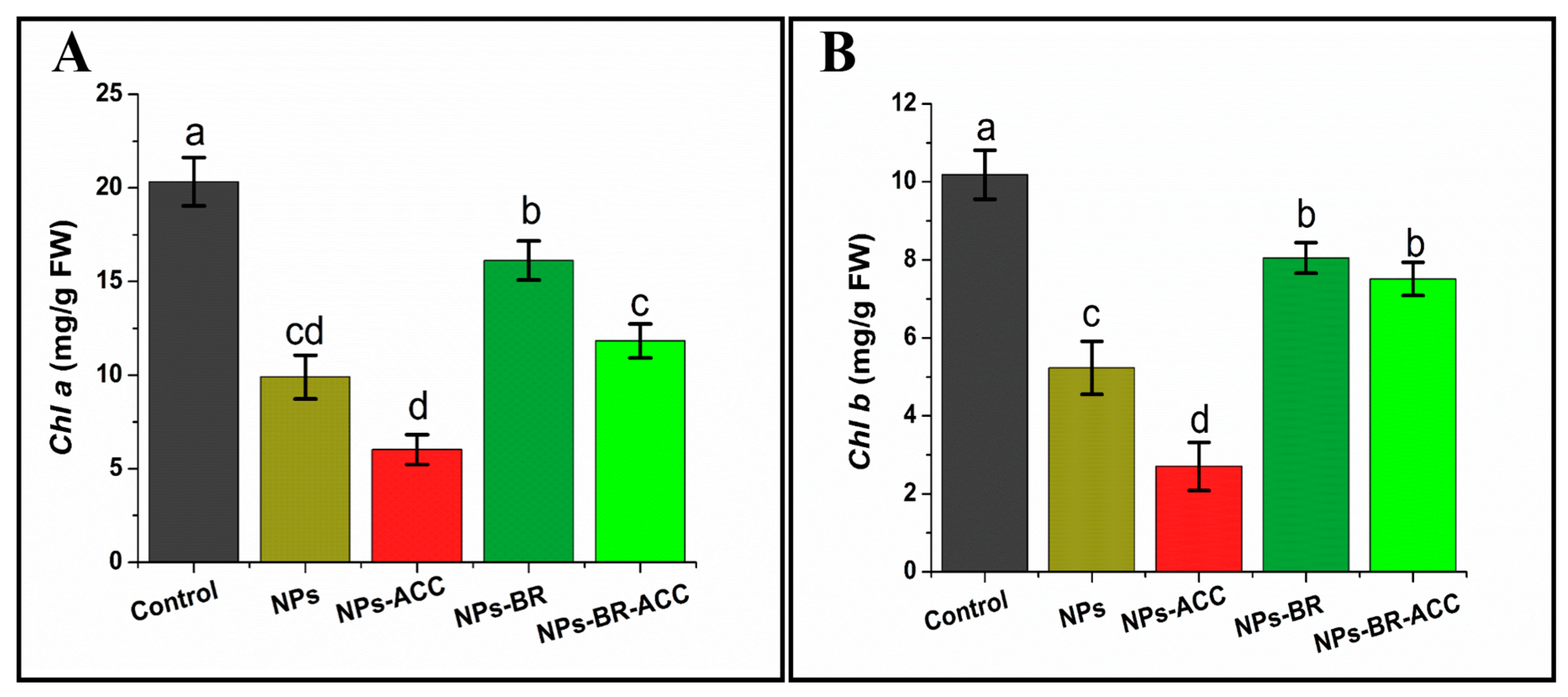
2.4. Photosynthetic Pigment Measurements
2.5. Measurements of Lipid Peroxidation
2.6. NBT and DAB Staining
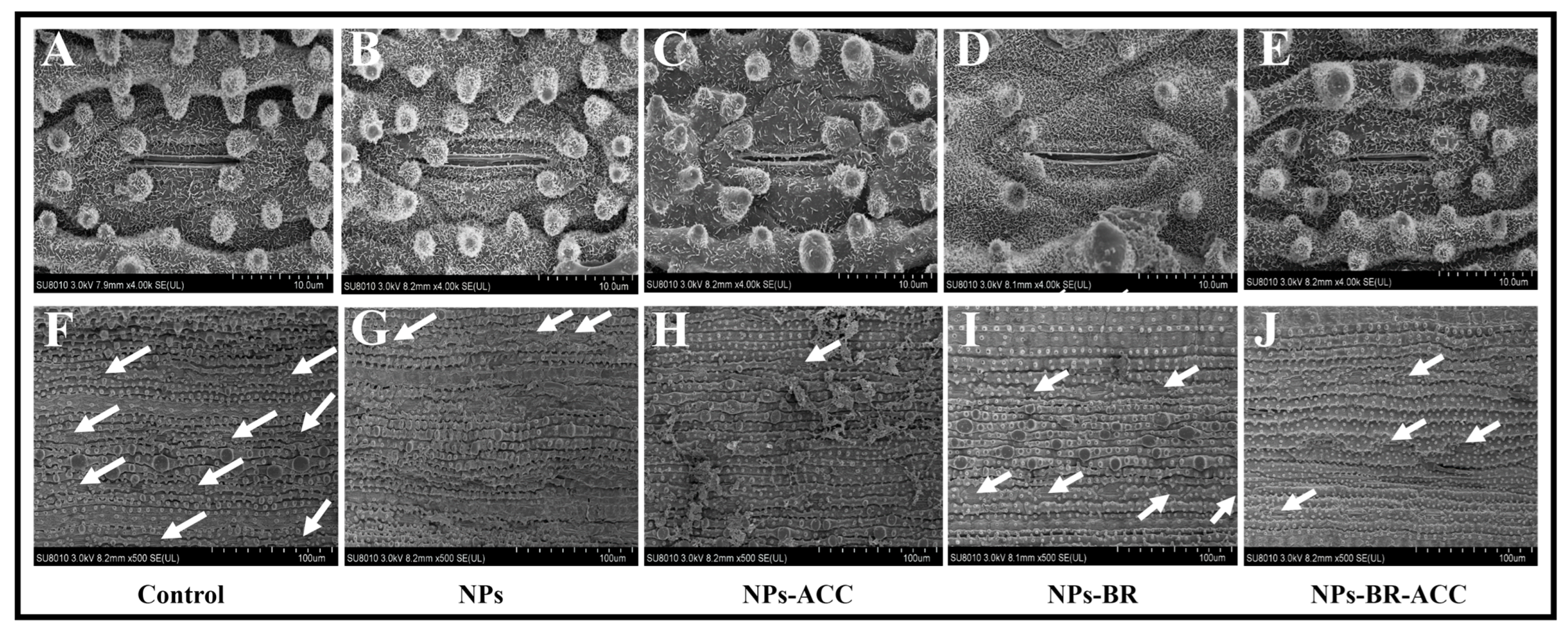
2.7. Scanning Electron Microscopy (SEM)
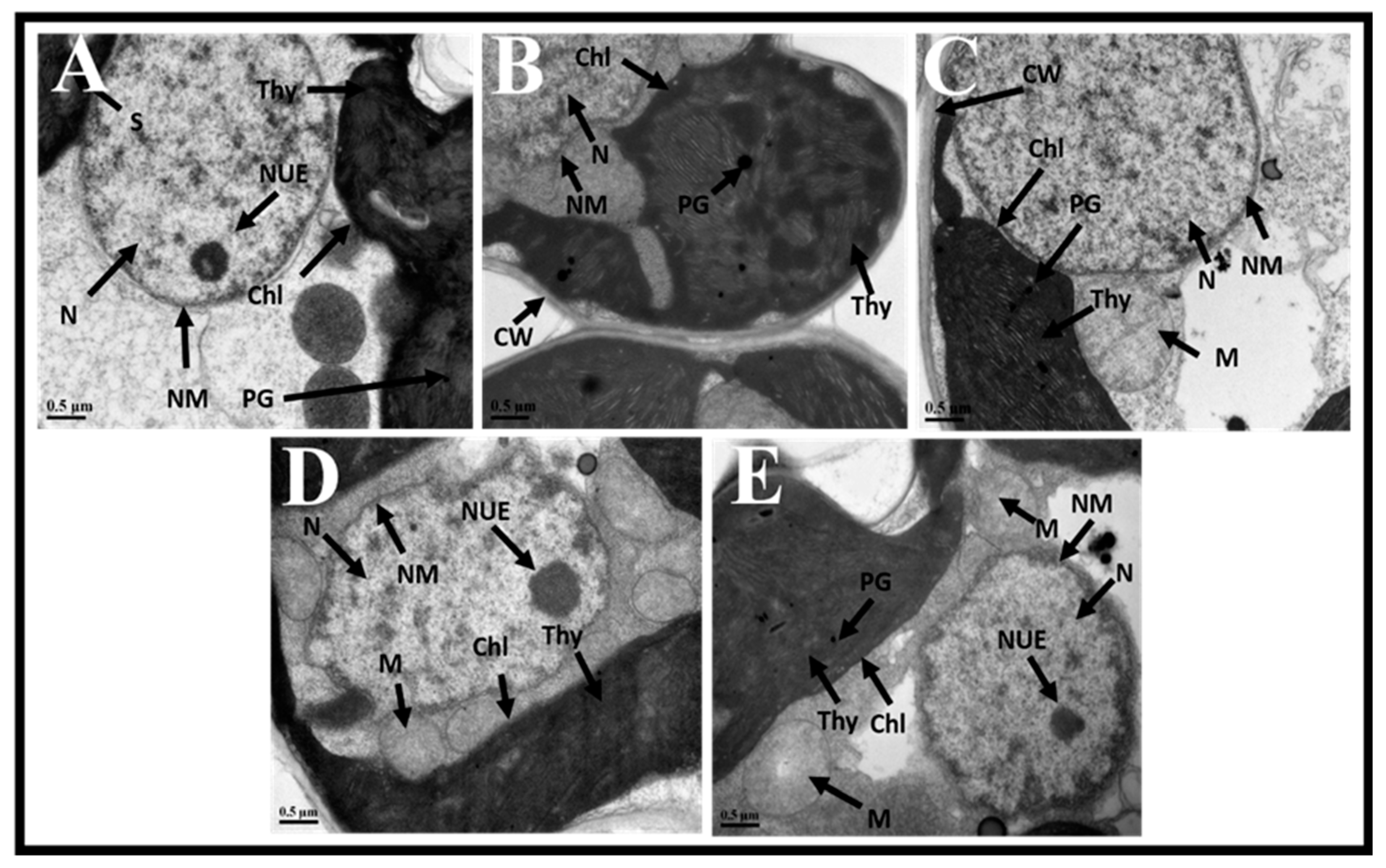
2.8. Transmission Electron Microscopy (TEM)
2.9. Measurement of Antioxidant Enzyme Activities
2.10. Statistical Analysis
3. Results
3.1. Characterization of CuO NPs
3.2. BRs Reverse Ethylene-Induced CuO NP Toxicity and Improves Plant Growth Attributes
3.3. BRs Inhibit Ethylene-Induced Chlorophyll Degradation in Rice Seedlings Under CuO NPs
3.4. BRs Minimize Ethylene-Induced Oxidative Damage in Rice Seedlings
3.5. BRs Reverse the Ethylene-Induced MDA Content Accumulation
3.6. BRs Ameliorate Ethylene-Induced Stomatal Damage in Rice Seedings
3.7. BRs Reduce Ethylene-Induced Ultrastructural Damages Under CuO NP Stress
3.8. BRs Improve the Surface of Rice Root Exposed to CuO NPs
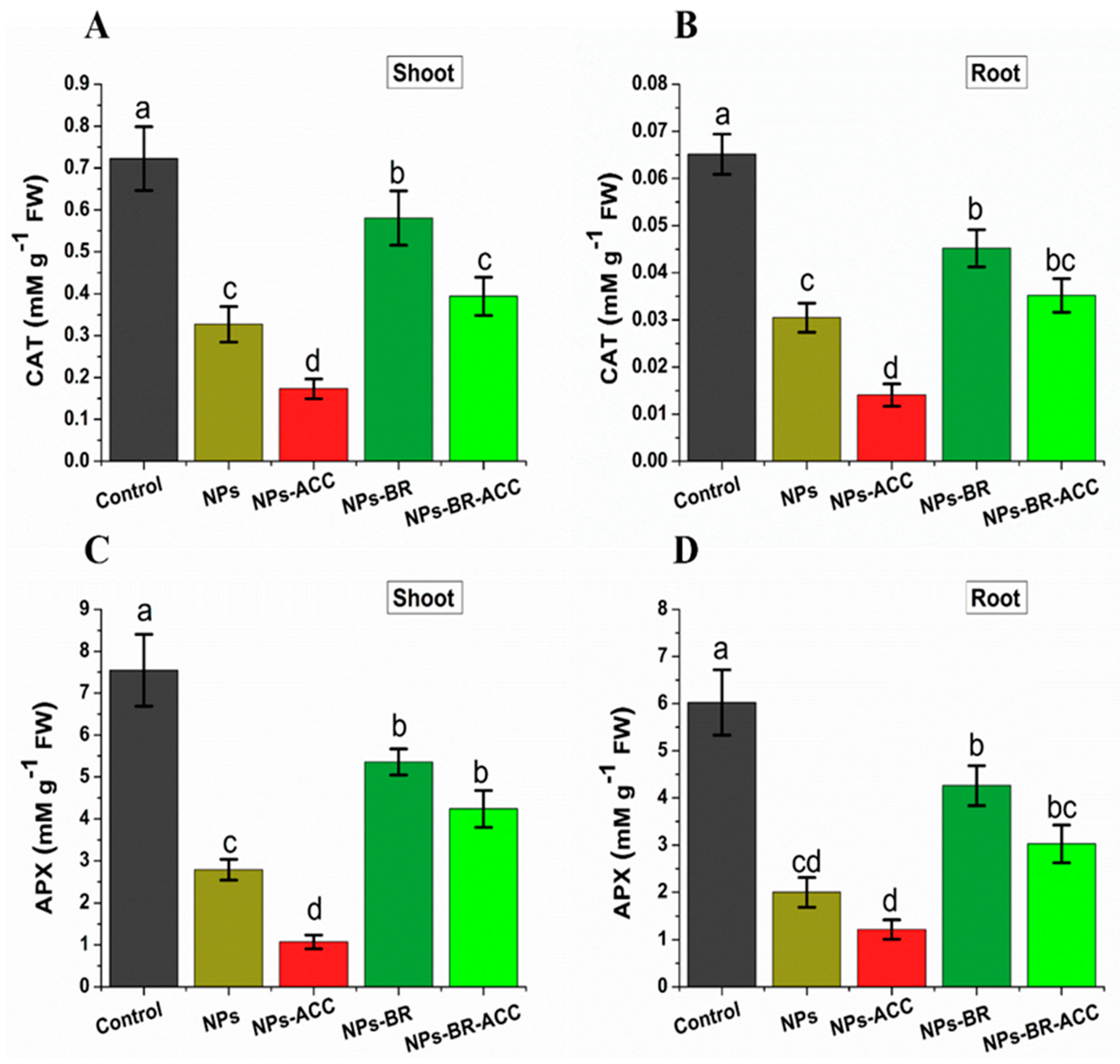
3.9. BRs Reversed Ethylene-Reduced Antioxidant Enzyme Activities Under CuO NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, K.; Li, Y.; Tu, C.; Guo, Y.; Yang, X.; Xia, C.; Hou, H. Nanotechnology in food packaging with implications for sustainable outlook and safety concerns. Food Biosci. 2024, 58, 103625. [Google Scholar] [CrossRef]
- Sandhu, Z.A.; Raza, M.A.; Alqurashi, A.; Sajid, S.; Ashraf, S.; Imtiaz, K.; Aman, F.; Alessa, A.H.; Shamsi, M.B.; Latif, M. Advances in the Optimization of Fe Nanoparticles: Unlocking Antifungal Properties for Biomedical Applications. Pharmaceutics 2024, 16, 645. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Zhang, Z.; Ke, M.; Qu, Q.; Peijnenburg, W.; Lu, T.; Zhang, Q.; Ye, Y.; Xu, P.; Du, B.; Sun, L.; et al. Impact of copper nanoparticles and ionic copper exposure on wheat (Triticum aestivum L.) root morphology and antioxidant response. Environ. Pollut. 2018, 239, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, M.; Anand, R.; Sharma, P.; Rajput, P.; Sharma, N.; Singh, K. Multifunctional Copper Nanoparticles: Synthesis and Applications. ECS J. Solid State Sci. Technol. 2021, 10, 063011. [Google Scholar] [CrossRef]
- Bakshi, M.; Kumar, A. Chapter 16—Applications of copper nanoparticles in plant protection and pollution sensing: Toward promoting sustainable agriculture. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–413. [Google Scholar]
- Ameta, R.K.; Malik, P.; Korgaokar, S.; Vanzara, P.; Soni, K. Contemporary advances in the plant resources mediated synthesis of copper oxide nanoparticles: Insights on structure-function-workability understanding. Plant Nano Biol. 2024, 7, 100065. [Google Scholar] [CrossRef]
- Ingle, P.U.; Shende, S.S.; Hande, D.; Rai, M.; Golinska, P.; Gade, A.K. Mycogenic Copper Oxide Nanoparticles for Fungal Infection Management in Agricultural Crop Plants. BioNanoScience 2024, 14, 359–367. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Luo, J.; Abdallah, Y.; Yu, S.; Wang, X.; Alkhalifah, D.H.M.; Hozzein, W.N.; Wang, F.; Bi, J.A.; Yan, C.; et al. Copper oxide nanoparticles: An effective suppression tool against bacterial leaf blight of rice and its impacts on plants. Pest Manag. Sci. 2024, 80, 1279–1288. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Fedorenko, A.; Lysenko, V.; Bederska-Błaszczyk, M.; Olchowik, J.; Tsitsuashvili, V.; Chaplygin, V. Structural and ultrastructural changes in nanoparticle exposed plants. In Nanoscience for Sustainable Agriculture; Springer: Cham, Switzerland, 2019; pp. 281–295. [Google Scholar]
- Rajput, V.D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V.; Fedorenko, A. Effects of copper nanoparticles (CuO NPs) on crop plants: A mini review. BioNanoScience 2018, 8, 36–42. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Fidalgo, F. Metal-based nanomaterials and oxidative stress in plants: Current aspects and overview. In Phytotoxicity of Nanoparticles; Springer: Cham, Switzerland, 2018; pp. 197–227. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. Nanoimpact 2017, 7, 28–40. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper nanoparticles: Synthesis and characterization, physiology, toxicity and antimicrobial applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Khan, A.R.; Fan, X.; Salam, A.; Azhar, W.; Ulhassan, Z.; Qi, J.; Liaquat, F.; Yang, S.; Gan, Y. Melatonin-mediated resistance to copper oxide nanoparticles-induced toxicity by regulating the photosynthetic apparatus, cellular damages and antioxidant defense system in maize seedlings. Environ. Pollut. 2023, 316, 120639. [Google Scholar] [CrossRef]
- Assadian, E.; Zarei, M.H.; Gilani, A.G.; Farshin, M.; Degampanah, H.; Pourahmad, J. Toxicity of copper oxide (CuO) nanoparticles on human blood lymphocytes. Biol. Trace Elem. Res. 2018, 184, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Ahmed, B.; Sushkova, S.; Singh, R.; Soldatov, M.; Laratte, B.; Fedorenko, A.; Mandzhieva, S.; Blicharska, E. Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: Critical review of the state of the science and future perspectives. Rev. Environ. Contam. Toxicol. 2020, 252, 51–96. [Google Scholar]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO nanoparticles: A threat to soil organisms, plants, and human health. Environ. Geochem. Health 2020, 42, 147–158. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, X.; Wu, H.; Zou, L.; Wang, L.; Shi, J. Effects and mechanisms of copper oxide nanoparticles with regard to arsenic availability in soil–rice systems: Adsorption behavior and microbial response. Environ. Sci. Technol. 2022, 56, 8142–8154. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ. Toxicol. Chem. 2018, 37, 11–20. [Google Scholar] [CrossRef]
- Małecki, J.J.; Kadzikiewicz-Schoeneich, M.; Szostakiewicz-Hołownia, M. Concentration and mobility of copper and zinc in the hypergenic zone of a highly urbanized area. Environ. Earth Sci. 2015, 75, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Qiu, Y.; Liu, Y. Bioavailability and Toxicity of nano Copper Oxide to Pakchoi (Brassica campestris L.) as Compared with bulk Copper Oxide and Ionic Copper. Bull. Environ. Contam. Toxicol. 2024, 112, 52. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Parada, J.; de Oliveira, H.C.; Benavides-Mendoza, A.; Leiva, S.; Fernandez-Baldo, M.; Seabra, A.B. Copper nanoparticles as a potential emerging pollutant: Divergent effects in the agriculture, risk-benefit balance and integrated strategies for its use. Emerg. Contam. 2024, 10, 100352. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Sabir, I.A.; Ashraf, M.; Liaquat, F.; Gulzar, S.; Chang, L.; Zhang, Y. Phytotoxic effects of chemically synthesized copper oxide nanoparticles induce physiological, biochemical, and ultrastructural changes in Cucumis melo. Environ. Sci. Pollut. Res. 2023, 30, 51595–51606. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka-Puchta, L.; Sawicka, D.; Zapor, L.; Miranowicz-Dzierzawska, K. Assessing cytotoxicity and endoplasmic reticulum stress in human blood–brain barrier cells due to silver and copper oxide nanoparticles. J. Appl. Genet. 2025, 66, 87–103. [Google Scholar] [CrossRef]
- Pourahmad, J.; Salami, M.; Zarei, M.H. Comparative Toxic Effect of Bulk Copper Oxide (CuO) and CuO Nanoparticles on Human Red Blood Cells. Biol. Trace Elem. Res. 2023, 201, 149–155. [Google Scholar] [CrossRef]
- Roy, D.; Adhikari, S.; Adhikari, A.; Ghosh, S.; Azahar, I.; Basuli, D.; Hossain, Z. Impact of CuO nanoparticles on maize: Comparison with CuO bulk particles with special reference to oxidative stress damages and antioxidant defense status. Chemosphere 2022, 287, 131911. [Google Scholar] [CrossRef]
- Shaw, A.K.; Ghosh, S.; Kalaji, H.M.; Bosa, K.; Brestic, M.; Zivcak, M.; Hossain, Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ. Exp. Bot. 2014, 102, 37–47. [Google Scholar] [CrossRef]
- Salam, A.; Rehman, M.; Qi, J.; Khan, A.R.; Yang, S.; Zeeshan, M.; Ulhassan, Z.; Afridi, M.S.; Yang, C.; Chen, N.; et al. Cobalt stress induces photosynthetic and ultrastructural distortion by disrupting cellular redox homeostasis in maize. Environ. Exp. Bot. 2024, 217, 105562. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Yang, S.; He, D.; Khan, A.R.; Salam, A.; Azhar, W.; Muhammad, S.; Ali, S.; Hamid, Y.; Khan, I.; et al. Seed priming with nano-silica effectively ameliorates chromium toxicity in Brassica napus. J. Hazard. Mater. 2023, 458, 131906. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, H.; Jiang, S.J.; Zhang, L.; He, Q.Y.; He, H.Q. Effects of the nitric oxide donor sodium nitroprusside on antioxidant enzymes in wheat seedling roots under nickel stress. Russ. J. Plant Physiol. 2010, 57, 833–839. [Google Scholar] [CrossRef]
- Shen, C.X.; Zhang, Q.F.; Li, J.; Bi, F.C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, J.; Praetorius, A.; Gondikas, A.; Fabienke, W.; von der Kammer, F.; Hofmann, T. Detection of Engineered Copper Nanoparticles in Soil Using Single Particle ICP-MS. Int. J. Environ. Res. Public Health 2015, 12, 15756–15768. [Google Scholar] [CrossRef]
- McVay, I.R.; Maher, W.A.; Krikowa, F.; Ubrihien, R. Metal concentrations in waters, sediments and biota of the far south-east coast of New South Wales, Australia, with an emphasis on Sn, Cu and Zn used as marine antifoulant agents. Environ. Geochem. Health 2019, 41, 1351–1367. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Guo, F.; Lv, M.; Zhang, J.; Li, J. Crosstalk between Brassinosteroids and Other Phytohormones during Plant Development and Stress Adaptation. Plant Cell Physiol. 2024, 65, 1530–1543. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Barker, J.; Liu, G.; Hasanuzzaman, M.; Li, Y.; Ramakrishnan, M.; Mokhberdoran, F. Co-Application of 24-Epibrassinolide and Titanium Oxide Nanoparticles Promotes Pleioblastus pygmaeus Plant Tolerance to Cu and Cd Toxicity by Increasing Antioxidant Activity and Photosynthetic Capacity and Reducing Heavy Metal Accumulation and Translocation. Antioxidants 2022, 11, 451. [Google Scholar] [CrossRef]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S.; et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Silva, A.P.S.D.; Alencar, A.A.D.S.; Sudré, C.P.; Araújo, M.D.S.B.D.; Lobato, A.K.D.S. Brassinosteroids: Relevant Evidence Related to Mitigation of Abiotic and Biotic Stresses in Plants. Agronomy 2024, 14, 840. [Google Scholar] [CrossRef]
- Anwar, A.; Di, Q.; Yan, Y.; He, C.; Li, Y.; Yu, X. Exogenous 24-epibrassinolide alleviates the detrimental effects of suboptimal root zone temperature in cucumber seedlings. Arch. Agron. Soil Sci. 2019, 65, 1927–1940. [Google Scholar] [CrossRef]
- Peng, R.; Sun, W.; Jin, X.; Yu, L.; Chen, C.; Yue, Z.; Dong, Y. Analysis of 2,4-epibrassinolide created an enhancement tolerance on Cd toxicity in Solanum nigrum L. Environ. Sci. Pollut. Res. 2020, 27, 16784–16797. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Rattan, A.; Gautam, V.; Bhardwaj, R. Alleviation of cadmium and mercury stress by supplementation of steroid hormone to Raphanus sativus seedlings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 661–666. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Wu, M.; Kkan, A.R.; Jan, M.; Ali, A.; Liu, Y.; Ge, S.; Wu, J.; Gan, Y.; et al. Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 166–179. [Google Scholar] [CrossRef]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009, 57, 606–614. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Jiroutova, P.; Oklestkova, J.; Strnad, M. Crosstalk between brassinosteroids and ethylene during plant growth and under abiotic stress conditions. Int. J. Mol. Sci. 2018, 19, 3283. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.M.M.; Senn, M.E.; Grozeff, G.E.G.; Fanello, D.D.; Carrión, C.A.; Núñez, M.; Bishop, G.J.; Bartoli, C.G. Impact of brassinosteroids and ethylene on ascorbic acid accumulation in tomato leaves. Plant Physiol. Biochem. 2014, 74, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.K.; Arshad, M. Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Ji, X.; Liu, Y.; Lin, Z.; Lin, Z.; Xiao, S.; Peng, B.; Tan, C.; Zhang, X. Effect of a novel Ca-Si composite mineral on Cd bioavailability, transport and accumulation in paddy soil-rice system. J. Environ. Manag. 2019, 233, 802–811. [Google Scholar] [CrossRef]
- Liu, J.; Hou, H.; Zhao, L.; Sun, Z.; Lu, Y.; Li, H. Mitigation of Cd accumulation in rice from Cd-contaminated paddy soil by foliar dressing of S and P. Sci. Total Environ. 2019, 690, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Azhar, W.; Khan, A.R.; Muhammad, N.; Liu, B.; Song, G.; Hussain, A.; Yasin, M.U.; Khan, S.; Munir, R.; Gan, Y. Ethylene mediates CuO NP-induced ultrastructural changes and oxidative stress in Arabidopsis thaliana leaves. Environ. Sci. Nano 2020, 7, 938–953. [Google Scholar] [CrossRef]
- Ali, I.; Liu, B.; Farooq, M.A.; Islam, F.; Azizullah, A.; Yu, C.; Su, W.; Gan, Y. Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Saf. 2016, 124, 277–284. [Google Scholar] [CrossRef]
- Qi, J.; Yang, S.; Salam, A.; Yang, C.; Khan, A.R.; Wu, J.; Azhar, W.; Gan, Y. OsRbohI Regulates Rice Growth and Development via Jasmonic Acid Signalling. Plant Cell Physiol. 2023, 64, 686–699. [Google Scholar] [CrossRef]
- Yoshida, S. Routine procedures for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1976; pp. 61–66. [Google Scholar]
- Wu, J.; Yu, C.; Hunag, L.; Wu, M.; Liu, B.; Liu, Y.; Song, G.; Liu, D.; Gan, Y. Overexpression of MADS-box transcription factor OsMADS25 enhances salt stress tolerance in Rice and Arabidopsis. Plant Growth Regul. 2020, 90, 163–171. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Wang, J.; Huang, Q.; Aziz, R.; Zhou, W. Dual behavior of selenium: Insights into physio-biochemical, anatomical and molecular analyses of four Brassica napus cultivars. Chemosphere 2019, 225, 329–341. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Ali, S.; Gill, R.A.; Mwamba, T.M.; Abid, M.; Li, L.; Zhang, N.; Zhou, W. Comparative orchestrating response of four oilseed rape (Brassica napus) cultivars against the selenium stress as revealed by physio-chemical, ultrastructural and molecular profiling. Ecotoxicol. Environ. Saf. 2018, 161, 634–647. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kumar, N.; Sharma, V.; Kaur, G.; Lata, C.; Dasila, H.; Perveen, K.; Khan, F.; Gupta, V.K.; Khanam, M.N. Brassinosteroids as promoters of seedling growth and antioxidant activity under heavy metal zinc stress in mung bean (Vigna radiata L.). Front. Microbiol. 2023, 14, 1259103. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide Alleviates the Injurious Effects of Cr(VI) Toxicity in Tomato Plants: Insights into Growth, Physio-Biochemical Attributes, Antioxidant Activity and Regulation of Ascorbate–Glutathione and Glyoxalase Cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Castañeda-Murillo, C.C.; Rojas-Ortiz, J.G.; Sánchez-Reinoso, A.D.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar brassinosteroid analogue (DI-31) sprays increase drought tolerance by improving plant growth and photosynthetic efficiency in lulo plants. Heliyon 2022, 8, e08977. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Huo, S.F.; Wang, L.T.; Meng, J.F.; Zhang, Z.W.; Xi, Z.M. Exogenous 24-Epibrassinolide alleviates oxidative damage from copper stress in grape (Vitis vinifera L.) cuttings. Plant Physiol. Biochem. 2018, 130, 555–565. [Google Scholar] [CrossRef]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ. Exp. Bot. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Avalbaev, A.; Fedyaev, V.; Lubyanova, A.; Yuldashev, R.; Allagulova, C. 24-Epibrassinolide Reduces Drought-Induced Oxidative Stress by Modulating the Antioxidant System and Respiration in Wheat Seedlings. Plants 2024, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- González-Melendi, P.; Fernández-Pacheco, R.; Coronado, M.J.; Corredor, E.; Testillano, P.S.; Risueño, M.C.; Marquina, C.; Ibarra, M.R.; Rubiales, D.; Pérez-de-Luque, A. Nanoparticles as smart treatment-delivery systems in plants: Assessment of different techniques of microscopy for their visualization in plant tissues. Ann. Bot. 2008, 101, 187–195. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Kevat, N.; Sharma, P.K. Copper Oxide Nanoparticle and Copper (II) Ion Exposure in Oryza sativa Reveals Two Different Mechanisms of Toxicity. Water Air Soil Pollut. 2020, 231, 258. [Google Scholar] [CrossRef]
- Kennedy, C.; Gonsalves, F. The action of divalent zinc, cadmium, mercury, copper and lead on the trans-root potential and H+, efflux of excised roots. J. Exp. Bot. 1987, 38, 800–817. [Google Scholar] [CrossRef]
- Soares, E.V.; Soares, H.M. Harmful effects of metal(loid) oxide nanoparticles. Appl. Microbiol. Biotechnol. 2021, 105, 1379–1394. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.H.; Liu, W.C.; Zhang, X.W.; Lu, Y.T. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol. 2015, 168, 1777–1791. [Google Scholar] [CrossRef]
- Hong, Z.; Ueguchi-Tanaka, M.; Shimizu-Sato, S.; Inukai, Y.; Fujioka, S.; Shimada, Y.; Takatsuto, S.; Agetsuma, M.; Yoshida, S.; Watanabe, Y.; et al. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 2002, 32, 495–508. [Google Scholar] [CrossRef]
- Caño-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-García, S.; Cheng, J.-C.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Sima, X.F.; Shen, X.C.; Fang, T.; Yu, H.Q.; Jiang, H. Efficiently reducing the plant growth inhibition of CuO NPs using rice husk-derived biochar: Experimental demonstration and mechanism investigation. Environ. Sci. Nano 2017, 4, 1722–1732. [Google Scholar] [CrossRef]
- Arteca, R.N.; Tsai, D.S.; Schlagnhaufer, C.; Mandava, N.B. The effect of brassinosteroid on auxin-induced ethylene production by etiolated mung bean segments. Physiol. Plant. 1983, 59, 539–544. [Google Scholar] [CrossRef]
- Lim, S.H.; Chang, S.C.; Lee, J.S.; Kim, S.K.; Kim, S.Y. Brassinosteroids affect ethylene production in the primary roots of maize (Zea mays L.). J. Plant Biol. 2002, 45, 148–153. [Google Scholar] [CrossRef]
- Khan, A.R.; Azhar, W.; Wu, J.; Ulhassan, Z.; Salam, A.; Zaidi, S.H.R.; Yang, S.; Song, G.; Gan, Y. Ethylene participates in zinc oxide nanoparticles induced biochemical, molecular and ultrastructural changes in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 226, 112844. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Fedorenko, A.; Sushkova, S.; Mandzhieva, S.; Lysenko, V.; Duplii, N.; Fedorenko, G.; Dvadnenko, K.; Ghazaryan, K. Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total Environ. 2018, 645, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Nwafor, C.C.; Han, Y.; Liu, L.; Rashid, M.; Batool, M.; El-Badri, A.M.; Cao, C.; Zhan, M. Spermidine and brassinosteroid regulate root anatomical structure, photosynthetic traits and antioxidant defense systems to alleviate waterlogging stress in maize seedlings. S. Afr. J. Bot. 2022, 144, 389–402. [Google Scholar] [CrossRef]
- Zhang, N.; Ali, S.; Huang, Q.; Yang, C.; Ali, B.; Chen, W.; Zhang, K.; Ali, S.; Ulhassan, Z.; Zhou, W. Seed pretreatment with brassinosteroids stimulates sunflower immunity against parasitic weed (Orobanche cumana) infection. Physiol. Plant. 2024, 176, e14324. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Xia, X.J.; Gao, C.J.; Song, L.X.; Zhou, Y.H.; Shi, K.; Yu, J.Q. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef]
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006, 47, 907–916. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Paul Bolwell, G. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Wang, C.; Yin, S.S.; Li, X.L.; Hu, W.J.; Simon, M.; Shen, Z.J.; Xiao, Q.; Chu, C.C.; et al. Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. J. Hazard. Mater. 2015, 297, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of nitrate reductase in brassinosteroid-induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by upregulating the ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2020, 196, 110483. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.A.; Hao, Y.; Mehmood, S.; Shu, H.; Zhou, Y.; Jin, W.; Chen, C.; Li, L.; Altaf, M.A.; Wang, Z. Physiological and transcriptomic analysis provide molecular insight into 24-epibrassinolide mediated Cr(VI)-toxicity tolerance in pepper plants. Environ. Pollut. 2022, 306, 119375. [Google Scholar] [CrossRef]
- Deslauriers, S.D.; Larsen, P.B. Feronia is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 2010, 3, 626–640. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, W.; Salam, A.; Khan, A.R.; Ahmad, I.; Gan, Y. Brassinosteroids Alleviate Ethylene-Induced Copper Oxide Nanoparticle Toxicity and Ultrastructural and Stomatal Damage in Rice Seedlings. Agriculture 2025, 15, 907. https://doi.org/10.3390/agriculture15080907
Azhar W, Salam A, Khan AR, Ahmad I, Gan Y. Brassinosteroids Alleviate Ethylene-Induced Copper Oxide Nanoparticle Toxicity and Ultrastructural and Stomatal Damage in Rice Seedlings. Agriculture. 2025; 15(8):907. https://doi.org/10.3390/agriculture15080907
Chicago/Turabian StyleAzhar, Wardah, Abdul Salam, Ali Raza Khan, Irshan Ahmad, and Yinbo Gan. 2025. "Brassinosteroids Alleviate Ethylene-Induced Copper Oxide Nanoparticle Toxicity and Ultrastructural and Stomatal Damage in Rice Seedlings" Agriculture 15, no. 8: 907. https://doi.org/10.3390/agriculture15080907
APA StyleAzhar, W., Salam, A., Khan, A. R., Ahmad, I., & Gan, Y. (2025). Brassinosteroids Alleviate Ethylene-Induced Copper Oxide Nanoparticle Toxicity and Ultrastructural and Stomatal Damage in Rice Seedlings. Agriculture, 15(8), 907. https://doi.org/10.3390/agriculture15080907







