Response of Different Perennial Ryegrass Varieties to Water Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Yield and Root Mass
2.3. Biochemical Analysis
2.4. Carbohydrate Analyses
2.5. Statistical Analysis
3. Results
3.1. Yield and Root Mass
3.2. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Formation
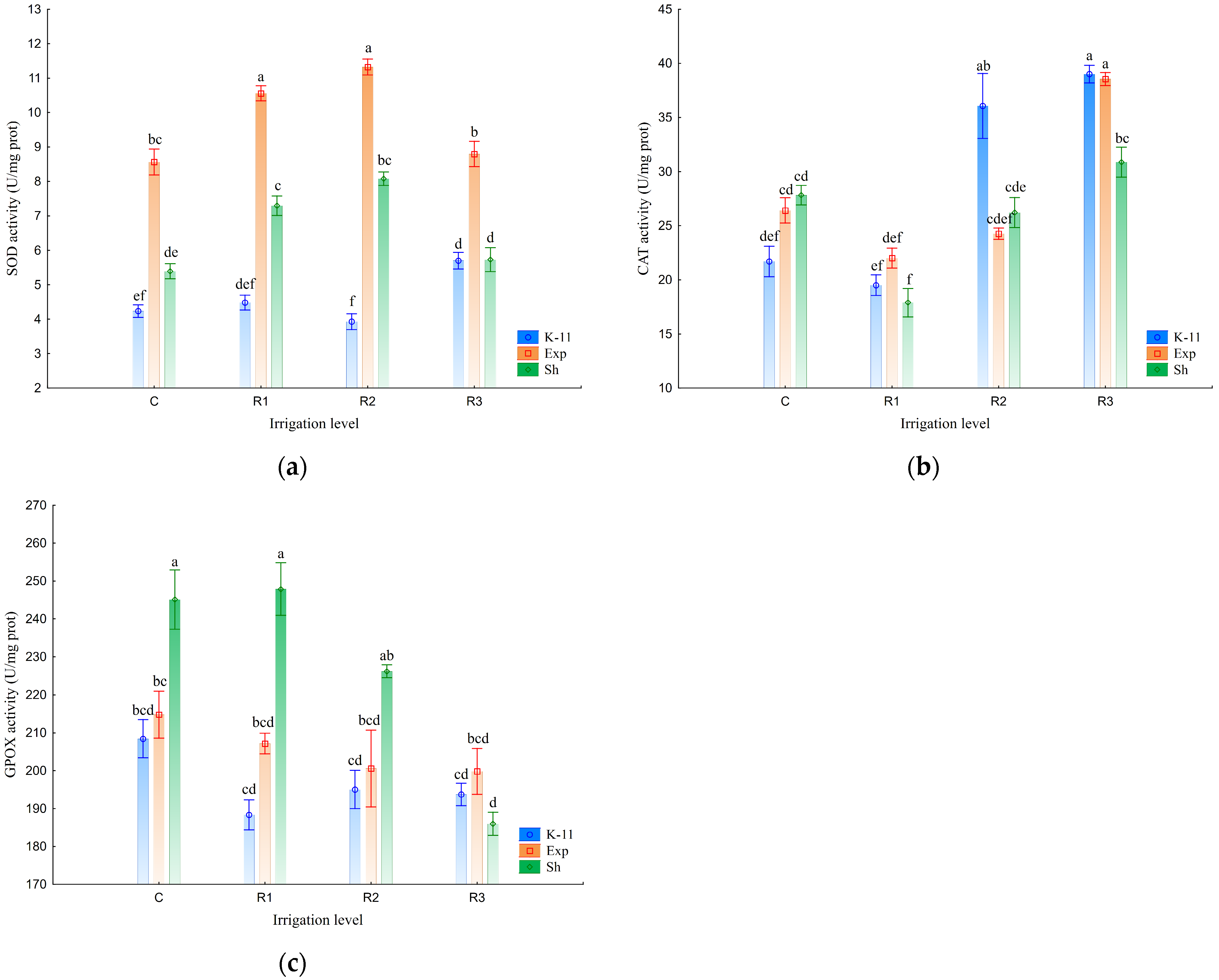
3.3. Antioxidant Enzyme Activity
3.4. Water-Soluble Sugars Content
4. Discussion
4.1. Yield and Root Mass
4.2. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Formation
4.3. Antioxidant Enzyme Activity
4.4. Water-Soluble Sugars Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meza, I.; Siebert, S.; Döll, P.; Kusche, J.; Herbert, C.; Eyshi Rezaei, E.; Nouri, H.; Gerdener, H.; Popat, E.; Frischen, J.; et al. Global-Scale Drought Risk Assessment for Agricultural Systems. Nat. Hazards Earth Syst. Sci. 2020, 20, 695–712. [Google Scholar] [CrossRef]
- Mahmoud, N.; Abdou, M.A.H.; Salaheldin, S.; Soliman, W.S. Lemongrass Growth, Essential Oil, and Active Substances as Affected by Water Deficit. Horticulturae 2022, 8, 250. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant Adaptations to the Combination of Drought and High Temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Zörb, C.; Geilfus, C.-M. Drought and Crop Yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Colas, V.; Barre, P.; Van Parijs, F.; Wolters, L.; Quitté, Y.; Ruttink, T.; Roldán-Ruiz, I.; Escobar Gutiérrez, A.J.; Muylle, H. Seasonal Differences in Structural and Genetic Control of Digestibility in Perennial Ryegrass. Front. Plant Sci. 2022, 12, 801145. [Google Scholar] [CrossRef]
- Wang, R.; Gao, Y.; Li, J.; Wang, X.; Yang, Y.; Huang, H.; Zhou, Z.; Wang, P.; Zhao, L. Drought and Heat Stress Studies in Perennial Ryegrass: A Bibliometric Analysis 1994–2024. Front. Sustain. Food Syst. 2024, 8, 1458552. [Google Scholar] [CrossRef]
- Fariaszewska, A.; Aper, J.; Van Huylenbroeck, J.; Baert, J.; De Riek, J.; Staniak, M.; Pecio, Ł. Mild Drought Stress-Induced Changes in Yield, Physiological Processes and Chemical Composition in Festuca, Lolium and Festulolium. J. Agron. Crop Sci. 2017, 203, 103–116. [Google Scholar] [CrossRef]
- Shahidi, R.; Yoshida, J.; Cougnon, M.; Reheul, D.; Van Labeke, M.-C. Morpho-Physiological Responses to Dehydration Stress of Perennial Ryegrass and Tall Fescue Genotypes. Funct. Plant Biol. 2017, 44, 612. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant Drought Survival under Climate Change and Strategies to Improve Perennial Grasses. A Review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef]
- Perlikowski, D.; Augustyniak, A.; Masajada, K.; Skirycz, A.; Soja, A.M.; Michaelis, Ä.; Wolter, G.; Kosmala, A. Structural and Metabolic Alterations in Root Systems under Limited Water Conditions in Forage Grasses of Lolium-Festuca Complex. Plant Sci. 2019, 283, 211–223. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative Role of Sugar Signaling and Transport during Drought Stress Responses in Plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Roitsch, T. Source-Sink Regulation by Sugar and Stress. Curr. Opin. Plant Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Vidossich, P.; Alfonso-Prieto, M.; Rovira, C. Catalases versus Peroxidases: DFT Investigation of H2O2 Oxidation in Models Systems and Implications for Heme Protein Engineering. J. Inorg. Biochem. 2012, 117, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Dragišić Maksimović, J.; Mojović, M.; Vučinić, Ž.; Maksimović, V. Spatial Distribution of Apoplastic Antioxidative Constituents in Maize Root. Physiol. Plant. 2021, 173, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Blamey, F.P.C.; Edmeades, D.C.; Asher, C.J.; Edwards, D.G.; Wheeler, D.M. Evaluation of Solution Culture Techniques for Studying Aluminium Toxicity in Plants. In Plant-Soil Interactions at Low pH; Wright, R.J., Baligar, V.C., Murrmann, R.P., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 905–912. ISBN 978-94-010-5520-8. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maksimović, J.J.D.; Živanović, B.D. Quantification of the Antioxidant Activity in Salt-Stressed Tissues. In Plant Salt Tolerance; Shabala, S., Cuin, T.A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 913, pp. 237–250. ISBN 978-1-61779-985-3. [Google Scholar]
- Fridovich, I. Superoxide Dismutases. In Advances in Enzymology—And Related Areas of Molecular Biology; Meister, A., Ed.; Wiley: Hoboken, NJ, USA, 1986; Volume 58, pp. 61–97. ISBN 978-0-471-88013-4. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of Enhanced Peroxidase Activity with Induced Systemic Resistance of Cucumber to Colletotrichum Lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Carrow, R.N. Drought Avoidance Characteristics of Diverse Tall Fescue Cultivars. Crop Sci. 1996, 36, 371–377. [Google Scholar] [CrossRef]
- Sokolovic, D.; Babic, S.; Radovic, J.; Milenkovic, J.; Lugic, Z.; Andjelkovic, S.; Vasic, T. Genetic Variation of Root Characteristics and Deep Root Production in Perennial Ryegrass Cultivars Contrasting in Field Persistency. In Breeding Strategies for Sustainable Forage and Turf Grass Improvement; Barth, S., Milbourne, D., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 275–281. ISBN 978-94-007-4554-4. [Google Scholar]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate Drought Stress Affected Root Growth and Grain Yield in Old, Modern and Newly Released Cultivars of Winter Wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing Root Phenotypes for Drought Resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef]
- Palta, J.A.; Chen, X.; Milroy, S.P.; Rebetzke, G.J.; Dreccer, M.F.; Watt, M. Large Root Systems: Are They Useful in Adapting Wheat to Dry Environments? Funct. Plant Biol. 2011, 38, 347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A Major Locus Controlling Malondialdehyde Content under Water Stress Is Associated with Fusarium Crown Rot Resistance in Wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Đurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Cingel, A.; Vujičić, M.; Milošević, S. Morpho-Physiological and Molecular Evaluation of Drought and Recovery in Impatiens Walleriana Grown Ex Vitro. Plants 2020, 9, 1559. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Wang, X.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Effects of Different Drought Degrees on Physiological Characteristics and Endogenous Hormones of Soybean. Plants 2022, 11, 2282. [Google Scholar] [CrossRef]
- Farooq, M.; Ahmad, R.; Shahzad, M.; Sajjad, Y.; Hassan, A.; Shah, M.M.; Naz, S.; Khan, S.A. Differential Variations in Total Flavonoid Content and Antioxidant Enzymes Activities in Pea under Different Salt and Drought Stresses. Sci. Hortic. 2021, 287, 110258. [Google Scholar] [CrossRef]
- Farfan-Vignolo, E.R.; Asard, H. Effect of Elevated CO2 and Temperature on the Oxidative Stress Response to Drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 2012, 59, 55–62. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, A.; Ullah, Z.; Sher, H.; Iqbal, J.; Iqbal, R. Exploring Antioxidant Potential and Microsatellite Based Genetic Diversity in Different Germplasm of Aegilops Tauschii. Genet. Resour. Crop Evol. 2024, 1–13. [Google Scholar] [CrossRef]
- DaCosta, M.; Huang, B. Osmotic Adjustment Associated with Variation in Bentgrass Tolerance to Drought Stress. J. Am. Soc. Hortic. Sci. 2006, 131, 338–344. [Google Scholar] [CrossRef]
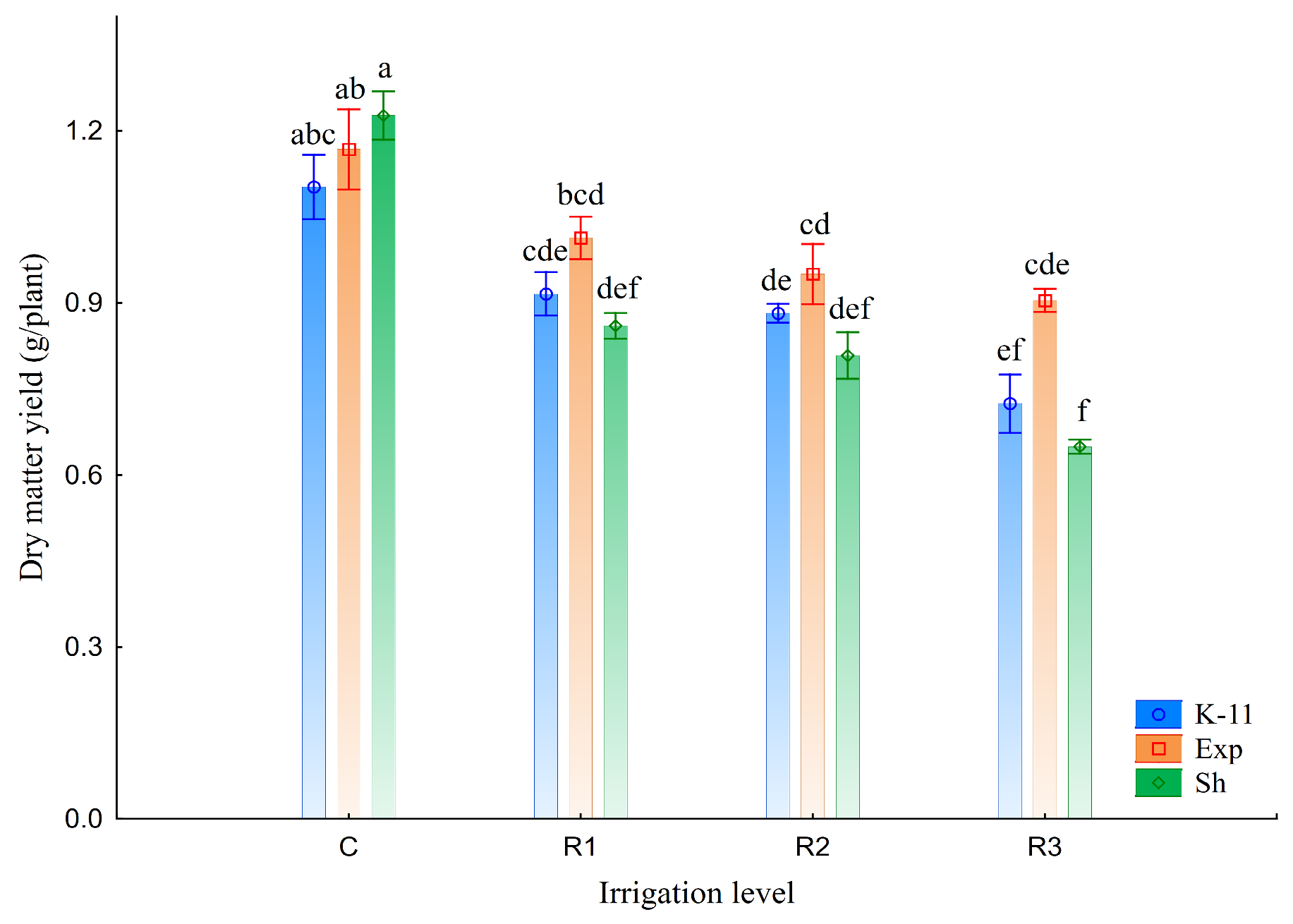




| DMY (g/Plant) | RDM from 0–50 cm (g/Plant) | RDM from 50–90 cm (g/Plant) | Root:Shoot Ratio | Root Mass Ratio (0–50/50–90) | |
|---|---|---|---|---|---|
| Irrigation (A) | |||||
| C | 1.17 ± 0.03 a | 0.95 ± 0.11 a | 0.23 ± 0.02 a | 1.01 ± 0.09 a | 4.23 ± 0.45 b |
| R1 | 0.93 ± 0.03 b | 0.85 ± 0.09 ab | 0.21 ± 0.01 ab | 1.16 ± 0.13 a | 3.98 ± 0.39 b |
| R2 | 0.88 ± 0.03 b | 0.77 ± 0.10 bc | 0.18 ± 0.01 b | 1.10 ± 0.14 a | 4.47 ± 0.68 b |
| R3 | 0.76 ± 0.04 c | 0.67 ± 0.07 c | 0.12 ± 0.01 c | 1.07 ± 0.12 a | 6.59 ± 1.40 a |
| Population (B) | |||||
| K-11 | 0.91 ± 0.04 b | 0.53 ± 0.03 c | 0.16 ± 0.01 b | 0.77 ± 0.02 c | 3.38 ± 0.18 b |
| Exp | 1.01 ± 0.04 a | 0.75 ± 0.04 b | 0.22 ± 0.01 a | 0.96 ± 0.04 b | 3.57 ± 0.15 b |
| Sh | 0.87 ± 0.07 b | 1.14 ± 0.06 a | 0.18 ± 0.02 b | 1.51 ± 0.05 a | 7.51 ± 0.87 a |
| ANOVA | |||||
| A | * | * | * | NS | * |
| B | * | * | * | * | * |
| A × B | * | NS | * | NS | * |
| H2O2 (µmol/g FW) | MDA (nmol/g FW) | SOD (U/mg Prot) | CAT (U/mg Prot) | GPOX (U/mg Prot) | |
|---|---|---|---|---|---|
| Irrigation (A) | |||||
| C | 31.78 ± 0.78 d | 53.97 ± 3.51 c | 6.06 ± 0.66 c | 25.31 ± 1.10 c | 222.8 ± 6.5 a |
| R1 | 34.97 ± 1.07 c | 63.16 ± 3.17 b | 7.45 ± 0.89 a | 19.81 ± 0.81 d | 214.5 ± 9.1 ab |
| R2 | 37.23 ± 0.95 b | 67.69 ± 5.87 a | 7.78 ± 1.08 a | 28.85 ± 2.07 b | 207.3 ± 5.8 b |
| R3 | 40.16 ± 1.27 a | 68.60 ± 4.35 a | 6.74 ± 0.54 b | 36.14 ± 1.41 a | 193.2 ± 2.9 c |
| Population (B) | |||||
| K-11 | 35.18 ± 1.15 b | 48.76 ± 1.69 c | 4.59 ± 0.22 c | 29.07 ± 2.69 a | 196.4 ± 2.9 b |
| Exp | 37.72 ± 0.90 a | 75.64 ± 3.11 a | 9.81 ± 0.37 a | 27.81 ± 1.96 ab | 205.6 ± 3.4 b |
| Sh | 35.21 ± 1.54 b | 65.67 ± 2.10 b | 6.62 ± 0.35 b | 25.70 ± 1.55 b | 226.3 ± 7.8 a |
| ANOVA | |||||
| A | * | * | * | * | * |
| B | * | * | * | * | * |
| A × B | * | * | * | * | * |
| Glucose (mg/g FW) | Fructose (mg/g FW) | Sucrose (mg/g FW) | |
|---|---|---|---|
| Irrigation (A) | |||
| C | 2.72 ± 0.12 a | 2.62 ± 0.11 ab | 6.46 ± 0.56 b |
| R1 | 2.76 ± 0.12 a | 2.53 ± 0.12 b | 6.07 ± 0.57 b |
| R2 | 2.73 ± 0.19 a | 2.52 ± 0.18 b | 6.99 ± 1.04 b |
| R3 | 2.82 ± 0.12 a | 2.76 ± 0.11 a | 11.31 ± 1.04 a |
| Population (B) | |||
| K-11 | 3.11 ± 0.07 a | 2.95 ± 0.05 a | 10.60 ± 0.94 a |
| Exp | 2.86 ± 0.07 b | 2.68 ± 0.07 b | 6.82 ± 0.50 b |
| Sh | 2.31 ± 0.05 c | 2.19 ± 0.09 c | 5.70 ± 0.63 c |
| ANOVA | |||
| A | NS | * | * |
| B | * | * | * |
| A × B | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prijović, M.; Sokolović, D.; Maksimović, J.D.; Maksimović, V.; Milosavljević, D.; Babić, S.; Stepić, M.; Sabovljević, A. Response of Different Perennial Ryegrass Varieties to Water Stress. Agriculture 2025, 15, 917. https://doi.org/10.3390/agriculture15090917
Prijović M, Sokolović D, Maksimović JD, Maksimović V, Milosavljević D, Babić S, Stepić M, Sabovljević A. Response of Different Perennial Ryegrass Varieties to Water Stress. Agriculture. 2025; 15(9):917. https://doi.org/10.3390/agriculture15090917
Chicago/Turabian StylePrijović, Mladen, Dejan Sokolović, Jelena Dragišić Maksimović, Vuk Maksimović, Dragica Milosavljević, Snežana Babić, Marija Stepić, and Aneta Sabovljević. 2025. "Response of Different Perennial Ryegrass Varieties to Water Stress" Agriculture 15, no. 9: 917. https://doi.org/10.3390/agriculture15090917
APA StylePrijović, M., Sokolović, D., Maksimović, J. D., Maksimović, V., Milosavljević, D., Babić, S., Stepić, M., & Sabovljević, A. (2025). Response of Different Perennial Ryegrass Varieties to Water Stress. Agriculture, 15(9), 917. https://doi.org/10.3390/agriculture15090917












