Impacts of Warming and Acidification on Coral Calcification Linked to Photosymbiont Loss and Deregulation of Calcifying Fluid pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Experimental Design
2.2. Coral Husbandry
2.3. Seawater Chemistry Manipulation and Measurement
2.4. Calcification Rates
2.5. Estimating Coral Photosymbiont Index
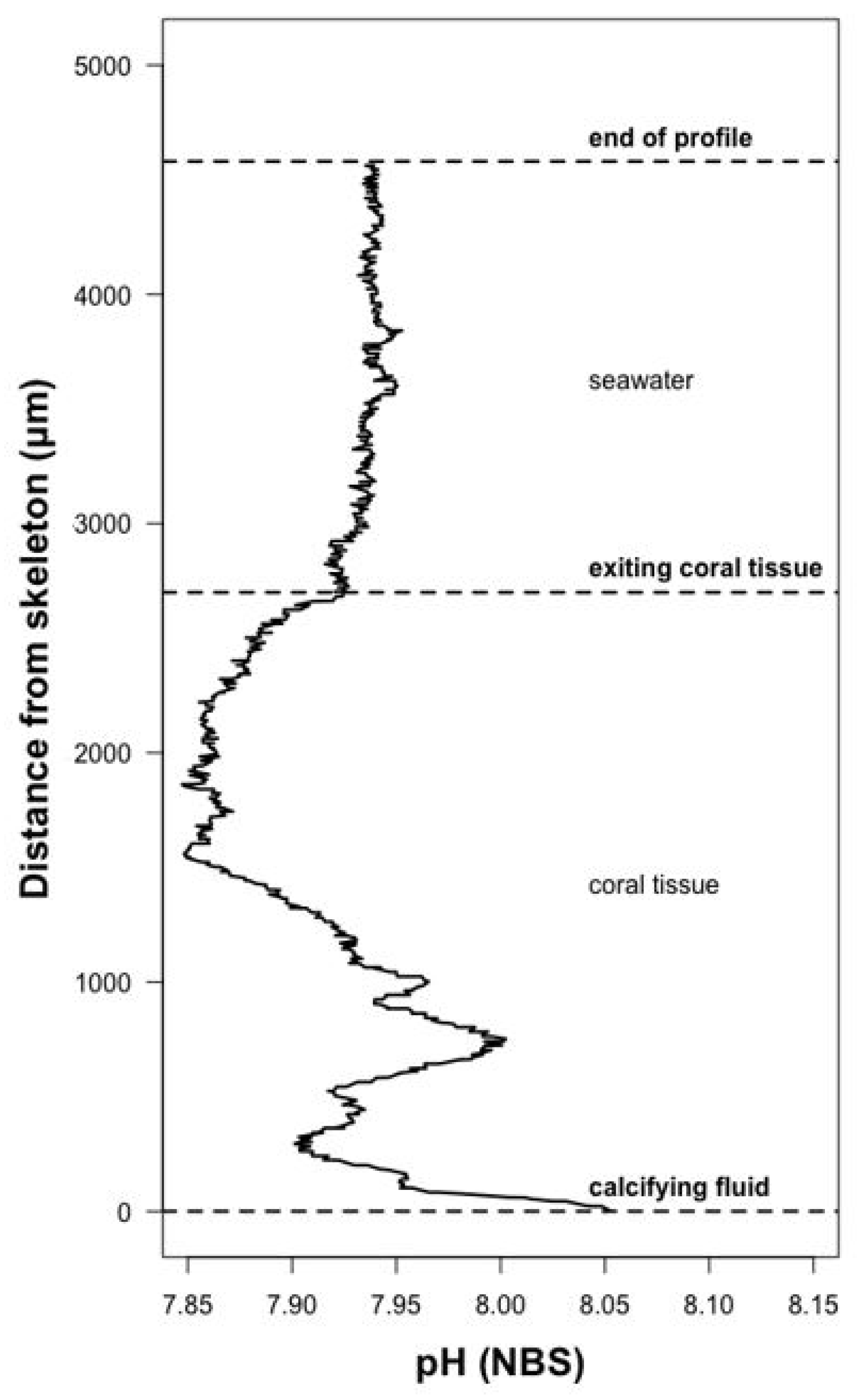
2.6. Measurement of Calcifying Fluid pH
2.7. Statistical Analysis
3. Results
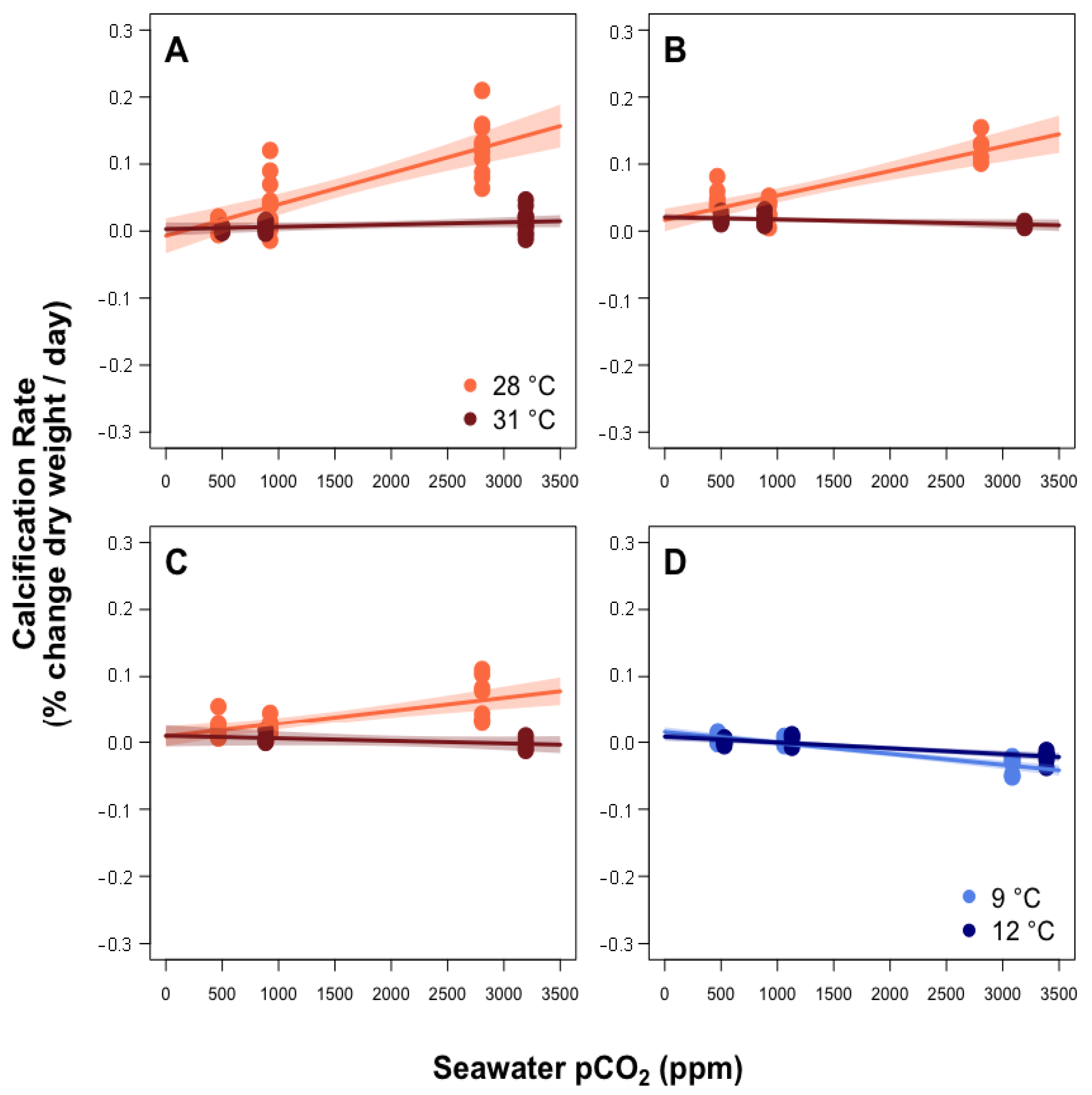
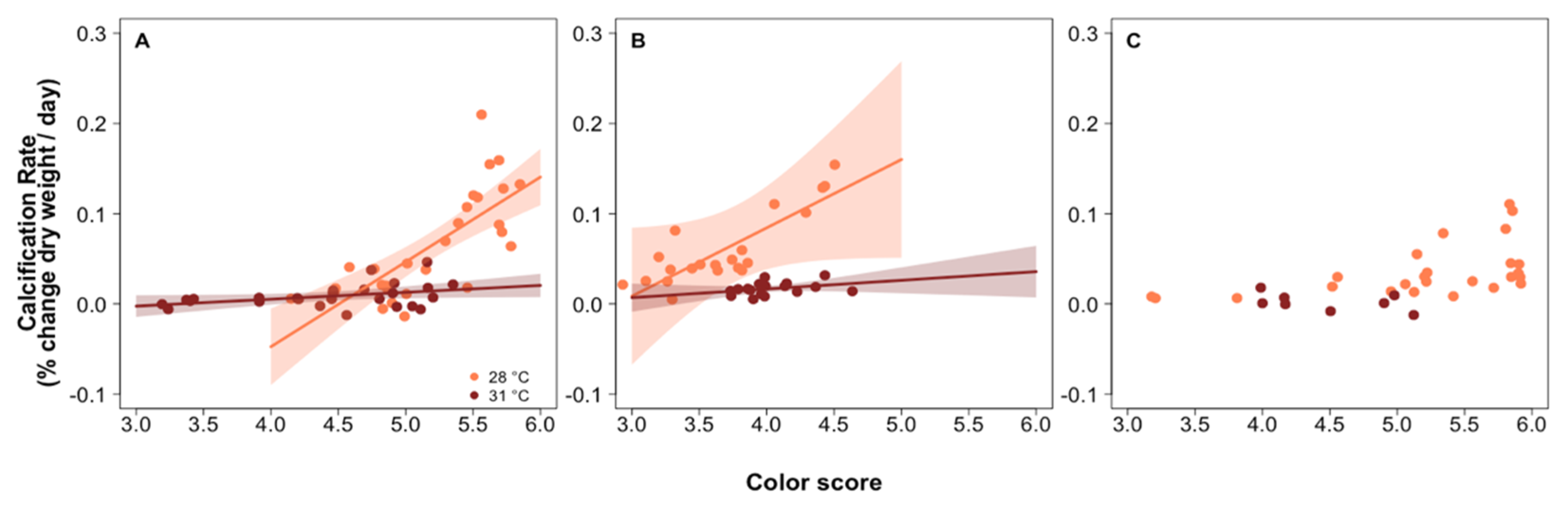
3.1. Predictors of Calcification Rate
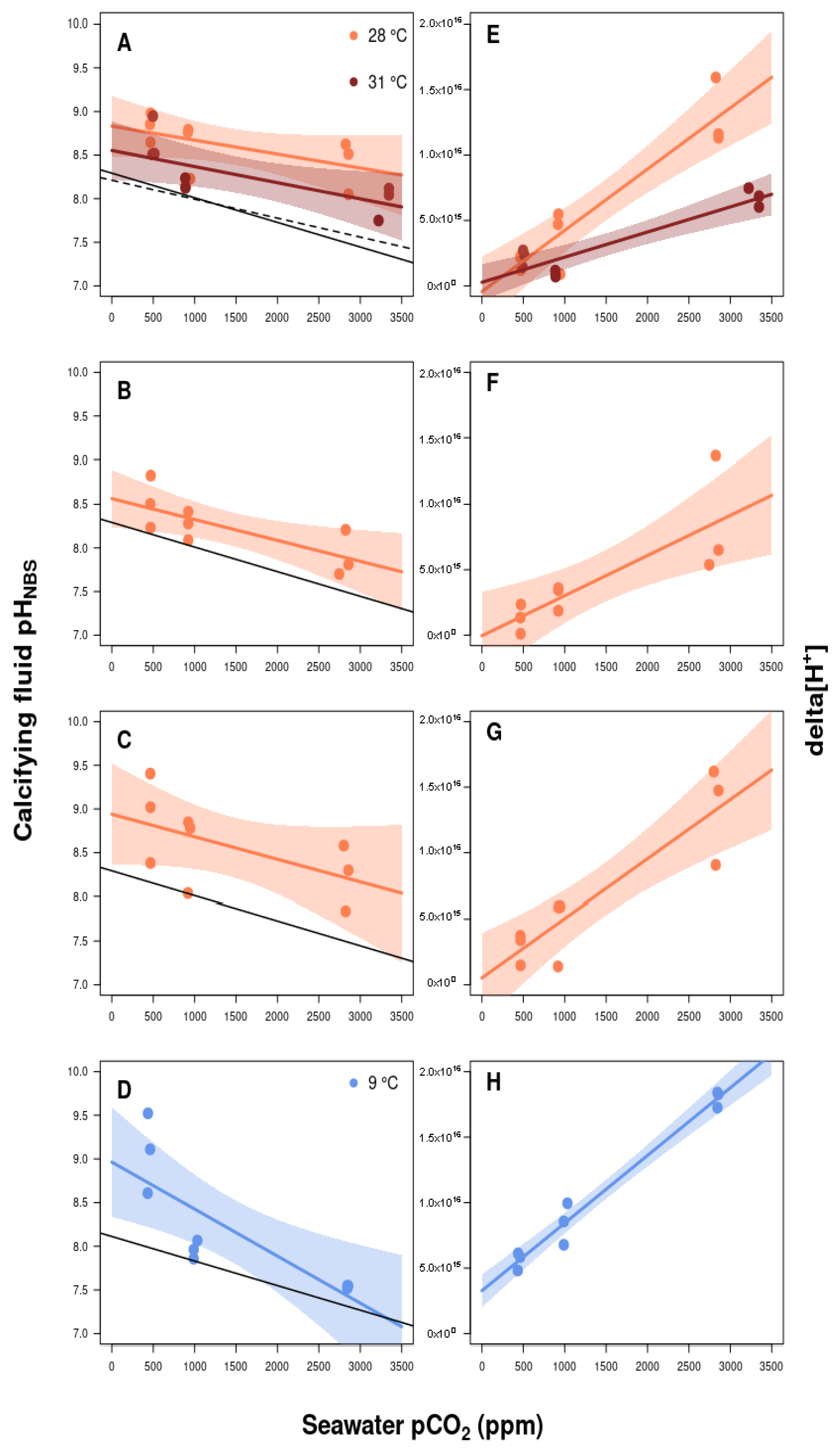
3.2. Calcifying Fluid Chemistry
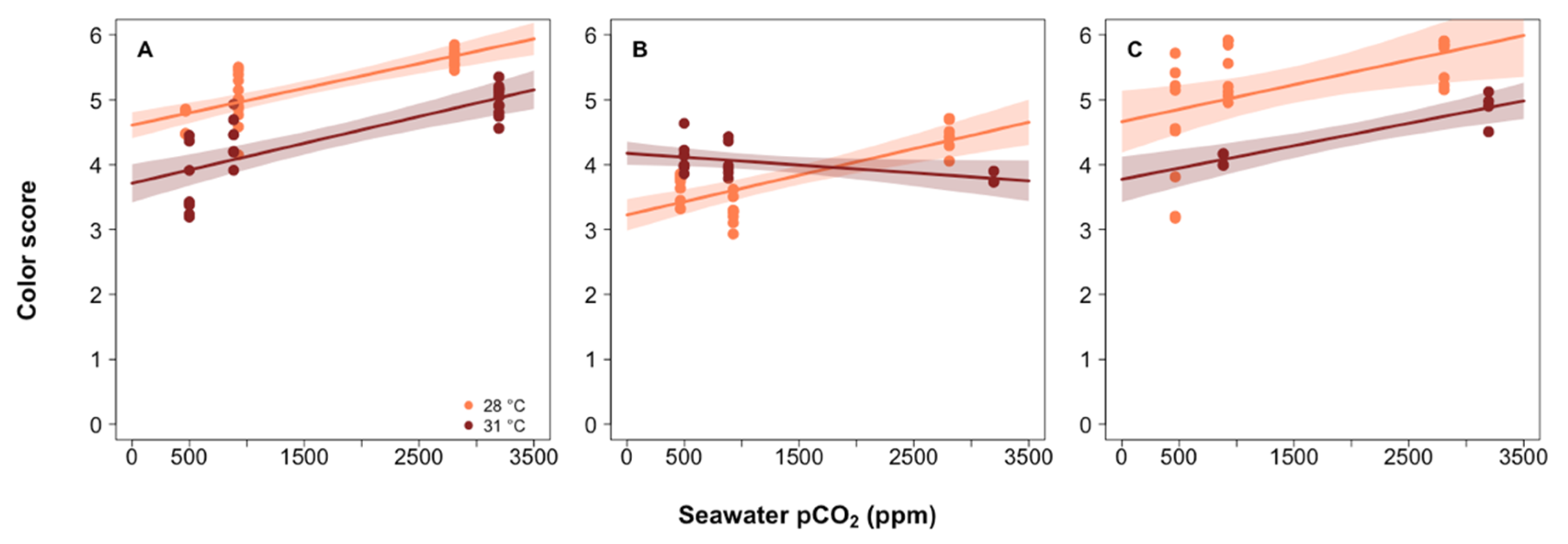
3.3. Estimated Coral Photosymbiont Index
3.4. Investigating the Role of Photosynthesis in Calcification
4. Discussion
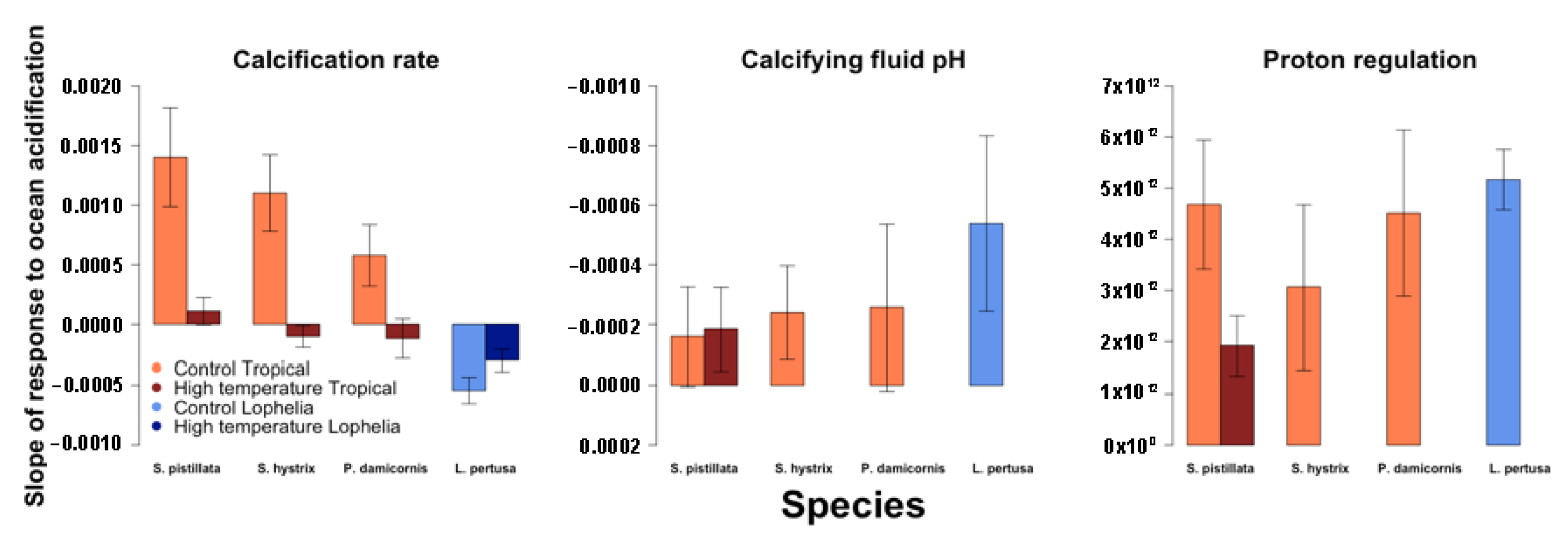
4.1. Calcification Response to pCO2 and Thermal Stress
4.2. Role of Calcifying Fluid pH Regulation in Coral Response to pCO2 and Thermal Stress
4.3. Role of Photosymbionts in Coral Response to pCO2 and Thermal Stress
4.4. Proposed Mechanistic Framework for Zooxanthellate Coral Response to pCO2 and Thermal Stress
4.5. Limitations of Laboratory-Based Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013—The Physical Science Basis; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107415324. [Google Scholar] [CrossRef]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.B.; Ghazaleh, M.N.; Connolly, B.; Westfield, I.; Castillo, K.D. Impacts of seawater saturation state state (ΩA = 0.4–4.6) and temperature (10, 25 °C) on the dissolution kinetics of whole-shell biogenic carbonates. Geochim. Cosmochim. Acta 2016, 192, 318–337. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Rodgers, K.S.; Kuffner, I.B.; Andersson, A.J.; Cox, E.F.; Mackenzie, F.T. Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs 2008, 27, 473–483. [Google Scholar] [CrossRef]
- Milliman, J.D. Production and accumulation of calcium carbonate in the ocean: Budget of a nonsteady state. Glob. Biogeochem. Cycles 1993, 7, 927–957. [Google Scholar] [CrossRef]
- Anthony, K.R.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Bio. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- McCulloch, M.; Falter, J.; Trotter, J.; Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2012, 2, 623–627. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; de Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003, 142, 419–426. [Google Scholar] [CrossRef]
- Allison, N.; Finch, A.A. 11B, Sr, Mg and B in a modern Porites coral: The relationship between calcification site pH and skeletal chemistry. Geochim. Cosmochim. Acta 2010, 74, 1790–1800. [Google Scholar] [CrossRef]
- Ries, J.B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 2011, 75, 4053–4064. [Google Scholar] [CrossRef]
- Venn, A.; Tambutté, E.; Holcomb, M.; Allemand, D.; Tambutté, S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 2011, 6, e20013. [Google Scholar] [CrossRef] [PubMed]
- Comeau, S.; Cornwall, C.E.; McCulloch, M.T. Decoupling between the response of coral calcifying fluid pH and calcification to ocean acidification. Sci. Rep. 2017, 7, 7573. [Google Scholar] [CrossRef] [PubMed]
- Sevilgen, D.S.; Venn, A.A.; Hu, M.Y.; Tambutté, E.; de Beer, D.; Planas-Bielsa, V.; Tambutté, S. Full in vivo characterization of carbonate chemistry at the site of calcification in corals. Sci. Adv. 2019, 5, eaau7447. [Google Scholar] [CrossRef] [PubMed]
- Allison, N.; Cohen, I.; Finch, A.A.; Erez, J.; Tudhope, A.W. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Commun. 2014, 5, 5741. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; D’Olivo, J.P.; Falter, J.; Holcomb, M.; Trotter, J.A. Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 2017, 8, 15686. [Google Scholar] [CrossRef]
- Ries, J.B. Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Biol. Ecol. 2011, 403, 54–64. [Google Scholar] [CrossRef]
- Spalding, C.; Finnegan, S.; Fischer, W.W. Energetic costs of calcification under ocean acidification. Glob. Biogeochem. Cycles 2017, 31, 866–877. [Google Scholar] [CrossRef]
- Castillo, K.D.; Ries, J.B.; Bruno, J.F.; Westfield, I.T. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. Biol. Sci. 2014, 281, 20141856. [Google Scholar] [CrossRef]
- Marubini, F.; Ferrier-Pages, C.; Furla, P.; Allemand, D. Coral calcification responds to seawater acidification: A working hypothesis towards a physiological mechanism. Coral Reefs 2008, 27, 491–499. [Google Scholar] [CrossRef]
- Bove, C.B.; Ries, J.B.; Davies, S.W.; Westfield, I.T.; Umbanhowar, J.; Castillo, K.D. Common Caribbean corals exhibit highly variable responses to future acidification and warming. Proc. Biol. Sci. 2019, 286, 20182840. [Google Scholar] [CrossRef]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 2010, 29, 661–674. [Google Scholar] [CrossRef]
- Reynaud, S.; Leclercq, N.; Romaine-Lioud, S.; Ferrier-Pagès, C.; Jaubert, J.; Gattuso, J.-P. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Chang. Biol. 2003, 9, 1660–1668. [Google Scholar] [CrossRef]
- Comeau, S.; Cornwall, C.E.; DeCarlo, T.M.; Doo, S.S.; Carpenter, R.C.; McCulloch, M.T. Resistance to ocean acidification in coral reef taxa is not gained by acclimatization. Nat. Clim. Chang. 2019, 9, 477. [Google Scholar] [CrossRef]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef]
- Mass, T.; Drake, J.L.; Peters, E.C.; Jiang, W.; Falkowski, P.G. Immunolocalization of skeletal matrix proteins in tissue and mineral of the coral Stylophora pistillata. Proc. Natl. Acad. Sci. USA 2014, 111, 12728–12733. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S.; Goffredo, S. Coral biomineralization: A focus on intra-skeletal organic matrix and calcification. Semin. Cell Dev. Biol. 2015, 35, 17–26. [Google Scholar] [CrossRef]
- Marin, F.; Smith, M.; Isa, Y.; Muyzer, G.; Westbroek, P. Skeletal matrices, muci, and the origin of invertebrate calcification. Proc. Natl. Acad. Sci. USA 1996, 93, 1554–1559. [Google Scholar] [CrossRef]
- Westbroek, P.; Marin, F. A marriage of bone and nacre. Nature 1998, 392, 861–862. [Google Scholar] [CrossRef]
- Mass, T.; Drake, J.L.; Haramaty, L.; Kim, J.D.; Zelzion, E.; Bhattacharya, D.; Falkowski, P.G. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr. Biol. 2003, 23, 1126–1131. [Google Scholar] [CrossRef]
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilberg, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, E7670–E7678. [Google Scholar] [CrossRef]
- Hohn, S.; Reymond, C.E. Coral calcification, mucus, and the origin of skeletal organic molecules. Coral Reefs 2019, 38, 973–984. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.; Freiwald, A.; Cairns, S. The Biology and Geology of Deep-Sea Coral Habitats; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Hennige, S.J.; Wicks, L.C.; Kamenos, N.A.; Perna, G.; Findlay, H.S.; Roberts, J.M. Hidden impacts of ocean acidification to live and dead coral framework. Proc. R. Soc. B 2015, 282, 20150990. [Google Scholar] [CrossRef] [PubMed]
- Büscher, J.V.; Form, A.U.; Riebesell, U. Interactive effects of ocean acidification and warming on growth, fitness and survival of the cold-water coral Lophelia pertusa under different food availabilities. Front. Mar. Sci. 2017, 4, 101. [Google Scholar] [CrossRef]
- Georgian, S.E.; Dupont, S.; Kurman, M.; Butler, A.; Strömberg, S.M.; Larsson, A.I.; Cordes, E.E. Biogeographic variability in the physiological response of the cold-water coral Lophelia pertusa to ocean acidification. Mar. Ecol. 2016, 37, 1345–1359. [Google Scholar] [CrossRef]
- Naumann, M.S.; Orejas, C.; Ferrier-Pagès, C. Species-specific physiological response by the cold-water corals Lophelia pertusa and Madrepora oculata to variations within their natural temperature range. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 36–41. [Google Scholar] [CrossRef]
- Holcomb, M.; Venn, A.A.; Tambutté, E.; Tambutté, S.; Allemand, D.; Trotter, J.; McCulloch, M. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 2014, 4, 5207. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Sutton, J.N.; Ries, J.B.; Eagle, R.A. Regulation of calcification site pH is a polyphyletic but not always governing response to ocean acidification. Sci. Adv. 2020, 6, eaax1314. [Google Scholar] [CrossRef]
- D’Olivo, J.P.; McCulloch, M.T. Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Nat. Sci. Rep. 2017, 7, 2207. [Google Scholar] [CrossRef]
- Guillermic, M.; Cameron, L.P.; De Corte, I.; Misra, S.; Bijma, J.; de Beer, D.; Reymond, C.E.; Westphal, H.; Ries, J.B.; Eagle, R.A. Thermal stress reduces pocilloporid coral resilience to ocean acidification by impairing control over calcifying fluid chemistry. Sci. Adv. 2021, 7, eaba9958. [Google Scholar] [CrossRef]
- Ellison, J.C.; Fiu, M. Vulnerability of Fiji’s mangroves and associated coral reefs to climate change. Ed. WWF S. Pac. Programme 2010, 50p. [Google Scholar]
- Cameron, L.P.; Reymond, C.E.; Müller-Lundin, F.; Westfield, I.; Grabowski, J.H.; Westphal, H.; Ries, J.B. Effects of temperature and ocean acidification on the extrapallial fluid pH, calcification rate, and condition factor of the king scallop Pecten maximus. J. Shellfish. Res. 2019, 38, 763–777. [Google Scholar] [CrossRef]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. MS excel program developed for CO2 system calculations. In ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center; Oak Ridge National Laboratory U.S. Department Energy: Oak Ridge, TN, USA, 2006. [Google Scholar]
- Siebeck, U.E.; Marshall, N.J.; Klüter, A.; Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Conti-Jerpe, I.E.; Thompson, P.D.; Wai Martin Wong, C.; Oliveira, N.L.; Duprey, N.N.; Moynihan, M.A.; Baker, D.M. Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 2020, 6, eaaz5443. [Google Scholar] [CrossRef] [PubMed]
- Morgans, C.A.; Hung, J.Y.; Bourne, D.G.; Quigley, K.M. Symbiodiniaceae probiotics for use in bleaching recovery. Restor. Ecol. 2020, 28, 282–288. [Google Scholar] [CrossRef]
- De Beer, D.E.; Schramm, A.; Santegoeds, C.M.; Kühl, M. A nitrite microsensor for profiling environmental biofilms. Appl. Environ. Microbiol. 1997, 63, 973–977. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Akaike, H. Factor analysis and AIC. In Selected Papers of Hirotugu Akaike. Springer Series in Statistics (Perspectives in Statistics); Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1987. [Google Scholar]
- Houlbrèque, F.; Rodolfo-Metalpa, R.; Jeffree, R.; Oberhänsli, F.; Teyssié, J.-L.; Boisson, F.; Al-Trabeen, K.; Ferrier-Pagès, C. Effects of increased pCO2 on zinc uptake and calcification in the tropical coral Stylophora pistillata. Coral Reefs 2012, 31, 101–109. [Google Scholar] [CrossRef]
- Venn, A.A.; Tambutté, E.; Caminiti-Segonds, N.; Techer, N.; Allemand, D.; Tambutté, S. Effects of light and darkness on pH regulation in three coral species exposed to seawater acidification. Sci. Rep. 2019, 9, 2201. [Google Scholar] [CrossRef]
- Rädecker, N.; Meyer, F.W.; Bednarz, V.N.; Cardini, U.; Wild, C. Ocean acidification rapidly reduces dinitrogen fixation associated with the hermatypic coral Seriatopora hystrix. Mar. Ecol. Prog. Ser. 2014, 511, 297–302. [Google Scholar] [CrossRef]
- Cantin, N.E.; Cohen, A.L.; Karnauskas, K.B.; Tarrant, A.M.; McCorkle, D.C. Ocean warming slows coral growth in the central Red Sea. Science 2010, 329, 322–325. [Google Scholar] [CrossRef]
- Horvath, K.M.; Castillo, K.D.; Armstrong, P.; Westfield, I.T.; Courtney, T.; Ries, J.B. Next-century ocean acidification and warming both reduce calcification rate, but only acidification alters skeletal morphology of reef-building coral Siderastrea siderea. Sci. Rep. 2016, 6, 29639. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, V.; Grottoli, A.G.; Warner, M.E.; Cai, W.-J.; Melman, T.F.; Hoadley, K.D.; Pettay, D.T.; Hu, X.; Li, Q.; Xu, H.; et al. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 2013, 8, e75049. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.R.; Towle, E.K.; van Hooidonk, R.; Mor, C.; Winter, R.N.; Piggot, A.M.; Cunning, R.; Baker, A.C.; Klaus, J.S.; Swart, P.K.; et al. Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs. Glob. Chang. Biol. 2016, 23, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Hönisch, B.; Ridgwell, A.; Schmidt, D.N.; Gibbs, S.J.; Sluijs, A.; Zeebe, R.; Kump, L.; Martindale, R.C.; Greene, S.E.; Kiessling, W.; et al. The geologic record of ocean acidification. Science 2012, 335, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Bijma, J.; Pörtner, H.-O.; Yesson, C.; Rogers, A.D. Climate change and the oceans–What does the future hold? Mar. Pollut. Bull. 2013, 74, 495–505. [Google Scholar] [CrossRef]
- Kurman, M.D.; Gómez, C.E.; Georgian, S.E.; Lunden, J.J.; Cordes, E.E. Intra-specific variation reveals potential adaptation to ocean acidification in a cold-water coral from the Gulf of Mexico. Front. Mar. Sci. 2017, 4, 111. [Google Scholar] [CrossRef]
- Fabry, V.J.; McClintock, J.B.; Mathis, J.T.; Grebmeier, J.M. Ocean acidification at high latitudes: The bellwether. Oceanography 2009, 22, 160–171. [Google Scholar] [CrossRef]
- Costello, M.J.; McCrea, M.; Freiwald, A.; Lundälv, T.; Jonsson, L.; Bett, B.J.; van Weering, T.C.E.; de Haas, H.; Roberts, J.M.; Allen, D. Role of cold-water Lophelia pertusa coral reefs as fish habitat in the NE Atlantic. In Cold-Water Corals and Ecosystems. Erlangen Earth Conference Series; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Germany, 2005; pp. 771–805. [Google Scholar]
- Mortensen, P.B.; Hovland, T.; Fossa, J.H.; Furevik, D.M. Distribution, abundance and size of Lophelia pertusa coral reefs in mid-Norway in relation to seabed characteristics. J. Mar. Biol. Assoc. UK 2001, 81, 581–597. [Google Scholar] [CrossRef]
- Freiwald, A. Geobiology of Lophelia pertusa (scleractinia) reefs in the North Atlantic. Habilitation Thesis, University of Bremen, Bremen, Germany, 1998. [Google Scholar]
- Rogers, A.D. The biology of Lophelia pertusa (linneaus 1758) and other deep-water reef-forming corals and impacts from human activities. Int. Rev. Hydrobiol. 1999, 84, 315–406. [Google Scholar] [CrossRef]
- Dodds, L.A.; Roberts, J.M.; Taylor, A.C.; Marubini, F. Metabolic tolerance of the cold-water coral Lophelia pertusa (scleractinia) to temperature and dissolved oxygen change. J. Exp. Mar. Biol. Ecol. 2007, 349, 205–214. [Google Scholar] [CrossRef]
- Fine, M.; Oren, U.; Loya, Y. Bleaching effect on regeneration and resource translocation in the coral Oculina patagonica. Mar. Ecol. Prog. Ser. 2002, 234, 119–125. [Google Scholar] [CrossRef]
- Tambutté, E.; Allemand, D.; Zoccola, D.; Meibom, A.; Lotto, S.; Caminiti, N.; Tambutté, S. Observations of the tissue-skeleton interface in the scleractinian coral Stylophora pistillata. Coral Reefs 2007, 26, 517–529. [Google Scholar] [CrossRef]
- Järnegren, J.; Kutti, T. Lophelia pertusa in Norwegian waters. What have we learned since 2008? NINA Rep. 2014, 1028, 40. [Google Scholar]
- Davies, P.S. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989, 101, 389–395. [Google Scholar] [CrossRef]
- Hennige, S.J.; Wolfram, U.; Wickes, L.; Murray, F.; Murray Roberts, J.; Kamenos, N.A.; Schofield, S.; Groetsch, A.; Spiesz, E.M.; Aubin-Tam, M.-E.; et al. Crumbling reefs and cold-water coral habitat loss in a future ocean: Evidence of “Coralporosis” as an indicator of habitat integrity. Front. Mar. Sci. 2020, 7, 668. [Google Scholar] [CrossRef]
- Chan, N.C.S.; Connelly, S.R. Sensitivity of coral calcification to ocean acidification: A meta-analysis. Glob. Chang. Biol. 2013, 19, 282–290. [Google Scholar] [CrossRef]
- Davies, S.W.; Marchetti, A.; Ries, J.B.; Castillo, K.D. Thermal and pCO2 stress elicit divergent transcriptomic responses in a resilient coral. Front. Mar. Sci. 2016, 3, 112. [Google Scholar] [CrossRef]
- Form, A.U.; Riebesell, U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Chang. Biol. 2012, 18, 843–853. [Google Scholar] [CrossRef]
- Tanaka, K.; Holcomb, M.; Takahashi, A.; Kurihara, H.; Asami, R.; Shinjo, R.; Sowa, K.; Rankenburg, K.; Watanabe, T.; McCulloch, M. Response of Acropora digitifera to ocean acidification: Constraints from 11B, Sr, Mg, and Ba compositions of aragonitic skeletons cultured under variable seawater pH. Coral Reefs 2015, 34, 1139–1149. [Google Scholar] [CrossRef]
- Comeau, S.; Cornwall, C.E.; DeCarlo, T.M.; Krieger, E.; McCulloch, M.T. Similar controls on calcification under ocean acidification across unrelated coral reef taxa. Glob. Chang. Biol. 2018, 24, 4857–4868. [Google Scholar] [CrossRef]
- Sutton, J.N.; Liu, Y.-W.; Ries, J.B.; Guillermic, M.; Ponzevera, E.; Eagle, R.A. 11B as monitor of calcification site pH in divergent marine organisms. Biogeosciences 2018, 15, 1447–1467. [Google Scholar] [CrossRef]
- Cai, W.-J.; Ma, Y.; Hopkinson, B.M.; Grottoli, A.G.; Warner, M.E.; Ding, Q.; Hu, X.; Yuan, X.; Schoepf, V.; Xu, H.; et al. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Commun. 2016, 7, 11144. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; McConnaughey, T.A. Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 2003, 54, 151–187. [Google Scholar] [CrossRef]
- Kooijman, B. Dynamic Energy Budget Theory for Metabolic Organization; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Holcomb, M.; McCorkle, D.C.; Cohen, A.L. Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J. Exp. Mar. Biol. Ecol. 2010, 386, 27–33. [Google Scholar] [CrossRef]
- Edmunds, P.J. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 2011, 56, 2402–2410. [Google Scholar] [CrossRef]
- Gates, R.D.; Baghdasarian, G.; Muscatine, L. Temperature stress causes host cell detachment in symbiotic cnidarians: Implications for coral bleaching. Biol. Bull. 1992, 182, 324–332. [Google Scholar] [CrossRef]
- Brading, P.; Warner, M.E.; Davey, P.; Smith, D.J.; Achterberg, E.P.; Suggett, D.J. Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). Limnol. Oceanogr. 2011, 56, 927–938. [Google Scholar] [CrossRef]
- Moya, A.; Tambutté, S.; Bertucci, A.; Tambutté, E.; Lotto, S.; Vullo, D.; Supuran, C.T.; Allemand, D.; Zoccola, D. Carbonic anhydrase in the scleractinian coral Stylophora pistillata characterization, localization, and role in biomineralization. J. Biol. Chem. 2008, 283, 25475–25484. [Google Scholar] [CrossRef]
- Chen, S.; Gagnon, A.C.; Adkins, J.F. Carbonic anhydrase, coral calcification and a new model of stable isotope vital effects. Geochim. Cosmochim. Acta 2018, 236, 179–197. [Google Scholar] [CrossRef]
- Muscatine, L.; McCloskey, L.R.; Marian, R.E. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 1981, 26, 601–611. [Google Scholar] [CrossRef]
- Dubinsky, Z.; Jokiel, P.L. Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac. Sci. 1994, 48, 313–324. [Google Scholar]
- Aichelman, H.E.; Bove, C.B.; Castillo, K.D.; Boulton, J.M.; Knowlton, A.C.; Nieves, O.C.; Ries, J.B.; Davies, S.W. Exposure duration modulates the response of Caribbean corals to global change stressors. Limnol. Oceanogr. Lett. 2021, 66, 8. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Comeau, S.; DeCarlo, T.M.; Moore, B.; D’alexis, Q.; McCulloch, M.T. Resistance of corals and coralline algae to ocean acidification: Physiological control of calcification under natural pH variability. Proc. R. Soc. B 2018, 285, 20181168. [Google Scholar] [CrossRef]
- Cohen, A.L.; Holcomb, M. Why corals care about ocean acidification: Uncovering the mechanism. Oceanography 2009, 22, 118–127. [Google Scholar] [CrossRef]
- Allemand, D.; Ferrier-Pagès, C.; Furla, P.; Houlbrèque, F.; Puverel, S.; Reynaud, S.; Tambutté, É.; Tambutté, S.; Zoccola, D. Biomineralization in reef-building corals: From molecular mechanisms to environmental control. Comptes Rendus Palevol 2004, 3, 453–467. [Google Scholar] [CrossRef]
- Gagliano, M.; McCormick, M.I.; Moore, J.A.; Depczynski, M. The basics of acidification: Baseline variability of pH on Australian coral reefs. Mar. Biol. 2010, 157, 1849–1856. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6, e28983. [Google Scholar] [CrossRef]
- Cyronak, T.; Takeshita, Y.; Courtney, T.A.; DeCarlo, E.H.; Eyre, B.D.; Kline, D.I.; Martz, T.; Page, H.; Price, N.N.; Smith, J.; et al. Diel temperature and pH variability scale with depth across diverse coral reef habitats. Limnol. Oceanogr. Lett. 2019, 5, 193–203. [Google Scholar] [CrossRef]
- Gray, S.E.C.; DeGrandpre, M.D.; Langdon, C.; Corredor, J.E. Short-term and seasonal pH, pCO2 and saturation state variability in a coral-reef ecosystem. Glob. Biogeochem. Cycles 2012, 26, GB3012. [Google Scholar] [CrossRef]
- Kline, D.I.; Teneva, L.; Hauri, C.; Schneider, K.; Miard, T.; Chai, A.; Marker, M.; Dunbar, R.; Caldeira, K.; Lazar, B.; et al. Six month in situ high-resolution carbonate chemistry and temperature study on a coral reef flat reveals asynchronous pH and temperature anomalies. PLoS ONE 2015, 10, e0127648. [Google Scholar] [CrossRef]
| 400 ppm (9 °C) | 400 ppm (12 °C) | 1000 ppm (9 °C) | 1000 ppm (12 °C) | 2800 ppm (9 °C) | 2800 ppm (12 °C) | ||
|---|---|---|---|---|---|---|---|
| CALCULATED PARAMETERS | |||||||
| pCO2 (gas-e) | (ppm-v) | 466 | 499 | 925 | 885 | 2807 | 3194 |
| SE | 8 | 9 | 15 | 12 | 119 | 135 | |
| Range | 362–540 | 425–607 | 808–1144 | 772–1050 | 1728–4302 | 2298–4945 | |
| n | 32 | 32 | 31 | 32 | 32 | 32 | |
| pHSW | 8.11 | 8.06 | 7.85 | 7.87 | 7.42 | 7.42 | |
| SE | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | |
| Range | 8.03–8.30 | 7.97–8.27 | 7.73–7.98 | 7.80–7.99 | 7.28–7.51 | 7.20–7.57 | |
| n | 32 | 31 | 31 | 32 | 32 | 32 | |
| [CO32−] | (µM) | 334 | 320 | 217 | 265 | 90 | 113 |
| SE | 9 | 11 | 6 | 4 | 3 | 4 | |
| Range | 235–395 | 211–442 | 159–274 | 226–309 | 64–113 | 76–149 | |
| n | 32 | 32 | 31 | 32 | 32 | 32 | |
| [HCO3−] | (µM) | 2133 | 2017 | 2424 | 2468 | 2697 | 3030 |
| SE | 33 | 36 | 35 | 35 | 77 | 54 | |
| Range | 1846–2433 | 1772–2389 | 2138–2748 | 2193–2842 | 2104–3289 | 2476–3475 | |
| n | 32 | 31 | 31 | 32 | 32 | 32 | |
| [CO2] (SW) | (µM) | 12.3 | 12.3 | 24.6 | 22.0 | 74.3 | 79.5 |
| SE | 0.2 | 0.3 | 0.4 | 0.4 | 3.2 | 3.4 | |
| Range | 9–14 | 10–17 | 21–30 | 19–27 | 45–112 | 56–121 | |
| n | 32 | 32 | 31 | 32 | 32 | 32 | |
| ΩA | 5.4 | 5.2 | 3.5 | 4.3 | 1.4 | 1.8 | |
| SE | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | 0.1 | |
| Range | 3.8–6.4 | 3.5–7.2 | 2.6–4.4 | 3.7–5.0 | 1.0–1.8 | 1.2–2.4 | |
| n | 32 | 32 | 31 | 32 | 32 | 32 | |
| MEASURED PARAMETERS | |||||||
| Sal | (psu) | 34.87 | 35.63 | 35.44 | 35.99 | 35.75 | 35.68 |
| SE | 0.06 | 0.08 | 0.05 | 0.06 | 0.05 | 0.06 | |
| Range | 33.75–36.25 | 34.45–37.55 | 34.55–36.65 | 34.85–37.25 | 34.55–36.75 | 34.35–37.05 | |
| n | 104 | 104 | 104 | 104 | 104 | 104 | |
| Temp | (°C) | 28.29 | 31.72 | 27.88 | 30.83 | 28.17 | 30.93 |
| SE | 0.01 | 0.06 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Range | 28.1–28.5 | 30.8–32.3 | 27.6–28.2 | 30.5–31.0 | 28.0–28.4 | 30.6–31.2 | |
| n | 104 | 104 | 104 | 104 | 104 | 104 | |
| pHNBS | 8.27 | 8.24 | 8.04 | 8.12 | 7.62 | 7.69 | |
| SE | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| Range | 8.02–8.47 | 8.12–8.48 | 7.88–8.33 | 7.92–8.34 | 7.47–7.98 | 7.48–7.97 | |
| n | 104 | 104 | 104 | 104 | 104 | 104 | |
| TA | (µM) | 2915 | 2774 | 2939 | 3083 | 2907 | 3290 |
| SE | 49 | 54 | 46 | 40 | 81 | 56 | |
| Range | 2420–3304 | 2309–3223 | 2524–3306 | 2735–3462 | 2260–3493 | 2705–3794 | |
| n | 32 | 32 | 32 | 32 | 32 | 32 | |
| DIC | (µM) | 2480 | 2350 | 2645 | 2755 | 2861 | 3223 |
| SE | 40 | 44 | 44 | 37 | 82 | 57 | |
| Range | 2097–2824 | 2009–2726 | 2013–3003 | 2445–3134 | 2231–3490 | 2634–3700 | |
| n | 32 | 32 | 32 | 32 | 32 | 32 | |
| 400 ppm (9 °C) | 400 ppm (12 °C) | 1000 ppm (9 °C) | 1000 ppm (12 °C) | 2800 ppm (9 °C) | 2800 ppm (12 °C) | ||
|---|---|---|---|---|---|---|---|
| CALCULATED PARAMETERS | |||||||
| pCO2 (gas-e) | (ppm-v) | 451 | 494 | 1096 | 1079 | 2864 | 3167 |
| SE | 24 | 16 | 99 | 60 | 222 | 202 | |
| Range | 238–580 | 399–595 | 672–1815 | 834–1496 | 1600–4162 | 1821–4669 | |
| n | 16 | 16 | 16 | 16 | 16 | 16 | |
| pHSW | 8.20 | 8.21 | 7.87 | 7.86 | 7.42 | 7.42 | |
| SE | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 | 0.03 | |
| Range | 8.01–8.54 | 7.96–8.40 | 7.62–8.12 | 7.70–8.06 | 7.17–7.67 | 7.19–7.63 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
| [CO32−] | (µM) | 244 | 289 | 121 | 140 | 52 | 60 |
| SE | 23 | 25 | 9 | 8 | 6 | 5 | |
| Range | 128–486 | 149–458 | 87–201 | 102–211 | 25–99 | 35–110 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
| [HCO3−] | (µM) | 2674 | 2775 | 2794 | 2835 | 3034 | 3058 |
| SE | 107 | 111 | 89 | 95 | 69 | 81 | |
| Range | 2028–3356 | 2215–3348 | 2299–3366 | 2350–3349 | 2655–3420 | 2604–3486 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
| [CO2] (SW) | (µM) | 20 | 19 | 45 | 41 | 127 | 118 |
| SE | 1 | 1 | 4 | 2 | 8 | 8 | |
| Range | 11–26 | 14–24 | 28–83 | 30–59 | 72–190 | 72–187 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
| ΩA | 3.7 | 4.4 | 1.8 | 2.1 | 0.8 | 0.9 | |
| SE | 0.3 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Range | 1.9–7.3 | 2.3–6.9 | 1.3–3.0 | 1.6–3.2 | 0.4–1.5 | 0.5–1.7 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
| MEASURED PARAMETERS | |||||||
| Sal | (psu) | 34.98 | 35.13 | 34.96 | 35.23 | 35.01 | 35.40 |
| SE | 0.02 | 0.03 | 0.03 | 0.06 | 0.02 | 0.05 | |
| Range | 34.55–35.25 | 34.65–35.65 | 34.55–35.65 | 34.20–36.15 | 34.45–35.25 | 34.35–36.05 | |
| n | 60 | 60 | 60 | 60 | 60 | 60 | |
| Temp | (°C) | 8.86 | 12.17 | 9.07 | 12.52 | 8.83 | 12.67 |
| SE | 0.03 | 0.04 | 0.03 | 0.04 | 0.02 | 0.03 | |
| Range | 8.5–9.4 | 11.6–12.6 | 8.7–9.4 | 12.0–12.9 | 8.5–9.1 | 12.0–13.1 | |
| n | 60 | 60 | 60 | 60 | 60 | 60 | |
| pHNBS | 8.07 | 8.13 | 7.80 | 7.82 | 7.39 | 7.40 | |
| SE | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Range | 7.82–8.39 | 7.85–8.43 | 7.48–8.15 | 7.52–8.01 | 7.13–7.75 | 7.12–7.63 | |
| n | 60 | 60 | 60 | 60 | 60 | 60 | |
| TA | (µM) | 3245 | 3442 | 3080 | 3163 | 3157 | 3198 |
| SE | 148 | 161 | 93 | 106 | 80 | 87 | |
| Range | 2355–4106 | 2601–4349 | 2598–3570 | 2609–3783 | 2752–3570 | 2759–3655 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
| DIC | (µM) | 2938 | 3083 | 2960 | 3016 | 3214 | 3235 |
| SE | 125 | 134 | 93 | 101 | 69 | 85 | |
| Range | 2180–3659 | 2395–3793 | 2449–3536 | 2494–3556 | 2805–3599 | 2741–3676 | |
| n | 20 | 20 | 20 | 20 | 20 | 20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cameron, L.P.; Reymond, C.E.; Bijma, J.; Büscher, J.V.; De Beer, D.; Guillermic, M.; Eagle, R.A.; Gunnell, J.; Müller-Lundin, F.; Schmidt-Grieb, G.M.; et al. Impacts of Warming and Acidification on Coral Calcification Linked to Photosymbiont Loss and Deregulation of Calcifying Fluid pH. J. Mar. Sci. Eng. 2022, 10, 1106. https://doi.org/10.3390/jmse10081106
Cameron LP, Reymond CE, Bijma J, Büscher JV, De Beer D, Guillermic M, Eagle RA, Gunnell J, Müller-Lundin F, Schmidt-Grieb GM, et al. Impacts of Warming and Acidification on Coral Calcification Linked to Photosymbiont Loss and Deregulation of Calcifying Fluid pH. Journal of Marine Science and Engineering. 2022; 10(8):1106. https://doi.org/10.3390/jmse10081106
Chicago/Turabian StyleCameron, Louise P., Claire E. Reymond, Jelle Bijma, Janina V. Büscher, Dirk De Beer, Maxence Guillermic, Robert A. Eagle, John Gunnell, Fiona Müller-Lundin, Gertraud M. Schmidt-Grieb, and et al. 2022. "Impacts of Warming and Acidification on Coral Calcification Linked to Photosymbiont Loss and Deregulation of Calcifying Fluid pH" Journal of Marine Science and Engineering 10, no. 8: 1106. https://doi.org/10.3390/jmse10081106
APA StyleCameron, L. P., Reymond, C. E., Bijma, J., Büscher, J. V., De Beer, D., Guillermic, M., Eagle, R. A., Gunnell, J., Müller-Lundin, F., Schmidt-Grieb, G. M., Westfield, I., Westphal, H., & Ries, J. B. (2022). Impacts of Warming and Acidification on Coral Calcification Linked to Photosymbiont Loss and Deregulation of Calcifying Fluid pH. Journal of Marine Science and Engineering, 10(8), 1106. https://doi.org/10.3390/jmse10081106









