Diversity of Fucales (Ochrophyta, Phaeophyceae) along the Coasts of Lipari and Vulcano (Aeolian Archipelago), Tyrrhenian Sea (Central Mediterranean Sea)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Previous and Current Distributions of Fucales
| Species | This Study | Past | Reference |
|---|---|---|---|
| Cystoseira compressa (Esper) Gerloff & Nizamuddin | x | x | [5] |
| Cystoseira foeniculacea (Linnaeus) Greville | x | x | as Cystoseira discors [4] |
| Cystoseira humilis var. myriophylloides (Sauvageau) J. H. Price & D. M. John | x | [5] | |
| Cystoseira cf. micheleae Verlaque, Blanfuné, Boudouresque, Thibaut & Sellam | x | ||
| Cystoseira pustulata (Ercegovic) Neiva & Serrão | x | x | as Cystoseira fimbriata v. pustulata [9] |
| Ericaria amentacea (C. Agardh) Molinari & Guiry | x | as Cystoseira amentacea v. stricta [5] | |
| Ericaria crinita (Duby) Molinari & Guiry | x | as Cystoseira crinita [5] | |
| Ericaria dubia (Valiante) Neiva & Serrão | x | ||
| Ericaria selaginoides (Linnaeus) Molinari & Guiry | x | as Cystoseira tamariscifolia [5] | |
| Ericaria zosteroides (C. Agardh) Molinari & Guiry | x | as Cystoseira zosteroides [5] | |
| Ericaria brachycarpa (J. Agardh) Molinari & Guiry | x | x | as Cystoseira brachycarpa v. brachycarpa [5] |
| Ericaria funkii (Gerloff & Nizamuddin) Molinari & Guiry | x | x | as Cystoseira jabukae [5] |
| Gongolaria barbata (Stackhouse) Kuntze | x | as Cystoseira barbata v. barbata [5] | |
| Gongolaria montagnei (J. Agardh) Kuntze | x | as Cystoseira spinosa v. spinosa [5] | |
| Gongolaria montagnei var. tenuior (Ercegović) Molinari & Guiry | x | as Cystoseira spinosa v. tenuior [5] | |
| Gongolaria montagnei var. compressa (Ercegović) Verlaque, Blanfuné, Boudouresque & Thibaut | x | x | as Cystoseira spinosa v. compressa [5] |
| Gongolaria sauvageauana (Hamel) Molinari & Guiry | x | x | as Cystoseira sauvageauana [5] |
| Sargassum acinarium (Linnaeus) Setchell | x | [5] | |
| Sargassum flavifolium Kützing | x | [5] | |
| Sargassum vulgare C. Agardh | x | x | [5] |
| Sargassum hornschuchii C. Agardh | x | x | [5] |
| Sargassum furcatum Kützing | x | ||
| Sargassum trichocarpum J. Agardh | x |
| Lipari | Vulcano | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | SV | PG | PM | SF | LF | MuZ | CG | PS | SQ | CGr | |
| Depth range (m) | 0–33 | 0–26 | 0–34 | 0–28 | 0–22 | 0–31 | 0–0.5 | 0–29 | 0–22 | 0–37 | 0–33 |
| Temperature range (°C) | 25°–16° | 25°–19° | 25°–18° | 25°–17° | 25°–18° | 25°–19° | 25° | 26°–17° | 26°–16° | 25°–16° | 26°–17° |
| Taxa | |||||||||||
| Cystoseira compressa | 0.5 | 8 * | 0–0.5 * | 0–0.5 | 0.5–1 | 0.5–1 | 7–10 | 0–5 * | |||
| Cystoseira foeniculacea | 5–29 | 8–19 | 12 | 13 | 7–21 | 11–13 | |||||
| Cystoseira cf. micheleae | 6–8 | 8–14 | |||||||||
| Cystoseira pustulata | 3–5 * | 4–9 * | |||||||||
| Ericaria dubia | 31 | 27 | 30 | ||||||||
| Ericaria brachycarpa | 5–10 | 3–6 | 8–13 | 0.5–15 | 12–17 | 0.2–15 | 0.8–1 | 7 | 0.5–13 | ||
| Ericaria funkii | 27 * | 16–26 * | 15–27 | 18–21 * | 17–22 | 23 * | 16–31 * | 24–31 | |||
| Gongolaria montagnei var. compressa | 28 | 6–28 | 7–20 | ||||||||
| Gongolaria sauvageauana | 16–23 | 20–27 | 17–19 | 9–16 | 11 * | 7–20 * | |||||
| Sargassum vulgare | 0.5–1 | 12–15 * | 15 | 2–9 * | |||||||
| Sargassum hornschuchii | 7–17 * | ||||||||||
| Sargassum furcatum | 12–28 * | 18–26 | 21 | 21 | 10–18 * | 8–12 | 12–24 | ||||
| Sargassum trichocarpum | 15–26 * | 17 | 15–21 * | ||||||||
3.2. Alpha and Beta Diversity
3.3. List of the Found Species
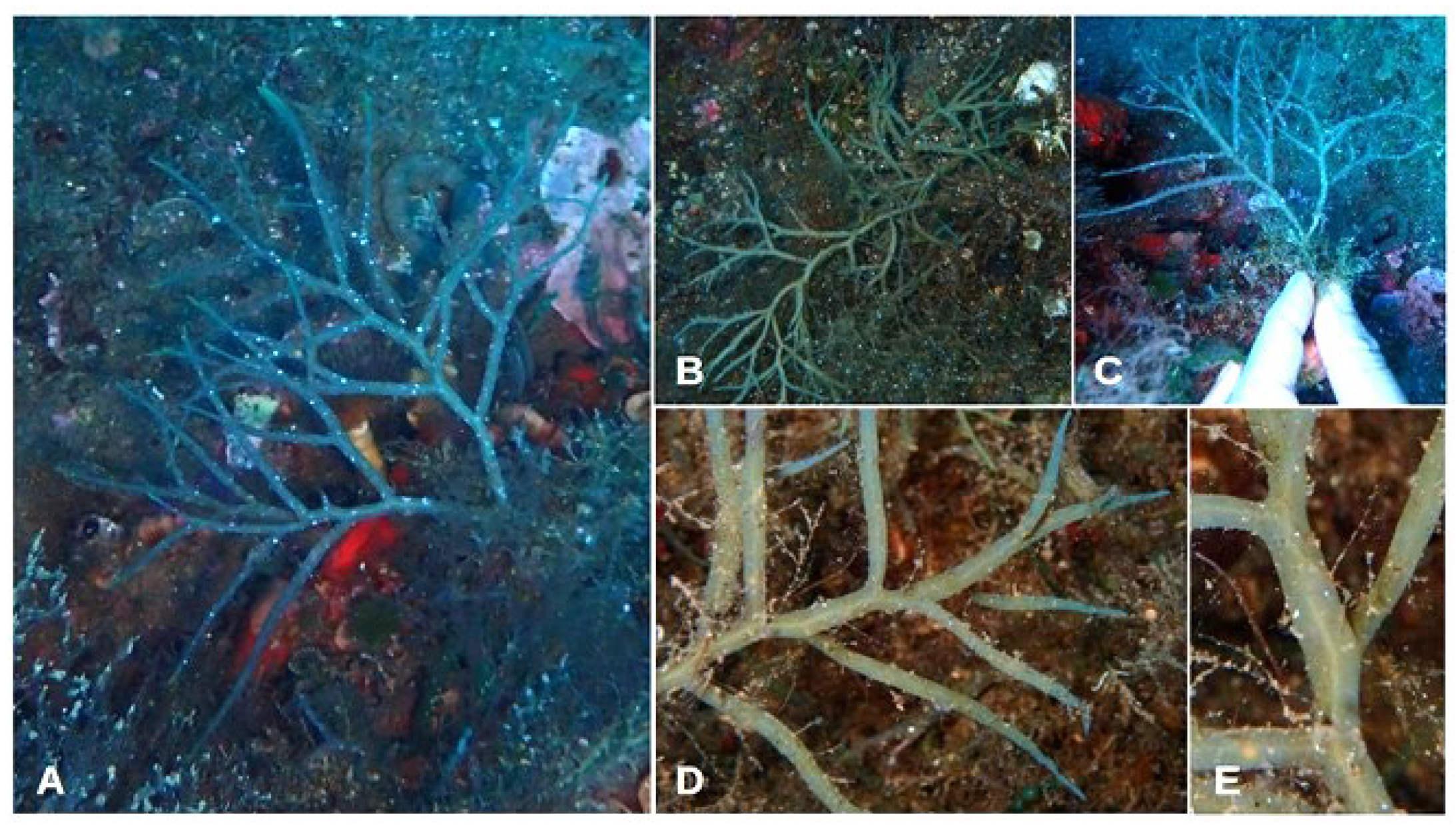
- Cystoseira compressa (Esper) Gerloff & Nizamuddin (Figure 3A–E)
- Basionym: Fucus compressus Esper, 1799.
- Synonyms: Cystoseira flicina Bory, Cystoseira abrotanifolia f. fimbriata Sauvageau, Fucus fimbriatus Desfontaines, 1799, Cystoseira fimbriata Bory, 1832.
- Morphological observations of Aeolian specimens: The thalli of this species are caespitose, almost completely flattened, without spiniform appendages and with a small discoid holdfast. The thalli are 4–12 cm in length. The apices are small, smooth and non-prominent. The primary branches are flattened and have with an alternating and distichous disposal. The higher-order branches are flattened or cylindrical, always with an alternate–distich arrangement. They feature pedicellate receptacles, which are lanceolate–fusiform and can be simple or branched.
- Habitat: This species shows great adaptability to different habitats and environments. During the monitoring activities, it was observed from the surface to a depth of 10 m, in both sheltered and exposed waters. In deeper waters, only scattered individuals were observed, while in shallower waters, almost continuous stands were observed.
- Distribution: This species was found in eight of the eleven explored sites, on both Lipari and Vulcano (Table 3).
- Further remarks: It was observed that this species has colonised the infralittoral fringe, replacing E. amentacea and E. selaginoides, which were previously documented at these islands (see Table 2). As already demonstrated by Mangialajo et al. [42], the reasons for this substitution could be related to a high level of pollution of the surface waters, due to the continuous passage and discharge of ships, particularly during summer. This replacement probably led to an alteration or adaptation in morphological form, characterised by long fronds with a three-dimensional, pyramidal shape, similar to those of E. amentacea, capable of withstanding a high degree of wave motion and prolonged air exposure. This hypothesis was also suggested by Mangialajo et al. [42], who observed that the lengths of the axes of C. compressa increased towards the urbanised shores as a result of their release from competition with E. amentacea. However, Mangialajo et al. [42] did not exclude the potential effects of other environmental factors.
- Cystoseira foeniculacea (Linnaeus) Greville (Figure 4A–C)
- Basionym: Fucus foeniculaceus Linnaeus
- Synonyms: Cystoseira abrotanifolia (Linnaeus) C. Agardh, C. concatenata (Linnaeus) C. Agardh, C. discors (Linnaeus) C. Agardh, C. ercegovicii Giaccone, Fucus abrotanifolius Linnaeus, F. barbatus Linnaeus, F. concatenatus Linnaeus, F. discors Linnaeus, Phyllacantha concatenata (Linnaeus) Kützing.
- Morphological observations of Aeolian specimens: This species is caespitose, with several axes entirely covered by small spiniform appendages, which provide a characteristic knotty habit. The thalli are 5–7 cm long. The holdfast is wide and discoid. The apices of this species are spinose and very prominent in relation to the insertions of the primary branches. The latter are cylindrical, covered with small spines and feature secondary branches with a distichous and alternating disposal. The last-order branches are filiform.
- Habitat: C. foeniculacea was observed in the infralittoral, at depths from 5 m to 21 m, as scattered individuals or forming small patches.
- Distribution: C. foeniculacea was found at six (three in Lipari and three in Vulcano) of the eleven studied sites (Table 3).
- Further remarks: This species was often observed growing on small pebbles (5–6 cm) on coarse gravel substrate.
- Basionym: Cystoseira micheleae Verlaque, Blanfuné, Boudouresque, Thibaut & Sellam.
- Synonyms: Cystoseira granulata var. turneri Montagne.
- Morphological observations of Aeolian specimens: This species has a characteristic foliose habit. It is non-caespitose with an axis originating from a robust discoid base, which can be single or branched. The thalli are 4–8 cm in length. The apex is spinose and not prominent, often surrounded by tophules. The latter are ovoid, from spinose to smooth–tuberculate with ageing. The primary branches are cylindrical at the base and then tend to be flattened with an inconspicuous midrib. They can be alternately branched in one or several planes, with simple or bifid-spine-like appendages. Receptacles were not observed.
- Habitat: This species was observed in the infralittoral, at depths from 6 m to 14 m, forming dense patches. According to Blanfuné et al. [35] and as observed during the monitoring, it prefers sub-horizontal to sloping bottoms, with slopes of up to 45°. We also observed small-sized thalli growing on pebbles.
- Distribution: This species was observed on the island of Vulcano at Capo Grillo and Parete della Sirena (Table 3). The latter site is characterised by gaseous emissions of H2S, SO2, SO3.
- Further remarks: This species was described by Montagne [43], from Algeria, as C. granulata var. turneri Montagne. Between 2014 and 2015, Sellam et al. [44] collected some samples in the regions of Tipaza and Algiers that corresponded to Montagne’s taxon. They elevated the Montagne variety to species status, giving it a new name, Cystoseira micheleae Verlaque (as Cystoseira michaelae Verlaque et al.), due to the existence of C. turneri (Yendo) Roberts, and they designed the lectotype. Furthermore, the authors distinguished C. micheleae from C. montagnei (now Gongolaria montagnei) through the following characteristics: in the former, the tophules are spinose when young and become tuberculate when older, while in the latter, they remain spinose; and in the former, the receptacles are both basal and intercalary near the tophules, and terminal on the branchlets, while in the latter, they are only in the terminal position. To date, this species has only been reported along the coasts of Algeria and Tunisia [35]. However, some of the reports of G. montagnei up until 2017, the date of the publication of the work by Sellam et al. [44], could have been misidentifications referring to this taxon and, therefore, the distribution of C. michelae could be wider.
- Cystoseira pustulata (Ercegovic) Neiva & Serrão (Figure 6A–E)
- Basionym: Cystoseira abrotanifolia subsp. pustulata Ercegovic.
- Synonym: Cystoseira compressa subsp. pustulata (Ercegovic) Verlaque.
- Morphological observations of Aeolian specimens: This species has a very delicate and minute habit. It is characterised by caespitose thalli that are 7–12 cm in length, which are attached to the substrate by a small discoid holdfast. The apices are smooth, tiny and not prominent. The primary branches are cylindrical-to-compressed, with an alternating and distichous arrangement. They are sprinkled with prominent cryptostomata that provide a pustulate aspect, from which the name “pustulata” derives. The secondary and tertiary branches are cylindrical and slender. They feature tiny, lanceolate and fusiform receptacles, which can be simple or bifurcated and may be sustained by a flattened aerocyst.
- Habitat: Scattered individuals from this species were observed in the infralittoral, at depths from 3 m to 9 m.
- Distribution: C. pustulata was found only at the sites of Pietra Menalda and Parete della Sirena, in Lipari and Vulcano, respectively (Table 3).
- Further remarks: This species was previously reported on the island of Vulcano as C. fimbriata v. pustulata by Giaccone [9]. Subsequently, Giaccone et al. [5] reported this entity as a synonym of C. humilis v. humilis in the list of phytobenthic species of the Aeolian archipelago. Recently, Neiva et al. [26] pointed out that the previous records of C. humilis could be misidentifications and might actually refer to C. pustulata, which has been defined as a separate and independent species.
- Ericaria dubia (Valiante) Neiva & Serrão (Figure 7A–E)
- Basionym: Cystoseira dubia Valiante.
- Synonym: Cystoseira fucoides Ercegovic.
- Morphological observations of Aeolian specimens: This species is pseudocaespitose, with an axis that prematurely divides. It can have an erect or more creeping habit. The thalli are 4–7 cm long. The apex is smooth and not prominent. In situ, it shows slight iridescence. The primary branches have a cylindrical basal part and a flattened distal part. The branching disposal is distichous and alternate, in one plane. All the branches have an entire margin and are run throughout by an evident midrib. Receptacles were not observed.
- Habitat: E. dubia was found as scattered individuals or as forming small patches in the lower infralittoral–circalittoral, at depths from 27 m to 31 m.
- Distribution: This species was recorded at three sites: one in Lipari (Punta Castagna) and two in Vulcano (Capo Grillo and Capo Grosso) (Table 3).
- Further remarks: The annual cycle of the development of a E. dubia population was well studied by Tita [45] in Cannizzaro (on the eastern coast of Sicily). Currently, in this area, this species is completely absent (according to our personal observation). As a deep species, E. dubia is particularly threatened by pollution, turbidity, sedimentation from watersheds, trawling and competition with non-indigenous species [35]. Despite its rarity, the status of this species in many Mediterranean areas is still unknown. Therefore, the finding of this species in the Aeolian islands is valuable and needs to be addressed in the context of the MPA-creation process.
- Ericaria brachycarpa (J. Agardh) Molinari & Guiry (Figure 8A–C)
- Basionym: Cystoseira brachycarpa J. Agardh
- Synonyms: Cystoseira brachycarpa J. Agardh, Cystoseira caespitosa Sauvageau, Carpodesmia brachycarpa (J. Agardh) Orellana & Sansón
- Morphological observations of Aeolian specimens: This species is caespitose, with several thalli that originate from a wide and irregular holdfast. The thalli are 10–14 cm in length. The axes are rough and knotty due to the fronds sinking to the sea floor during the resting period. The apices are not prominent and they are covered by spinose appendages. The primary branches are cylindrical and can feature spines. The higher-order branches are cylindrical, without spines. Receptacles were not observed.
- Habitat: E. brachycarpa was observed in the infralittoral, at depths from 0.8 m to 17 m, forming well-structured and continuous stands.
- Distribution: This species is widely distributed in Lipari and Vulcano, indeed, it was observed at nine of the eleven examined sites (Table 3).
- Further remarks: Recently, Neiva et al. [26] highlighted the existence of the two cryptic species, E. brachycarpa and E. balearica (Sauvageau) Neiva, Ballesteros & Serrão, which are well genetically differentiated and geographically separated. The E. balearica is distributed in the Balearic Sea and on the Sicilian Island of Pantelleria, while E. brachycarpa is present in Greece and along the northern coast of Sicily. Since one of these Sicilian localities (Capo Milazzo) is near the Aeolian archipelago, it is likely that the specimens found in Lipari and Vulcano belong to the entity E. brachycarpa. Future molecular analyses could confirm this hypothesis.
- Ericaria funkii (Gerloff & Nizamuddin) Molinari & Guiry (Figure 9A–F)
- Basionym: Cystoseira funkii Schiffner ex Gerloff & Nizamuddin
- Synonym: Carpodesmia funkii (Schiffner ex Gerloff & Nizamuddin) Orellana & Sansón
- Morphological observations of Aeolian specimens: This species is non-caespitose, with an axis that has a coralloid aspect. The thalli are 13–15 cm in length. In situ, this species shows strong iridescence. The thalli are attached to the substrate through haptera or a digitiform holdfast. The apex is smooth and not prominent. The primary branches are cylindrical and spiniform. They feature higher-order branches with an alternate disposal. The tophules are oblong and spinose or rough. They are distributed along the axis, near the base, further contributing to providing the thallus with this coralloid aspect. The receptacles are positioned on the terminal branchlets and are diffuse. Conceptacles are located at the base of the spine.
- Habitat: This species was found in the lower infralittoral–circalittoral, at depths from 15 m to 31 m, forming dense and continuous stands.
- Distribution: This species was found at eight of the eleven explored sites, in both Lipari and Vulcano (Table 3).
- Further remarks: In the past, in the Aeolian archipelago, the presence of Cystoseira jabukae Ercegovic was documented [5]. Some authors considered E. funkii to be a synonym of C. jabukae. However, Verlaque et al. [46], by studying specimens of C. jabukae collected from Corsica and comparing them with the description by Ercegovic [47], concluded that C. jabukae and C. funkii are two distinct species, and considered the previous reports from the western Mediterranean and the Ionian Sea to refer to C. funkii [33]. This C. funkii is distinguished by the following characters: a haptera-like holdfast; a short primary axis that branches out into divaricated and radially arranged secondary axes; spiny tophules spaced along the axis; the lower portion of the primary branches bearing spines; abundant spinose appendages; and receptacles with spinose appendages [46]. Given the aforementioned features, we believe that the specimens observed during this study belong to E. funkii.
- Gongolaria montagnei var. compressa (Ercegović) Verlaque, Blanfuné, Boudouresque & Thibaut (Figure 10A–C)
- Basionym: Cystoseira adriatica subsp. compressa Ercegovic.
- Synonyms: Cystoseira adriatica subsp. compressa Ercegovic, Cystoseira platyramosa Ercegovic, Cystoseira adriatica subsp. intermedia Ercegovic, Cystoseira adriatica var. intermedia (Ercegovic) Giaccone, Cystoseira spinosa var. compressa (Ercegovic) Cormaci, G. Furnari, Giaccone, Scammacca & D. Serio, Cystoseira montagnei var. compressa (Ercegovic) M. Verlaque, Blanfuné, Boudouresque, Thibaut & Sellam.
- Morphological observations of Aeolian specimens: This species is non-caespitose, with an axis that rarely divides, attached to the substrate by a compact discoid holdfast. The thalli are 12–15 cm long. The apex is spinose and not prominent. The primary branches originate from the tophules; they are initially cylindrical and then become flattened with serrated margins and a central midrib. All the branches have an alternating disposal and can feature spiniform appendages. The tophules are distributed along the length of the thallus, especially in its upper part. They are ovoid and covered by spines. Receptacles were not observed.
- Habitat: This species was found in the infralittoral, at depths from 6 m to 28 m, as both scattered individuals and sparse patches.
- Distribution: G. montagnei var. compressa was reported at one site (Punta Castagna) in Lipari and at two sites (Capo Grillo and Parete della Sirena) in Vulcano (Table 3).
- Further remarks: In the literature consulted, it was observed that this species is still present in Lipari and Vulcano (Table 2), demonstrating a quite stable trend.
- Gongolaria sauvageauana (Hamel) Molinari & Guiry (Figure 11A–C)
- Basionym: Cystoseira sauvageauana Hamel.
- Synonyms: Cystoseira selaginoides var. polyoedematis Sauvageau, Cystoseira sauvageauana Hamel, Cystoseira sauvageauana var. polyoedematis (Sauvageau) Hamel, Cystoseira sicula Schiffner ex Gerloff & Nizamuddin, Treptacantha sauvageauana (Hamel) Orellana & Sansón.
- Morphological observations of Aeolian specimens: This species is non-caespitose, with an axis that can be simple or branched, attached to the substrate by a discoid holdfast. The thalli are 12–16 cm in length. The apex is spinose and very prominent in relation to the insertion of the primary branches. The primary branches are long and cylindrical, and they are slender in relation to the axis. They can feature spiniform appendages in their lower portions. The higher-order branches are cylindrical and widely spaced, and they decrease in length towards the top. The receptacles are on the terminal branchlets and they are compact and cylindrical, with a few spiniform appendages. Conceptacles can be observed at the base of the spine, when present.
- Habitat: This species was found forming patches or almost continuous stands in the infralittoral, at depths from 9 m to 27 m.
- Distribution: This species was found at six sites: four in Lipari (Punta Castagna, Pietra Menalda, Secca delle Formiche and Le Formiche) and two in Vulcano (Capo Grillo and Scoglio del Quaglietto) (Table 3).
- Further remarks: The decline of G. sauvageauana in several Mediterranean areas has been widely documented [25] and, thus, it can now be considered a rare species. In the Aeolian archipelago, this taxon was reported in the past, and it is still present today. Therefore, the presence of this species on these islands deserves special consideration, particularly in light of the establishment of the MPA.
- Sargassum vulgare C. Agardh (Figure 12A–C)
- Basionym: Sargassum vulgare C. Agardh, nom. illeg.
- Synonyms: Fucus salicifolius S. G. Gmelin, Sargassum megalophyllum Montagne, Sargassum coarctatum Kützing, Sargassum vulgare var. megalophyllum (Montagne) Vickers.
- Morphological observations of Aeolian specimens: This species has a single knotty axis, simple or branched, which is attached to the substrate through a discoid holdfast. The thalli are 5–8 cm long. The primary branches are cylindrical and feature secondary branches, which have a distichous–alternating arrangement. The foliose branches are lanceolate with an evident midrib, with toothed or wavy margins. Aerocysts were not observed. The receptacles are composed (with a sterile pedicel) and are fusiform, warty, simple or branched.
- Habitat: Scattered individuals of this species were found from the upper infralittoral (0.5–1 m) to a depth of 15 m.
- Distribution: The S. vulgare was found at four sites: one in Lipari (Le Formiche) and three in Vulcano (Parete della Sirena, Scoglio del Quaglietto and Capo Grosso) (Table 3).
- Further remarks: This species has a stable trend on these islands.
- Sargassum hornschuchii C. Agardh (Figure 13A–D)
- Basionym: Sargassum hornschuchii C. Agardh.
- Synonym: Stichophora hornschuchii (C. Agardh) Kützing.
- Morphological observations of Aeolian specimens: This species is erect, with a single smooth axis, attached to the substrate by a discoid holdfast. The thalli are 5–8 cm in length. The primary branches are flattened at the base, and sometimes slightly toothed at the margins. The foliose branchlets are narrow, lanceolate with a distichous–alternating disposal. They have a central midrib and entire, smooth or slightly toothed margins. Aerocysts were not observed. The receptacles are warty, flattened or triangular. They are composed, brought by a sterile pedicel, which is well developed and branched.
- Habitat: This species was found in the infralittoral at depths from 7 to 17 m, as scattered individuals or small patches.
- Distribution: The S. hornschuchii was only recorded at the site of Scoglio del Quaglietto, on the island of Vulcano (Table 3).
- Sargassum furcatum Kützing (Figure 14A–C)
- Basionym: Sargassum furcatum Kützing
- Synonyms: Sargassum vulgare f. furcatum (Kützing) J. Agardh
- Morphological observations of Aeolian specimens: This species has a creeping habit. The thalli, which are 5–8 cm long, are composed of a single axis, attached to the substrate by a discoid holdfast. The primary and secondary branches are knotty and feature foliose branches, which can be lanceolate or lobate, with pointed apexes and smooth or toothed margins, with an evident midrib that follows the bifurcation of the branches. Indeed, this species takes the name “furcatum” because its foliose branches can be divided up to four times. Aerocysts were not observed. The receptacles are cylindrical, lanceolate, warty and branched, forming a bunch. They are usually situated at the axilla of the foliose branches.
- Habitat: Scattered individuals were found in the infralittoral, at depths from 8 m to 28 m.
- Distribution: The S. furcatum was recorded at seven of the eleven examined sites: four in Lipari (Punta Castagna, Parete dei Gabbiani, Punta Menalda and Le Formiche) and three in Vulcano (Capo Grillo, Parete della Sirena and Capo Grosso).
- Further remarks: This species, with an Atlantic and Pacific distribution [41], was first reported in the Mediterranean by Flores-Moya and Conde [50], in Spain. Subsequently, in 2021, it was found in several locations along the eastern coast of Sicily [51]. Through this monitoring activity, S. furcatum was also reported in the Aeolian archipelago, representing a further record of this non-indigenous species in Sicily.
- Sargassum trichocarpum J. Agardh (Figure 15A–C)
- Basionym: Sargassum trichocarpum J. Agardh.
- Synonyms: Sargassum boryanum Montagne, Sargassum vulgare var. trichocarpum J. Agardh.
- Morphological observations of Aeolian specimens: This species is erect, with a single smooth axis, which is attached to the substrate by a discoid holdfast. The thalli are 7–12 cm in length. The primary branches are cylindrical and smooth. The foliose branches are elongated and narrow, with wavy or serrated margins and a central midrib. The aerocysts are spherical and brought by a slender pedicel. The receptacles are cylindrical, slender and branched several times in one plane.
- Habitat: This species was found in the infralittoral, at depths from 15 m to 26 m, forming small patches on the seabed.
- Distribution: This species was recorded at three sites (Capo Grillo, Parete della Sirena and Capo Grosso) of Vulcano (Table 3).
- Further remarks: In the literature consulted, it was observed that this species was not previously documented in the Aeolian archipelago (Table 2). Thus, the record presented here is the first for these islands. Since, as reported by Thibaut et al. [49] and Ballesteros and Weitzmann [52], S. trichocarpum is a regressing and rare species in the Mediterranean Sea, this record should receive special consideration in the context of the creation of MPAs.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Álvarez, H.; Perry, A.L.; Blanco, J.; García, S.; Aguilar, R. Towards the Creation of a Marine Protected Area in the Aeolian Islands. Results of the 2018 Aeolian Expediton, 1st ed.; Oceana: Madrid, Spain, 2019; pp. 1–136. [Google Scholar]
- Francalanci, L.; Avanzinelli, R.; Tommasini, S.; Heuman, A. A west east geochemical and isotopic traverse along the volcanism of the Aeolian Island arc, southern Tyrrhenian Sea, Italy: Inferences on mantle source processes. Geol. Soc. Am. Spec. Pap. 2007, 418, 235. [Google Scholar] [CrossRef]
- Esposito, V.; Andaloro, F.; Canese, S.; Bortoluzzi, G.; Bo, M.; Di Bella, M.; Giordano, P.; Spagnoli, F.; La Cono, V.; Yakimov, M.M.; et al. Exceptional discovery of a shallow-water hydrothermal site in the SW area of Basiluzzo islet (Aeolian archipelago, South Tyrrhenian Sea): An environment to preserve. PLoS ONE 2018, 13, e0190710. [Google Scholar] [CrossRef]
- Giaccone, G. Associazioni algali e fenomeni secondari di vulcanismo nelle acque marine di Vulcano (Mar Tirreno). Plant Biosyst. 1969, 103, 353–366. [Google Scholar] [CrossRef]
- Giaccone, G.; Cormaci, M.; Furnari, G.; Scammacca, B.; Alongi, G.; Catra, M.; Di Martino, V.; Marino, G.; Serio, D. Biodiversità vegetale marina dell’arcipelago “Isole Eolie”. Boll. Accad. Gioenia Nat. Sci. Catania 1999, 32, 191–242. [Google Scholar]
- Cavaliere, A. Ricerche sulla flora algologica dello Stretto di Messina. Primo contributo. Boll. Ist. Bot. Univ. Catania 1957, 1, 155–179. [Google Scholar]
- Cavaliere, A. Quelques notes sur les algues recueillies aux Isoles Eoliennes pendant una breve campagne thalassographique réalisée de Novembre 1957 a Fevrier 1958. Rapp. Commis. Int. Explor. Sci. Mer Médit. 1958, 15, 185–186. [Google Scholar]
- Cavaliere, A. Ricerche sulla flora algologica dello Stretto di Messina. Secondo contributo. Boll. Ist. Bot. Univ. Catania 1959, 3, 79–88. [Google Scholar]
- Giaccone, G. Raccolte di fitobenthos sulla banchina continentale Italiana. Plant Biosyst. 1969, 103, 485514. [Google Scholar] [CrossRef]
- Cormaci, M.; Furnari, G.; Scammacca, B.; Serio, D.; Pizzuto, F.; Alongi, G.; Dinaro, R. La vegetazione marina di substrato duro dell’isola di Salina (Isole Eolie). Boll. Accad. Gioenia Sci. Nat. Catania 1992, 25, 115–144. [Google Scholar]
- Cormaci, M.; Furnari, G.; Serio, D.; Pizzuto, F. Osservazioni sulle fitocenosi bentoniche dell’isola di Salina (Isole Eolie). In Parchi Naturali e Aree Protette; Guerrini, A., Ed.; Atti del Secondo Workshop del Progetto Strategico “Clima Ambiente e Territorio del Mezzogiorno” del C.N.R. Salina 20–30 Maggio 1990; Litografia Idonea G.: Catania, Italy, 1994; pp. 339–365. [Google Scholar]
- Alongi, G.; Pizzuto, F.; Scammacca, B. La flora sommersa dell’isola di Vulcano (Isole Eolie). Boll. Accad. Gioenia Sci. Nat. Catania 1993, 26, 273–291. [Google Scholar]
- Acunto, S.; Matagliati, F.; Rindi, F.; Rossi, F. Osservazioni floristiche sui popolamenti bentonici della baia di levante dell’Isola di Vulcano (Isole Eolie). Biol. Mar. Medit. 1997, 4, 348–350. [Google Scholar]
- Catra, M.; Alongi, G.; Giaccone, G. La flora sommersa dell’Isola di Filicudi (Isole Eolie). Boll. Accad. Gioenia Sci. Nat. Catania 1999, 32, 99–114. [Google Scholar]
- Giaccone, G. Contributo allo studio dei popolamenti algali del Basso Tirreno. Ann. Dell’Univ. Di Ferrara Nuova Ser. Bot. 1971, 4, 17–43. [Google Scholar]
- Acunto, S.; Matagliati, F.; Cinelli, F. Osservazioni sui popolamenti bentonici di un’area interessata da attività idrotermale nei pressi dell’Isola di Panarea (Isole Eolie). Biol. Mar. Medit. 1996, 3, 434–436. [Google Scholar]
- Acunto, S.; Rindi, F. Variabilità spaziale di popolamenti fitobentonici in relazione ad attività idrotermali nella Baia di Levante dell’isola di Vulcano (Isole Eolie): Studio preliminare. Biol. Mar. Medit. 1997, 4, 351–352. [Google Scholar]
- Bellissimo, G.; Rull Lluch, J.; Di Maida, G.; Pirrotta, M.; Tomasello, A.; Calvo, S. Influence of hydrothermal vents on phytobenthic communities in the Aeolian Islands (Tyrrhenian Sea): Preliminary results. In Proceedings of the 4th Mediterranean Symposium on Marine Vegetation, Yasmine-Hammamet, Tunisia, 2–4 December 2010. [Google Scholar]
- Bellissimo, G.; Rull Lluch, J.; Tomasello, A.; Calvo, S. The community of Cystoseira brachycarpa J. Agardh emend. Giaccone (Fucales, Phaeophyceae) in a shallow hydrothermal vent area of the Aeolian Islands (Tyrrhenian Sea, Italy). Plant Biosyst. 2014, 148, 21–26. [Google Scholar] [CrossRef]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; de Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Mangialajo, L.; Ruggieri, N.; Asnaghi, V.; Chiantore, M.; Povero, P.; Cattaneo-Vietti, R. Ecological status in the Ligurian Sea: The effect of coastline urbanisation and the importance of proper reference sites. Mar. Pollut. Bull. 2007, 55, 30–41. [Google Scholar] [CrossRef]
- Asnaghi, V.; Chiantore, M.; Bertolotto, R.M.; Parravicini, V.; Cattaneo-Vietti, R.; Gaino, F.; Moretto, P.; Privitera, D.; Mangialajo, L. Implementation of the European water framework directive: Natural variability associated with the CARLIT method on the rocky shores of the Ligurian Sea (Italy). Mar. Ecol. 2009, 30, 505–513. [Google Scholar] [CrossRef]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfune, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in the French Riviera: The harbinger of future extinctions? Mediterr. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef]
- Neiva, J.; Bermejo, R.; Medrano, A.; Capdevila, P.; MillaFigueras, D.; Afonso, P.; Ballesteros, E.; Sabour, B.; Serio, D.; Nóbrega, E.; et al. DNA barcoding reveals cryptic diversity, taxonomic conflicts and novel biogeographical insights in Cystoseira s.l. (Phaeophyceae). Eur. J. Phycol. 2022, 58, 351–375. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A. The Fucales (Ochrophyta, Phaeophyceae) of the Island of Pantelleria (Sicily Channel, Mediterranean Sea): A new contribution. Ital. Bot. 2023, 15, 137–163. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Chauvet, C.; Duval, C.; Galzin, R.; Lejeune, P.; Barnabé, G.; Blanc, F.; Chevalier, R.; Duclerc, J.; et al. The underwater observation of fish communities and fish populations: Methods and problems. Rev. Ecol. Terre Vie 1985, 40, 467–540. [Google Scholar]
- La Mesa, G.; Salvati, E.; Agnesi, S.; Tunesi, L. Assessment of coastal fish assemblages before the establishment of a new marine protected area in central Mediterranean: Its role in formulating zoning proposal. Mediterr. Mar. Sci. 2017, 18, 11–21. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A. Assessment of grazing impact on deep canopy-forming species in the western Ionian Sea, Central Mediterranean. Int. J. Aquat. Biol. 2020, 8, 365–376. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Cottalorda, J.M.; Hereu, B.; Susini, M.L.; Verlaque, M. Unexpected temporal stability of Cystoseira and Sargassum forests in Port-Cros, one of the oldest Mediterranean marine National Parks. Cryptogam. Algol. 2016, 37, 61–90. [Google Scholar] [CrossRef]
- Gómez-Garreta, A.; Barceló-Martí, M.C.; Ribera-Siguan, M.A.; Rull-Lluch, J. Cystoseira C. Agardh. In Flora Phycologica Iberica, 1st ed.; Gómez-Garreta, A., Ed.; Universidade de Murcia: Múrcia, Spania, 2001; Volume 1, Fucales; pp. 99–166. [Google Scholar]
- Cormaci, M.; Furnari, G.; Catra, M.; Alongi, G.; Giaccone, G. Flora marina bentonica del Mediterraneo: Phaeophyceae. Boll. Accad. Gioenia Sci. Nat. Catania 2012, 45, 1–508. [Google Scholar]
- Rodríguez-Prieto, C.; Ballesteros, E.; Boisset, F.; Afonso Carrilo, J. Guía de Las Macroalgas y Fanerógamas Marinas del Mediterráneo Occidental; Ediciones Omega: Barcelona, Spain, 2013; pp. 1–656. [Google Scholar]
- Blanfuné, A.; Verlaque, M.; Boudouresque, C.F.; Rozis, E.; Thibaut, T. Les Forêts Marines de France et de Méditerranée. Guide de Détermination des Espèces-Ingénieurs. Sargassaceae, Fucales, Phaeophyceae; Presses Universitaries de Provence: Marseille, France, 2022; pp. 1–207. [Google Scholar]
- Gray, J.S. The measurement of marine species diversity, with an application to the benthic fauna of the Norwegian continental shelf. J. Exp. Mar. Biol. Ecol. 2000, 250, 23–49. [Google Scholar] [CrossRef]
- Danovaro, R.; Bianchelli, S.; Gambi, C.; Mea, M.; Zeppilli, D. α-, β-, γ-, δ-and ε-diversity of deep-sea nematodes in canyons and open slopes of Northeast Atlantic and Mediterranean margins. Mar. Ecol. Prog. Ser. 2009, 396, 197–209. [Google Scholar] [CrossRef]
- Bianchelli, S.; Buschi, E.; Danovaro, R.; Pusceddu, A. Biodiversity loss and turnover in alternative states in the Mediterranean Sea: A case study on meiofauna. Sci. Rep. 2016, 6, 34544. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Gamito, S. Caution is needed when applying Margalef diversity index. Ecol. Indic. 2010, 10, 550–551. [Google Scholar] [CrossRef]
- AlgaeBase. Available online: https://www.algaebase.org (accessed on 30 July 2023).
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Montagne, C. Cryptogames algériennes, ou plantes cellulaires recueillies par M. Roussel aux environs d’Alger, et publiées par le Docteur Camille Montagne. Ann. Sci. Nat. Bot. 1838, 2, 268–279 (+ Suppl. 337–345, plates 8–9). [Google Scholar]
- Sellam, L.N.; Blanfuné, A.; Boudouresque, C.F.; Thibaut, T.; Rebzani Zahaf, C.; Verlaque, M. Cystoseira montagnei J. Agardh and C. spinosa Sauvageau (Phaeophyceae, Sargassaceae): A taxonomic reappraisal of misused names, with the proposal of Cystoseira michaelae Verlaque et al. nom. et stat. nov. Cryptogam. Algol. 2017, 38, 133–157. [Google Scholar] [CrossRef]
- Tita, G. Aspects écologiques d’un peuplement à Cystoseira dubia Valiante. Mar. Life 1994, 4, 9–17. [Google Scholar]
- Verlaque, M.; Ballesteros, E.; Sala, E.; Garrabou, J. Cystoseira jabukae (Cystoseiraceae, Fucophyceae) from Corsica (Mediterranean) with notes on the previously misunderstood species C. funkii. Phycologia 1999, 38, 77–86. [Google Scholar] [CrossRef]
- Ercegovic, A. Sur les Cystoseira Adriatiques, leur Morphologie, Ecologie et Evolution. Fauna i Flora Jadrana, 1st ed.; Institut Oceanographie et Peche: Split, Croatia, 1952; Volume 2, pp. 1–212. [Google Scholar]
- Rendina, F.; Falace, A.; Alongi, G.; Buia, M.C.; Neiva, J.; Appolloni, L.; Marletta, G.; Russo, G.F. The Lush Fucales Underwater Forests off the Cilento Coast: An Overlooked Mediterranean Biodiversity Hotspot. Plants 2023, 12, 1497. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Verlaque, M.; Boudouresque, C.F.; Ruitton, S. The Sargassum conundrum: Very rare, threatened or locally extinct in the NW Mediterranean and still lacking protection. Hydrobiologia 2016, 781, 3–23. [Google Scholar] [CrossRef]
- Flores-Moya, A.; Conde, F. Fragmentos taxonómicos, corológicos, nomenclaturales y fitosociológicos (67–74). Acta Bot. Malacit. 1998, 23, 197–228. [Google Scholar] [CrossRef]
- Tiralongo, F.; Akyol, O.; Al Mabruk, S.A.; Battaglia, P.; Beton, D.; Bitlis, B.; Borg, J.A.; Bouchoucha, M.; Çinar, M.E.; Crocetta, F.; et al. New Alien Mediterranean Biodiversity Records (August 2022). Mediterr. Mar. Sci. 2022, 23, 725–747. [Google Scholar] [CrossRef]
- Ballesteros, E.; Weitzmann, B. On the presence of a small population of Sargassun trichocarpum J. Agardh (Phaeophyceae: Fucales) in Catalonia (Northwestern Mediterranean). Butll. Inst. Catalana Hist. Nat. 2021, 85, 83–85. [Google Scholar]
- Verlaque, M.; Boudouresque, C.F.; Perret-Boudouresque, M. Mediterranean seaweeds listed as threatened under the Barcelona Convention: A critical analysis. Sci. Rep. Port Cros Natl. Park 2019, 33, 179–214. [Google Scholar]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Lombardo, A.; Marletta, G. The marine Heterobranchia (Mollusca: Gastropoda) fauna of the Aeolian archipelago (Tyrrhenian Sea). First contribution: Lipari and Vulcano. Int. J. Aquat. Biol. 2023, 11, 288–300. [Google Scholar] [CrossRef]
- Civitarese, G.; Gačić, M.; Lipizer, M.; Eusebi Borzelli, G.L. On the impact of the Bimodal Oscillating System (BiOS) on the biogeochemistry and biology of the Adriatic and Ionian Seas (eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Tomasini, J.A.; Romdhane, M.S.; Do Chi, T.; Mouillot, D. Historical colonization of the Mediterranean Sea by Atlantic fishes: Do biological traits matter? Hydrobiologia 2008, 607, 51–62. [Google Scholar] [CrossRef]
- Darling, E.S.; Côté, I.M. Seeking resilience in marine ecosystems. Science 2018, 359, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Serio, D.; Alongi, G.; Catra, M.; Cormaci, M.; Furnari, G. Changes in the benthic algal flora of Linosa Island (Straits of Sicily, Mediterranean Sea). Bot. Mar. 2006, 49, 135–144. [Google Scholar] [CrossRef]
- ISPRA, Istituto Superiore per la Protezione e la Ricerca Ambientale. Available online: https://www.mareografico.it/it/stazioni.html (accessed on 11 November 2023).
- Verdura, J.; Santamaría, J.; Ballesteros, E.; Smale, D.; Cefalì, M.E.; Golo, R.; de Caralt, S.; Vergés, A.; Cebrian, E. Local-scale climatic refugia offer sanctuary for a habitat-forming species during a marine heatwave. J. Ecol. 2021, 109, 1758–1773. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Martinez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Ecophysiological responses to elevated CO2 and temperature in Cystoseira tamariscifolia (Phaeophyceae). Clim. Chang. 2017, 142, 67–81. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Savonitto, G.; Lipizer, M.; Mancuso, P.; Ciriaco, S.; Srijemsi, M.; Falace, A. Climatic anomalies may create a long-lasting ecological phase shift by altering the reproduction of a foundation species. Ecology 2019, 100, e02838. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef] [PubMed]
- Orfanidis, S.; Rindi, F.; Cebrian, E.; Fraschetti, S.; Nasto, I.; Taskin, E.; Bianchelli, S.; Papathanasiou, V.; Kosmidou, M.; Caragnano, A.; et al. Effects of Natural and Anthropogenic Stressors on Fucalean Brown Seaweeds Across Different Spatial Scales in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 658417. [Google Scholar] [CrossRef]
- Arévalo, R.; Pinedo, S.; Ballesteros, E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: Descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar. Pollut. Bull. 2007, 55, 104–113. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E. Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): Relationships with environmental factors and anthropogenic pressures. Estuar. Coast. Shelf Sci. 2009, 84, 476–482. [Google Scholar] [CrossRef]
- Tsiamis, K.; Panayotidis, P.; Salomidi, M.; Pavlidou, A.; Kleinteich, J.; Balanika, K.; Küpper, F.C. Macroalgal community response to re-oligotrophication in Saronikos Gulf. Mar. Ecol. Prog. Ser. 2013, 472, 73–85. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Capdevila, P.; Hereu, B.; Salguero-Gómez, R.; Rovira, G.; Medrano, A.; Cebrian, E.; Garrabou, J.; Kersting, D.K.; Linares, C. Warming impacts on early life stages increase the vulnerability and delay the population recovery of a long-lived habitat forming macroalga. J. Ecol. 2019, 107, 1129–1140. [Google Scholar] [CrossRef]
- Falace, A.; Marletta, G.; Savonitto, G.; Carniel, F.C.; Srijemsi, M.; Bevilacqua, S.; Tretiach, M.; Alongi, G. Is the South-Mediterranean Canopy-Forming Ericaria giacconei (=Cystoseira hyblaea) a loser from Ocean Warming? Front. Mar. Sci. 2021, 8, 760637. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Strain, E.M.A.; Piccioni, E.; De Clerck, O.; Sarà, G.; Airoldi, L. Status of vulnerable Cystoseira populations along the Italian infralittoral fringe, and relationships with environmental and anthropogenic variables. Mar. Pollut. Bull. 2018, 129, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Marletta, G. Status of shallow Fucales (Ochrophyta) assemblages in the bay of Brucoli, eastern coast of Sicily (Ionian Sea). Biodivers. J. 2021, 12, 1011–1026. [Google Scholar] [CrossRef]
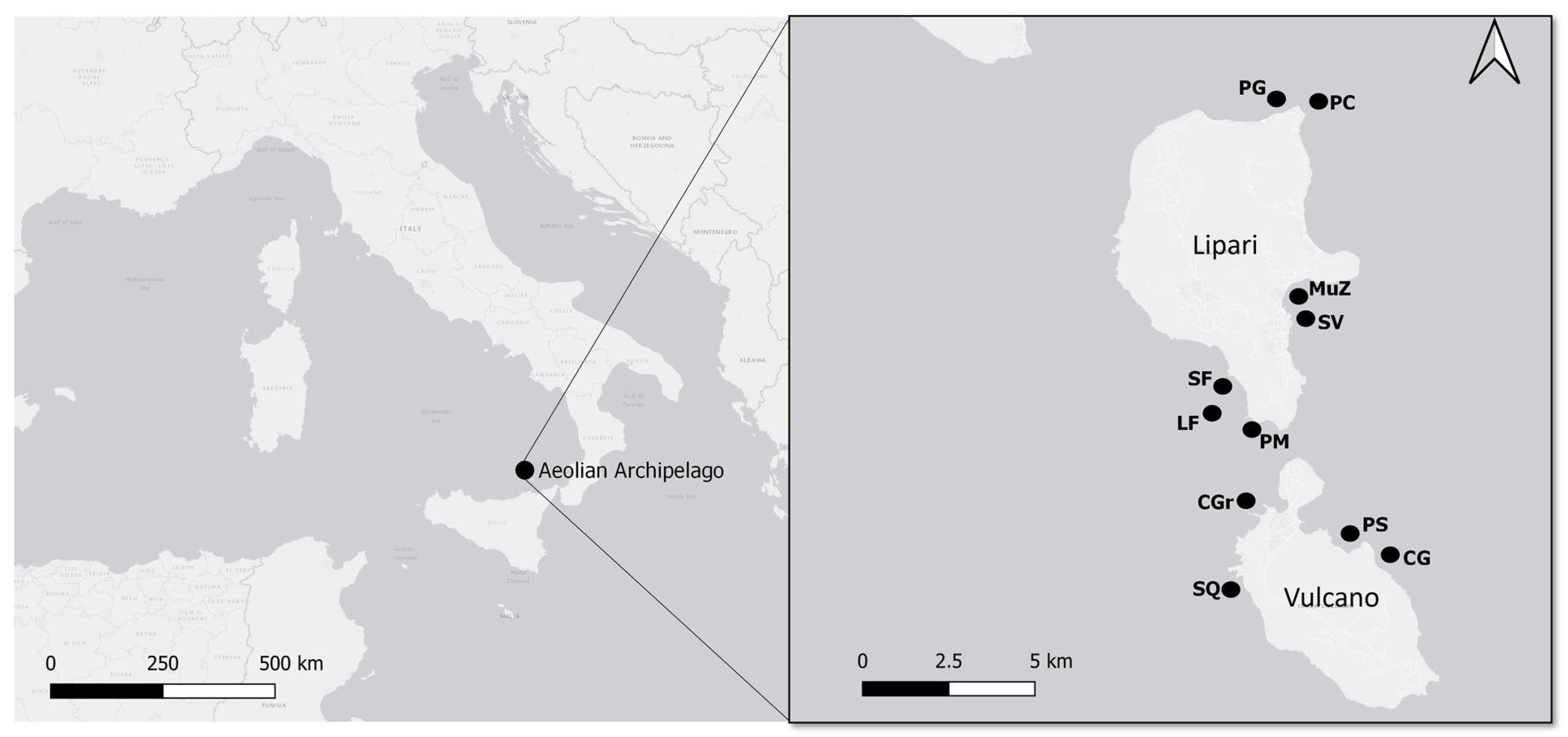













| Island | Site | Abbreviation | Coordinates | Date | Activity |
|---|---|---|---|---|---|
| Lipari | Punta Castagna | PC | 38.522157, 14.963679 | 3 July | Diving |
| Lipari | Secca del Villaggio | SV | 38.461403, 14.957582 | 4 July | Diving |
| Lipari | Parete dei Gabbiani | PG | 38.523374, 14.954881 | 5 July | Diving |
| Lipari | Pietra Menalda | PM | 38.439146, 14.941681 | 6 July | Snorkelling, Diving |
| Lipari | Secca delle Formiche | SF | 38.443401, 14.940077 | 6 July | Diving |
| Lipari | Le Formiche | LF | 38.441079, 14.939076 | 7 July | Snorkelling, Diving |
| Lipari | Zona Municipio | Muz | 38.469284, 14.957667 | 8 July | Snorkelling |
| Vulcano | Capo Grillo | CG | 38.409227, 14.984991 | 3 July | Diving |
| Vulcano | Parete della Sirena | PS | 38.409583, 14.974225 | 4 July | Diving |
| Vulcano | Scoglio del Quaglietto | SQ | 38.399449, 14.938724 | 5 July | Diving |
| Vulcano | Capo Grosso | CGr | 38.419163, 14.941785 | 7 July | Snorkelling, Diving |
| Island | Site | Number of Species | Simpson | Shannon | Margalef |
|---|---|---|---|---|---|
| Lipari | PC | 7 | 0.14 | 1.95 | 3.08 |
| SV | 1 | 1 | 0 | 0 | |
| PG | 4 | 0.25 | 1.39 | 2.16 | |
| PM | 6 | 0.17 | 1.79 | 2.79 | |
| SF | 4 | 0.25 | 1.39 | 2.16 | |
| LF | 7 | 0.14 | 1.95 | 3.08 | |
| MuZ | 1 | 1 | 0 | 0 | |
| Total | 30 | 2.95 | 8.47 | 13.27 | |
| Vulcano | CG | 10 | 0.1 | 2.30 | 3.91 |
| PS | 8 | 0.13 | 2.08 | 3.37 | |
| SQ | 6 | 0.17 | 1.79 | 2.79 | |
| CGr | 8 | 0.13 | 2.08 | 3.37 | |
| Total | 32 | 0.53 | 8.25 | 13.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marletta, G.; Lombardo, A.; Serio, D.; Bianchelli, S. Diversity of Fucales (Ochrophyta, Phaeophyceae) along the Coasts of Lipari and Vulcano (Aeolian Archipelago), Tyrrhenian Sea (Central Mediterranean Sea). J. Mar. Sci. Eng. 2023, 11, 2222. https://doi.org/10.3390/jmse11122222
Marletta G, Lombardo A, Serio D, Bianchelli S. Diversity of Fucales (Ochrophyta, Phaeophyceae) along the Coasts of Lipari and Vulcano (Aeolian Archipelago), Tyrrhenian Sea (Central Mediterranean Sea). Journal of Marine Science and Engineering. 2023; 11(12):2222. https://doi.org/10.3390/jmse11122222
Chicago/Turabian StyleMarletta, Giuliana, Andrea Lombardo, Donatella Serio, and Silvia Bianchelli. 2023. "Diversity of Fucales (Ochrophyta, Phaeophyceae) along the Coasts of Lipari and Vulcano (Aeolian Archipelago), Tyrrhenian Sea (Central Mediterranean Sea)" Journal of Marine Science and Engineering 11, no. 12: 2222. https://doi.org/10.3390/jmse11122222





