The Bryozoan Margaretta cereoides as Habitat-Former in the Coralligenous of Marzamemi (SE Sicily, Mediterranean Sea)
Abstract
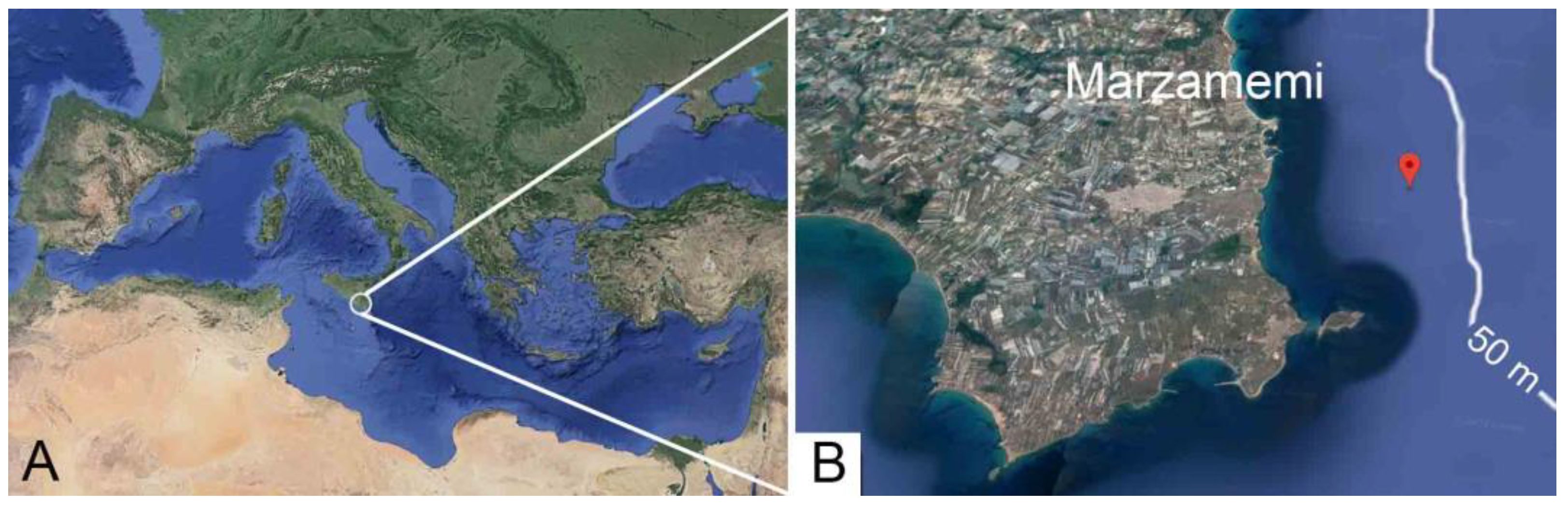
:1. Introduction
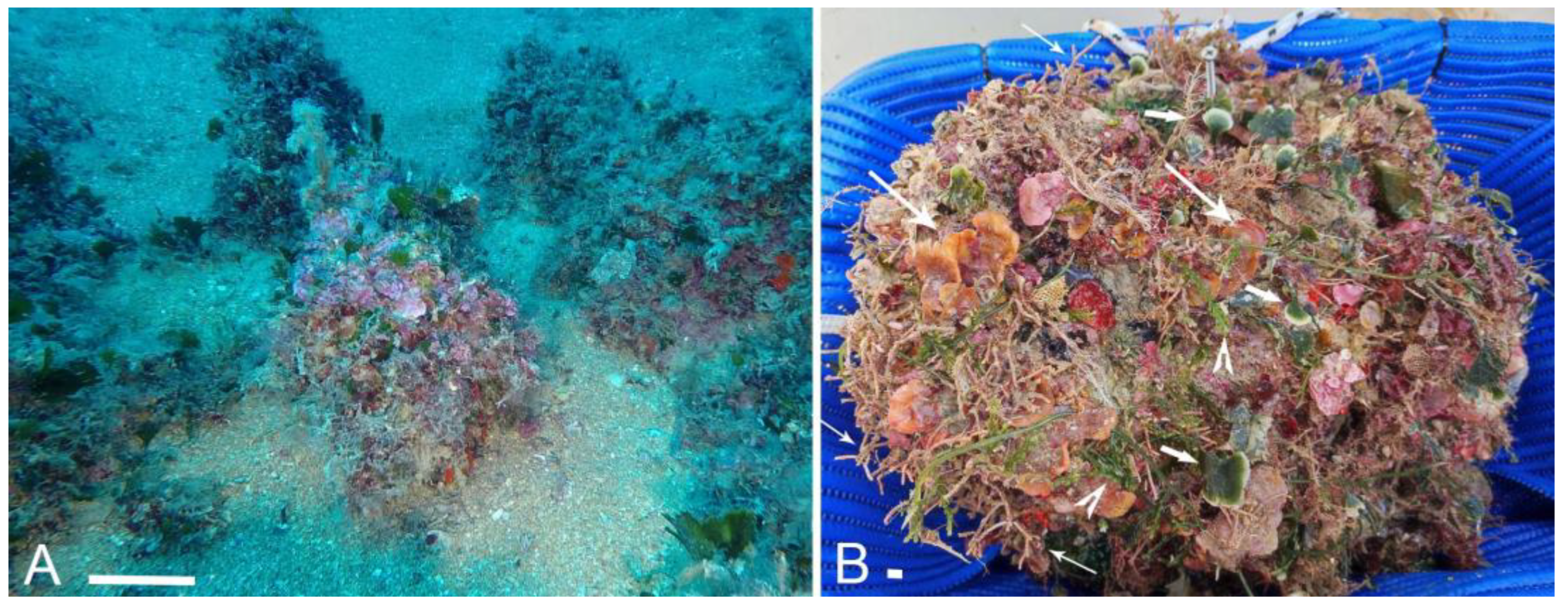
2. Materials and Methods
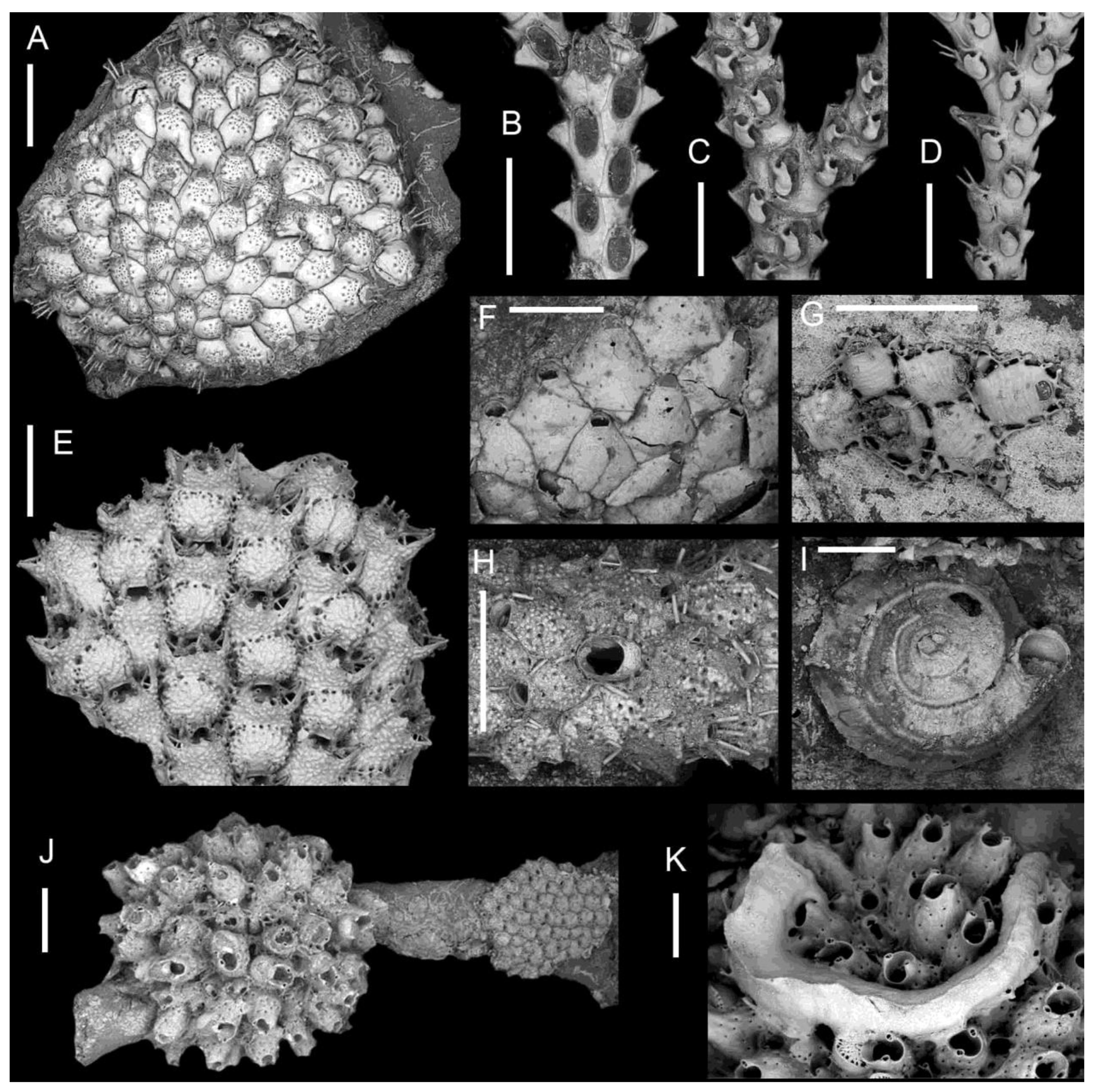
3. Results
4. Discussion
4.1. Diversity
4.2. Habitat-Forming Role of M. cereoides and Algae in the Canopy of the Studied Coralligenous Build-Up
4.3. Bryozoan and Serpulid Assemblages on M. cereoides and other Large Habitat-Forming Bryozoans
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, A.C.L.; Probert, P.K.; Rowden, A.A.; Smith, A.M. Complex Habitat Generated by Marine Bryozoans: A Review of Its Distribution, Structure, Diversity, Threats and Conservation. Aquat. Conserv. 2012, 22, 547–563. [Google Scholar]
- Lombardi, C.; Taylor, P.D.; Cocito, S. Bryozoan Constructions in a Changing Mediterranean Sea. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, I., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 373–384. [Google Scholar]
- Lombardi, C.; Taylor, P.D.; Cocito, S. Bryozoans: The ‘Forgotten’ Bioconstructors. In Perspectives on the Marine Animal Forests of the World; Rossi, S., Bramanti, L., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 193–217. [Google Scholar]
- Cocito, S. Bioconstruction and Biodiversity: Their Mutual Influence. Sci. Mar. 2004, 68, 137–144. [Google Scholar]
- Holon, F.; Harmelin, J.-G. Bryozoan Reef, an Unknown Mediterranean Habitat? Int. Bryozool. Assoc. Bull. 2014, 10, 10–11. [Google Scholar]
- Novosel, M. Bryozoans of the Adriatic Sea. In Denisia 16; Neue Serie 28; Landesmuseen: Zurich, Switzerland, 2005; pp. 231–246. [Google Scholar]
- Cocito, S.; Novosel, M.; Pasarić, Z.; Key, M.M., Jr. Growth of the Bryozoan Pentapora fascialis (Cheilostomata, Ascophora) around Submarine Freshwater Springs in the Adriatic Sea. Linzer Biol. Beitr. 2006, 38, 15–24. [Google Scholar]
- Ferdeghini, F.; Cocito, S. Biologically Generated Diversity in Two Bryozoan Buildups. Biol. Mar. Mediterr. 1999, 6, 191–197. [Google Scholar]
- Maluquer, P. Algunas Consideraciones sobre la Fauna Asociada a las Colonias de Schizoporella errata (Waters, 1878) del Puerto de Mahón (Menorca, Baleares). Publ. Dept. Zool. Barc. 1985, 11, 23–28. [Google Scholar]
- Cocito, S. Le Biocostruzioni a Briozoi. Biol. Mar. Mediterr. 2009, 16, 19–30. [Google Scholar]
- Stebbing, A.R.D. The Epizoic Fauna of Flustra foliacea (Bryozoa). J. Mar. Biol. Ass. UK 1971, 51, 283–300. [Google Scholar]
- Connor, D.W.; Allen, J.H.; Golding, N.; Howell, K.L.; Lieberknecht, L.M.; Northen, K.O.; Reker, J.B. The Marine Habitat Classification for Britain and Ireland. Version 04.05 Infralittoral Rock Section; JNCC: Peterborough, UK, 2004. [Google Scholar]
- Stebbing, A.R.D. Growth of Flustra Foliacea (Bryozoa). Mar. Biol. 1971, 9, 267–273. [Google Scholar]
- Bitschofsky, F.; Forster, S.; Scholz, J. Regional and Temporal Changes in Epizoobiontic Bryozoan-Communities of Flustra foliacea (Linnaeus, 1758) and Implications for North Sea Ecology. Estuar. Coast. Shelf Sci. 2011, 91, 423–433. [Google Scholar]
- McKinney, F.K.; Jaklin, A. Spatial Niche Partitioning in the Cellaria Meadow Epibiont Association, Northern Adriatic Sea. Cah. Biol. Mar. 2000, 41, 1–17. [Google Scholar]
- Gautier, Y.V. Recherches Écologiques Sur Les Bryozoaires Chilostomes En Méditerranée Occidentale. Rec. Trav. Stat. Mar. Endoume 1962, 38, 1–434. [Google Scholar]
- Ferdeghini, F.; Acunto, S.; Cocito, S.; Cinelli, F. Variability at Different Spatial Scales of a Coralligenous Assemblage at Giannutri Island (Tuscan Archipelago, Northwest Mediterranean). In Island, Ocean and Deep-Sea Biology, Developments in Hydrobiology; Jones, M.B., Azevedo, J.M.N., Neto, A.I., Costa, A.C., Martins, A.M.F., Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 152, pp. 27–36. [Google Scholar]
- McKinney, F.K.; Jaklin, A. Sediment Accumulation in a Shallow-Water Meadow Carpeted by a Small Erect Bryozoan. Sediment. Geol. 2001, 145, 397–410. [Google Scholar]
- Rosso, A.; Di Martino, E.; Sanfilippo, R.; Di Martino, V. Bryozoan Communities and Thanatocoenoses from Submarine Caves in the Plemmirio Marine Protected Area (SE Sicily) Bryozoans from Submarine Caves. In Bryozoan Studies 2010, Proceedings of the 15th IBA Conference, Kiel, Germany, 2–7 August 2010; Ernst, A., Schäfer, P., Scholz, J., Eds.; Lecture Notes in Earth System Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 143, pp. 251–269. [Google Scholar]
- Rosso, A.; Altieri, C.; Bazzicalupo, P.; Bertolino, M.; Bracchi, V.A.; Bruno, F.; Cipriani, M.; Costa, G.; D’Alpa, F.; Donato, G.; et al. Bridging Together Research and Technological Innovation: First Results and Expected Bearings of the Project CRESCIBLUREEF on Mediterranean Coralligenous. In Proceedings of the 4th Mediterranean Symposium on the Conservation of the Coralligenous and Other Calcareous Bio-Concretions, Genova, Italy, 20–21 September 2022; p. 108. [Google Scholar]
- Violanti, V.; di Geronimo, I.; Saccà, R. Foraminiferal Thanatocoenoses from the Gulf of Noto (Southeastern Sicily). Boll. Mus. Reg. Sci. Nat. Torino. Spec. Vol. 1990, 773–799. [Google Scholar]
- Di Geronimo, I.; Di Geronimo, R.; Improta, S.; Rosso, A.; Sanfilippo, R. Preliminary Observation on a Columnar Coralline Build-up from off SE Sicily. Biol. Mar. Mediterr. 2001, 8, 229–237. [Google Scholar]
- Di Geronimo, I.; Di Geronimo, R.; Rosso, A.; Sanfilippo, R. Structural and Taphonomic Analysis of a Columnar Coralline Algal Build-up from SE Sicily. Geobios 2002, 35, 86–95. [Google Scholar]
- Rosso, A.; Sanfilippo, R. The Contribution of Bryozoans and Serpuloideans to Coralligenous Concretions from SE Sicily. In UNEP-MAP-RAC/SPA, Proceedings of the First Symposium on the Coralligenous and Other Calcareous Bio-Concretions of the Mediterranean Sea, Tabarka, Tunisia, 15–16 January 2009; Pergent-Martini, C., Brichet, M., Eds.; RAC/SPA Publication: Tunis, Tunisia, 2009; pp. 123–128. [Google Scholar]
- Varzi, A.G.; Fallati, L.; Savini, A.; Bracchi, V.A.; Bazzicalupo, P.; Rosso, A.; Sanfilippo, R.; Bertolino, M.; Muzzupappa, M.; Basso, D. Geomorphological Mapping of Coralligenous Reefs Offshore Southeastern Sicily (Ionian Sea). J. Maps 2023. [Google Scholar] [CrossRef]
- Bracchi, V.A.; Bazzicalupo, P.; Fallati, L.; Varzi, A.G.; Savini, A.; Negri, M.P.; Rosso, A.; Sanfilippo, R.; Guido, A.; Bertolino, M. The Main Builders of Mediterranean Coralligenous: 2D and 3D Quantitative Approaches for Its Identification. In Crustose Coralline Red Algae Frameworks and Rhodoliths: Past and Present; Frontiers: London, UK, 2022; Volume 16648714, p. 41. [Google Scholar]
- Donato, G.; Sanfilippo, R.; Sciuto, F.; D’Alpa, F.; Serio, D.; Bracchi, V.A.; Bazzicalupo, P.; Negri, P.; Guido, A.; Bertolino, M.; et al. Biodiversity of a Coralligenous Build-up off Marzamemi (SE Sicily, Ionian Sea). In UNEP SPA/RAC, Proceedings of the 4th Mediterranean Symposium on the Conservation of the Coralligenous and Other Calcareous Bio-Concretions, Genova, Italy, 20–21 September 2022; pp. 151–152.
- Sanfilippo, R.; Rosso, A.; Guido, A.; Gerovasileiou, V. Serpulid Communities from Two Marine Caves in the Aegean Sea, Eastern Mediterranean. J. Mar. Biol. Assoc. UK 2017, 97, 1059–1068. [Google Scholar]
- Rosso, A.; Di Martino, E. Bryozoan Diversity in the Mediterranean Sea: An Update. Medit. Mar. Sci. 2016, 17, 567–607. [Google Scholar]
- Castelli, A.; Bianchi, C.; Cantone, G.; Çinar, M.; Gambi, M.; Giangrande, A.; Iraci Sareri, D.; Lanera, P.; Licciano, M.; Musco, L.; et al. Annelida Polychaeta. In Relini G. Checklist of the Flora and Fauna in Italian Seas. Biol. Mar. Medit. 2008, 15 (Suppl. S1), 323–373. [Google Scholar]
- Ben-Eliahu, M.N.; Fiege, D. Serpulid Tube-Worms (Annelida: Polychaeta) of the Central and Eastern Mediterranean with Particular Attention to the Levant Basin. Senckenberg. Marit. 1996, 28, 1–51. [Google Scholar]
- Çinar, M.E.; Bilecenoglu, M.; Öztürk, B.; Can, A. New Records of Alien Species on the Levantine Coast of Turkey. Aquat. Invasions 2006, 1, 84–90. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoğlu, M.; Yokeş, M.B.; Öztürk, B.; Taşkin, E.; Bakir, K.; Doğan, A.; Açik, Ş. Current Status (as of End of 2020) of Marine Alien Species in Turkey. PLoS ONE 2021, 16, e0251086. [Google Scholar]
- Casellato, S.; Stefanon, A. Coralligenous Habitat in the Northern Adriatic Sea: An Overview. Mar. Ecol. 2008, 29, 321–341. [Google Scholar]
- Laubier, L. Le Coralligène Des Albères-Monographie Biocénotique. Ann. Inst. Oceanogr. 1966, 43, 137–316. [Google Scholar]
- Liuzzi, M.G.; Lopez-Gappa, J. Algae as Hosts for Epifaunal Bryozoans: Role of Functional Groups and Taxonomic Relatedness. J. Sea Res. 2011, 65, 28–32. [Google Scholar]
- Hayward, P.J. Invertebrate Epiphytes of Coastal Marine Algae. Shore Environ. 1980, 2, 761–787. [Google Scholar]
- Gallardo, D.; Oliva, F.; Ballesteros, M. Marine Invertebrate Epibionts on Photophilic Seaweeds: Importance of Algal Architecture. Mar. Biodivers. 2021, 51, 16. [Google Scholar]
- Ryland, J.S. Experiments on the Selection of Algal Subsrates by Polyzoan Larvae. J. Exp. Biol. 1959, 36, 613–631. [Google Scholar]
- Steinberg, P.D.; de Nys, R. Chemical Mediation of Colonization of Seaweed Surfaces. J. Phycol. 2002, 38, 621–629. [Google Scholar] [CrossRef]
- Gama, B.A.P.; Plouguerné, E.; Pereira, R.C. The Antifouling Defence Mechanisms of Marine Macroalgae. In Advances in Botanical Research; Elsevier: Oxford, UK, 2014; Volume 71, pp. 413–440. ISBN 0065-2296. [Google Scholar]
- Uriz, M.J.; Martin, D.; Turon, X.; Ballesteros, E.; Hughes, R.; Acebal, C. An Approach to the Ecological Significance of Chemically Mediated Bioactivity in Mediterranean Benthic Communities. Mar. Ecol. Prog. Ser. 1991, 70, 175–188. [Google Scholar] [CrossRef]
- Koçak, F.; Aydin-Önen, S. Epiphytic Bryozoan Community of Posidonia oceanica (L.) Delile Leaves in Two Different Meadows at Disturbed and Control Locations. Mediterr. Mar. Sci. 2014, 15, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Dick, M.H.; Grischenko, A.V. Rocky-Intertidal Cheilostome Bryozoans from the Vicinity of the Sesoko Biological Station, West-Central Okinawa, Japan. J. Nat. Hist. 2017, 51, 141–266. [Google Scholar]
- Subías-Baratau, A.; Sanchez-Vidal, A.; di Martino, E.; Figuerola, B. Marine Biofouling Organisms on Beached, Buoyant and Benthic Plastic Debris in the Catalan Sea. Mar. Pollut. Bull. 2022, 175, 113405. [Google Scholar]
- Martí, R.; Uriz, M.J.; Turon, X. Seasonal and Spatial Variation of Species Toxicity in Mediterranean Seaweed Communities: Correlation to Biotic and Abiotic Factors. Mar. Ecol. Prog. Ser. 2004, 282, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Rossbach, F.I.; Casoli, E.; Plewka, J.; Schmidt, N.; Wild, C. New Insights into a Mediterranean Sea Benthic Habitat: High Diversity of Epiphytic Bryozoan Assemblages on Phyllophora crispa (Rhodophyta) Mats. Diversity 2022, 14, 346. [Google Scholar]
- Rossbach, F.I.; Casoli, E.; Beck, M.; Wild, C. Mediterranean Red Macro Algae Mats as Habitat for High Abundances of Serpulid Polychaetes. Diversity 2021, 13, 265. [Google Scholar]
- Yepes-Narváez, V. Environmental Ecology of Marine Bryozoans (Phylum Bryozoa) and Ascidians (Tunicata: Ascidiacea) under Multistressor Scenarios. Ph.D. Thesis, The Manchester Metropolitan University, Manchester, UK, 2020; 288p. [Google Scholar]
- Rosso, A.; Sanfilippo, R.; Sciuto, F.; Serio, D.; Catra, M.; Alongi, G.; Viola, A.; Leonardi, R. Preliminary Information on Bryozoans Associated with Selected Infralittoral Algae Communities from Eastern Sicily (Mediterranean). Australas. Palaeontol. Mem. 2019, 52, 115–129. [Google Scholar]
- Manriquez, P.H.; Cancino, J.M. Bryozoan-Macroalgal Interactions: Do Epibionts Benefit? Mar. Ecol. Prog. Ser. 1996, 138, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.; Cancino, J.M.; Molina, M.X. Effect of Encrusting Bryozoans on the Physiology of Their Algal Substratum. J. Mar. Biolog. Assoc. UK 1991, 71, 877–882. [Google Scholar] [CrossRef]
- Taylor, P.D.; Wilson, M.A. Palaeoecology and Evolution of Marine Hard Substrate Communities. Earth Sci. Rev. 2003, 62, 1–103. [Google Scholar]
- McKinney, F.K.; Jackson, J.B.C. Bryozoan Evolution; Unwin Hyman: Boston, MA, USA, 1989; p. 238. [Google Scholar]
- López de la Cuadra, C.M.; García-Gómez, J.C. New and Little-Known Ascophoran Bryozoans from the Western Mediterranean, Collected by ‘Fauna Iberica’ Expeditions. J. Nat. Hist. 2001, 35, 1717–1732. [Google Scholar]
- Kupriyanova, E.K.; Nishi, E.; ten Hove, H.; Rzhavsky, A.V. Life-History Patterns in Serpulimorph Polychaetes: Ecological and Evolutionary Perspectives. Oceanogr. Mar. Biol. Ann. Rev. 2001, 39, 1–101. [Google Scholar]
- Fava, F.; Ponti, M.; Abbiati, M. Role of Recruitment Processes in Structuring Coralligenous Benthic Assemblages in the Northern Adriatic Continental Shelf. PLoS ONE 2016, 11, e0163494. [Google Scholar]
- Taylor, P.D. Bryozoan Paleobiology; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 1118455002. [Google Scholar]
- Harmelin, J.-G.; Rosso, A. On Some “Hemicyclopora” and “Escharella” Species (Bryozoa, Cheilostomatida) from the Atlantic-Mediterranean Region. Re-Examination of Their Generic Status and Description of New Species and a New Genus. Zookeys 2023, in press. [Google Scholar]
- Kidwell, S.M.; Bosence, D.W.J.; Allison, P.A.; Briggs, D.E.G. Taphonomy and Time-Averaging of Marine Shelly Faunas. In Taphonomy: Releasing the Data Locked in the Fossil Record; Allison, P.A., Briggs, D., Eds.; Plenum: New York, NY, USA, 1991; pp. 115–209. [Google Scholar]
- Rosso, A. Popolamenti e Tanatocenosi a Briozoi di Fondi Mobili Circalitorali del Golfo di Noto (Sicilia, Italia). Nat. Sicil. 1996, 20, 189–225. [Google Scholar]
- Nylund, G.M.; Pavia, H. Chemical versus Mechanical Inhibition of Fouling in the Red Alga Dilsea aarnosa. Mar. Ecol. Prog. Ser. 2005, 299, 111–121. [Google Scholar] [CrossRef]
- Rosso, A. Contributo alla Conoscenza di Alcuni Popolamenti, Tanatocenosi e Tafocenosi a Briozoi di Alcuni Fondi Mobili Circalitorali. Ph.D. Thesis, University of Messina, Messina, Italy, 1989. Volume 331. [Google Scholar]
- Mazzella, L.; Scipione, M.B.; Buia, M.C. Spatio-temporal Distribution of Algal and Animal Communities in a Posidonia oceanica Meadow. Mar. Ecol. 1989, 10, 107–129. [Google Scholar] [CrossRef]
- Nesti, U.; Piazzi, L.; Balata, D. Variability in the Structure of Epiphytic Assemblages of the Mediterranean Seagrass Posidonia oceanica in Relation to Depth. Mar. Ecol. 2009, 30, 276–287. [Google Scholar]
- Di Martino, E.; Taylor, P.D. A Brief Review of Seagrass-Associated Bryozoans, Recent and Fossil. Studi Trentini Sci. Nat. 2014, 94, 79–94. [Google Scholar]
- Di Geronimo, I.; Giacobbe, S.; Rosso, A.; Sanfilippo, R. Popolamenti e Tanatocenosi del Banco Apollo (Ustica, Mar Tirreno Meridionale). 1990, pp. 697–729. Available online: https://www.researchgate.net/publication/283302225_Popolamenti_e_tanatocenosi_del_Banco_Apollo_Ustica_Mar_Tirreno_meridionale (accessed on 7 February 2023).
- Harmelin, J.-G. Bryozoan Facies in the Coralligenous Community: Two Assemblages with Contrasting Features at Port-Cros Archipelago (Port-Cros National Park, France, Mediterranean). Sci. Rep. Port-Cros Natl. Park 2017, 31, 105–123. [Google Scholar]
- Giampaoletti, J.; Cardone, F.; Corriero, G.; Gravina, M.F.; Nicoletti, L. Sharing and Distinction in Biodiversity and Ecological Role of Bryozoans in Mediterranean Mesophotic Bioconstructions. Front. Mar. Sci. 2020, 7, 581292. [Google Scholar]
- Pica, D.; Berning, B.; Calicchio, R. Cheilostomatida (Bryozoa) from the Ionian Apulian Coast (Italy) with the Description of New Species. Eur. Zool. J. 2022, 89, 371–422. [Google Scholar]
- Ryland, J.S. Physiology and Ecology of Marine Bryozoans. Adv. Mar. Biol. 1977, 14, 285–443. [Google Scholar]
- Hiscock, K. Water Movement. In The Ecology of Shallow Sublittoral Benthos; Earll, R., Erwin, D.G., Eds.; Sublittoral Ecology; Clarendon Press: Oxford, UK, 1983; pp. 58–96. [Google Scholar]
- Bianchi, C.N.; Morri, C. The Battle Is Not to the Strong: Serpulid Reefs in the Lagoon of Orbetello (Tuscany, Italy). Estuar Coast. Shelf Sci. 2001, 53, 215–220. [Google Scholar]
- Zibrowius, H. Remarques sur Trois Espèces de Serpulidae Acclimatées en Méditerranée: Hydroides Dianthus (Verrill, 1873), Hydroides dirampha Mörch, 1863, et Hydroides elegans (Haswell, 1883). Rapp. Comm. Int. Mer. Médit. 1973, 21, 683–686. [Google Scholar]
- Ben-Eliahu, M.N.; ten Hove, H.A. Serpulidae (Annelida: Polychaeta) from the Suez Canal—From a Lessepsian Migration Perspective (a Monograph). Zootaxa 2011, 2848, 1–147. [Google Scholar] [CrossRef] [Green Version]





| Engineer Species of the Canopy | Margaretta cereoides | Flabellia petiolata | Peyssonnelia rubra | Osmundaria volubilis | Further Fleshy Algae | Total Canopy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonies/Thalli | 87 | 82 + 121 Stems | 54 | 20 | Several, Not Counted | |||||||
| Approximate Available Surface (cm2) | 440 | 576 | 217 | 161 | Not Estimated | |||||||
| L | D | L | D | L | D | L | D | L | D | L | D | |
| Bryozoan number of species | 47 | 9 | 37 | 4 | 19 | 2 | 19 | 1 | 41 | 4 | 63 | 14 |
| Bryozoan total number of colonies | 457 | 83 | 324 | 12 | 81 | 2 | 48 | 1 | 220 | 11 | 1132 | 103 |
| Bryozoan colonies/cm2 | 1.02 | 0.18 | 0.56 | 0.02 | 0.37 | 0.01 | 0.30 | 0.01 | NA | NA | NA | NA |
| Bryozoan relative species percentages (%) | 74.60 | 14.29 | 58.73 | 6.35 | 30.16 | 3.17 | 30.16 | 1.59 | 65.08 | 6.35 | 100 | 100 |
| Bryozoan relative colony percentages (%) | 40.37 | 80.58 | 28.62 | 11.65 | 7.16 | 1.94 | 4.24 | 0.97 | 19.43 | 10.68 | 100 | 100 |
| Serpulid number of species | 11 | 9 | 8 | 3 | 3 | 3 | 5 | 4 | 12 | 9 | ||
| Serpulid total number of specimens | 158 | 161 | 139 | 3 | 19 | 4 | 58 | 7 | 378 | 169 | ||
| Serpulid specimens/cm2 | 0.36 | 0.37 | 0.24 | 0.01 | 0.09 | 0.02 | NA | NA | NA | NA | ||
| Serpulid relative species percentages (%) | 91.67 | 100 | 66.67 | 33.33 | 25.00 | 25.00 | 41.67 | 44.44 | 100 | 100 | ||
| Serpulid relative specimen percentages (%) | 41.80 | 100 | 36.77 | 1.78 | 5.03 | 1.06 | 15.34 | 4.14 | 100 | 100 | ||
| Engineer Species of the Canopy | Margaretta cereoides | Flabellia petiolata | Peyssonnelia rubra | Osmundaria volubilis | Further Flesy Algae | Total Canopy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epibiont Bryozoan Species | L | D | L | D | L | D | L | D | L | D | L | D |
| Total number of cyclostome species | 10 | 2 | 8 | 2 | 3 | 6 | 9 | 11 | 4 | |||
| Cyclostome species % | 21.3 | 22.2 | 21.6 | 50 | 15.8 | 31.6 | 22 | 17.5 | 30.8 | |||
| Total number of cyclostome colonies | 97 | 4 | 80 | 4 | 10 | 9 | 56 | 251 | 7 | |||
| Cyclostome colonies % | 21.2 | 4.82 | 24.7 | 33.3 | 12.3 | 18.8 | 25.5 | 22.2 | 6.8 | |||
| Total number of ctenostome species | 5 | 2 | 2 | 1 | 3 | 5 | ||||||
| Ctenostome species % | 10.6 | 5.41 | 10.5 | 5.26 | 7.32 | 7.94 | ||||||
| Total number of ctenostome colonies | 38 | 18 | 5 | 1 | 5 | 66 | ||||||
| Ctenostome colonies % | 8.3 | 5.6 | 6.2 | 2.1 | 2.3 | 5.8 | ||||||
| Total number of cheilostome species | 32 | 7 | 26 | 2 | 14 | 2 | 12 | 1 | 29 | 4 | 47 | 9 |
| Cheilostome species % | 68.1 | 77.8 | 70.3 | 50 | 73.7 | 100 | 63.2 | 100 | 70.7 | 100 | 74.6 | 69.2 |
| Total number of cheilostome colonies | 322 | 79 | 226 | 8 | 66 | 2 | 38 | 1 | 159 | 11 | 815 | 96 |
| Cheilostome colonies % | 70.5 | 95.2 | 69.8 | 66.7 | 81.5 | 100 | 79.2 | 100 | 72.3 | 100 | 72.0 | 93.2 |
| Total number of bryozoan species | 47 | 9 | 37 | 4 | 19 | 2 | 19 | 1 | 41 | 4 | 63 | 13 |
| Total number of bryozoan colonies | 457 | 83 | 324 | 12 | 81 | 2 | 48 | 1 | 220 | 11 | 1132 | 103 |
| Engineer Species of the Canopy | Margaretta cereoides | Flabellia petiolata | Peyssonnelia rubra | Osmundaria volubilis | Further Fleshy Algae | Total Canopy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epibiont Bryozoan Species | L | D | L | D | L | D | L | D | L | D | L | D |
| Total number of erect flexible species | 16 | 10 | 1 | 6 | 4 | 11 | 17 | 1 | ||||
| Erect flexible species % | 34 | 27 | 25 | 31.6 | 21.1 | 26.8 | 27 | 7.7 | ||||
| Total number of erect flexible colonies | 133 | 72 | 2 | 21 | 10 | 53 | 289 | 2 | ||||
| Erect flexible colonies % | 29.1 | 22.2 | 16.7 | 25.9 | 20.8 | 24.1 | 25.5 | 1.94 | ||||
| Total number of encrusting species | 27 | 9 | 26 | 3 | 12 | 2 | 12 | 1 | 25 | 4 | 41 | 12 |
| Encrusting species % | 57.4 | 100 | 70.3 | 75 | 63.2 | 100 | 63.2 | 100 | 61 | 100 | 65.1 | 92.3 |
| Total number of encrusting colonies | 285 | 83 | 244 | 10 | 56 | 2 | 33 | 1 | 132 | 11 | 752 | 101 |
| Encrusting colonies % | 62.4 | 100 | 75.3 | 83.3 | 69.1 | 100 | 68.8 | 100 | 60 | 100 | 64.4 | 98.1 |
| Total number of erect rigid species | 4 | 1 | 1 | 3 | 5 | 5 | ||||||
| Erect rigid species % | 8.51 | 2.7 | 5.26 | 15.8 | 12.2 | 7.94 | ||||||
| Total number of erect rigid colonies | 39 | 8 | 4 | 5 | 35 | 91 | ||||||
| Erect rigid colonies % | 8.53 | 2.47 | 4.94 | 10.4 | 15.9 | 8.1 | ||||||
| Diversity (number of species) | 47 | 9 | 37 | 4 | 19 | 2 | 19 | 1 | 41 | 4 | 63 | 13 |
| Total abundance (number of colonies including undetermined ones) | 457 | 83 | 324 | 12 | 81 | 2 | 48 | 1 | 220 | 11 | 1132 | 103 |
| Engineer Species of the Canopy | Margaretta cereoides | Flabellia petiolata | Peyssonnelia rubra | Osmundaria volubilis | Further Fleshy Algae | Total Canopy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epibiont Serpulids | L | D | L | D | L | D | L | D | L | D | L | D |
| Total number of serpuline species | 8 | 7 | 5 | 3 | 3 | 3 | 5 | 4 | 9 | 7 | ||
| Serpuline species % | 72.7 | 77.8 | 62.5 | 100 | 100 | 100 | 100 | 100 | 75 | 77.8 | ||
| Total number of spirorbine species | 3 | 2 | 3 | 3 | 2 | |||||||
| Spirorbid species % | 27.3 | 22.2 | 37.5 | 25 | 22.2 | |||||||
| Total number of species | 11 | 9 | 8 | 3 | 3 | 3 | 5 | 4 | 12 | 9 | ||
| Total number of serpuline specimens | 129 | 87 | 71 | 3 | 19 | 4 | 57 | 7 | 292 | 95 | ||
| Serpuline specimens % | 81.6 | 54.0 | 51.1 | 100 | 100 | 100 | 98.3 | 100 | 77.2 | 56.2 | ||
| Total number of spirorbine specimens | 29 | 74 | 68 | 1 | 86 | 74 | ||||||
| Spirorbine specimens % | 18.4 | 46.0 | 48.9 | 1.72 | 22.8 | 43.8 | ||||||
| Total number of specimens | 158 | 161 | 139 | 3 | 19 | 4 | 58 | 7 | 378 | 169 | ||
| M. cereoides | F. petiolata | P. rubra | O. volubilis | Further Algae | All Canopy | |
|---|---|---|---|---|---|---|
| d | 8.979201569 | 7.234201966 | 4.633102932 | 5.424673103 | 8.568186705 | 10.39512588 |
| J’ | 0.727544638 | 0.844863311 | 0.869820647 | 0.942942333 | 0.828398275 | 0.771925758 |
| H’ | 2.990844237 | 3.216109475 | 2.688652546 | 2.914674783 | 3.223977221 | 3.363055763 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, A.; Donato, G.; Sanfilippo, R.; Serio, D.; Sciuto, F.; D’Alpa, F.; Bracchi, V.A.; Negri, M.P.; Basso, D. The Bryozoan Margaretta cereoides as Habitat-Former in the Coralligenous of Marzamemi (SE Sicily, Mediterranean Sea). J. Mar. Sci. Eng. 2023, 11, 590. https://doi.org/10.3390/jmse11030590
Rosso A, Donato G, Sanfilippo R, Serio D, Sciuto F, D’Alpa F, Bracchi VA, Negri MP, Basso D. The Bryozoan Margaretta cereoides as Habitat-Former in the Coralligenous of Marzamemi (SE Sicily, Mediterranean Sea). Journal of Marine Science and Engineering. 2023; 11(3):590. https://doi.org/10.3390/jmse11030590
Chicago/Turabian StyleRosso, Antonietta, Gemma Donato, Rossana Sanfilippo, Donatella Serio, Francesco Sciuto, Francesco D’Alpa, Valentina Alice Bracchi, Mauro Pietro Negri, and Daniela Basso. 2023. "The Bryozoan Margaretta cereoides as Habitat-Former in the Coralligenous of Marzamemi (SE Sicily, Mediterranean Sea)" Journal of Marine Science and Engineering 11, no. 3: 590. https://doi.org/10.3390/jmse11030590






