Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus licheniformis B3-15 and Their Potential Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacillus licheniformis Strain B3-15 and Exopolymer Production
2.2. Bacterial Pathogens
2.3. Antibacterial Activity of BPS B3-15 and Antiseptic Solution for Contact Lens Care
2.4. Antibiofilm Activity of BPS B3-15
2.4.1. BPS B3-15 Addition to Polystyrene at Different Concentrations
2.4.2. BPS B3-15 Addition to Polystyrene at Different Times
2.4.3. BPS B3-15 Addition to a PVC Medical Device
2.4.4. BPS B3-15 Addition on Contact Lenses (CLs)
2.5. BPS B3-15 Surface-Active Properties and Effects on Polystyrene Surface Adhesion
2.5.1. Surface-Active Properties
2.5.2. Surface Coating Assay
2.5.3. Cell-Surface Charges and Hydrophobicity Properties
3. Results
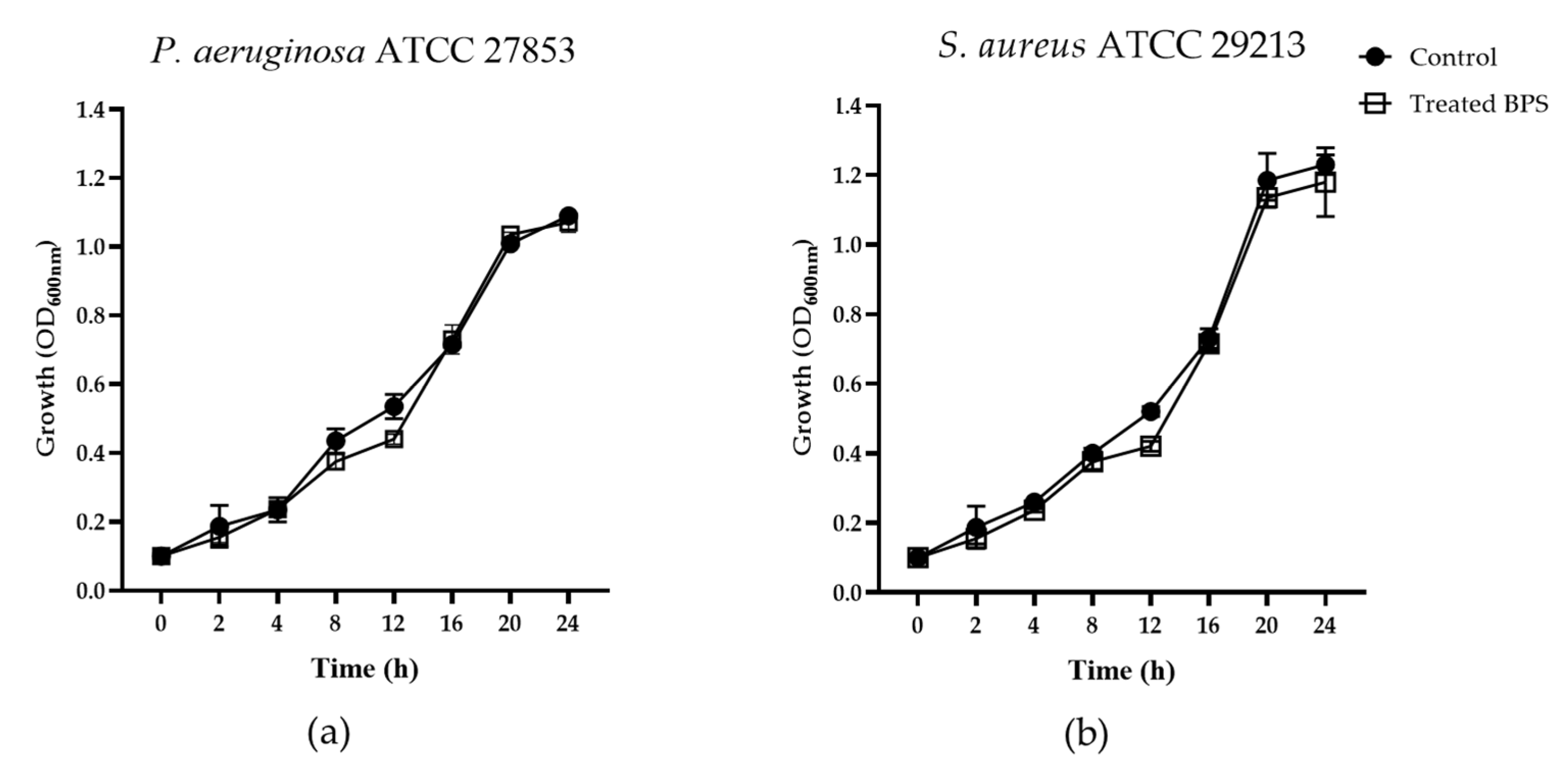
3.1. Antibacterial Activity of BPS B3-15
3.2. Antibiofilm Activity of BPS B3-15
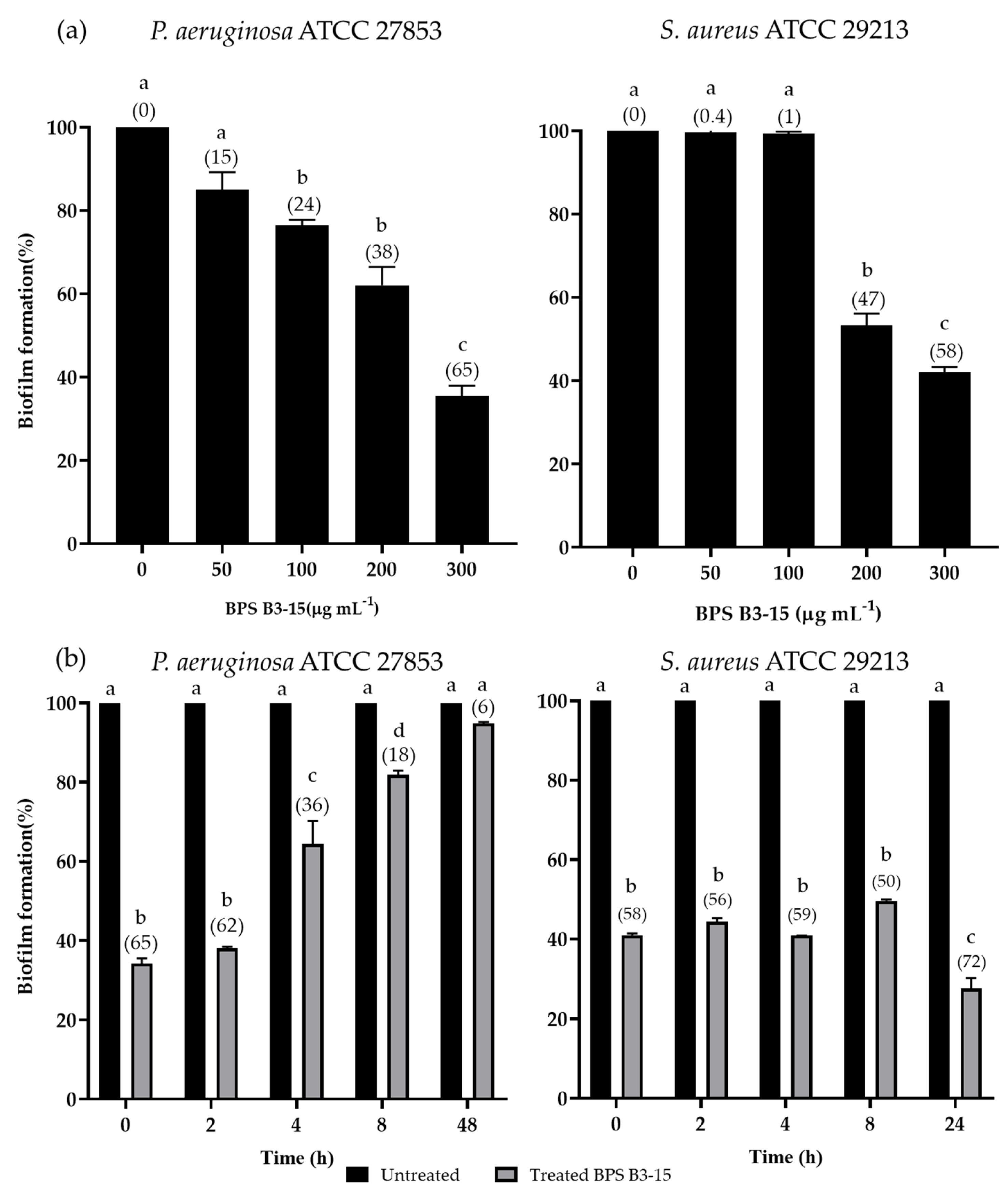
3.2.1. BPS B3-15 Addition on Polystyrene Surfaces at Increasing Concentrations and at Different Times of Bacterial Growth
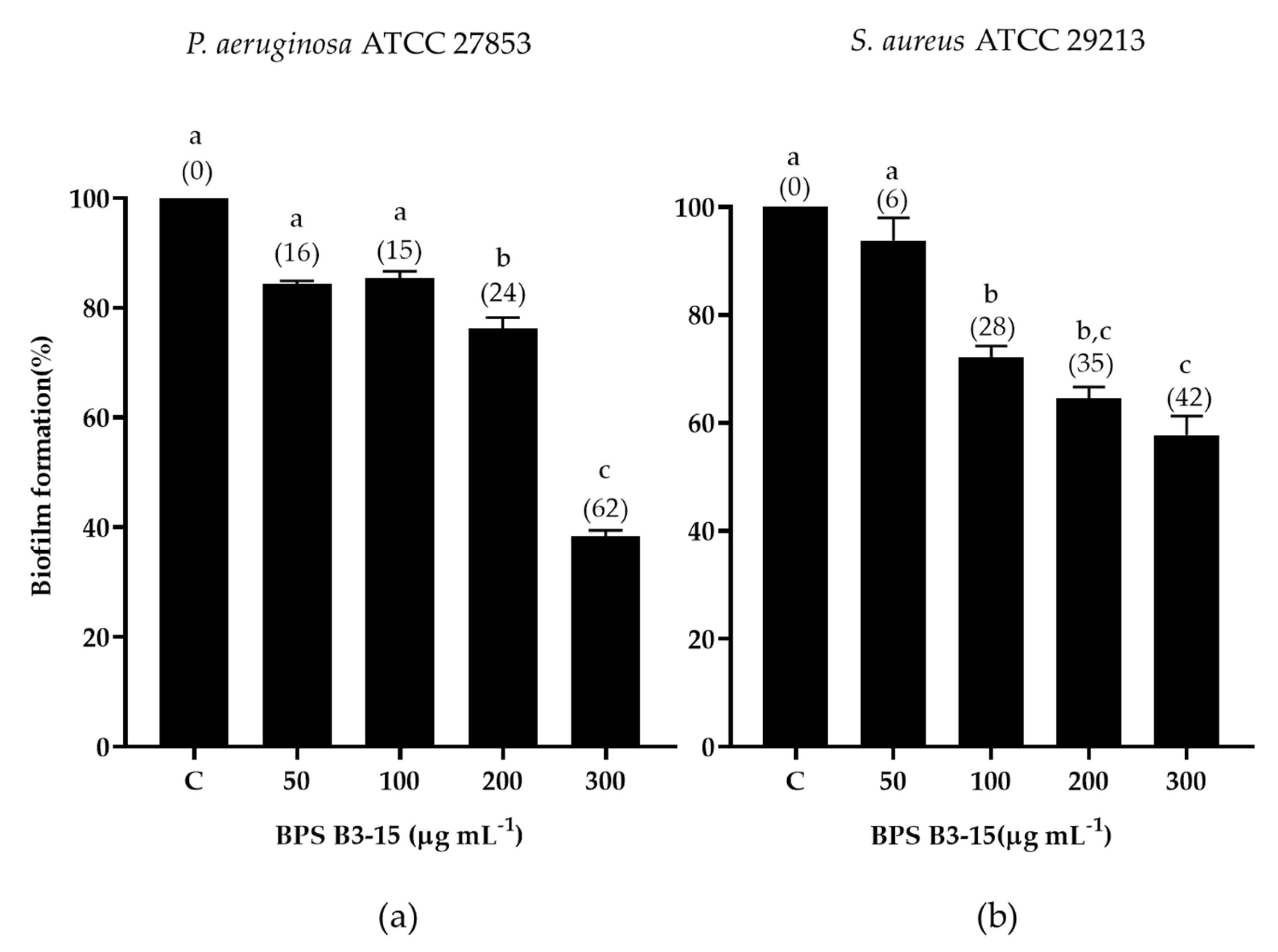
3.2.2. BPS B3-15 Addition to a Polyvinyl Chloride Medical Device
3.3. BPS B3-15 Addition on Contact Lenses (CL) and CL-Care Solution (CS)
3.3.1. Antibacterial Activity of CL-Care Solution (CS)
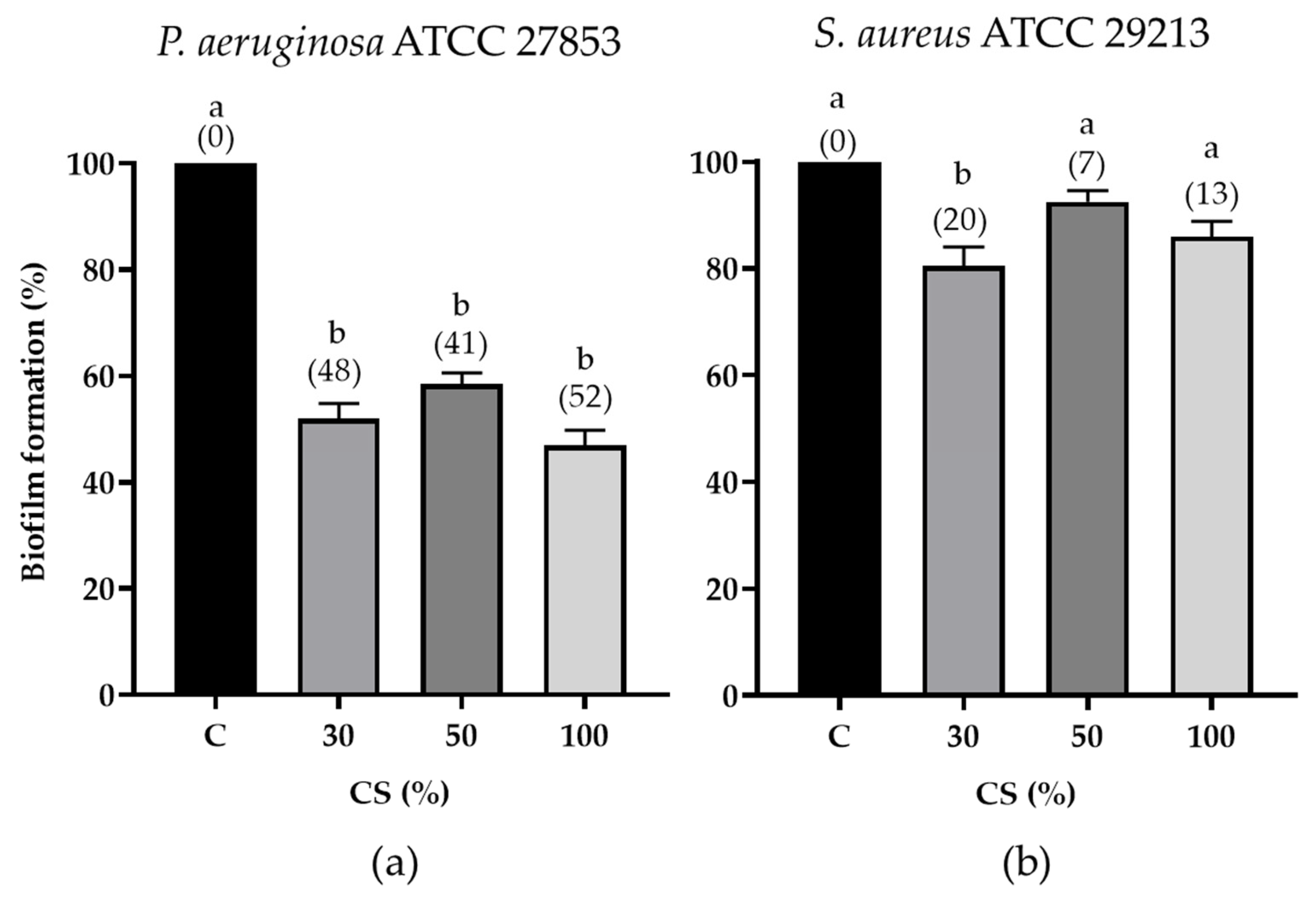
3.3.2. CL-Care Solution Antibiofilm Activity
3.3.3. Antibiofilm Effects of EPS B3-15, BS B3-15, and BPS B3-15 in Combination with CS on Contact Lenses
3.4. BPS B3-15 Surface-Active Properties and Effects on Polystyrene Surface Adhesion
3.4.1. Surface-Active Properties
3.4.2. Coating Assay
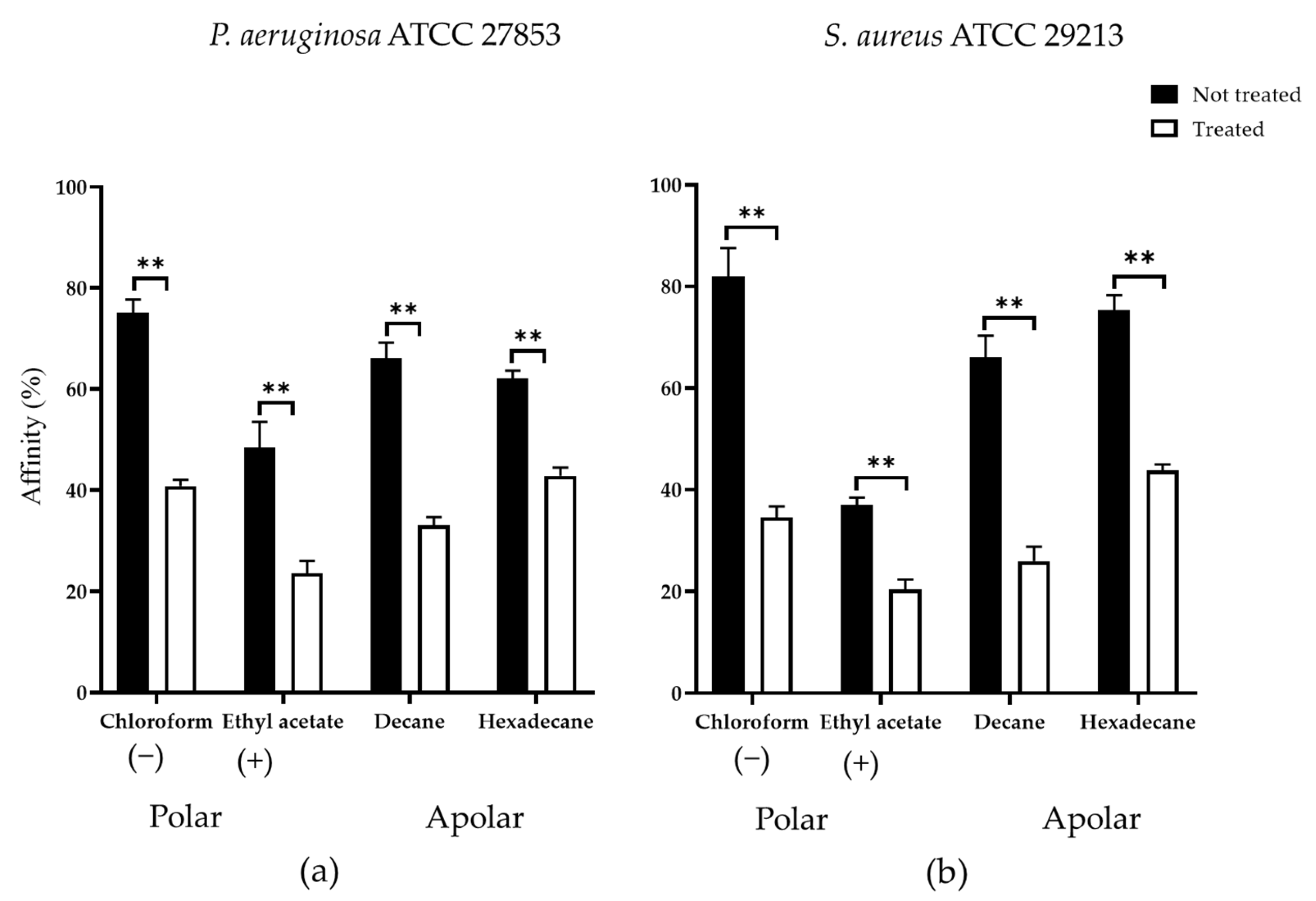
3.4.3. Cell-Surface Charges and Hydrophobicity Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Laganà, P.; Melcarne, L.; Delia, S. Acinetobacter baumannii and endocarditis, rare complication but important clinical relevance. Int. J. Cardiol. 2015, 187, 678–679. [Google Scholar] [CrossRef]
- Zammuto, V.; Rizzo, M.G.; Spanò, A.; Genovese, G.; Morabito, M.; Spagnuolo, D.; Capparucci, F.; Gervasi, C.; Smeriglio, A.; Trombetta, D.; et al. In vitro evaluation of antibiofilm activity of crude extracts from macroalgae against pathogens relevant in aquaculture. Aquaculture 2022, 549, 737729. [Google Scholar] [CrossRef]
- Zammuto, V.; Rizzo, M.G.; Spanò, A.; Spagnuolo, D.; Di Martino, A.; Morabito, M.; Gugliandolo, C. Effects of crude polysaccharides from marine macroalgae on the adhesion and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus. Algal Res. 2022, 63, 102646. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by Bacterial Human Pathogens: Clinical Relevance—Development, Composition and Regulation—Therapeutical Strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Rizzo, C.; Zammuto, V.; Lo Giudice, A.; Rizzo, M.G.; Spanò, A.; Laganà, P.; Martinez, M.; Guglielmino, S.; Gugliandolo, C. Antibiofilm activity of Antarctic sponge-associated bacteria against Pseudomonas aeruginosa and Staphylococcus aureus. J. Mar. Sci. Eng. 2021, 9, 243. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Balebona, M.C.; Moriñigo, M.A.; Borrego, J.J. Hydrophobicity and adhesion to fish cells and mucus of Vibrio strains isolated from infected fish. Int. Microbiol. 2001, 4, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Tuson, H.H.; Weibel, D.B. Bacteria–surface interactions. Soft Matter. 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Bagge, N.; Schuster, M.; Hentzer, M.; Ciofu, O.; Givskov, M.; Greenberg, E.P.; Høiby, N. Pseudomonas aeruginosa Biofilms Exposed to Imipenem Exhibit Changes in Global Gene Expression and β-Lactamase and Alginate Production. Antimicrob. Agents Chemother. 2004, 48, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Kaplan, J.B.; Ghigo, J.M. Antibiofilm polysaccharides. Environ. Microbiol. 2013, 15, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Zammuto, V.; Spanò, A.; Nicolò, M.S.; Grillo, E.; Caccamo, M.T.; Magazù, S.; Cappello, S.; Gugliandolo, C. Thermophilic hydrocarbon-utilizing bacilli from marine shallow hydrothermal vents as producers of biosurfactants. J. Mar. Sci. Eng. 2022, 10, 1077. [Google Scholar] [CrossRef]
- Cruz Mendoza, I.; Villavicencio-Vasquez, M.; Aguayo, P.; Coello Montoya, D.; Plaza, L.; Romero-Peña, M.; Marqués, A.M.; Coronel-León, J. Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing. Microorganisms 2022, 10, 1518. [Google Scholar] [CrossRef]
- Joshi, S.J.; Geetha, S.J.; Desai, A.J. Characterization and application of biosurfactant produced by Bacillus licheniformis R2. Appl. Biochem. Biotechnol. 2015, 177, 346–361. [Google Scholar] [CrossRef]
- Janek, T.; Lukaszewicz, M.; Krasowska, A. Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids Surf. B Biointerfaces 2013, 110, 379–386. [Google Scholar] [CrossRef] [PubMed]
- De França, Í.W.L.; Lima, A.P.; Lemos, J.A.M.; Lemos, C.G.F.; Melo, V.M.M.; de Sant’ana, H.B.; Gonçalves, L.R.B. Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal. Today 2015, 255, 10–15. [Google Scholar] [CrossRef]
- Zammuto, V.; Spanò, A.; Agostino, E.; Macrì, A.; De Pasquale, C.; Ferlazzo, G.; Rizzo, M.G.; Nicolò, M.S.; Guglielmino, S.; Gugliandolo, C. Anti-bacterial adhesion on abiotic and biotic surfaces of the exopolysaccharide from the marine Bacillus licheniformis B3-15. Mar. Drugs 2023, 21, 313. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Rizzo, M.G.; De Pasquale, C.; Ferlazzo, G.; Caccamo, M.T.; Magazù, S.; Guglielmino, S.P.P.; Gugliandolo, C. Lichenysin-like polypeptide production by Bacillus licheniformis B3-15 and Its antiadhesive and antibiofilm properties. Microorganisms 2023, 11, 1842. [Google Scholar] [CrossRef]
- Spanò, A.; Zammuto, V.; Macrì, A.; Agostino, E.; Nicolò, M.S.; Scala, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Caccamo, M.T.; et al. Arsenic adsorption and toxicity reduction of an exopolysaccharide produced by Bacillus licheniformis B3-15 of shallow hydrothermal vent origin. J. Mar. Sci. Eng. 2023, 11, 325. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Panico, A.; Lama, L.; Gambacorta, A.; Nicolaus, B. A halophilic thermotolerant Bacillus isolated from a marine hot spring able to produce a new exopolysaccharide. Biotechnol. Lett. 2002, 24, 515–519. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of Minimum Inhibitory Concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 509–515. [Google Scholar]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar]
- Mordmuang, A.; Udomwech, L.; Karnjana, K. Influence of contact lens materials and cleaning procedures on bacterial adhesion and biofilm formation. Clin. Ophthalmol. 2021, 15, 2391. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacilllus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Daerr, A.; Mogne, A. Pendent_Drop: An ImageJ Plugin to Measure the Surface Tension. J. Open Res. Softw. 2016, 4, 2–6. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Zammuto, V.; Spanò, A.; Gugliandolo, C.; Magazù, S. Hydrating capabilities of the biopolymers produced by the marine thermophilic Bacillus horneckiae SBP3 as evaluated by ATR-FTIR spectroscopy. Materials 2022, 15, 5988. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, M.T.; Kadouri, D.E.; Bendaoud, M.; Izano, E.A.; Sampathkumar, V.; Inzana, T.J.; Kaplan, J. Antibiofilm activity of Actinobacillus pleuropneumoniae serotype 5 capsular polysaccharides. PLoS ONE 2013, 8, e63844. [Google Scholar]
- Bellon-Fontaine, M.N.; Rault, J.; Van Oss, C.J. Microbial adhesion to solvents: A novel method to determine the electrondonor/ electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B Biointerfaces 1996, 7, 47–53. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterization and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef]
- Coronel-León, J.; de Grau, G.; Grau-Campistany, A.; Farfan, M.; Rabanal, F.; Manresa, A.; Marqués, A.M. Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: Production, chemical characterization and properties. Ann. Microbiol. 2015, 65, 2065–2078. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Moryl, M.; Płaza, G.; Bhagat, D.; Satpute, S.K.; Bernat, P. Surfactants of microbial origin as antibiofilm agents. Int. J. Environ. Health Res. 2019, 31, 401–420. [Google Scholar] [CrossRef]
- Quadriya, H.; Ali, S.A.M.; Parameshwar, J.; Manasa, M.; Khan, M.Y.; Hameeda, B. Microbes Living Together: Exploiting the Art for Making Biosurfactants and Biofilms. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Springer: Berlin/Heidelberg, Germany, 2018; pp. 161–177. [Google Scholar]
- Katsikogianni, M.; Missirlis, Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to combat medical device-associated biofilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Di Onofrio, V.; Gesuele, R.; Maione, A.; Liguori, G.; Liguori, R.; Guida, M.; Nigro, R.; Galdiero, E. Prevention of Pseudomonas aeruginosa biofilm formation on soft contact lenses by Allium sativum fermented extract (BGE) and Cannabinol oil extract (CBD). Antibiotics 2019, 8, 258. [Google Scholar] [CrossRef]
- Kilvington, S.; Huang, L.; Kao, E.; Powell, C.H. Development of a new contact lens multipurpose solution: Comparative analysis of microbiological, biological, and clinical performance. J. Optom. 2010, 13, 134–142. [Google Scholar] [CrossRef]
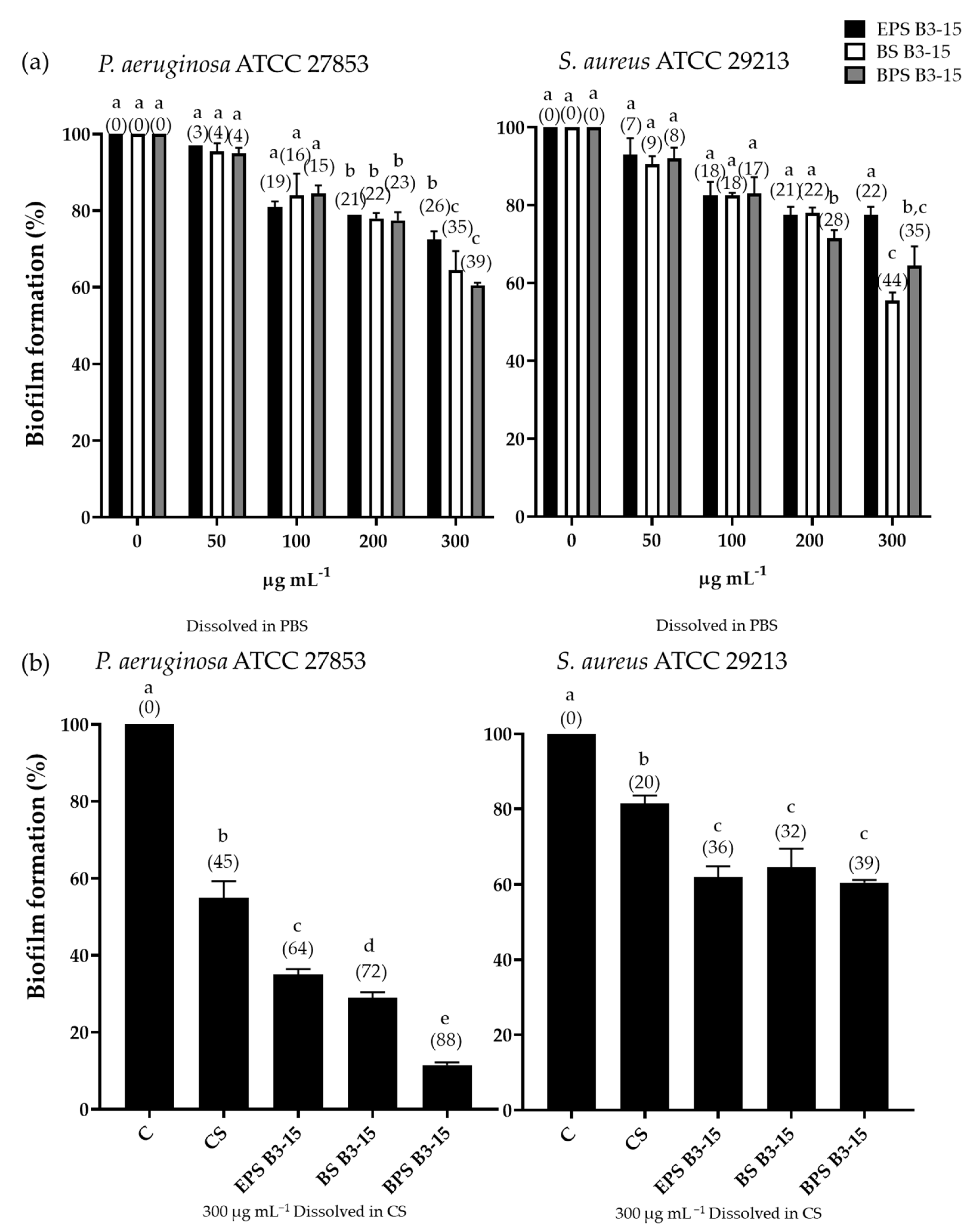


| CL-Care Solution (%) | Strain | Bacterial Biomass (OD600 nm) | Viable Cells (CFU mL−1) |
|---|---|---|---|
| 0 | P. aeruginosa | 1.23 | 5.05 × 108 |
| S. aureus | 1.27 | 9.60 × 108 | |
| 10 | P. aeruginosa | 0.97 | 4.13 × 108 |
| S. aureus | 1.17 | 8.79 × 108 | |
| 20 | P. aeruginosa | 0.77 | 3.24 × 108 |
| S. aureus | 0.54 | 3.95 × 108 | |
| 30 | P. aeruginosa | 0.60 | 2.50 × 108 |
| S. aureus | 0.40 | 3.00 × 108 | |
| 50 | P. aeruginosa | 0.07 | 1.00 × 104 |
| S. aureus | 0.06 | 3.00 × 103 | |
| 100 | P. aeruginosa | 0.07 | 3.50 × 102 |
| S. aureus | 0.06 | 2.00 × 102 |
| Antibiofilm Activity (%) | |||
|---|---|---|---|
| P. aeruginosa | S. aureus | ||
| Polystyrene | EPS B3-15 | 51 | 52 |
| BS B3-15 | 47 | 36 | |
| BPS B3-15 | 65 | 58 | |
| PVC | EPS B3-15 | 53 | 32 |
| BS B3-15 | 48 | 30 | |
| BPS B3-15 | 62 | 42 | |
| Contact lenses | EPS B3-15 | 26 | 22 |
| BS B3-15 | 35 | 44 | |
| BPS B3-15 | 39 | 35 | |
| CS | 48 | 20 | |
| EPS B3-15 + CS | 72 | 36 | |
| BS B3-15 + CS | 64 | 32 | |
| BPS B3-15 + CS | 88 | 39 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammuto, V.; Agostino, E.; Macrì, A.; Spanò, A.; Grillo, E.; Nicolò, M.S.; Gugliandolo, C. Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus licheniformis B3-15 and Their Potential Medical Applications. J. Mar. Sci. Eng. 2023, 11, 1660. https://doi.org/10.3390/jmse11091660
Zammuto V, Agostino E, Macrì A, Spanò A, Grillo E, Nicolò MS, Gugliandolo C. Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus licheniformis B3-15 and Their Potential Medical Applications. Journal of Marine Science and Engineering. 2023; 11(9):1660. https://doi.org/10.3390/jmse11091660
Chicago/Turabian StyleZammuto, Vincenzo, Eleonora Agostino, Angela Macrì, Antonio Spanò, Emanuela Grillo, Marco Sebastiano Nicolò, and Concetta Gugliandolo. 2023. "Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus licheniformis B3-15 and Their Potential Medical Applications" Journal of Marine Science and Engineering 11, no. 9: 1660. https://doi.org/10.3390/jmse11091660
APA StyleZammuto, V., Agostino, E., Macrì, A., Spanò, A., Grillo, E., Nicolò, M. S., & Gugliandolo, C. (2023). Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus licheniformis B3-15 and Their Potential Medical Applications. Journal of Marine Science and Engineering, 11(9), 1660. https://doi.org/10.3390/jmse11091660








