Research Progress on Preparation of Superhydrophobic Surface and Its Application in the Field of Marine Engineering
Abstract
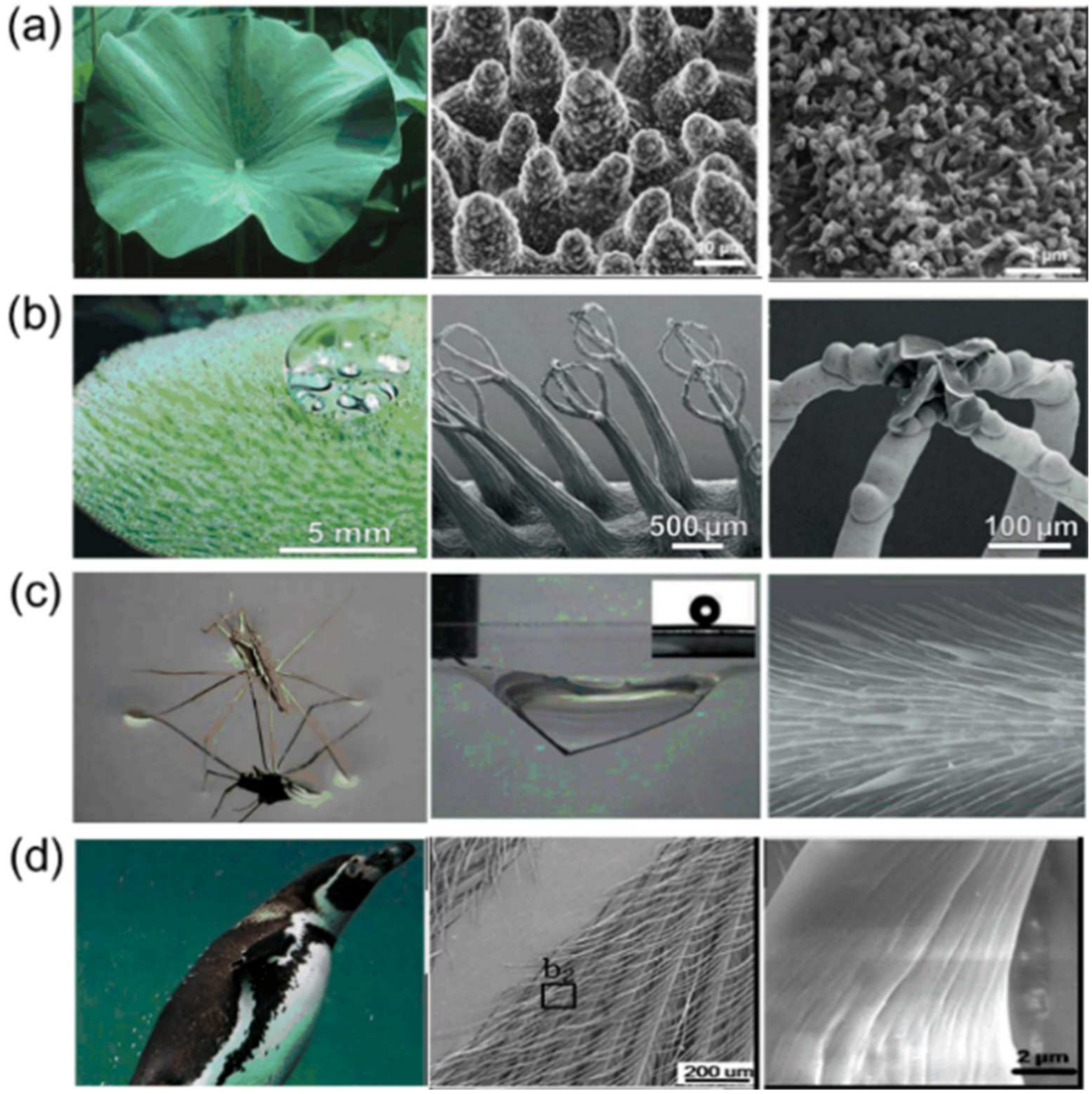
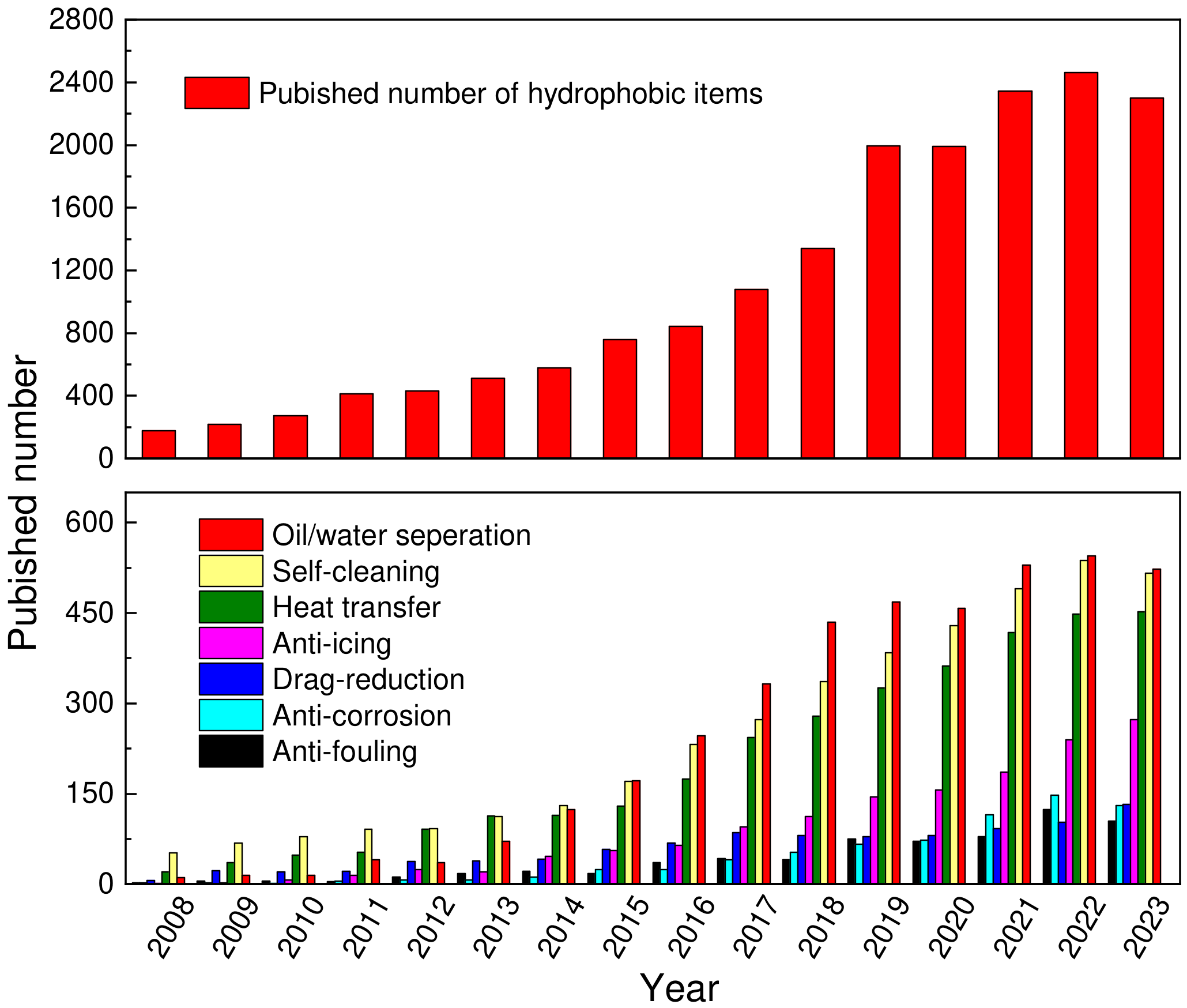
:1. Introduction
2. Basic Wetting Theories of Superhydrophobic Surface
3. Preparation Methods of Superhydrophobic Surfaces
3.1. Template Method
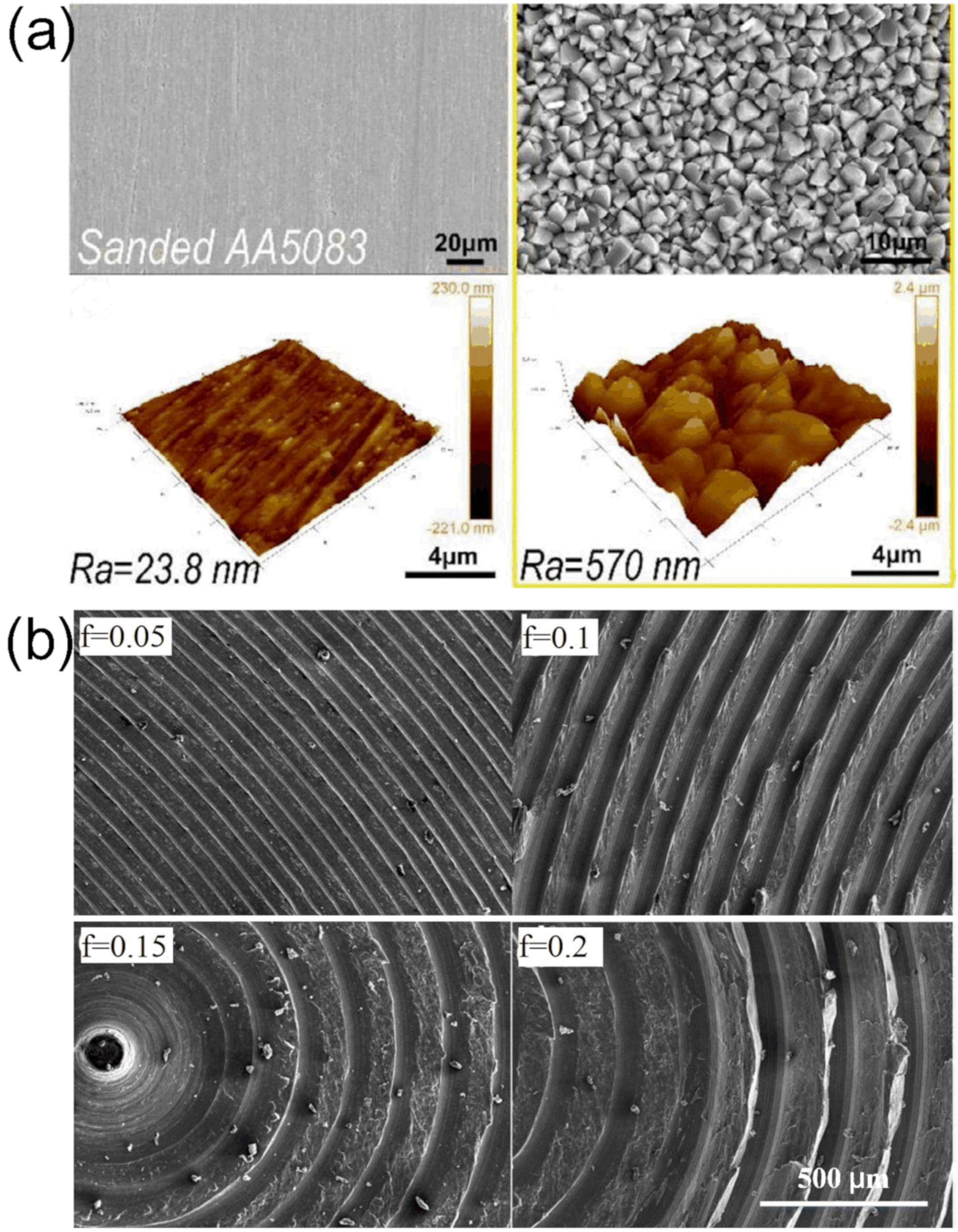
3.2. Etching Method
3.3. Electrochemical Method
3.4. Spraying Method
3.5. Other Methods
4. Application of Superhydrophobic Surface in Marine Engineering
4.1. Application in Self-Cleaning
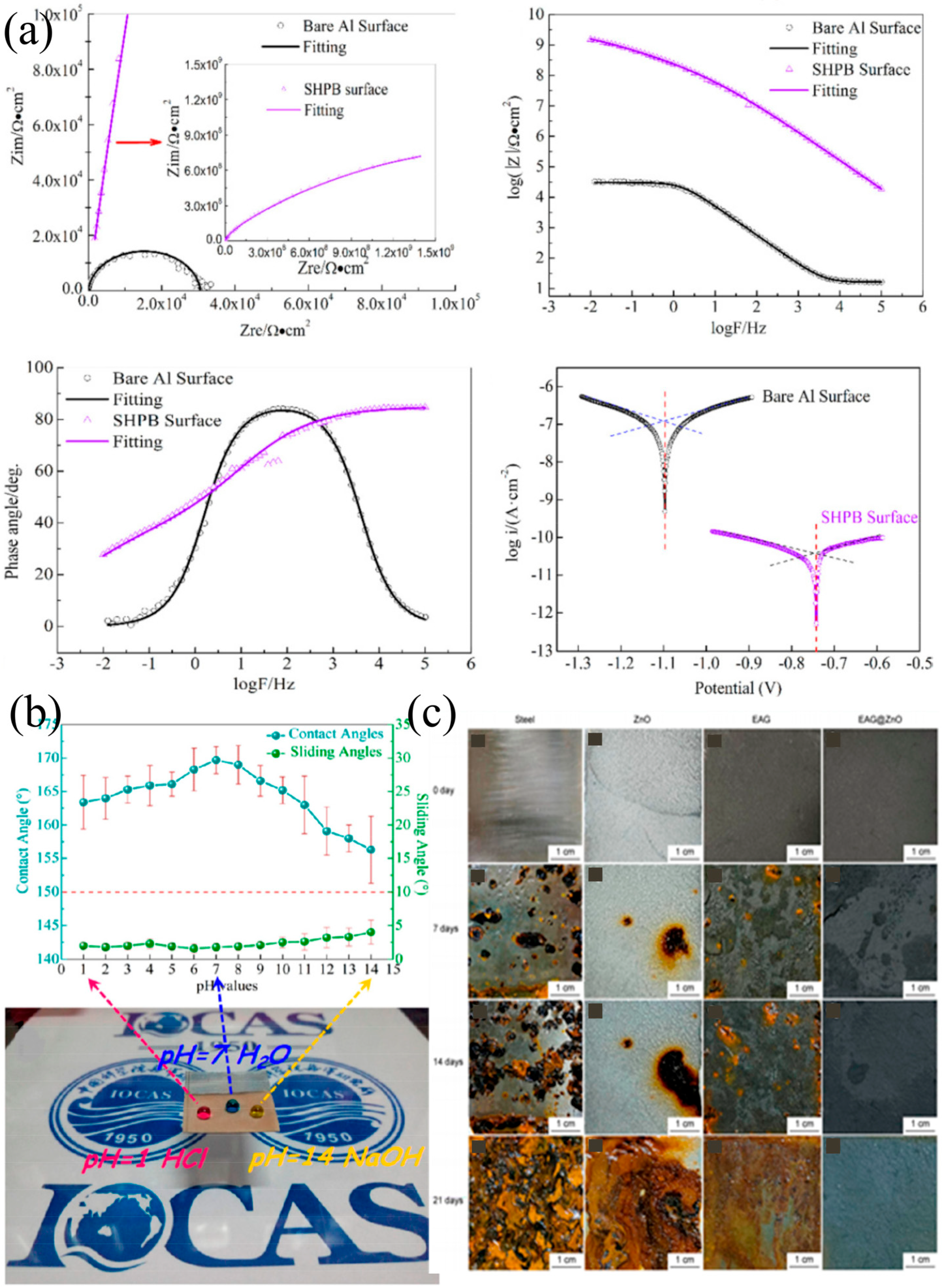
4.2. Application in Anti-Corrosion
4.3. Application in Heat Transfer
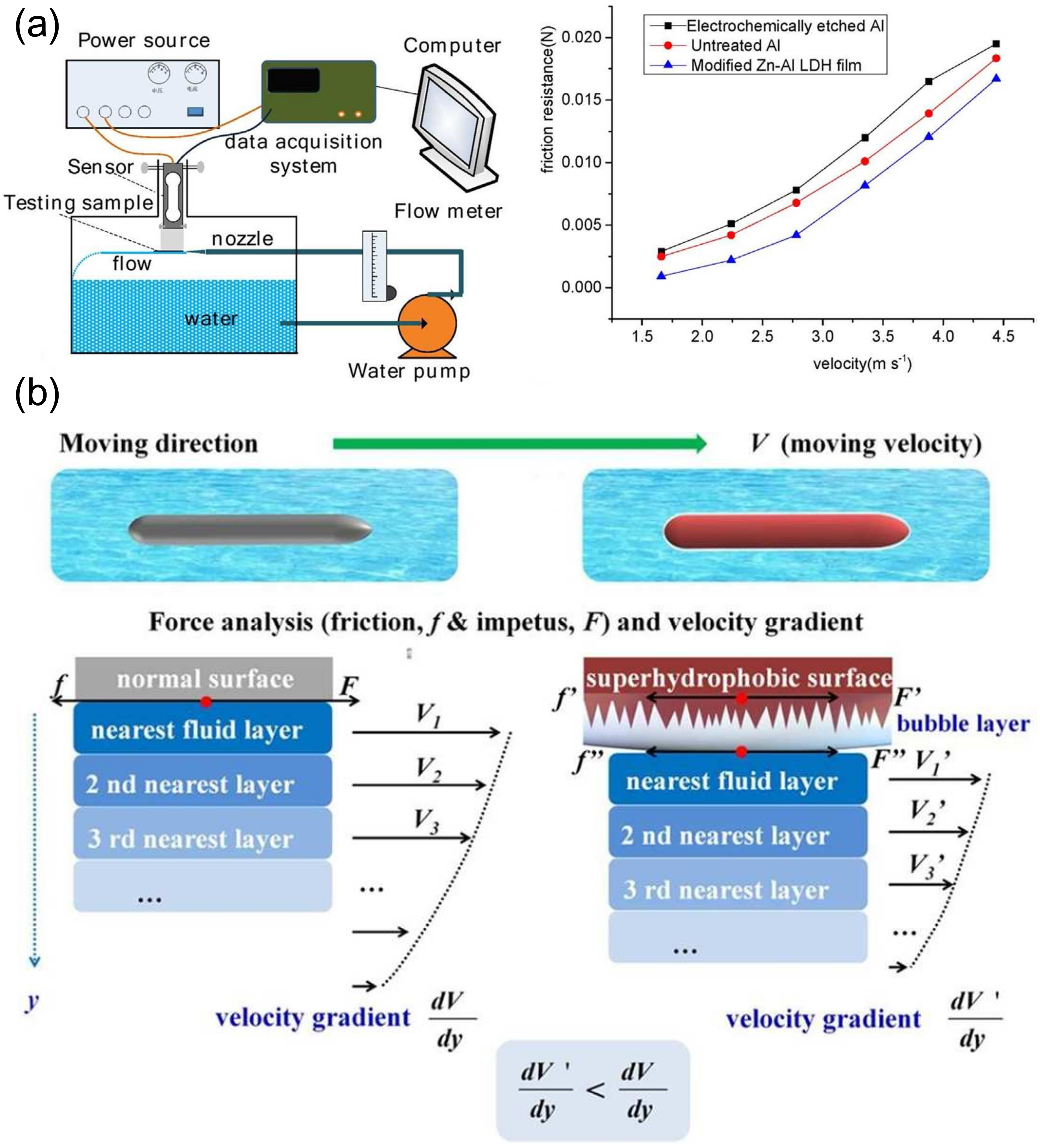
4.4. Application in Fluid Drag Reduction
4.5. Application in Anti-Fouling
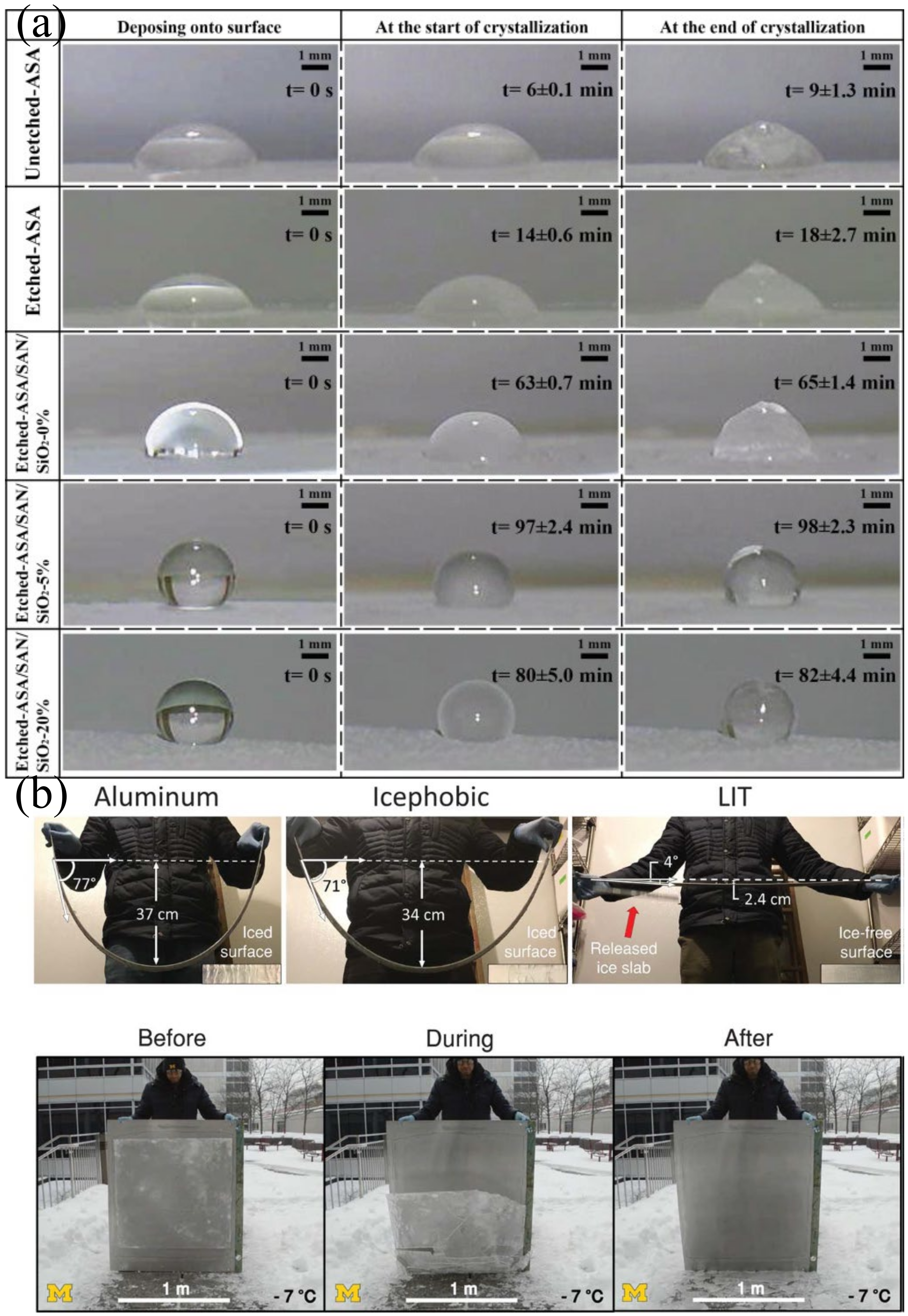
4.6. Application in Anti-Icing
4.7. Application in Oil/Water Separation
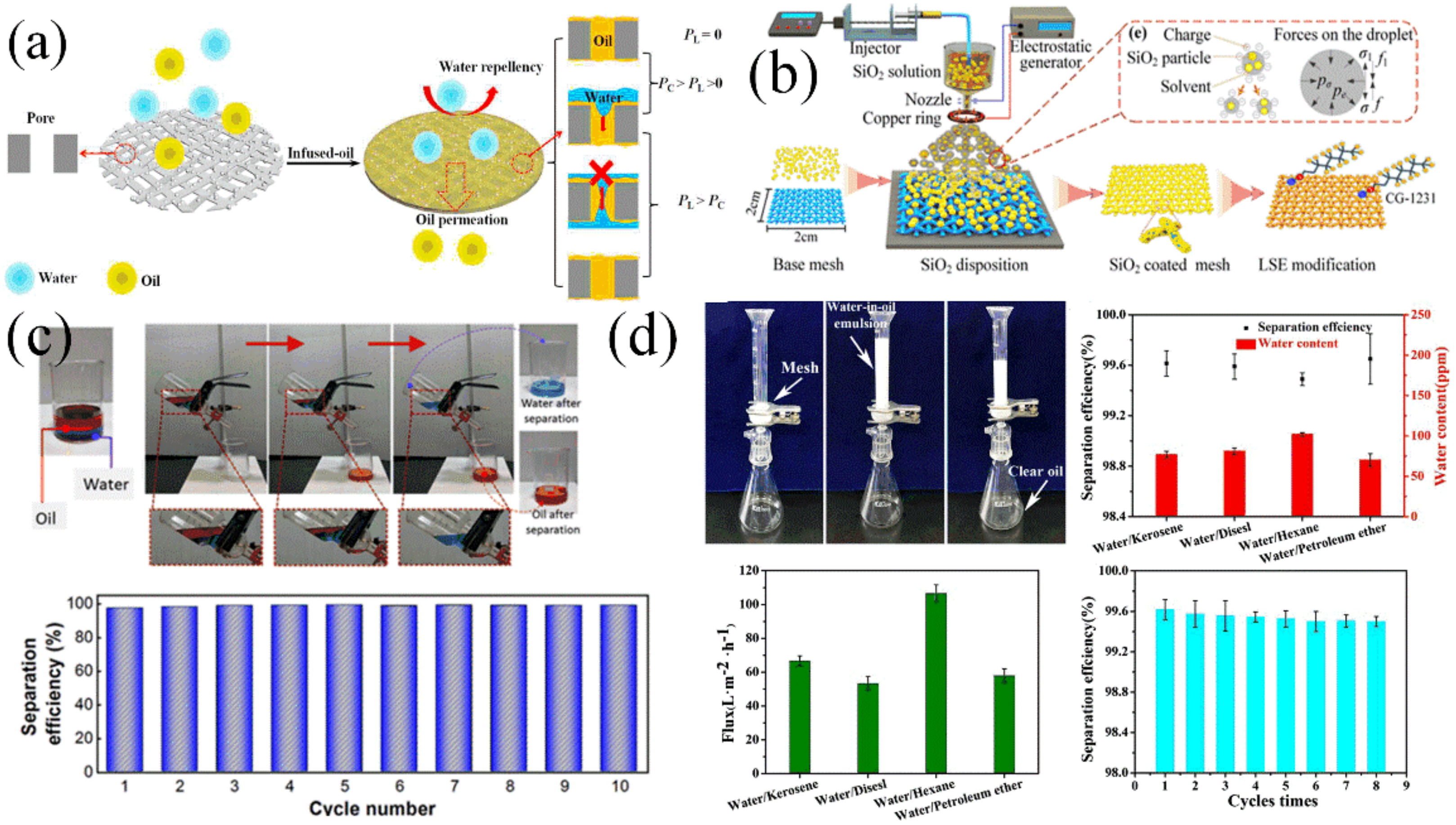
5. Future Challenges and Strategies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, T. An essay on the cohesion of fliuds. Philos. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Kock-Yee, L. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity, getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar]
- Rather, A.M.; Shome, A.; Bhunia, B.K.; Panuganti, A.; Mandal, B.B.; Manna, U. Correction: Simultaneous and controlled release of two different bioactive small molecules from nature inspired single material. J. Mater. Chem. B 2019, 7, 346. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A.; Cirisano, F. Superhydrophobic Coatings from Recyclable Materials for Protection in a Real Sea Environment. Coatings 2019, 9, 303. [Google Scholar] [CrossRef]
- Selim, M.S.; Hamouda, H.I.; Fatthallah, N.A.; Mostafae, M.S.; Higazy, S.A.; Shabana, S.; EL-Saeed, A.M.; Hao, Z. Advanced Bioinspired Superhydrophobic Marine Antifouling Coatings. Superhydrophobic Coating-Recent Advances in Theory and Applications; IntechOpen: London, UK, 2023. [Google Scholar]
- Cho, E.; Chang-Jian, C.; Chen, H.; Chuang, K.; Zheng, J.; Hsiao, Y.; Lee, K.; Huang, J. Robust Multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion, and anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Hsu, C.; Nazari, M.H.; Li, Q.; Shi, X. Enhancing degradation and corrosion resistance of AZ31 magnesium alloy through hydrophobic coating. Mater. Chem. Phys. 2019, 225, 426–432. [Google Scholar] [CrossRef]
- Truesdell, R.; Mammoli, A.; Vorobieff, P.; van Swol, F.; Brinker, C.J. Drag reduction on a patterned superhydrophobic surface. Phys. Rev. Lett. 2006, 4, 97. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Rather, M.; Jana, N.; Hazarika, P.; Manna, U. Sustainable polymeric material for the facile and repetitive removal of oil-spills through the complementary use of both selective-absorption and active-filtration processes. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 23339–23348. [Google Scholar] [CrossRef]
- Rather, M.; Manna, U. Stretchable and durable superhydrophobicity that acts both in air and under oil. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 15208–15216. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Barthlott, W.; Schimmel, T.; Wiersch, S.; Koch, K.; Brede, M.; Barczewski, M.; Walheim, S.; Weis, A.; Kaltenmaier, A.; Leder, A.; et al. The salvinia paradox, superhydrophobic surfaces with hydrophilic pins for air retention under water. Adv. Mater. 2010, 22, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 7013–7036. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Guo, G.; Wang, J.; Wu, J.; Yang, S.; Jiang, L. Icephobicity of penguins spheniscus humboldti and an artificial replica of penguin feather with air-infused hierarchical rough structures. J. Phys. Chem. C 2016, 120, 15923–15929. [Google Scholar] [CrossRef]
- Chen, H.; Ran, T.; Gan, Y.; Zhou, J.; Zhang, Y.; Zhang, L.; Zhang, D.; Jiang, L. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater. 2018, 17, 935–942. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Fluid drag reduction with shark-skin riblet inspired microstructured surfaces. Adv. Funct. Mater. 2013, 23, 4507–4528. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.; Xu, X.; Green, L. Precise cutting microstructured superhydrophobic surface. Surf. Eng. 2016, 32, 119–124. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Zhu, Q.; Wang, X.; Zhao, X.; Sun, C.; Li, Y.; Hou, B. Controllable dianthus caryophyllus-like superhydro-philic/superhydrophobic hierarchical structure based on self-congregated nanowires for corrosion inhibition and bio-fouling mitigation. Chem. Eng. J. 2017, 312, 317–327. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, J.; Lee, C.; Bae, K.; Sohn, S.; Kim, H. Optimization of super-hydrophobic property by two-step surface modification. J. Nanosci. Nanotechnol. 2019, 19, 7192–7197. [Google Scholar] [CrossRef]
- Peng, S.; Deng, W. A simple method to prepare superamphiphobic aluminum surface with excellent stability. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 143–150. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Li, Z.; Qin, N.; Mo, J.; Pan, Y.; Luo, D. Robust superhydrophobic aluminum alloy surfaces with anti-icing ability, thermostability, and mechanical durability. Prog. Org. Coat. 2020, 147, 105745. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Ji, Y.; Zhang, J. Fabrication of robust ceramic based superhydrophobic coating on aluminum substrate via plasma electrolytic oxidation and chemical vapor deposition methods. J. Mater. Process. Technol. 2022, 306, 117641. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, Z.; Xu, K.; Zhu, H.; Liu, Y.; Wu, Y.; Yang, S. Reproducible PDMS flexible superhydrophobic films: A method utilizing picosecond laser-etched templates. Prog. Org. Coat. 2024, 189, 108344. [Google Scholar] [CrossRef]
- Chen, T.L.; Huang, C.Y.; Xie, Y.T.; Chiang, Y.Y.; Chen, Y.M.; Hsueh, H.Y. Bioinspired Durable Superhydrophobic Surface from Hierarchically Wrinkled Nanoporous Polymer. ACS Appl. Mater. Interfaces 2019, 11, 40875–40885. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Vanithakumari, S.C.; Krishna, D.N.G.; George, R.P.; Srinivasan, R.; Philip, J. Facile fabrication of robust superhydrophobic aluminum surfaces with enhanced corrosion protection and antifouling properties. Prog. Org. Coat. 2022, 162, 106560. [Google Scholar] [CrossRef]
- ECHA (European Chemical Agency). Candidate List of Substances of Very High Concern for Authorisation. 2013. Available online: http://echa.europa.eu/web/guest/candidate-list-table (accessed on 1 May 2014).
- Wenzel, R.N. Resistance of solid surface to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Vu, H.H.; Nguyen, N.T.; Kashaninejad, N. Re-Entrant Microstructures for Robust Liquid Repellent Surfaces. Adv. Mater. Technol. 2023, 8, 2201836. [Google Scholar] [CrossRef]
- Milne, A.J.B.; Amirfazli, A. The Cassie equation: How it is meant to be used. Adv. Colloid Interface Sci. 2012, 170, 48–55. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.Y.; Zhang, D. Recent advances in chemical durability and mechanical stability of superhydrophobic materials: Multi-strategy design and strengthening. J. Mater. Sci. Technol. 2022, 129, 40–69. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, Y.B.; He, Q. Durable and robust superhydrophobic fluororubber surface fabricated by template method with exceptional thermostability and mechanical stability. Sep. Purif. Technol. 2023, 306, 122423. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Momen, G.; Jafari, R.; Farzaneh, M. Direct replication of micro-nanostructures in the fabrication of superhydrophobic silicone rubber surfaces by compression molding. Appl. Surf. Sci. 2018, 458, 619–628. [Google Scholar] [CrossRef]
- Yun, X.; Xiong, Z.; He, Y.; Wang, X. Superhydrophobic lotus-leaf-like surface made from reduced graphene oxide through soft-lithographic duplication. RSC Adv. 2020, 10, 5478–5486. [Google Scholar] [CrossRef]
- Peng, P.; Ke, Q.; Zhou, G.; Tang, T. Fabrication of microcavity-array superhydrophobic surfaces using an improved template method. J. Colloid Interface Sci. 2013, 395, 326–328. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Wang, T.; Xu, J.; Ke, Q. Significantly improving strength and oil-adsorption performance of regular porous polydimethylsiloxane via soft template approach. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 10–17. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Matti, J.H.; Zhang, C.; Lin, F.; Liu, Q.; Zhu, S.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–66. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Facile fabrication of superhydrophobic surfaces from austenitic stainless steel (AISI 304) by chemical etching. Appl. Surf. Sci. 2018, 439, 598–604. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, X. A novel and facile fabrication of superhydrophobic surfaces on copper substrate via machined operation. Mater. Lett. 2017, 190, 115–118. [Google Scholar] [CrossRef]
- Pou, P.; Del Val, J.; Riveiro, A.; Comesaña, R.; Arias-González, F.; Lusquiños, F.; Bountinguiza, M.; Quintero, F.; Pou, J. Laser texturing of stainless steel under different processing atmospheres, from superhydrophilic to superhydrophobic surfaces. Appl. Surf. Sci. 2019, 475, 896–905. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Gogolides, E. Atmospheric plasma etching of polymers, a palette of applications in cleaning/ashing, pattern formation, nanotexturing and superhydrophobic surface fabrication. Microelectron. Eng. 2018, 194, 109–115. [Google Scholar] [CrossRef]
- Zhang, B.; Guan, F.; Zhao, X.; Zhang, Y.; Li, Y.; Duan, J.; Hou, B. Micro-nano textured superhydrophobic 5083 aluminum alloy as a barrier against marine corrosion and sulfate-reducing bacteria adhesion. J. Taiwan Inst. Chem. Eng. 2019, 97, 433–440. [Google Scholar] [CrossRef]
- Zhu, J. A novel fabrication of superhydrophobic surfaces on aluminum substrate. Appl. Surf. Sci. 2018, 447, 363–367. [Google Scholar] [CrossRef]
- Han, J.; Wang, Z.; Zhi, A.; Li, Y.; Zhao, S.; Yan, H.; Han, Q. A smart electroplating approach to fabricate mechanically robust and fluorine-free Ni-W alloys based superhydrophobic coating on Al alloy. Vacuum 2023, 217, 112501. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Q.; Li, Y. Facile fluorine-free one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chem. Eng. J. 2018, 352, 625–633. [Google Scholar] [CrossRef]
- Li, S.; Xiang, X.; Ma, B.; Meng, X. Facile preparation of diverse alumina surface structures by anodization and superhydrophobic surfaces with tunable water droplet adhesion. J. Alloys Compd. 2019, 779, 219–228. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, F.; Song, L.; Zeng, R.C.; Li, S.Q.; Han, E.H. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Cui, L.; Liu, H.; Zhang, W.; Han, Z.; Deng, M.; Zeng, R.; Li, S.; Wang, Z. Corrosion resistance of a superhydrophobic micro-arc oxidation coating on Mg-4Li-1Ca alloy. J. Mater. Sci. Technol. 2017, 33, 1263–1271. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Zou, Y.; Jia, D.; Zhou, Y. A fractal-patterned coating on titanium alloy for stable passive heat dissipation and robust superhydrophobicity. Chem. Eng. J. 2019, 374, 231–241. [Google Scholar] [CrossRef]
- Wang, H.; Di, D.; Zhao, Y.; Yuan, R.; Zhu, Y. A multifunctional polymer composite coating assisted with pore-forming agent, preparation, superhydrophobicity and corrosion resistance. Prog. Org. Coat. 2019, 132, 370–378. [Google Scholar] [CrossRef]
- Xie, Y.; Xiong, W.; Kareem, S.; Qiu, C.; Hu, Y.; Parkin, I.P.; Wang, S.; Wang, H.; Chen, J.; Li, L.; et al. Robust superamphiphobic coatings with gradient and hierarchical architecture and excellent anti-flashover performances. Nano Res. 2022, 15, 7565–7576. [Google Scholar] [CrossRef]
- Xin, L.; Li, H.; Gao, J.; Wang, Z.; Zhou, K.; Yu, S. Large-scale fabrication of decoupling coatings with promising robustness and superhydrophobicity for antifouling, drag reduction, and organic photodegradation. Friction 2023, 11, 716–736. [Google Scholar] [CrossRef]
- Li, Z.; Cao, M.; Li, P.; Zhao, Y.; Bai, H.; Wu, Y.; Jiang, L. Surface-embedding of functional micro-nanoparticles for achieving versatile superhydrophobic interfaces. Matter 2019, 1, 661–673. [Google Scholar] [CrossRef]
- Raimondo, M.; Veronesi, F.; Boveri, G.; Motta, A.; Zanoni, R. Superhydrophobic properties induced by sol-gel routes on copper surfaces. Appl. Surf. Sci. 2017, 422, 1022–1029. [Google Scholar] [CrossRef]
- Cui, M.; Xu, C.; Shen, Y.; Tian, H.; Feng, H.; Li, J. Electrospinning superhydrophobic nanofibrous poly(vinylidene fluoride)/stearic acid coatings with excellent corrosion resistance. Thin Solid Film. 2018, 657, 88–94. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, S.; Huang, T.Y.; Dong, A.; Duan, H. Superrepellency of underwater hierarchical structures on salvinia leaf. Proc. Natl. Acad. Sci. USA 2020, 117, 2282–2287. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, F.; He, Q.; Dai, F. Preparation of a superhydrophobic surface by a one-step powder pressing method with liquid silicone rubber as the carrier. ACS Omega 2023, 8, 8548–8556. [Google Scholar] [CrossRef]
- Jang, N.S.; Ha, S.H.; Kim, K.H.; Kim, J.M. Facile one-step photopatterning of hierarchical polymer structures for highly transparent, flexible superhydrophobic films. Prog. Org. Coat. 2019, 130, 24–30. [Google Scholar] [CrossRef]
- Rather, M.; Manna, U. Facile Synthesis of Tunable and Durable Bulk Superhydrophobic Material from Amine “Reactive” Polymeric Gel. Chem. Mater. 2016, 28, 8689–8869. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier, a review. Surf. Coat. Technol. 2019, 375, 100–111. [Google Scholar] [CrossRef]
- Qian, S.; Chen, Q.; Liu, J.; Hu, C.; Hu, Y.; Yang, L.; Zhou, X.; Hong, Y. Facile fabrication of environmentally friendly bio-based superhydrophobic surfaces via UV-polymerization for self-cleaning and high efficient oil/water separation. Prog. Org. Coat. Int. Rev. J. 2019, 137, 105346. [Google Scholar]
- Kumar, A.; Gogoi, B. Development of durable self-cleaning superhydrophobic coatings for aluminium surfaces via chemical etching method. Tribol. Int. 2018, 122, 114–118. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Q.; Fang, Y.; Zhang, J.; Yong, J.; Hou, X.; Chen, F. Superhydrophobicity-memory surfaces prepared by a femtosecond laser. Chem. Eng. J. 2019, 383, 123143. [Google Scholar] [CrossRef]
- Wang, L.; Cui, P.; Bi, Z.; Wang, C.; Zhou, B.; Zheng, L.; Niu, H.; Wang, D.; Li, Q. Superhydrophobic ultra-high molecular weight polyethylene porous material with self-cleaning ability, long-term stability, and high durability. Surf. Coat. Technol. 2022, 446, 128792. [Google Scholar] [CrossRef]
- Wang, C.; Tian, F.; Zhang, X. Feasible fabrication of durable superhydrophobic SiO2 coatings with translucency and self-cleaning performance. Mater. Res. Express 2020, 7, 106403. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Wang, J.; Zhang, H.; Zhang, J.; Ji, Y. Fabrication of fluorine-free superhydrophobic surface on aluminum substrate for corrosion protection and drag reduction. J. Mar. Sci. Eng. 2023, 11, 520. [Google Scholar] [CrossRef]
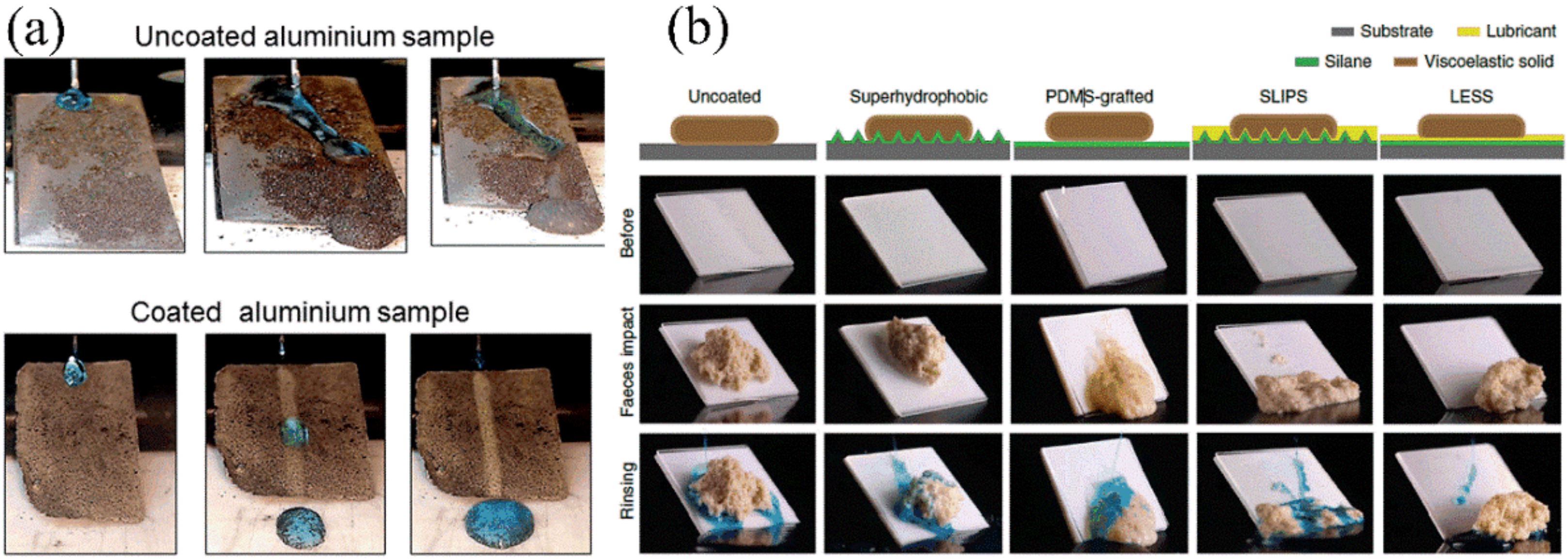
- Wang, J.; Wang, L.; Sun, N.; Tierney, R.; Li, H.; Corsetti, M.; Williams, L.; Wong, P.; Wong, T. Viscoelastic solid-repellent coatings for extreme water saving and global sanitation. Nat. Sustain. 2019, 2, 1097–1105. [Google Scholar] [CrossRef]
- Su, B.; Li, Y.; Wu, Z.; Shi, C.; Xu, Y.; Chen, A.; Yan, C.; Shi, Y. Abrasion-resistant super-slippery flush toilets fabricated by a selective laser sintering 3D printing technology. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Bi, P.; Li, H.; Zhao, G.; Ran, M.; Cao, L.; Guo, H.; Xue, Y. Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 2019, 9, 452. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Z.; Shi, T.; Yu, J.; Tang, M.; Huang, X. Fabrication of superhydrophobic surface with improved corrosioninhibition on 6061 aluminum alloy substrate. Appl. Surf. Sci. 2015, 342, 76–83. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Luo, D.; Liu, Y.; Han, Z.; Ren, L. Corrosion resistance controllable of biomimetic superhydrophobic microstructured magnesium alloy by controlled adhesion. Surf. Coat. Technol. 2018, 347, 173–180. [Google Scholar] [CrossRef]
- Ma, Q.; Tong, Z.; Wang, W.; Dong, G. Fabricating robust and repairable superhydrophobic surface on carbon steel by nanosecond laser texturing for corrosion protection. Appl. Surf. Sci. 2018, 455, 748–757. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhao, X.; Hu, X.; Yang, L.; Wang, N.; Li, Y.; Hou, B. Biomimetic one step fabrication of manganese stearate superhydrophobic surface as an efficient barrier against marine corrosion and Chlorella vulgaris-induced biofouling. Chem. Eng. J. 2016, 306, 441–451. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Yuan, S.; Li, Y.; Zhu, Y. Durable and anti-corrosion superhydrophobic coating with bistratal structure prepared by ambient curing. Prog. Org. Coat. 2020, 149, 105922. [Google Scholar] [CrossRef]
- Wu, S.W.; Jiang, Q.T.; Yuan, S.; Zhao, Q.K.; Liu, C.; Tang, H. Environmentally friendly expanded graphite-doped ZnO superhydrophobic coating with good corrosion resistance in marine environment. Rare Met. 2023, 42, 3075–3087. [Google Scholar] [CrossRef]
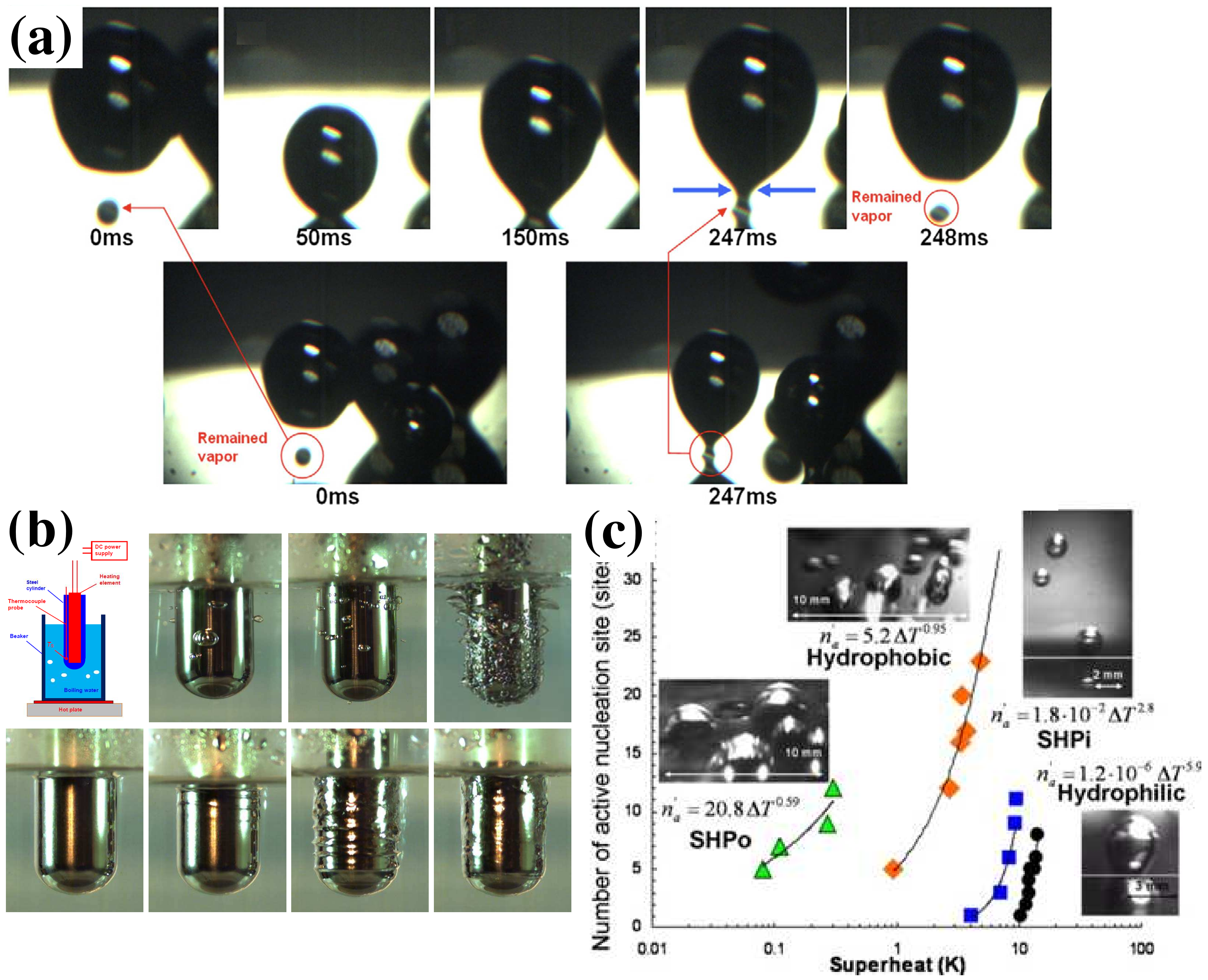
- Jo, H.; Ahn, H.S.; Kang, S.; Kim, M.H. A study of nucleate boiling heat transfer on hydrophilic, hydrophobic and heterogeneous wetting surfaces. Int. J. Heat Mass Transf. 2011, 54, 5643–5652. [Google Scholar] [CrossRef]
- Betz, A.R.; Jenkins, J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef]
- Vakarelski, I.U.; Patankar, N.A.; Marston, J.O.; Chan, D.Y.C.; Thoroddsen, S.T. Stabilization of Leidenfrost vapour layer by textured superhydrophobic surfaces. Nature 2012, 489, 274.e7. [Google Scholar] [CrossRef]
- Searle, M.; Emerson, P.; Crockett, J.; Maynes, D. Influence of microstructure geometry on pool boiling at superhydrophobic surfaces. Int. J. Heat Mass Transf. 2018, 127, 772–783. [Google Scholar] [CrossRef]
- Qin, L.; Li, S.; Zhao, X.; Zhang, X. Experimental research on flow boiling characteristics of micro pin-fin arrays with different hydrophobic coatings. Int. Commun. Heat Mass Transf. 2021, 126, 105456. [Google Scholar] [CrossRef]
- Miljkovic, N.; Enright, R.; Nam, Y.; Lopez, K.; Dou, N.; Sack, J.; Wang, E.N. Jumping-droplet-enhanced condensation on scalable superhydrophobic nanostructured surfaces. Nano Lett. 2013, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Mou, L.W.; Zhang, J.Y.; Fan, L.W.; Li, J.Q. A visualized study of enhanced steam condensation heat transfer on a honeycomb-like microporous superhydrophobic surface in the presence of a non-condensable gas. Int. J. Heat Mass Transf. 2020, 150, 119352. [Google Scholar] [CrossRef]
- Khatir, Z.; Kubiak, K.J.; Jimack, P.K.; Mathia, T.G. Dropwise condensation heat transfer process optimization on superhydrophobic surfaces using a multi-disciplinary approach. Appl. Therm. Eng. 2016, 106, 1337–1344. [Google Scholar] [CrossRef]
- Hao, T.; Wang, K.; Chen, Y.; Ma, X.; Lan, Z.; Bai, T. Multiple bounces and oscillatory movement of a microdroplet in superhydrophobic minichannels. Ind. Eng. Chem. Res. 2018, 57, 4452–4461. [Google Scholar] [CrossRef]
- Taghvaei, E.; Moosavi, A.; Nouri-Borujerdi, A.; Daeian, M.A.; Vafaeinejad, S. Superhydrophobic surfaces with a dual-layer micro- and nanoparticle coating for drag reduction. Energy 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Zhao, X.; Zong, Z.; Jiang, Y.; Sun, T. A numerical investigation of the mechanism of air-injection drag reduction. Appl. Ocean Res. 2020, 94, 101978. [Google Scholar] [CrossRef]
- Mäkiharju, S.A.; Ceccio, S.L. On multi-point gas injection to form an air layer for frictional drag reduction. Ocean. Eng. 2018, 147, 206–214. [Google Scholar] [CrossRef]
- Park, H.J.; Tasaka, Y.; Oishi, Y.; Murai, Y. Drag reduction promoted by repetitive bubble injection in turbulent channel flows. Int. J. Multiph. Flow 2015, 75, 12–25. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, L.; Liu, X.; Weng, R.; Wang, Y.; Wu, Z. Wetting behavior and drag reduction of superhydrophobic layered double hydroxides films on aluminum. Appl. Surf. Sci. 2016, 380, 178–184. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Mo, J.; Yu, N.; Zhou, Z. Study of the drag reduction performance on steel spheres with superhydrophobic ER/ZnO coating. Mater. Sci. Eng. B 2023, 288, 116144. [Google Scholar] [CrossRef]
- Abu Rowin, W.; Hou, J.; Ghaemi, S. Turbulent channel flow over riblets with superhydrophobic coating. Exp. Therm. Fluid Sci. 2018, 94, 192–204. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.; Cai, Y.; Tong, W.; Xiong, D. Scalable superhydrophobic coating with controllable wettability and investigations of its drag reduction. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 290–295. [Google Scholar] [CrossRef]
- Zhang, S.; Ouyang, X.; Li, J.; Gao, S.; Han, S.; Liu, L.; Wei, H. Underwater Drag-Reducing Effect of Superhydrophobic Submarine Model. Langmuir 2015, 31, 587–593. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, R.; Liu, C.; Mo, J. Application of ZnO/epoxy resin superhydrophobic coating for buoyancy enhancement and drag reduction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129714. [Google Scholar] [CrossRef]
- Guan, N.; Liu, Z.; Jiang, G.; Zhang, C.; Ding, N. Experimental and theoretical investigations on the flow resistance reduction and slip flow in super-hydrophobic micro tubes. Exp. Therm. Fluid Sci. 2015, 69, 45–57. [Google Scholar] [CrossRef]
- Hao, Y.; Wong, P.L.; Mao, J.H. Solving coupled boundary slip and heat transfer EHL problem under large slide-roll ratio conditions. Tribol. Int. 2019, 133, 73–87. [Google Scholar]
- Liu, Y.; Zhang, L.; Zhang, C.; Liu, L. Bioinspired antifouling Fe-based amorphous coating via killing-resisting dual surface modifications. Sci. Rep. 2022, 12, 819. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Krishna, N.G.; Anandkumar, B.; Vanithakumari, S.C.; Philip, J. A comprehensive review on an-ticorrosive/antifouling superhydrophobic coatings: Fabrication, assessment, applications, challenges and future perspec-tives. Adv. Colloid Interface Sci. 2024, 324, 103090. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Bazzoli, D.G.; Overton, T.W.; Mendes, P.M. Plasma Activation and its Nanoconfinement Effects Boost Surface Anti-Biofouling Performance. Adv. Mater. Interfaces 2023, 10, 2202087. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Y.; Li, Q.; Liu, Y.; She, Z.; Chen, F.; Li, L.; Zhang, X.; Zhang, P. Researching a highly anti-corrosion superhydrophobic film fabricated on AZ91D magnesium alloy and its anti-bacteria adhesion effect. Mater. Charact. 2015, 99, 200–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, C.; Fan, Z.; Wang, Y. A multifunctional composite membrane with photocatalytic, self-cleaning, oil/water separation and antibacterial properties. Nanotechnology 2022, 33, 355703. [Google Scholar] [CrossRef] [PubMed]
- Seyfi, J.; Panahi-Sarmad, M.; OraeiGhodousi, A.; Goodarzi, V.; Khonakdar, H.A.; Asefnejad, A.; Shojaei, S. Antibacterial superhydrophobic polyvinyl chloride surfaces via the improved phase separation process using silver phosphate nanoparticles. Colloids Surf. B Biointerfaces 2019, 183, 110438. [Google Scholar] [CrossRef] [PubMed]
- Bruzaud, J.; Tarrade, J.; Celia, E.; Thierry, D.; Elisabeth, T.G.; Frédéric, G.; Jean-Marie, H.; Morgan, G.; Marie-Noëlle, B. The design of superhydrophobic stainless steel surfaces by controlling nanostructures, a key parameter to reduce the implantation of pathogenic bacteria. Mater. Sci. Eng. C 2017, 73, 40–47. [Google Scholar] [CrossRef]
- Zhang, X.; Han, H.; Kuang, W.; Tian, H.; Wang, X.; Cheng, W. Facile fabrication of fluorine-free slippery antifouling coatings with self-cleaning and anti-microorganism properties. J. Mater. Sci. 2023, 21, 8969–8980. [Google Scholar] [CrossRef]
- Selim, M.; Yang, H.; Wang, F.; Fatthallah, N.; Nesreen, A.; Li, X.; Li, Y.; Huang, Y. Superhydrophobic silicone/SiC nanowire composite as a fouling release coating material. J. Coat. Technol. Res. 2019, 16, 1165–1180. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Sofia, S.; Anandkumar, B.; Philip, J. Long term antifouling performance of superhydrophobic surfaces in seawater environment: Effect of substrate material, hierarchical surface feature and surface chemistry. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129194. [Google Scholar] [CrossRef]
- Suryaprabha, T.; Ha, H.; Hwang, B.; Sethuraman, M.G. Self-cleaning, superhydrophobic, and antibacterial cotton fabrics with chitosan-based composite coatings. Int. J. Biol. Macromol. 2023, 250, 126217. [Google Scholar] [CrossRef]
- Ghamari, N.; Ahmadi, R.; Sheikhzadeh, M.S.; Afshar, A. Development of PDMS/TiO2/Ag3PO4 antibacterial coating on 316L/PDMS implants, evaluation of superhydrophobicity, bio-corrosion, mechanical behaviour, surface nanostructure and chemistry. J. Mech. Behav. Biomed. Mater. 2024, 150, 106315. [Google Scholar] [CrossRef]
- Wang, B.; An, W.; Wang, L.; Jiao, L.; Zhang, H.; Song, H.; Liu, S. Superhydrophobic and antibacterial hierarchical surface fabricated by femtosecond laser. Sustainability 2022, 14, 12412. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Perumal, G.; Arora, H.S.; Popat, K.C. Enhanced antibacterial properties on superhydrophobic micro-nano structured titanium surface. J. Biomed. Mater. Res. A 2022, 110, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, L.; Wang, Y.; Tao, H.; Liu, Z.; Wang, T.; Ye, F.; He, Y.; Lin, J. Air pocket-optimization strategy for micro/nanostructures fabricated by femtosecond laser technology for anti-icing per-formance improvement. Appl. Surf. Sci. 2024, 655, 159454. [Google Scholar] [CrossRef]
- Shen, Y.; Tao, J.; Wang, G.; Zhu, C.; Chen, H.; Jin, M.; Xie, Y. Bioinspired fabrication of hierarchical-structured superhydrophobic surfaces to understand droplet bouncing dynamics for enhancing water repellency. J. Phys. Chem. C 2018, 122, 7312–7320. [Google Scholar] [CrossRef]
- Farokhirad, S.; Lee, T. Computational study of microparticle effect on self-propelled jumping of droplets from superhydrophobic substrates. Int. J. Multiph. Flow 2017, 95, 220–234. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Z.; Chen, T.; Xi, Y.; Zhang, J. Fabrication of superhydrophobic surface with desirable anti-icing performance based on micro/nano-structures and organosilane groups. Appl. Surf. Sci. 2020, 501, 144165. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; He, Z.; Chen, J.; Gao, W.; Cao, P. Superhydrophobic Ni nanocone surface prepared by electrodeposition and its overall performance. Surf. Coat. Technol. 2023, 464, 129548. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, B.; Zhou, X.; Liu, R.; Hu, J.; Zheng, H.; Chen, Z. An abrasion resistant TPU/SH–SiO2 superhydrophobic coating for anti-icing and anti-corrosion applications. J. Renew. Mater. 2022, 10, 1239–1255. [Google Scholar] [CrossRef]
- Zeng, D.; Li, Y.; Huan, D.; Liu, H.; Wang, J. Robust epoxy-modified superhydrophobic coating for aircraft anti-icing systems. Colloids Surf. A Physicochem. Eng. Asp. 2021, 3, 127377. [Google Scholar] [CrossRef]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low-interfacial toughness materials for effective large-scale deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef]
- Barthwal, S.; Lee, B.; Lim, S.-H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil. Appl. Surf. Sci. 2019, 496, 143677. [Google Scholar] [CrossRef]
- He, Z.; Wu, C.; Hua, M.; Wu, S.; Wu, D.; Zhu, X.; Wang, J.; He, X. Bioinspired multifunctional anti-icing hydrogel. Matter 2020, 2, 723–734. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. One-step facile fabrication of sustainable cellulose membrane with superhydrophobicity via a sol-gel strategy for efficient oil/water separation. Surf. Coat. Technol. 2019, 361, 19–26. [Google Scholar] [CrossRef]
- Li, H.; Zhu, G.; Shen, Y.; Han, Z.; Zhang, J.; Li, J. Robust superhydrophobic attapulgite meshes for effective separation of water-in-oil emulsions. J. Colloid Interface Sci. 2019, 557, 84–93. [Google Scholar] [CrossRef]
- Liu, P.; Niu, L.; Tao, X.; Li, X.; Zhang, Z. Facile preparation of superhydrophobic quartz sands with micro-nano-molecule hierarchical structure for controlling the permeability of oil and water phase. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 1–9. [Google Scholar] [CrossRef]
- Chen, F.; Hao, S.; Huang, S.; Lu, Y. Nanoscale SiO2-coated superhydrophobic meshes via electro-spray deposition for oil-water separation. Powder Technol. 2020, 373, 82–92. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Hu, Y.; Huang, Q.; Li, X.; Qing, Y.; Wu, Y. Rosin acid and SiO2 modified cotton fabric to prepare fluorine-free durable superhydrophobic coating for oil-water separation. J. Hazard. Mater. 2022, 440, 129797. [Google Scholar] [CrossRef]
- Sow, P.; Singhal, R.; Sahoo, P.; Radhakanth, S. Fabricating low-cost, robust superhydrophobic coatings with re-entrant topology for self-cleaning, corrosion inhibition, and oil-water separation. J. Colloid Interface Sci. 2021, 600, 358–372. [Google Scholar] [CrossRef]
- Wu, M.; An, N.; Li, Y.; Sun, J. Layer-by-layer assembly of fluorine-free polyelectrolyte-surfactant complexes for the fabrication of self-healing superhydrophobic films. Langmuir 2016, 32, 12361–12369. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, D.; Tong, L.; Lai, H.; Zhang, E.; Kang, H.; Wang, Y.; Liu, P.; Liu, Y.; Du, Y.; et al. Superhydrophobic Shape Memory Polymer Arrays with Switchable Isotropic/Anisotropic Wetting. Adv. Funct. Mater. 2017, 28, 1705002. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, P.; Zhang, D.; Wang, S. Near-Infrared Responsive Smart Superhydrophobic Coating with Self-Healing and Robustness Enhanced by Disulfide-Bonded Polyurethane. ACS Appl. Mater. Interfaces 2022, 14, 45988–46000. [Google Scholar] [CrossRef]
- Yan, J.; Liu, G.; Wang, T.; Zhao, J.; Ding, Y.; Chen, N. Analysis of superhydrophobic material performance based on molecular dynamics simulations. Surf. Eng. 2016, 32, 147–156. [Google Scholar] [CrossRef]
- He, X.; Lou, T.; Cao, P.; Bai, X.; Yuan, C.; Wang, C.; Neville, A. Experimental and molecular dynamics simulation study of chemically stable superhydrophobic surfaces. Surf. Coat. Technol. 2021, 418, 127236. [Google Scholar] [CrossRef]
- Bhushan, B.; Wang, Y.; Maali, A. Boundary Slip Study on Hydrophilic, Hydrophobic, and Superhydrophobic Sur-faces with Dynamic Atomic Force Microscopy. Langmuir 2009, 25, 8117–8121. [Google Scholar] [CrossRef]
- Erbil, Y. Practical Applications of Superhydrophobic Materials and Coatings: Problems and Perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, J.; Alodhayb, A.; Ma, G. Carbon-based nanotube and graphene thermal stable materials generated via surface oxidation and chemical modification. Inorg. Chem. Commun. 2024, 160, 111972. [Google Scholar] [CrossRef]



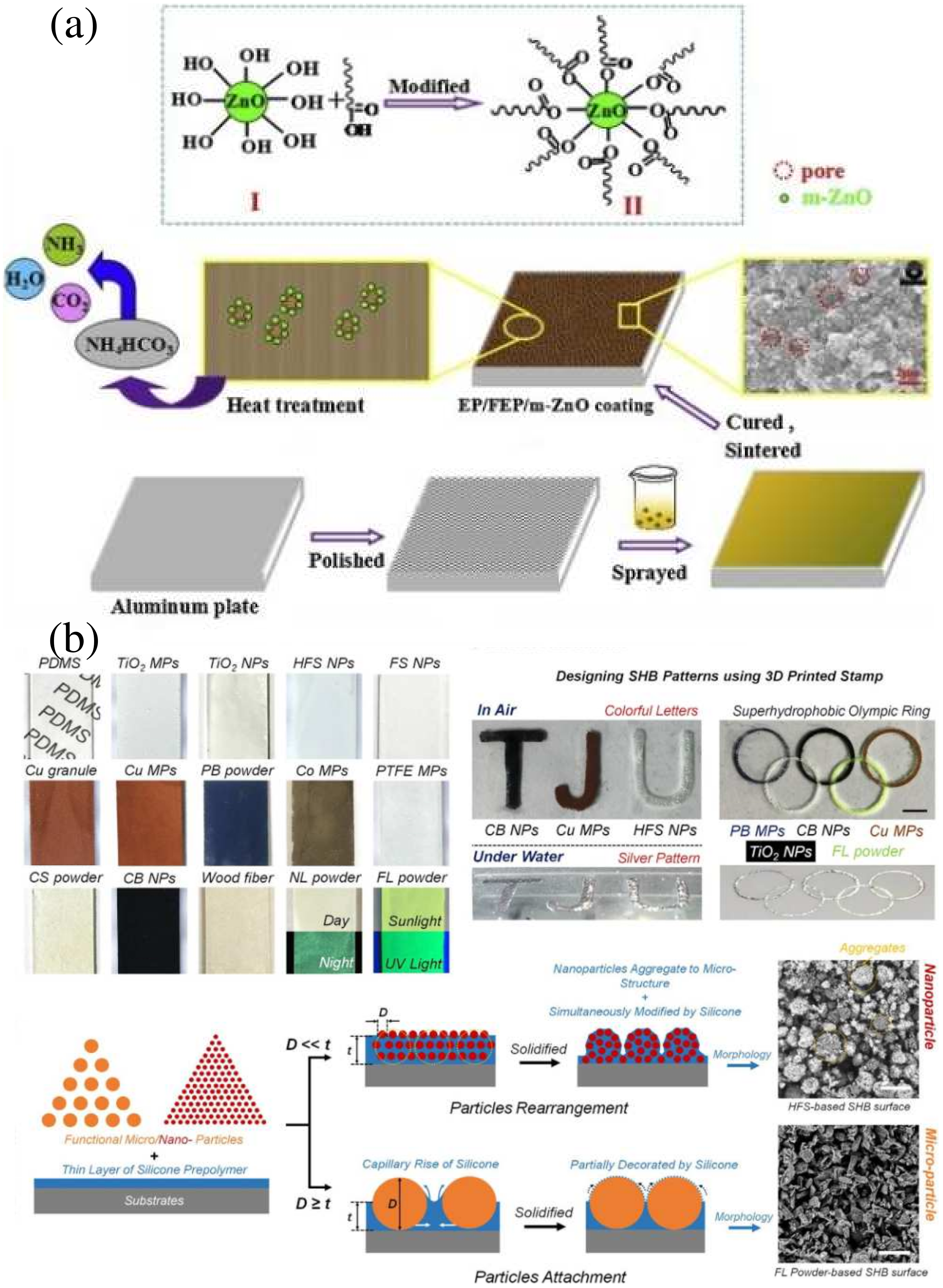


| Prepare Methods | Low Surface Energy Substance | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Template methods | Hard template method | 1H, 1H, 2H, 2H-perfluorooctyl | Low cost and simple preparation | Poor mechanical durability, resistance to degradation | [35] |
| Soft template method | PDMS | Low cost and simple preparation and high mechanical elongation | Poor mechanical durability | [38] | |
| Etching methods | Chemical etching | 1H, 1H, 2H, 2H-Perfluorooctanesulfonic acid | Fast and simple preparation | Uncontrollable microstructure size and environmental pollution, resistance to degradation | [40] |
| Mechanical etching | Stearic acid (SA) | Controllable microstructure size, Low cost | Requires special equipment and higher cost, bioaccumulation | [41] | |
| Laser micro-machining | Octafluorocycloboutane | Controllable microstructure size, Low cost | Requires special equipment and higher cost, high energy consumption, resistance to degradation | [42] | |
| Electrochemical methods | Electroplating | Stearic acid (SA) | Simple and controllable preparation | Time-consuming, limited to small areas, and environmental pollution | [46] |
| Electrochemical deposition | Stearic acid (SA) | Controllable reaction process | Small bonding force and limited to small areas | [47] | |
| Anodizing | Stearic acid (SA) | Simple and controllable preparation, Low cost | Time-consuming, limited applicable substrates, and environmental pollution | [48] | |
| Micro-arc oxidation (MAO) | Stearic acid (SA) | Fast and controllable preparation | Limited applicable substrates and high energy consumption | [49] | |
| Spraying methods | Direct spraying | Stearic acid (SA) | Simple and applicable to different substrates and shapes, Low cost | Poor mechanical durability | [52] |
| Glue + powder | Stearic acid (SA), fluorinated ethylene propylene (FEP) | Simple, applicable to different substrates and well-distributed particles | Poor mechanical durability | [55] | |
| Any other methods | Sol−gel technique | PDMS | Simple reaction process and well distribution, Low cost | Time-consuming and environmental pollution | [56] |
| Electrospinning | Stearic acid | Controllable microstructure size | Needed complex operations, high cost | [57] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Liao, X.; Ji, Y.; Mo, Y.; Zhang, J. Research Progress on Preparation of Superhydrophobic Surface and Its Application in the Field of Marine Engineering. J. Mar. Sci. Eng. 2024, 12, 1741. https://doi.org/10.3390/jmse12101741
Fu J, Liao X, Ji Y, Mo Y, Zhang J. Research Progress on Preparation of Superhydrophobic Surface and Its Application in the Field of Marine Engineering. Journal of Marine Science and Engineering. 2024; 12(10):1741. https://doi.org/10.3390/jmse12101741
Chicago/Turabian StyleFu, Jingguo, Xiaogang Liao, Yulong Ji, Yanqiang Mo, and Jifeng Zhang. 2024. "Research Progress on Preparation of Superhydrophobic Surface and Its Application in the Field of Marine Engineering" Journal of Marine Science and Engineering 12, no. 10: 1741. https://doi.org/10.3390/jmse12101741
APA StyleFu, J., Liao, X., Ji, Y., Mo, Y., & Zhang, J. (2024). Research Progress on Preparation of Superhydrophobic Surface and Its Application in the Field of Marine Engineering. Journal of Marine Science and Engineering, 12(10), 1741. https://doi.org/10.3390/jmse12101741









