Preliminary Assessment of Macrobenthos Associated with Red Coral Corallium rubrum (Linnaeus, 1758) Populations in the Northeastern Ionian Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Megabenthos Characterization
2.3. Red Coral Population Characterization
2.4. Data Analysis
3. Results
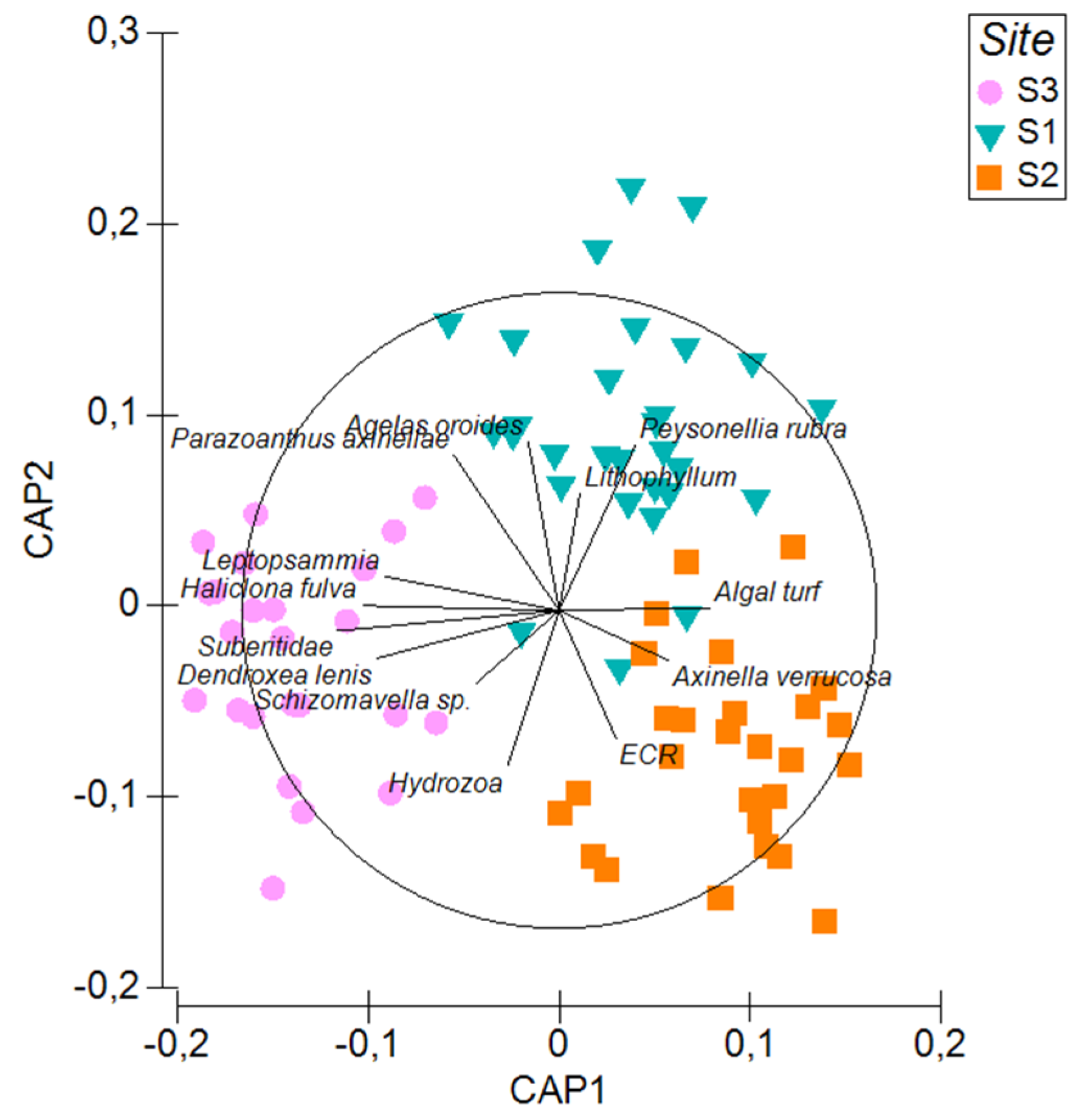
3.1. Megabenthos Features
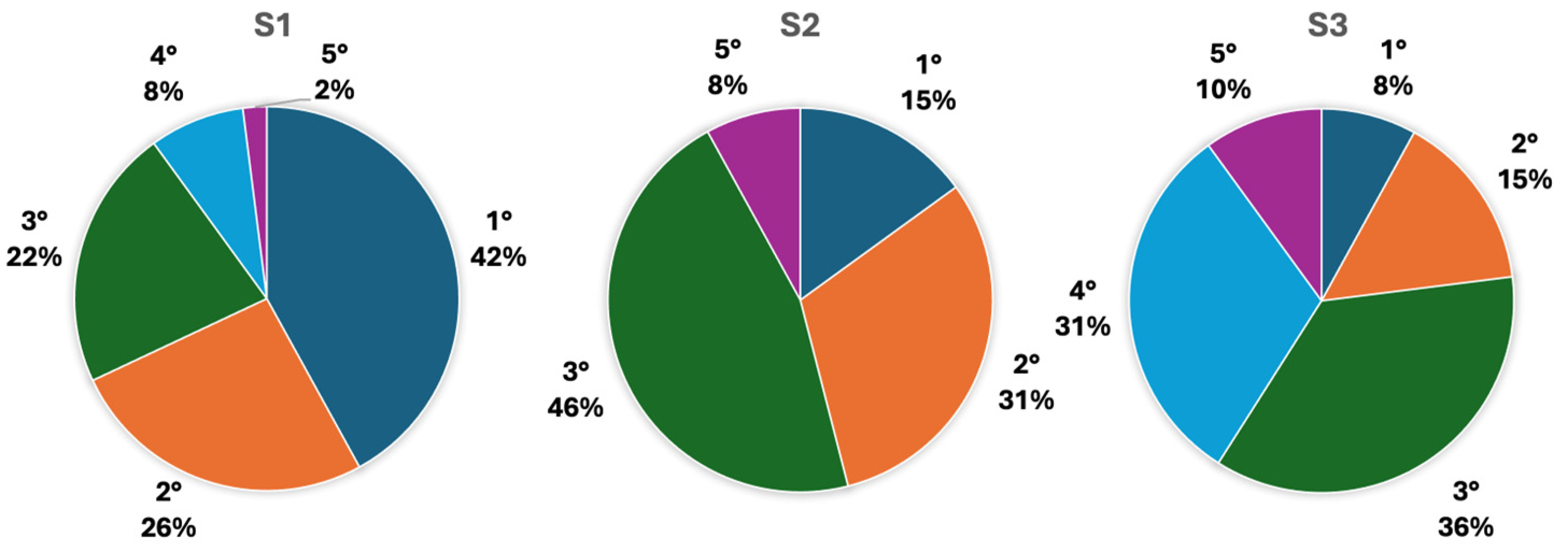
3.2. Red Coral Population Features
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beaumont, N.J.; Austen, M.C.; Atkins, J.P.; Burdon, D.; Degraer, S.; Dentinho, T.P.; Derous, S.; Holm, P.; Horton, T.; Van Ierland, E. Identification, Definition and Quantification of Goods and Services Provided by Marine Biodiversity: Implications for the Ecosystem Approach. Mar. Pollut. Bull. 2007, 54, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, P.V.; Soetaert, K.; Solan, M.; Thrush, S.; Wei, C.-L.; Danovaro, R.; Fulweiler, R.W.; Kitazato, H.; Ingole, B.; Norkko, A. Global Carbon Cycling on a Heterogeneous Seafloor. Trends Ecol. Evol. 2018, 33, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, E.; Grande, U.; Franzese, P.P.; Russo, G.F. Trends and Evolution in the Concept of Marine Ecosystem Services: An Overview. Water 2021, 13, 2060. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Vanreusel, A.; Gooday, A.J.; Levin, L.A.; Priede, I.G.; Buhl-Mortensen, P.; Gheerardyn, H.; King, N.J.; Raes, M. Biological Structures as a Source of Habitat Heterogeneity and Biodiversity on the Deep Ocean Margins. Mar. Ecol. 2010, 31, 21–50. [Google Scholar] [CrossRef]
- Galparsoro, I.; Borja, A.; Uyarra, M.C. Mapping Ecosystem Services Provided by Benthic Habitats in the European North Atlantic Ocean. Front. Mar. Sci. 2014, 1, 23. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Roberts, C.M.; O’Leary, B.C.; McCauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J.; Pauly, D.; Sáenz-Arroyo, A.; Sumaila, U.R.; Wilson, R.W.; et al. Marine Reserves Can Mitigate and Promote Adaptation to Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175. [Google Scholar] [CrossRef]
- Lester, S.E.; Halpern, B.S.; Grorud-Colvert, K.; Lubchenco, J.; Ruttenberg, B.I.; Gaines, S.D.; Airamé, S.; Warner, R.R. Biological Effects within No-Take Marine Reserves: A Global Synthesis. Mar. Ecol. Prog. Ser. 2009, 384, 33–46. [Google Scholar] [CrossRef]
- UNEP-WCMC Protected Area Profile for Italy from the World Database on Protected Areas (WDPA) and World Database on Other Effective Area-based Conservation Measures (WD-OECM), September 2024. Cambridge, UK. Available online: www.protectedplanet.net. (accessed on 12 September 2024).
- MedPAN. UNEP/MAP-SPA/RAC The System of Mediterranean MPAs in 2020. Available online: https://medpan.org/en/system-mediterranean-mpas-2020 (accessed on 4 October 2024).
- IUCN International Union for Conservation of Nature. Guidelines for Applying the IUCN Protected Area Management Categories to Marine Protected Areas; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2012; Available online: https://portals.iucn.org/library/sites/library/files/documents/PAG-019-2nd%20ed.-En.pdf (accessed on 9 August 2024).
- UNEP/MAP The Barcelona Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean. Protocol Concerning Specially Protected Areas and Biological Diversity in the Mediterranean; Barcelona Convention. 1995. Available online: https://web.unep.org/unepmap/who-we-are/legal-framework (accessed on 9 August 2024).
- EEC Habitats Directive. Council Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora. 1992. Available online: https://eur-lex.europa.eu/eli/dir/1992/43/2007-01-01 (accessed on 12 September 2024).
- Marine Strategy Framework Directive. 2008. Available online: https://eurlex.europa.eu/eli/dir/2008/56/oj (accessed on 9 August 2024).
- Greathead, C.; Magni, P.; Vanaverbeke, J.; Buhl-Mortensen, L.; Janas, U.; Blomqvist, M.; Craeymeersch, J.A.; Dannheim, J.; Darr, A.; Degraer, S.; et al. A Generic Framework to Assess the Representation and Protection of Benthic Ecosystems in European Marine Protected Areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1253–1275. [Google Scholar] [CrossRef]
- Klein, C.J.; Brown, C.J.; Halpern, B.S.; Segan, D.B.; McGowan, J.; Beger, M.; Watson, J.E. Shortfalls in the Global Protected Area Network at Representing Marine Biodiversity. Sci. Rep. 2015, 5, 17539. [Google Scholar] [CrossRef]
- Bavestrello, G.; Bo, M.; Canese, S.; Sandulli, R.; Cattaneo-Vietti, R. The Red Coral Populations of the Gulfs of Naples and Salerno: Human Impact and Deep Mass Mortalities. Ital. J. Zool. 2014, 81, 552–563. [Google Scholar] [CrossRef]
- Parenzan, P. Il Mar Piccolo e Il Mar Grande Di Taranto. Thalass. Salentina 1969, 3, 19–36. [Google Scholar]
- Mastrototaro, F.; Giove, A.; D’Onghia, G.; Tursi, A.; Matarrese, A.; Gadaleta, M.V. Benthic Diversity of the Soft Bottoms in a Semi-Enclosed Basin of the Mediterranean Sea. J. Mar. Biol. Assoc. UK 2008, 88, 247–252. [Google Scholar] [CrossRef]
- Scalera Liaci, L.; Sciscioli, M.; Fiordiponti, F. Distribuzione Dei Poriferi Del Mar Piccolo Di Taranto. Oebalia 1976, 2, 3–19. [Google Scholar]
- Pulitzer-Finali, G. A Collection of Mediterranean Demospongiae (Porifera) with, in Appendix, a List of the Demospongiae Hitherto Recorded from the Mediterranean Sea. Ann. Mus. Civ. Stor. Nat. Giacomo Doria 1983, 84, 445–621. [Google Scholar]
- Scalera-Liaci, L.; Corriero, G. Distribution of the Sponge Fauna from the Mar Piccolo and the Mar Grande (Taranto, Ionian Sea). Biol. Mar. Suppl. Al Not. SIBM 1993, 1, 317–318. [Google Scholar]
- Parenzan, P. Puglia Marittima; Congedo Editore: Galatina, Italy, 1983; Volume I and II. [Google Scholar]
- Tursi, A.R.; Matarrese, A.; Piscitelli, G.; Gherardi, M. Biocenosi Del Mar Grande Di Taranto. Quad. Lab. Tecnol. Pesca 1981, 3, 563–576. [Google Scholar]
- Tursi, A.R.; Matarrese, A.; Scalera Liaci, L.; Gherardi, M.; Lepore, E.; Sciscioli, M.; Piscitelli, G.; Chieppa, M. Associazioni Bentoniche Del Mar Grande Di Taranto: Primi Risultati Di Una Analisi Multivariata. Mem. Biol. Mar. Oceanogr. 1980, 10, 333–340. [Google Scholar]
- Tursi, A.R.; Piscitelli, G.; Gherardi, M.; Matarrese, A. Aspetti Ecologici Del Porto Di Taranto (Mar Grande). Oebalia 1978, 4, 41–78. [Google Scholar]
- Panetta, P. Importanza Dei Molluschi Nella Definizione Delle Biocenosi Del Mar Grande Di Taranto [Mar Mediterraneo]. Mem. Biol. Mar. Oceanogr. 1980, 10, 423–424. [Google Scholar]
- Chieppa, M.; Tursi, A.R. Premiers Résultats d’un Essai d’analyse Multivariée Sur Les Peuplements Bénthiques de Le Mar Grande de Taranto. In Actes de Journée de Travail sur l’Analyse des Données, INRIA; INRIA: Paris, France, 1980; pp. 195–208. [Google Scholar]
- Matarrese, A.; Mastrototaro, F.; D’onghia, G.; Maiorano, P.; Tursi, A. Mapping of the Benthic Communities in the Taranto Seas Using Side-Scan Sonar and an Underwater Video Camera. Chem. Ecol. 2004, 20, 377–386. [Google Scholar] [CrossRef]
- Longo, C.; Scalera Liaci, L.; Corriero, G. I Poriferi Del Mar Grande e Del Mar Piccolo Di Taranto. Biol. Mar. Mediterr. 2004, 11, 440–443. [Google Scholar]
- Pierri, C.; Longo, C.; Giangrande, A. Variability of Fouling Communities in the Mar Piccolo of Taranto (Northern Ionian Sea, Mediterranean Sea). J. Mar. Biol. Assoc. UK 2010, 90, 159–167. [Google Scholar] [CrossRef]
- Pierri, C.; Longo, C.; Falace, A.; Gravina, M.F.; Gristina, M.; Kaleb, S.; Lazic, T.; Lisco, S.; Moretti, M.; Putignano, M.; et al. Invertebrate Diversity Associated with a Shallow Rhodolith Bed in the Mediterranean Sea (Mar Piccolo of Taranto, South-east Italy). Aquat. Conserv. 2024, 34, e4054. [Google Scholar] [CrossRef]
- Bedulli, D.; Bianchi, C.; Zurlini, G.; Morri, C. Caratterizzazione Biocenotica e Strutturale Del Macrobenthos Delle Coste Pugliesi. In Indagine Ambientale del Sistema Marino Costiero della Regione Puglia; Enea: Rome, Italy, 1986; pp. 227–255. [Google Scholar]
- Capezzuto, F.; Carlucci, R.; Maiorano, P.; Sion, L.; Battista, D.; Giove, A.; Indennidate, A.; Tursi, A.; D’Onghia, G. The Bathyal Benthopelagic Fauna in the North-Western Ionian Sea: Structure, Patterns and Interactions. Chem. Ecol. 2010, 26, 199–217. [Google Scholar] [CrossRef]
- Longo, C.; Cardone, F.; Pierri, C.; Mercurio, M.; Mucciolo, S.; Marzano, C.N.; Corriero, G. Sponges Associated with Coralligenous Formations along the Apulian Coasts. Mar. Biodivers. 2018, 48, 2151–2163. [Google Scholar] [CrossRef]
- Parenzan, P. Biocenosi Bentoniche Della Costa Neretina Da Porto Cesareo a Gallipoli (Golfo Di Taranto). In Proceedings of the 5 Congresso Nazionale della Società Italiana di Biologia Marina, Nardò, Italy, 17–20 May 1973. [Google Scholar]
- Corriero, G.; Pierri, C.; Mercurio, M.; Nonnis Marzano, C.; Onen Tarantini, S.; Gravina, M.F.; Lisco, S.; Moretti, M.; De Giosa, F.; Valenzano, E.; et al. A Mediterranean Mesophotic Coral Reef Built by Non-Symbiotic Scleractinians. Sci. Rep. 2019, 9, 3601. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 79, pp. 61–136. ISBN 978-0-12-815101-3. [Google Scholar]
- De Luca, A.; Lisco, S.; Acquafredda, P.; Gimenez, G.; Moretti, M. The Coralligenous in the Present-Day System from the Salento Coast (Southern Adriatic Sea). Rend. Online Soc. Geol. Ital. 2023, 59, 105–111. [Google Scholar] [CrossRef]
- Cardone, F.; Corriero, G.; Longo, C.; Pierri, C.; Gimenez, G.; Gravina, M.F.; Giangrande, A.; Lisco, S.; Moretti, M.; De Giosa, F.; et al. A System of Marine Animal Bioconstructions in the Mesophotic Zone along the Southeastern Italian Coast. Front. Mar. Sci. 2022, 9, 948836. [Google Scholar] [CrossRef]
- Corriero, G.; Maria, M.; Longo, C. Studio Della Biodiversità Del Coralligeno Profondo Pugliese Con Particolare Riguardo Alla Facies a Corallo Rosso; CeRB Edizioni: Bari, Italy, 2012; 24p. [Google Scholar]
- AA.VV Uso Del ROV (Remotely Operated Vehicle) Nella Definizione Applicativa Di Piani Di Gestione per Il Corallo Rosso (Corallium rubrum). Strategie Gestionali per La Conservazione Della Specie e Valutazione Della Compatibilità Della Risorsa Con Un Potenziale Sfruttamento Commerciale Lungo Le Coste Italiane Del Tirreno Centro-Settentrionale; Progetto di Ricerca 7- Tematica A2-Uo1 D.M. Mipaaf N.298/11 Del 15 Dicembre 2011 (CUP J85E11000660001). 2013. Available online: https://www.remare.org/progetti/corallo (accessed on 15 July 2024).
- Pérès, J.-M.; Picard, J. Nouveau Manuel de Bionomie Benthique de la Mer Méditerranée; Station Marine d’Endoume: Marseille, France, 1964. [Google Scholar]
- Ballesteros, E. Mediterranean Coralligenous Assemblages: A Synthesis of Present Knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar]
- Montefalcone, M.; Tunesi, L.; Ouerghi, A. A Review of the Classification Systems for Marine Benthic Habitats and the New Updated Barcelona Convention Classification for the Mediterranean. Mar. Environ. Res. 2021, 169, 105387. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Bo, M.; Giudice, D.; Canese, S.; Cau, A.; Andaloro, F.; Angiolillo, M.; Greco, S.; Bavestrello, G. Structure and Status of the Italian Red Coral Forests: What Can a Large-Scale Study Tell? Front. Mar. Sci. 2022, 9, 1073214. [Google Scholar] [CrossRef]
- Angiolillo, M.; Gori, A.; Canese, S.; Bo, M.; Priori, C.; Bavestrello, G.; Salvati, E.; Erra, F.; Greenacre, M.; Santangelo, G. Distribution and Population Structure of Deep-dwelling Red Coral in the Northwest Mediterranean. Mar. Ecol. 2016, 37, 294–310. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Betti, F.; Coppari, M.; Toma, M.; Pronzato, R.; Canese, S.; Bertolino, M.; Costa, G.; Pansini, M.; et al. Keratose-Dominated Sponge Grounds from Temperate Mesophotic Ecosystems (NW Mediterranean Sea). Mar. Ecol. 2020, 41, e12620. [Google Scholar] [CrossRef]
- Bo, M.; Coppari, M.; Betti, F.; Massa, F.; Gay, G.; Cattaneo-Vietti, R.; Bavestrello, G. Unveiling the Deep Biodiversity of the Janua Seamount (Ligurian Sea): First Mediterranean Sighting of the Rare Atlantic Bamboo Coral Chelidonisis aurantiaca Studer, 1890. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 156, 103186. [Google Scholar] [CrossRef]
- Cau, A.; Paliaga, E.; Cannas, R.; Deiana, G.; Follesa, M.; Sacco, F.; Todde, S.; Orru, P. Preliminary Data on Habitat Characterization Relevance for Red Coral Conservation and Management. Ital. J. Geosci. 2015, 134, 60–68. [Google Scholar] [CrossRef]
- Moccia, D.; Cau, A.; Alvito, A.; Canese, S.; Cannas, R.; Bo, M.; Angiolillo, M.; Follesa, M.C. New Sites Expanding the “Sardinian Cold-water Coral Province” Extension: A New Potential Cold-water Coral Network? Aquat. Conserv. 2019, 29, 153–160. [Google Scholar] [CrossRef]
- Civitarese, G.; Gačić, M.; Lipizer, M.; Eusebi Borzelli, G.L. On the Impact of the Bimodal Oscillating System (BiOS) on the Biogeochemistry and Biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Pinardi, N.; Lyubartsev, V.; Cardellicchio, N.; Caporale, C.; Ciliberti, S.; Coppini, G.; De Pascalis, F.; Dialti, L.; Federico, I.; Filippone, M. Marine Rapid Environmental Assessment in the Gulf of Taranto: A Multiscale Approach. Nat. Hazards Earth Syst. Sci. 2016, 16, 2623–2639. [Google Scholar] [CrossRef]
- Trygonis, V.; Sini, M. photoQuad: A Dedicated Seabed Image Processing Software, and a Comparative Error Analysis of Four Photoquadrat Methods. J. Exp. Mar. Biol. Ecol. 2012, 424, 99–108. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, M.; Mass, C. Estudio Biometrico de Poblaciones de Coral Rajo (Corallium rubrum L.) Dellitoral de Gerona (NE de Espana). Bol. Inst. Esp. Ocean 1986, 3, 61–64. [Google Scholar]
- Brazeau, D.A.; Lasker, H.R. Inter- and Intraspecific Variation in Gorgonian Colony Morphology: Quantifying Branching Patterns in Arborescent Animals. Coral Reefs 1988, 7, 139–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Chapman, M.G. On Resemblance Measures for Ecological Studies, Including Taxonomic Dissimilarities and a Zero-Adjusted Bray–Curtis Coefficient for Denuded Assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. For PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Ban, N.C.; Adams, V.M.; Almany, G.R.; Ban, S.; Cinner, J.E.; McCook, L.J.; Mills, M.; Pressey, R.L.; White, A. Designing, Implementing and Managing Marine Protected Areas: Emerging Trends and Opportunities for Coral Reef Nations. J. Exp. Mar. Biol. Ecol. 2011, 408, 21–31. [Google Scholar] [CrossRef]
- Cardone, F.; Corriero, G.; Longo, C.; Mercurio, M.; Onen Tarantini, S.; Gravina, M.F.; Lisco, S.; Moretti, M.; De Giosa, F.; Giangrande, A.; et al. Massive Bioconstructions Built by Neopycnodonte Cochlear (Mollusca, Bivalvia) in a Mesophotic Environment in the Central Mediterranean Sea. Sci. Rep. 2020, 10, 6337. [Google Scholar] [CrossRef] [PubMed]
- Ferdeghini, F.; Acunto, S.; Cocito, S.; Cinelli, F. Variability at Different Spatial Scales of a Coralligenous Assemblage at Giannutri Island (Tuscan Archipelago, Northwest Mediterranean). In Island, Ocean and Deep-Sea Biology; Jones, M.B., Azevedo, J.M.N., Neto, A.I., Costa, A.C., Martins, A.M.F., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 27–36. [Google Scholar]
- Balata, D.; Piazzi, L.; Cecchi, E.; Cinelli, F. Variability of Mediterranean Coralligenous Assemblages Subject to Local Variation in Sediment Deposition. Mar. Environ. Res. 2005, 60, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Gallmetzer, I.; Haselmair, A.; Velimirov, B. Slow Growth and Early Sexual Maturity: Bane and Boon for the Red Coral Corallium rubrum. Estuar. Coast. Shelf Sci. 2010, 90, 1–10. [Google Scholar] [CrossRef]
- Boavida, J.; Paulo, D.; Aurelle, D.; Arnaud-Haond, S.; Marschal, C.; Reed, J.; Goncalves, J.M.; Serrao, E.A. A Well-Kept Treasure at Depth: Precious Red Coral Rediscovered in Atlantic Deep Coral Gardens (SW Portugal) after 300 Years. PLoS ONE 2016, 11, e0147228. [Google Scholar]
- Marschal, C.; Garrabou, J.; Harmelin, J.-G.; Pichon, M. A New Method for Measuring Growth and Age in the Precious Red Coral Corallium rubrum (L.). Coral Reefs 2004, 23, 423–432. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cannas, R.; Cau, A.; Pedoni, C.; Pesci, P.; Cau, A. Deep-Water Red Coral from the Island of Sardinia (North-Western Mediterranean): A Local Example of Sustainable Management. Mar. Freshw. Res. 2013, 64, 706–715. [Google Scholar] [CrossRef]
- Cau, A.; Follesa, M.C.; Cannas, R.; Sacco, F.; Todde, S.; Paliaga, E.; Orru, P.E. Multidisciplinary and Multiscalar Approach for a Sustainable Management of Red Coral (Corallium rubrum, L. 1758) from the Island of Sardinia (West Mediterranean Sea). In Proceedings of the ABSTRACT-GeoHab (Marine Geological and Biological Habitat Mapping) International Conference, Rome, Italy, 6–10 May 2013; p. 11. [Google Scholar]
- Tsounis, G.; Rossi, S.; Gili, J.-M.; Arntz, W.E. Red Coral Fishery at the Costa Brava (NW Mediterranean): Case Study of an Overharvested Precious Coral. Ecosystems 2007, 10, 975–986. [Google Scholar] [CrossRef]
- Santangelo, G.; Carletti, E.; Maggi, E.; Bramanti, L. Reproduction and Population Sexual Structure of the Overexploited Mediterranean Red Coral Corallium rubrum. Mar. Ecol. Prog. Ser. 2003, 248, 99–108. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Galparsoro, I.; Fernandez, T.V.; Johnson, K.; D’Anna, G.; Badalamenti, F.; Garofalo, G.; Carlström, J.; Piwowarczyk, J.; Rabaut, M. Maritime Ecosystem-Based Management in Practice: Lessons Learned from the Application of a Generic Spatial Planning Framework in Europe. Mar. Policy 2017, 75, 174–186. [Google Scholar] [CrossRef]
- Loh, T.; Archer, S.K.; Dunham, A. Monitoring Program Design for Data-limited Marine Biogenic Habitats: A Structured Approach. Ecol. Evol. 2019, 9, 7346–7359. [Google Scholar] [CrossRef]
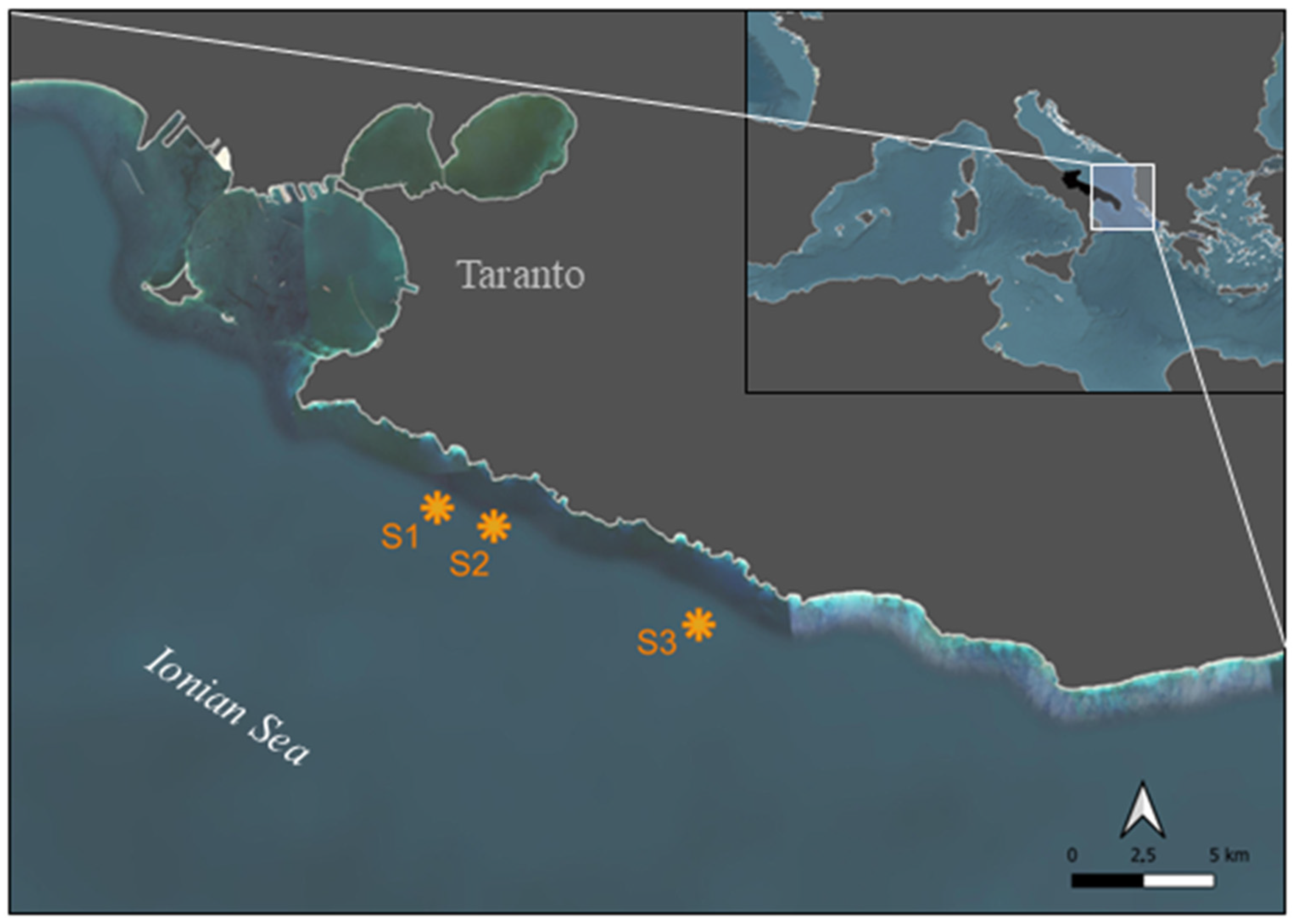
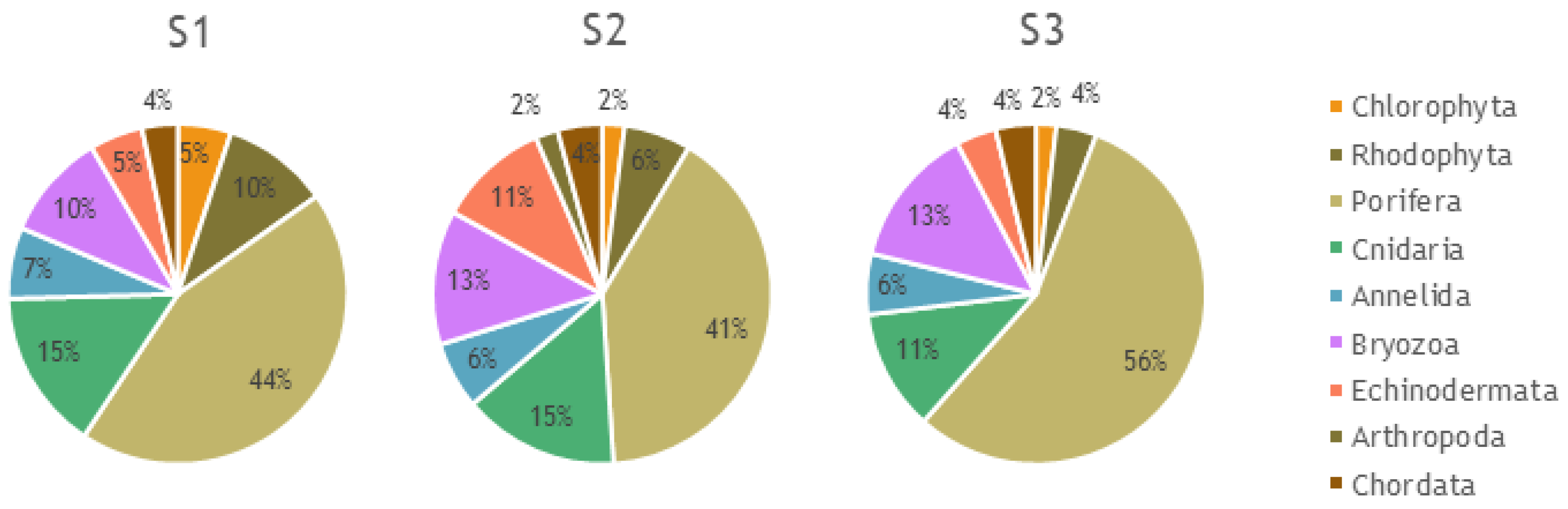
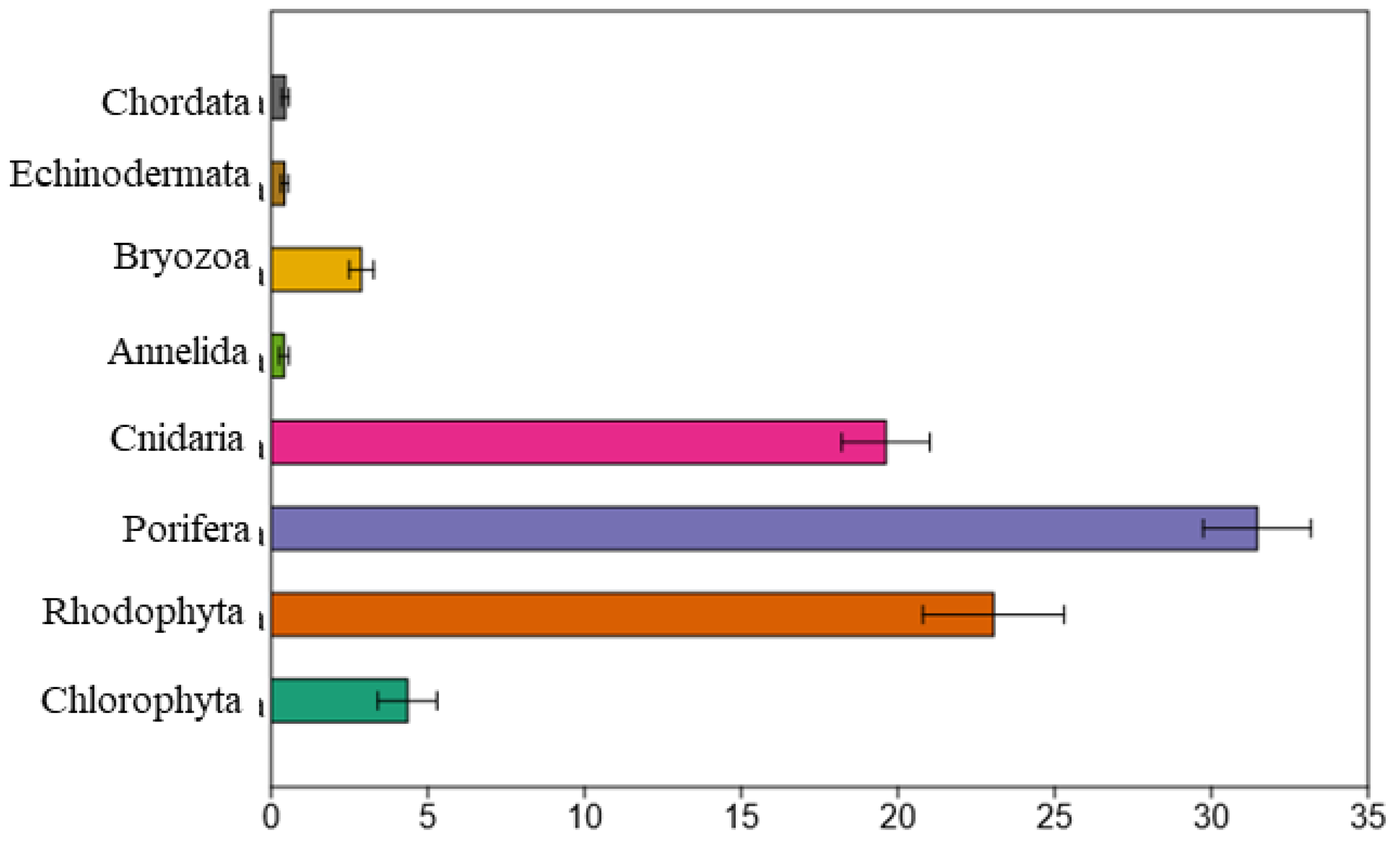
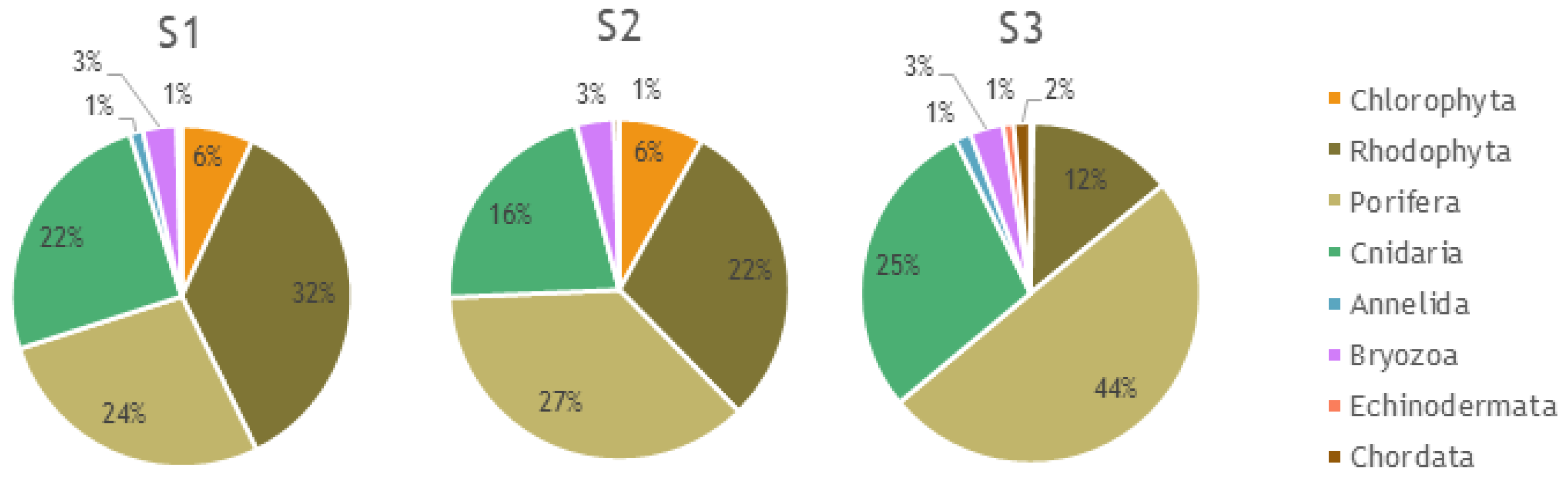
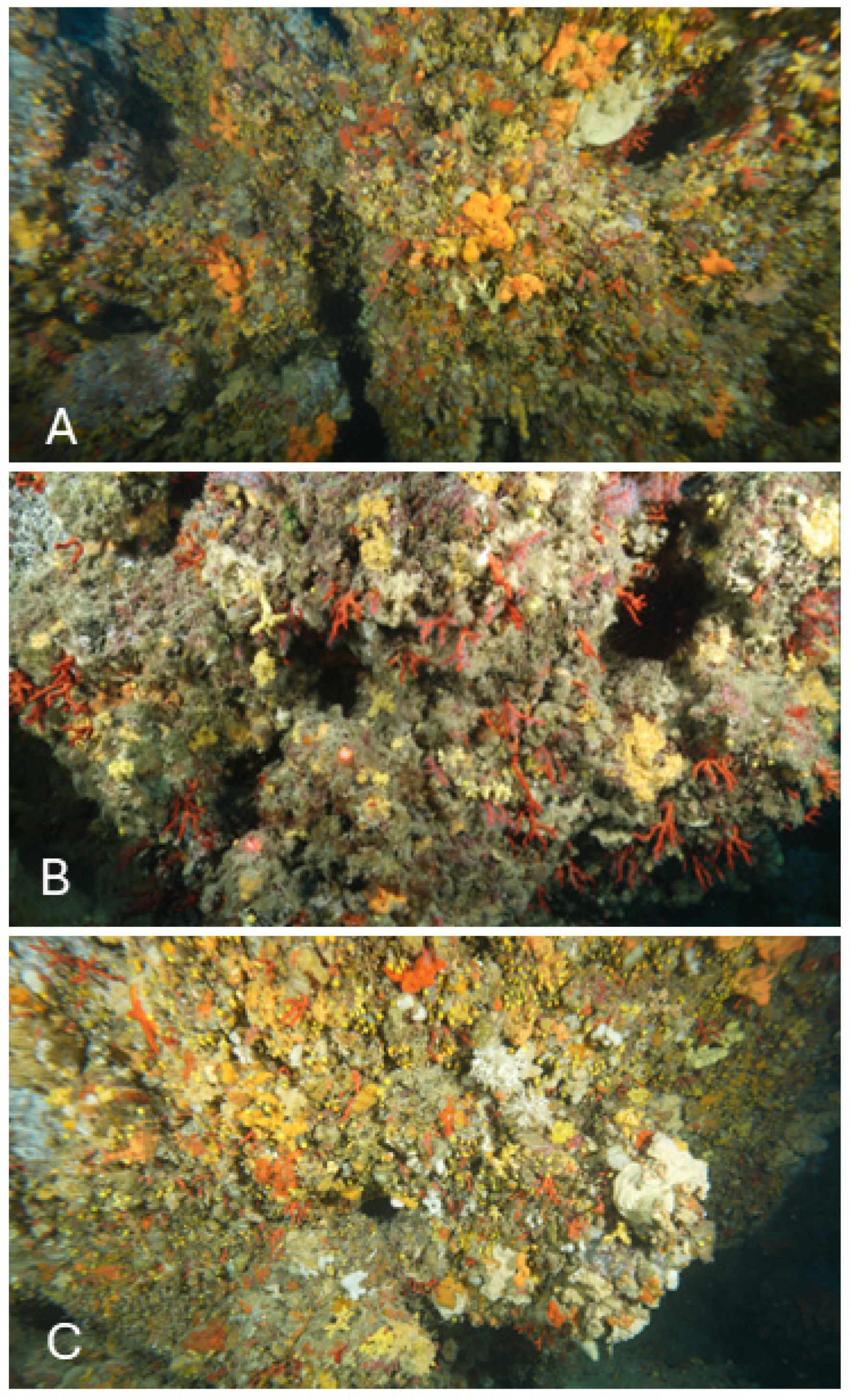

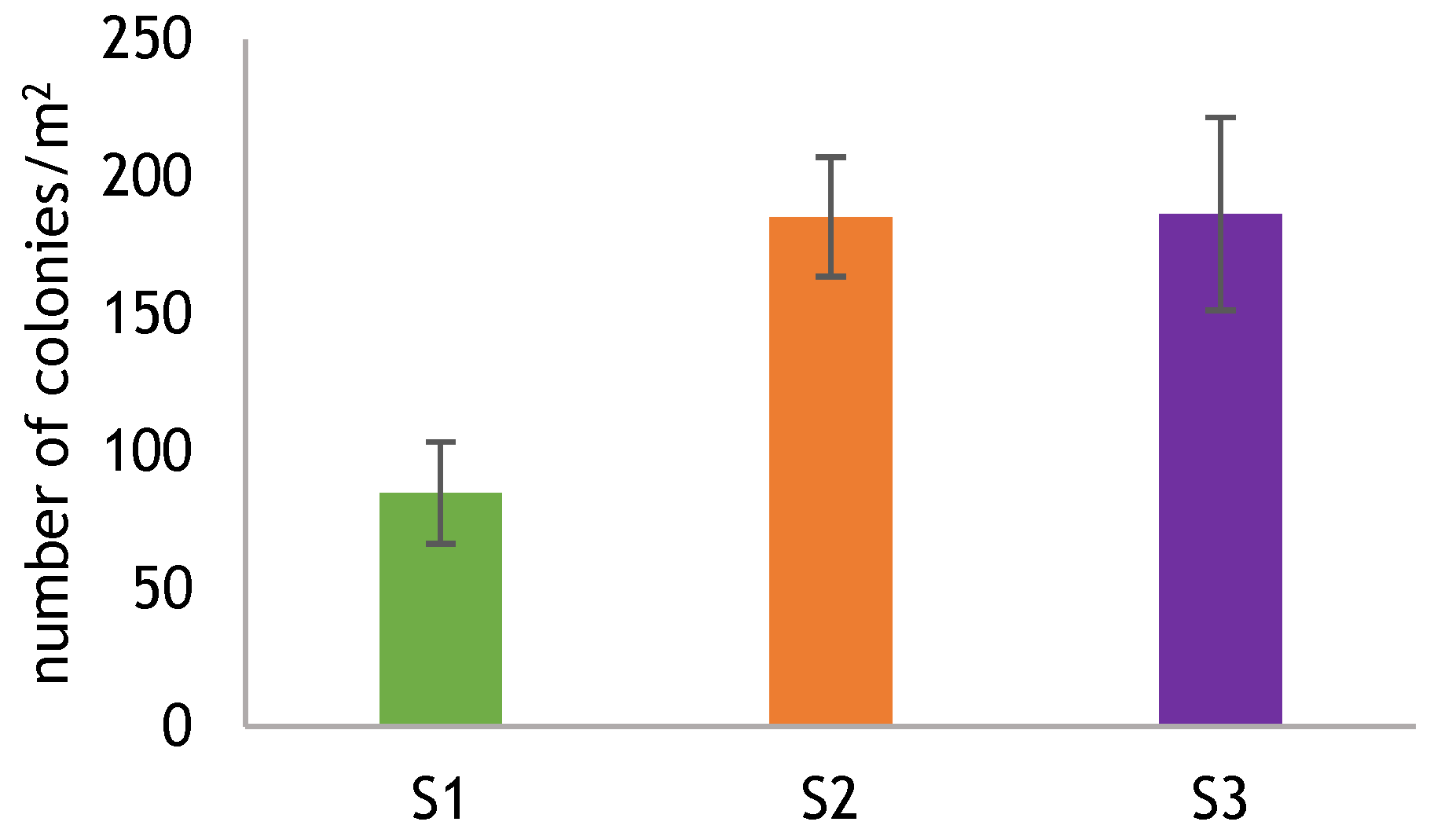
| Site | Maximum Depth | Bottom Morphology | Transects Depth |
|---|---|---|---|
| S1 | 52 m | Cliff with ravines | 51 ± 1 m |
| S2 | 65 m | Boulder ridge 4 m high | 56 ± 1 m |
| S3 | 60 m | Cliff with ravines and fractures | 54 ± 1 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercurio, M.; Corriero, G.; Giménez, G.A.; Dadamo, M.; Pierri, C. Preliminary Assessment of Macrobenthos Associated with Red Coral Corallium rubrum (Linnaeus, 1758) Populations in the Northeastern Ionian Sea. J. Mar. Sci. Eng. 2024, 12, 1825. https://doi.org/10.3390/jmse12101825
Mercurio M, Corriero G, Giménez GA, Dadamo M, Pierri C. Preliminary Assessment of Macrobenthos Associated with Red Coral Corallium rubrum (Linnaeus, 1758) Populations in the Northeastern Ionian Sea. Journal of Marine Science and Engineering. 2024; 12(10):1825. https://doi.org/10.3390/jmse12101825
Chicago/Turabian StyleMercurio, Maria, Giuseppe Corriero, Guadalupe Anahi Giménez, Marco Dadamo, and Cataldo Pierri. 2024. "Preliminary Assessment of Macrobenthos Associated with Red Coral Corallium rubrum (Linnaeus, 1758) Populations in the Northeastern Ionian Sea" Journal of Marine Science and Engineering 12, no. 10: 1825. https://doi.org/10.3390/jmse12101825
APA StyleMercurio, M., Corriero, G., Giménez, G. A., Dadamo, M., & Pierri, C. (2024). Preliminary Assessment of Macrobenthos Associated with Red Coral Corallium rubrum (Linnaeus, 1758) Populations in the Northeastern Ionian Sea. Journal of Marine Science and Engineering, 12(10), 1825. https://doi.org/10.3390/jmse12101825







