Changing Humboldt Squid Abundance and Distribution at Different Stages of Oceanic Mesoscale Eddies
Abstract
1. Introduction
2. Materials and Methods
2.1. Fisheries Data
2.2. Environmental Data
2.3. Mesoscale Eddy Analysis
2.4. Normalized Eddy Lifetime
2.5. The Relationship between Eddies and the Fishing Effort, Catch, and CPUE of D. gigas
2.6. Changes in Suitable Environments for D. gigas in Eddies
3. Results
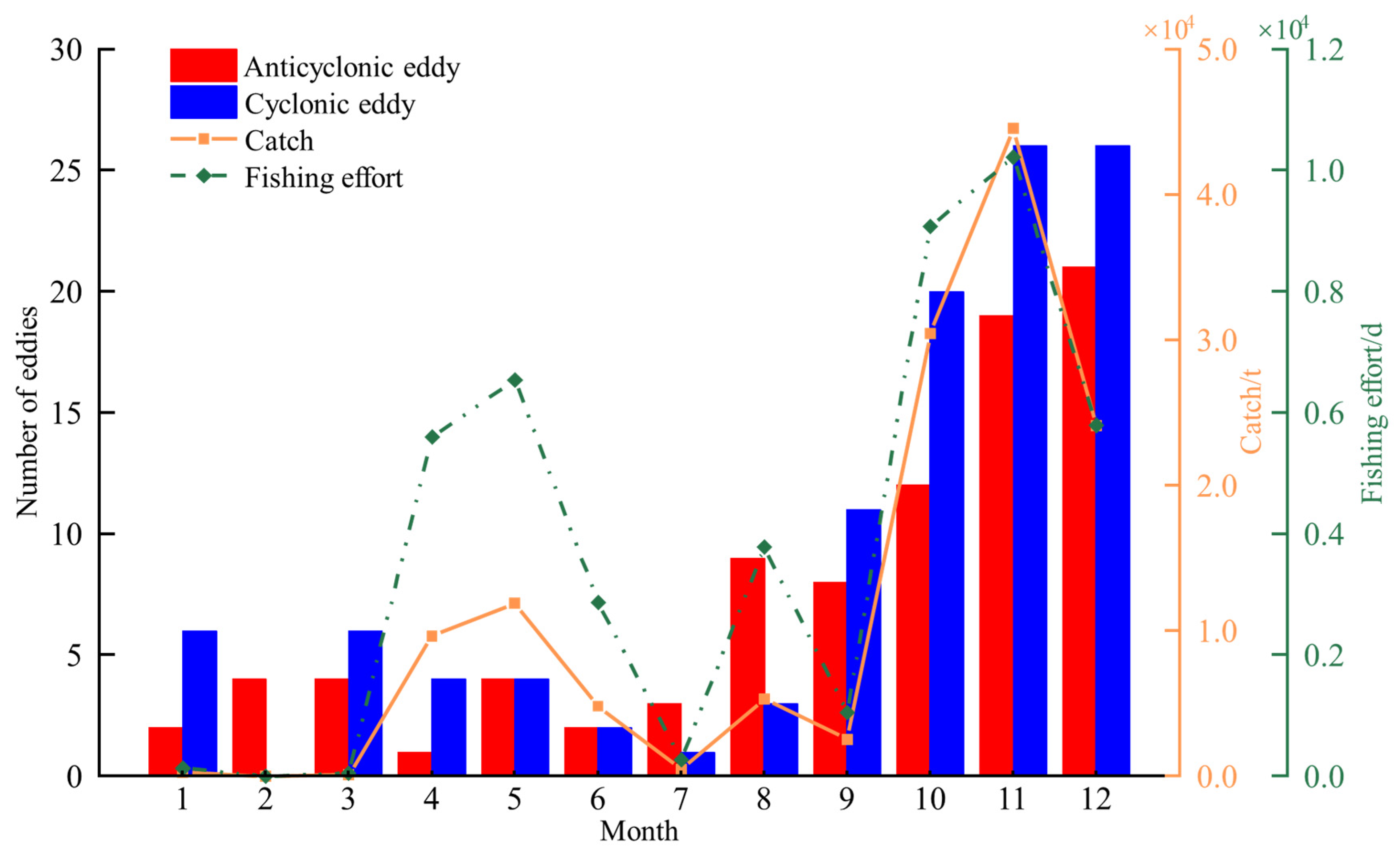
3.1. Monthly Variation in the Number of Eddies, D. gigas Catch, and Fishing Effort
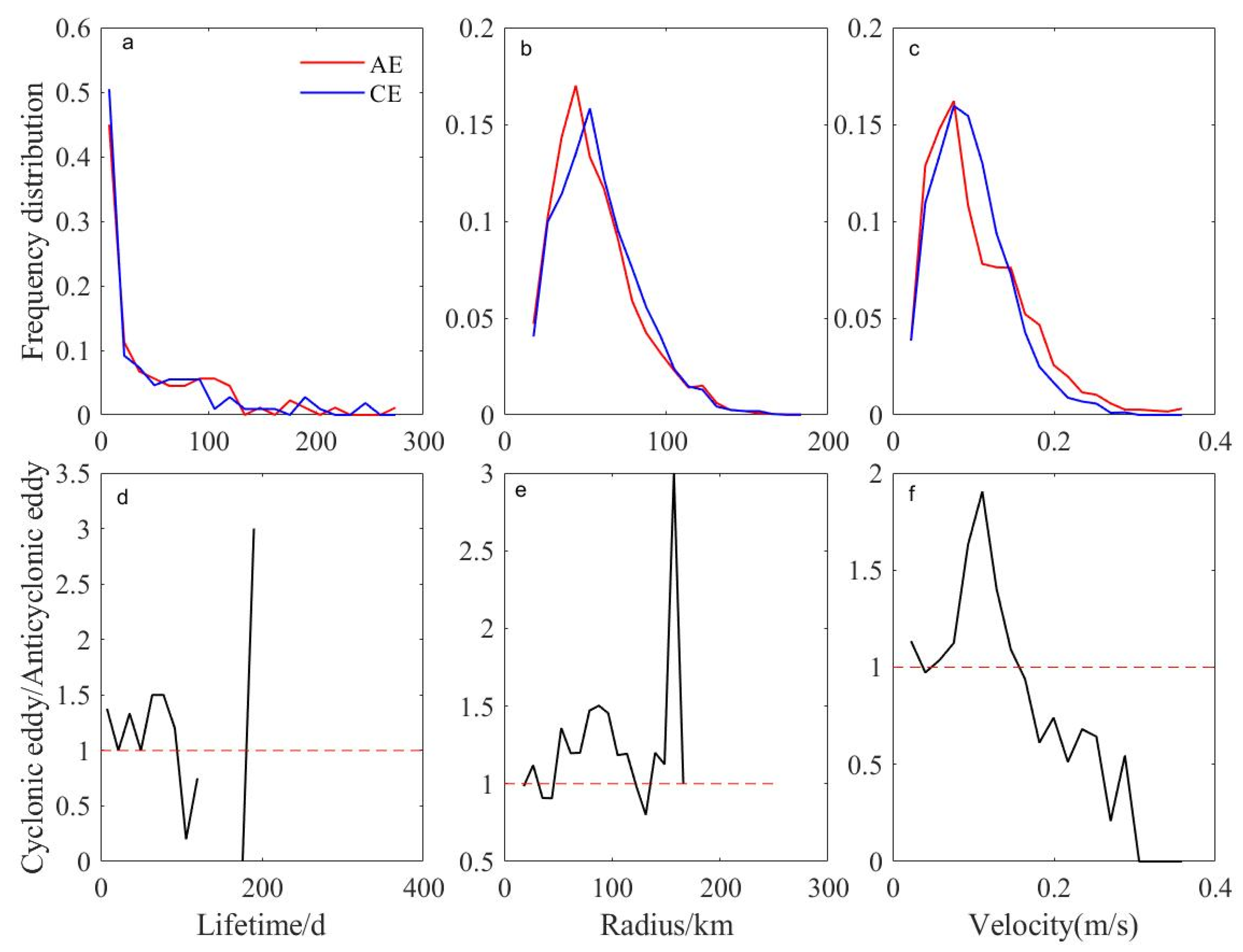
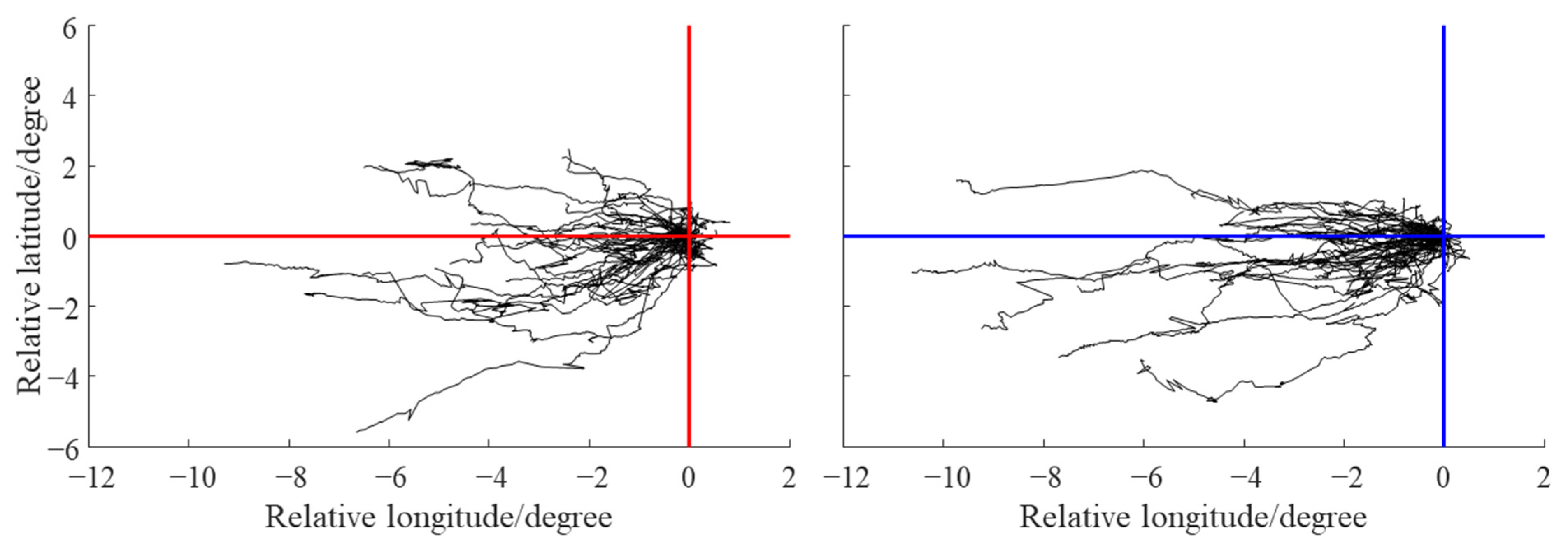
3.2. Characteristic Parameters and Trajectories of Eddies
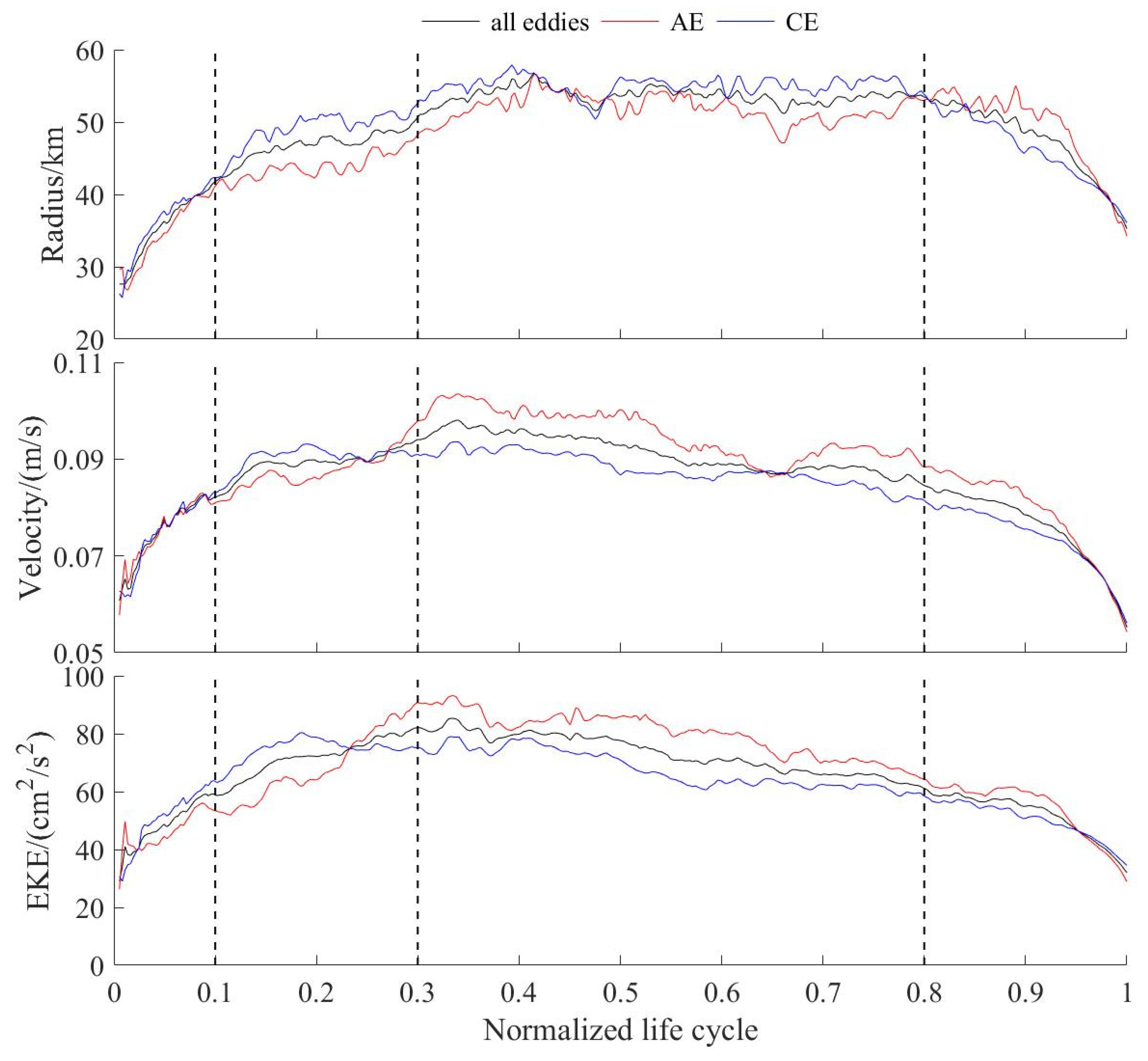
3.3. Changes in Eddies Characteristics over Different Stages
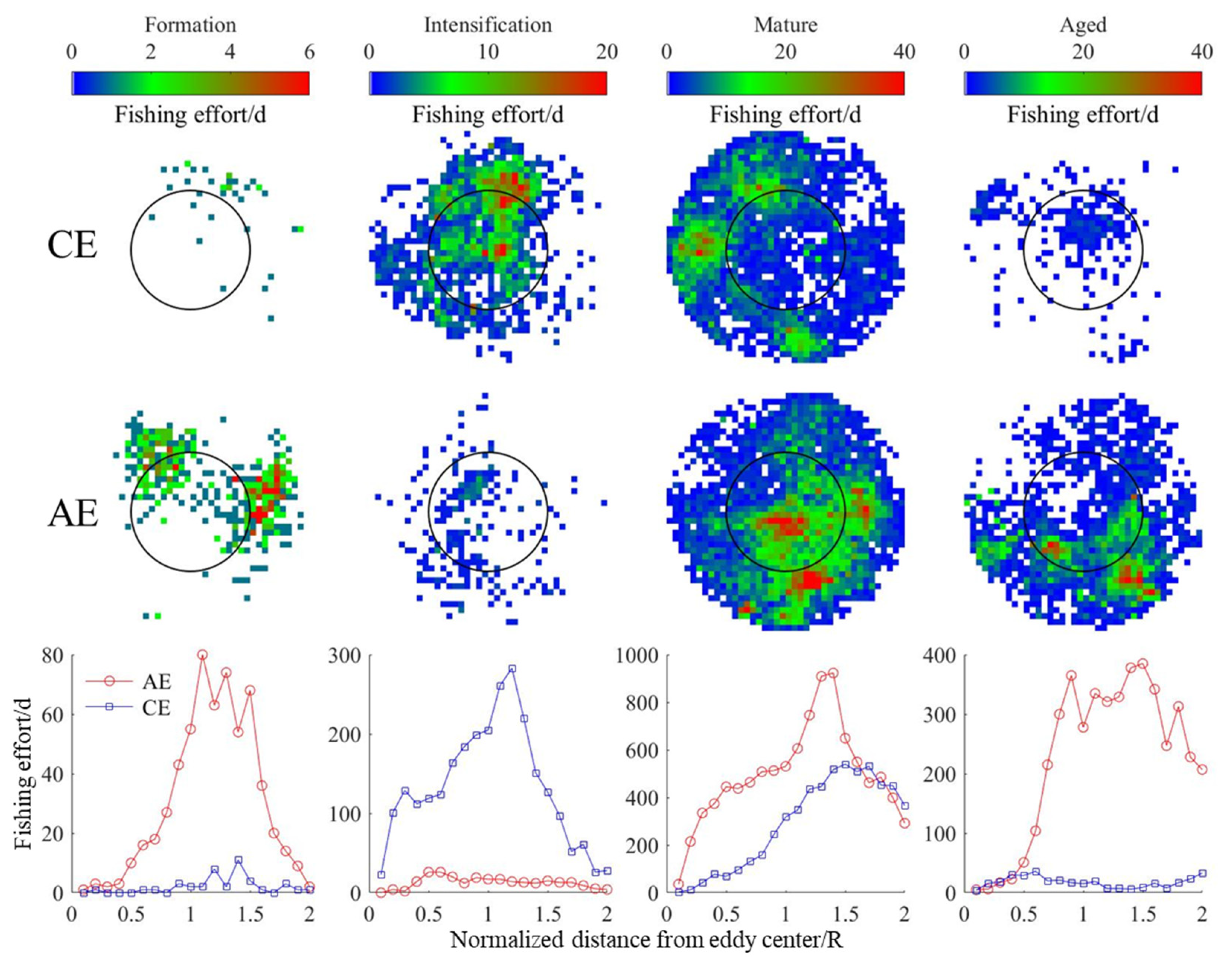
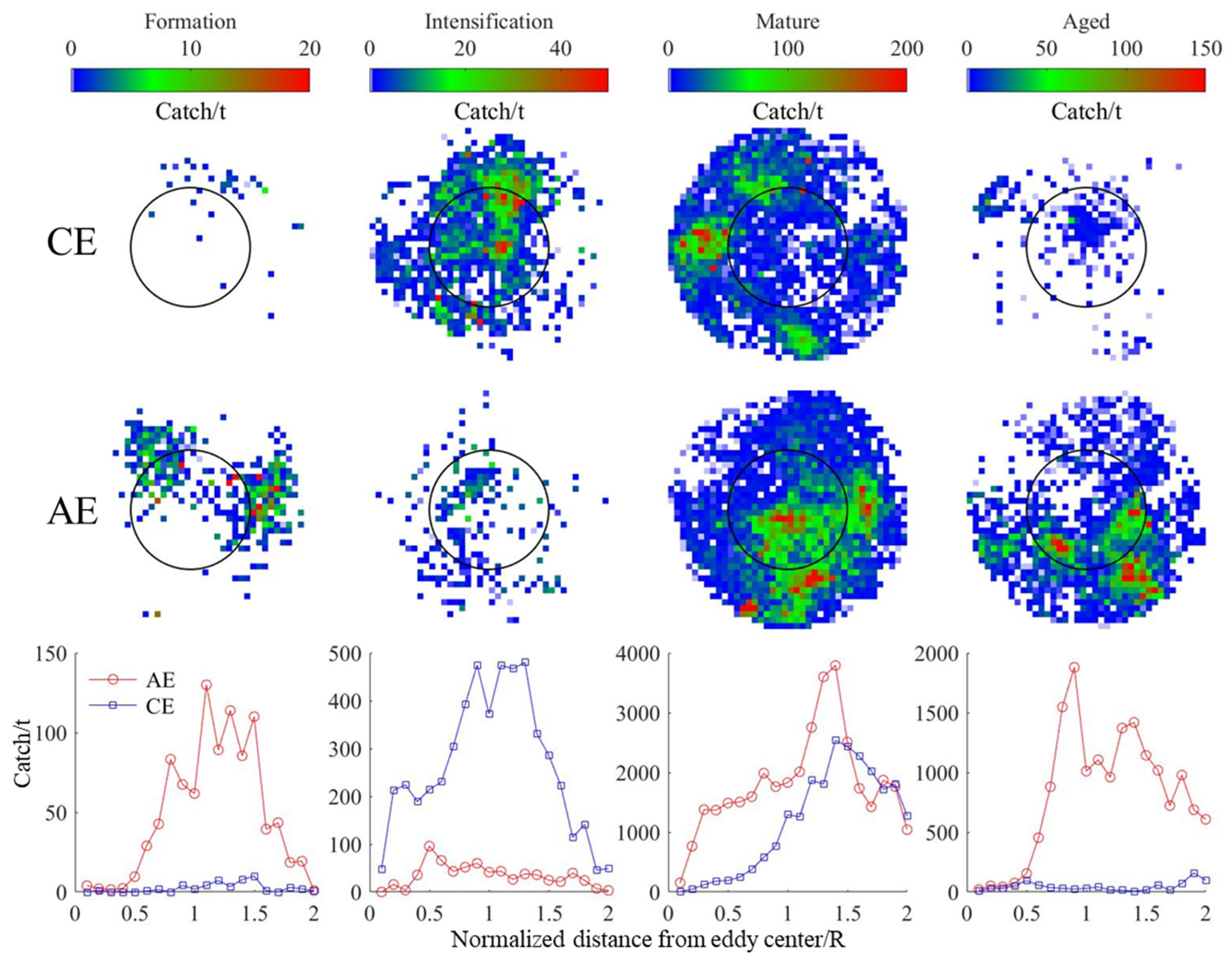
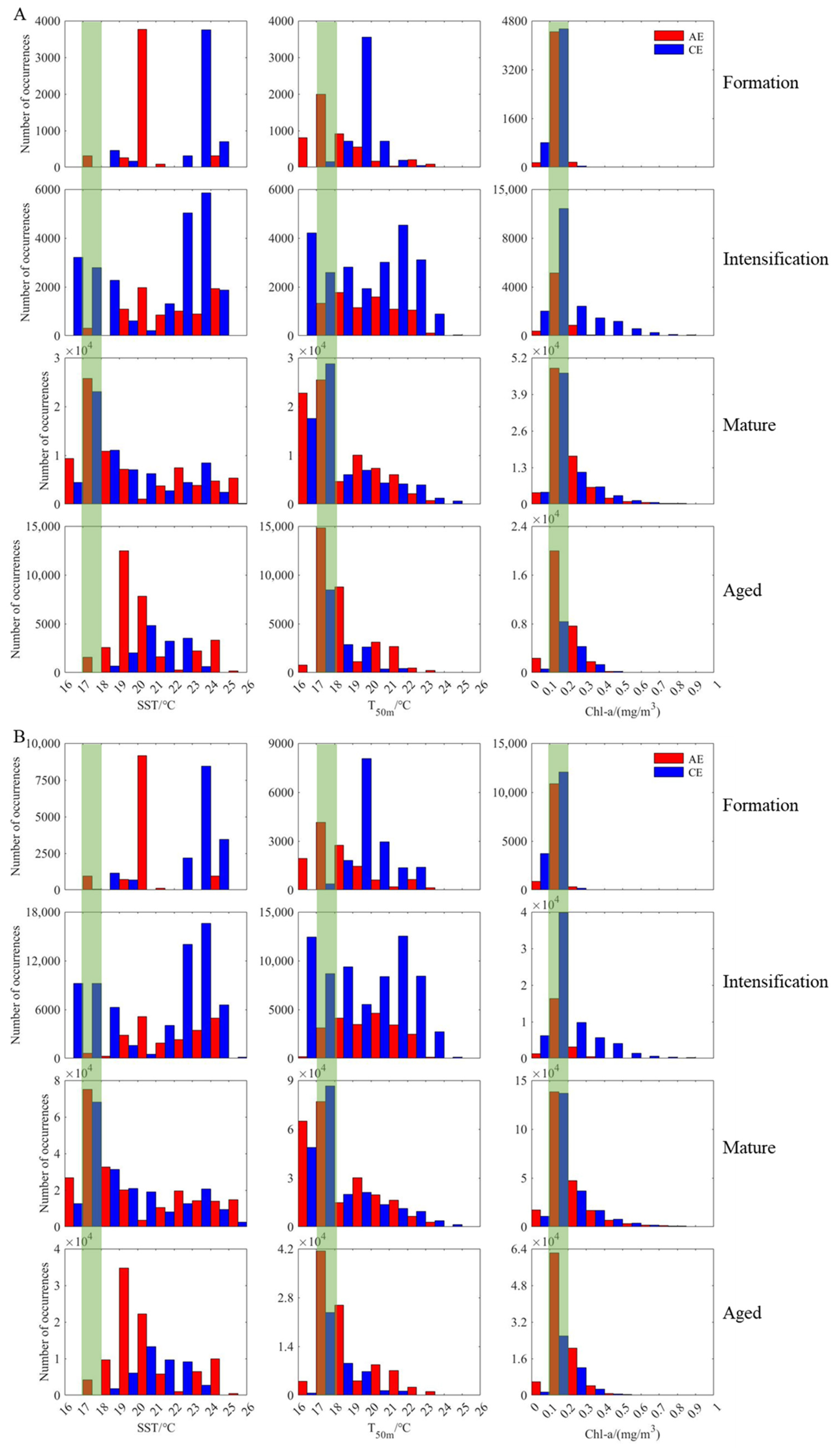
3.4. Changes in D. gigas and Suitable Environments during Different Eddy Stages
4. Discussion
4.1. Evolution and Spatial and Temporal Distribution Characteristics of Eddies off Peruvian Waters
4.2. Correlation Analysis of Eddies and D. gigas Distribution
4.3. Effects of Eddies on Seawater Temperature and Chlorophyll Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chelton, D.B.; Schlax, M.G.; Samelson, R.M.; De Szoeke, R.A. Global Observations of Large Oceanic Eddies. Geophys. Res. Lett. 2007, 34, 2007GL030812. [Google Scholar] [CrossRef]
- Xing, Q.; Yu, H.; Wang, H.; Ito, S.; Chai, F. Mesoscale Eddies Modulate the Dynamics of Human Fishing Activities in the Global Midlatitude Ocean. Fish Fish. 2023, 24, 527–543. [Google Scholar] [CrossRef]
- Janout, M.A.; Weingartner, T.J.; Okkonen, S.R.; Whitledge, T.E.; Musgrave, D.L. Some Characteristics of Yakutat Eddies Propagating along the Continental Slope of the Northern Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 2444–2459. [Google Scholar] [CrossRef]
- Lévy, M.; Klein, P.; Treguier, A.-M. Impact of Sub-Mesoscale Physics on Production and Subduction of Phytoplankton in an Oligotrophic Regime. J. Mar. Res. 2001, 59, 535–565. [Google Scholar] [CrossRef]
- Zhou, K.; Dai, M.; Xiu, P.; Wang, L.; Hu, J.; Benitez-Nelson, C.R. Transient Enhancement and Decoupling of Carbon and Opal Export in Cyclonic Eddies. JGR Ocean. 2020, 125, e2020JC016372. [Google Scholar] [CrossRef]
- McGillicuddy, D.J. Mechanisms of Physical-Biological-Biogeochemical Interaction at the Oceanic Mesoscale. Annu. Rev. Mar. Sci. 2016, 8, 125–159. [Google Scholar] [CrossRef]
- Durán Gómez, G.S.; Nagai, T.; Yokawa, K. Mesoscale Warm-Core Eddies Drive Interannual Modulations of Swordfish Catch in the Kuroshio Extension System. Front. Mar. Sci. 2020, 7, 680. [Google Scholar] [CrossRef]
- Godø, O.R.; Samuelsen, A.; Macaulay, G.J.; Patel, R.; Hjøllo, S.S.; Horne, J.; Kaartvedt, S.; Johannessen, J.A. Mesoscale Eddies Are Oases for Higher Trophic Marine Life. PLoS ONE 2012, 7, e30161. [Google Scholar] [CrossRef] [PubMed]
- Chelton, D.B.; Gaube, P.; Schlax, M.G.; Early, J.J.; Samelson, R.M. The Influence of Nonlinear Mesoscale Eddies on Near-Surface Oceanic Chlorophyll. Science 2011, 334, 328–332. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Ziemann, D.; Kolber, Z.; Bienfang, P.K. Role of Eddy Pumping in Enhancing Primary Production in the Ocean. Nature 1991, 352, 55–58. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Gaube, P.; Woodworth-Jefcoats, P.A.; Kobayashi, D.R.; Braun, C.D. Anticyclonic Eddies Aggregate Pelagic Predators in a Subtropical Gyre. Nature 2022, 609, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.C.; Boustany, A.M.; Roberts, J.J.; Chang, J.; Halpin, P.N. Tuna and Swordfish Catch in the U.S. Northwest Atlantic Longline Fishery in Relation to Mesoscale Eddies. Fish. Oceanogr. 2015, 24, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Montes, I.; Colas, F.; Capet, X.; Schneider, W. On the Pathways of the Equatorial Subsurface Currents in the Eastern Equatorial Pacific and Their Contributions to the Peru-Chile Undercurrent. J. Geophys. Res. 2010, 115, C09003. [Google Scholar] [CrossRef]
- Thiel, M.; Macaya, E.; Acuna, E.; Arntz, W.; Bastias, H.; Brokordt, K.; Camus, P.; Castilla, J.; Castro, L.; Cortes, M.; et al. The Humboldt Current System of Northern and Central Chile: Oceanographic Processes, Ecological Interactions And Socioeconomic Feedback. In Oceanography and Marine Biology—An Annual Review; Gibson, R., Atkinson, R., Gordon, J., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Chaigneau, A.; Dominguez, N.; Eldin, G.; Vasquez, L.; Flores, R.; Grados, C.; Echevin, V. Near-Coastal Circulation in the Northern Humboldt Current System from Shipboard ADCP Data: Circulation of the nhcs from SADCP data. J. Geophys. Res.-Oceans 2013, 118, 5251–5266. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Qian, W.; Jin, Y.; Li, J. δ13C and δ15N in Humboldt Squid Beaks: Understanding Potential Geographic Population Connectivity and Movement. Acta Oceanol. Sin. 2019, 38, 53–59. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World Squid Fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Morales-Bojórquez, E.; Pacheco-Bedoya, J.L. Jumbo Squid Dosidicus gigas: A New Fishery in Ecuador. Rev. Fish. Sci. Aquac. 2016, 24, 98–110. [Google Scholar] [CrossRef]
- Gilly, W.; Markaida, U.; Baxter, C.; Block, B.; Boustany, A.; Zeidberg, L.; Reisenbichler, K.; Robison, B.; Bazzino, G.; Salinas, C. Vertical and Horizontal Migrations by the Jumbo Squid Dosidicus gigas Revealed by Electronic Tagging. Mar. Ecol. Prog. Ser. 2006, 324, 1–17. [Google Scholar] [CrossRef]
- Markaida, U.; Sosa-Nishizaki, O. Food and Feeding Habits of Jumbo Squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. J. Mar. Biol. Ass. 2003, 83, 507–522. [Google Scholar] [CrossRef]
- Alegre, A.; Ménard, F.; Tafur, R.; Espinoza, P.; Argüelles, J.; Maehara, V.; Flores, O.; Simier, M.; Bertrand, A. Comprehensive Model of Jumbo Squid Dosidicus gigas Trophic Ecology in the Northern Humboldt Current System. PLoS ONE 2014, 9, e85919. [Google Scholar] [CrossRef]
- Field, J.C.; Baltz, K.; Phillips, A.J. Range Expansion and Trophic Interactions of the Jumbo Squid, Dosidicus gigas, in the California Current. CalCOFI Rep. 2007, 48, 131–146. [Google Scholar]
- Nigmatullin, C. A Review of the Biology of the Jumbo Squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fish. Res. 2001, 54, 9–19. [Google Scholar] [CrossRef]
- Chaigneau, A.; Gizolme, A.; Grados, C. Mesoscale Eddies off Peru in Altimeter Records: Identification Algorithms and Eddy Spatio-Temporal Patterns. Prog. Oceanogr. 2008, 79, 106–119. [Google Scholar] [CrossRef]
- Chaigneau, A.; Le Texier, M.; Eldin, G.; Grados, C.; Pizarro, O. Vertical Structure of Mesoscale Eddies in the Eastern South Pacific Ocean: A Composite Analysis from Altimetry and Argo Profiling Floats. J. Geophys. Res. 2011, 116, 2011JC007134. [Google Scholar] [CrossRef]
- Csirke, J.; Argüelles-torres, J.; Alegre, A.R.P. Biología, estructura poblacional y pesquería de pota o calamar gigante (Dosidicus gigas) en el Peru. Boletín Inst. Mar. Peru 2018, 33, 302–364. [Google Scholar]
- Ibáñez, C.M.; Sepúlveda, R.D.; Ulloa, P.; Keyl, F.; Pardo-Gandarillas, M.C. The Biology and Ecology of the Jumbo Squid Dosidicus gigas (Cephalopoda) in Chilean Waters: A Review. Lat. Am. J. Aquat. Res. 2016, 43, 402–414. [Google Scholar] [CrossRef]
- Waluda, C.; Rodhouse, P. Remotely Sensed Mesoscale Oceanography of the Central Eastern Pacific and Recruitment Variability in Dosidicus gigas. Mar. Ecol. Prog. Ser. 2006, 310, 25–32. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Yu, W.; Lin, D. Interannual Abundance Fluctuations of Two Oceanic Squids in the Pacific Ocean Can Be Evaluated Through Their Habitat Temperature Variabilities. Front. Mar. Sci. 2021, 8, 770224. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Yu, W.; Chen, X. Geographical Distribution Variations of Humboldt Squid Habitat in the Eastern Pacific Ocean. Ecosyst. Health Sustain. 2023, 9, 0010. [Google Scholar] [CrossRef]
- Fang, X.; Yu, W.; Chen, X.; Zhang, Y. Response of Abundance and Distribution of Humboldt Squid (Dosidicus gigas) to Short-Lived Eddies in the Eastern Equatorial Pacific Ocean from April to June 2017. Front. Mar. Sci. 2021, 8, 721291. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X. Ocean Warming-Induced Range-Shifting of Potential Habitat for Jumbo Flying Squid Dosidicus gigas in the Southeast Pacific Ocean off Peru. Fish. Res. 2018, 204, 137–146. [Google Scholar] [CrossRef]
- Paulino, C.; Segura, M.; Chacón, G. Spatial Variability of Jumbo Flying Squid (Dosidicus gigas) Fishery Related to Remotely Sensed SST and Chlorophyll—A Concentration (2004–2012). Fish. Res. 2016, 173, 122–127. [Google Scholar] [CrossRef]
- Le Vu, B.; Stegner, A.; Arsouze, T. Angular Momentum Eddy Detection and Tracking Algorithm (AMEDA) and Its Application to Coastal Eddy Formation. J. Atmos. Ocean. Technol. 2018, 35, 739–762. [Google Scholar] [CrossRef]
- Juza, M.; Escudier, R.; Pascual, A.; Pujol, M.-I.; Taburet, G.; Troupin, C.; Mourre, B.; Tintoré, J. Impacts of Reprocessed Altimetry on the Surface Circulation and Variability of the Western Alboran Gyre. Adv. Space Res. 2016, 58, 277–288. [Google Scholar] [CrossRef]
- Xu, G.; Dong, C.; Liu, Y.; Gaube, P.; Yang, J. Chlorophyll Rings around Ocean Eddies in the North Pacific. Sci Rep. 2019, 9, 2056. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Wan, R.; Zhang, C. Model Selection between Traditional and Popular Methods for Standardizing Catch Rates of Target Species: A Case Study of Japanese Spanish Mackerel in the Gillnet Fishery. Fish. Res. 2015, 161, 312–319. [Google Scholar] [CrossRef]
- Zhou, K.; Benitez-Nelson, C.R.; Huang, J.; Xiu, P.; Sun, Z.; Dai, M. Cyclonic Eddies Modulate Temporal and Spatial Decoupling of Particulate Carbon, Nitrogen, and Biogenic Silica Export in the North Pacific Subtropical Gyre. Limnol. Oceanogr. 2021, 66, 3508–3522. [Google Scholar] [CrossRef]
- Gaube, P.; Barceló, C.; McGillicuddy, D.J.; Domingo, A.; Miller, P.; Giffoni, B.; Marcovaldi, N.; Swimmer, Y. The Use of Mesoscale Eddies by Juvenile Loggerhead Sea Turtles (Caretta caretta) in the Southwestern Atlantic. PLoS ONE 2017, 12, e0172839. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Chen, X.; Zhou, M.; Zhang, C. Evaluating the Impacts of Mesoscale Eddies on Abundance and Distribution of Neon Flying Squid in the Northwest Pacific Ocean. Front Mar. Sci. 2022, 9, 862273. [Google Scholar] [CrossRef]
- Xiao, Y.; Punt, A.E.; Millar, R.B.; Quinn, T.J. Models in Fisheries Research: GLMs, GAMS and GLMMs. Fish. Res. 2004, 70, 137–139. [Google Scholar] [CrossRef]
- Kabacoff, R.I. R in Action: Data Analysis and Graphics with R; Manning Publications: Shelter Island, NY, USA, 2011. [Google Scholar]
- Chaigneau, A.; Pizarro, O. Mean Surface Circulation and Mesoscale Turbulent Flow Characteristics in the Eastern South Pacific from Satellite Tracked Drifters. J. Geophys. Res. 2005, 110, 2004JC002628. [Google Scholar] [CrossRef]
- Chaigneau, A.; Pizarro, O. Eddy Characteristics in the Eastern South Pacific. J. Geophys. Res. 2005, 110, 2004JC002815. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, C.; Guan, Y.; Chen, D.; McWilliams, J.; Nencioli, F. Eddy Analysis in the Subtropical Zonal Band of the North Pacific Ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2012, 68, 54–67. [Google Scholar] [CrossRef]
- Samelson, R.M.; Schlax, M.G.; Chelton, D.B. Randomness, Symmetry, and Scaling of Mesoscale Eddy Life Cycles. J. Phys. Oceanogr. 2014, 44, 1012–1029. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Tian, S.; Liu, B.; Qian, W. An Assessment of the West Winter–Spring Cohort of Neon Flying Squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Fish. Res. 2008, 92, 221–230. [Google Scholar] [CrossRef]
- Salthaug, A.; Aanes, S. Catchability and the Spatial Distribution of Fishing Vessels. Can. J. Fish. Aquat. Sci. 2003, 60, 259–268. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Chen, Y.; Xu, L.; Dai, X. Evaluating Habitat Suitability Indices Derived from CPUE and Fishing Effort Data for Ommatrephes bratramii in the Northwestern Pacific Ocean. Fish. Res. 2009, 95, 181–188. [Google Scholar] [CrossRef]
- Ziegler, P.E.; Frusher, S.D.; Johnson, C.R. Space–Time Variation in Catchability of Southern Rock Lobster Jasus edwardsii in Tasmania Explained by Environmental, Physiological and Density-Dependent Processes. Fish. Res. 2003, 61, 107–123. [Google Scholar] [CrossRef]
- Rijnsdorp, A. Effects of Fishing Power and Competitive Interactions among Vessels on the Effort Allocation on the Trip Level of the Dutch Beam Trawl Fleet. ICES J. Mar. Sci. 2000, 57, 927–937. [Google Scholar] [CrossRef]
- Swain, D.P.; Wade, E.J. Spatial Distribution of Catch and Effort in a Fishery for Snow Crab (Chionoecetes opilio): Tests of Predictions of the Ideal Free Distribution. Can. J. Fish. Aquat. Sci. 2003, 60, 897–909. [Google Scholar] [CrossRef]
- Braun, C.D.; Gaube, P.; Sinclair-Taylor, T.H.; Skomal, G.B.; Thorrold, S.R. Mesoscale Eddies Release Pelagic Sharks from Thermal Constraints to Foraging in the Ocean Twilight Zone. Proc. Natl. Acad. Sci. USA 2019, 116, 17187–17192. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.R.; Cheng, I.-J.; Parker, D.M.; Polovina, J.J.; Kamezaki, N.; Balazs, G.H. Loggerhead Turtle (Caretta caretta) Movement off the Coast of Taiwan: Characterization of a Hotspot in the East China Sea and Investigation of Mesoscale Eddies. ICES J. Mar. Sci. 2011, 68, 707–718. [Google Scholar] [CrossRef]
- Davis, R.W.; Ortega-Ortiz, J.G.; Ribic, C.A.; Evans, W.E.; Biggs, D.C.; Ressler, P.H.; Cady, R.B.; Leben, R.R.; Mullin, K.D.; Würsig, B. Cetacean Habitat in the Northern Oceanic Gulf of Mexico. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 121–142. [Google Scholar] [CrossRef]
- Correa-Ramirez, M.A.; Hormazábal, S.; Yuras, G. Mesoscale Eddies and High Chlorophyll Concentrations off Central Chile (29–39°S). Geophys. Res. Lett. 2007, 34, 2007GL029541. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Jin, P.; Zhang, Y.; Yu, W. Changing Humboldt Squid Abundance and Distribution at Different Stages of Oceanic Mesoscale Eddies. J. Mar. Sci. Eng. 2024, 12, 626. https://doi.org/10.3390/jmse12040626
Wu X, Jin P, Zhang Y, Yu W. Changing Humboldt Squid Abundance and Distribution at Different Stages of Oceanic Mesoscale Eddies. Journal of Marine Science and Engineering. 2024; 12(4):626. https://doi.org/10.3390/jmse12040626
Chicago/Turabian StyleWu, Xiaoci, Pengchao Jin, Yang Zhang, and Wei Yu. 2024. "Changing Humboldt Squid Abundance and Distribution at Different Stages of Oceanic Mesoscale Eddies" Journal of Marine Science and Engineering 12, no. 4: 626. https://doi.org/10.3390/jmse12040626
APA StyleWu, X., Jin, P., Zhang, Y., & Yu, W. (2024). Changing Humboldt Squid Abundance and Distribution at Different Stages of Oceanic Mesoscale Eddies. Journal of Marine Science and Engineering, 12(4), 626. https://doi.org/10.3390/jmse12040626





