Extracts from Microalgae and Archaea from the Andalusian Coast: A Potential Source of Antiproliferative, Antioxidant, and Preventive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Halophilic Microalgae
2.2. Microbial Biomass and Culture Conditions
2.3. Extract Preparation
2.3.1. Ethanolic Extracts
2.3.2. Protein Extraction (T3)
2.3.3. Protein Hydrolysis (T4)
2.3.4. Methanolic and Acetonic Fractions (T5)
2.4. Cell Lines and In Vitro Culture
2.5. Cytotoxicity Assay
2.6. Wound-Healing Assay
2.7. Chicken Chorioallantoic Membrane (CAM) Assay
2.8. In Vitro Antioxidant Analysis
2.9. Analysis of the Potential to Induce Detoxifying Enzymes
2.9.1. Obtention of Cytosolic Fractions
2.9.2. Glutathione S-Transferase Measuring
2.9.3. NAD(P)H: Quinone Oxidoreductase Determination
2.10. Statistical Evaluation
3. Results
3.1. Selection and Identification of Halophilic Microalgae
3.2. Antitumor Effects of Functional Extracts
3.2.1. Antiproliferative Effect against Cancer Cells
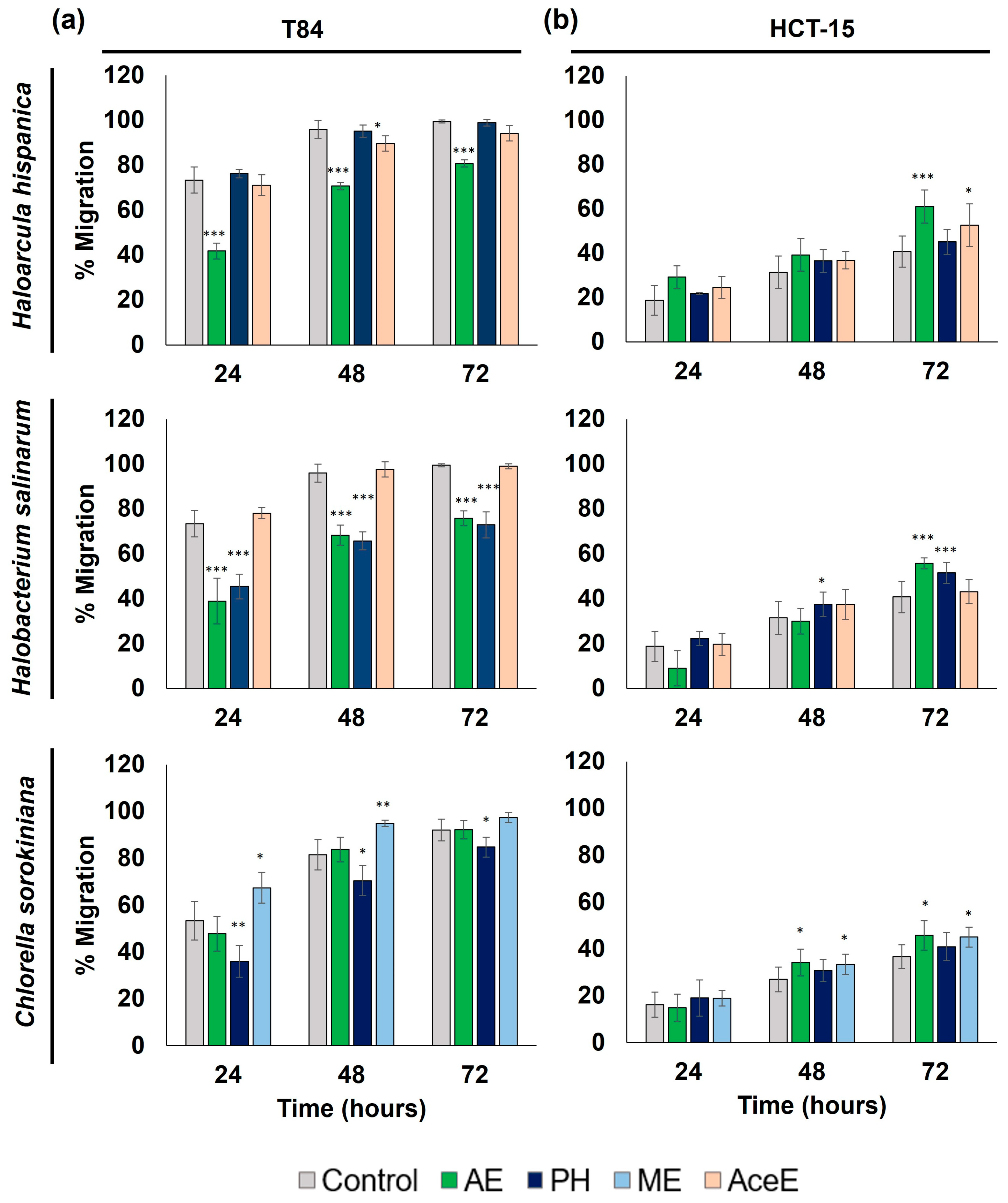
3.2.2. Alteration of Cell Migration Capacity Study
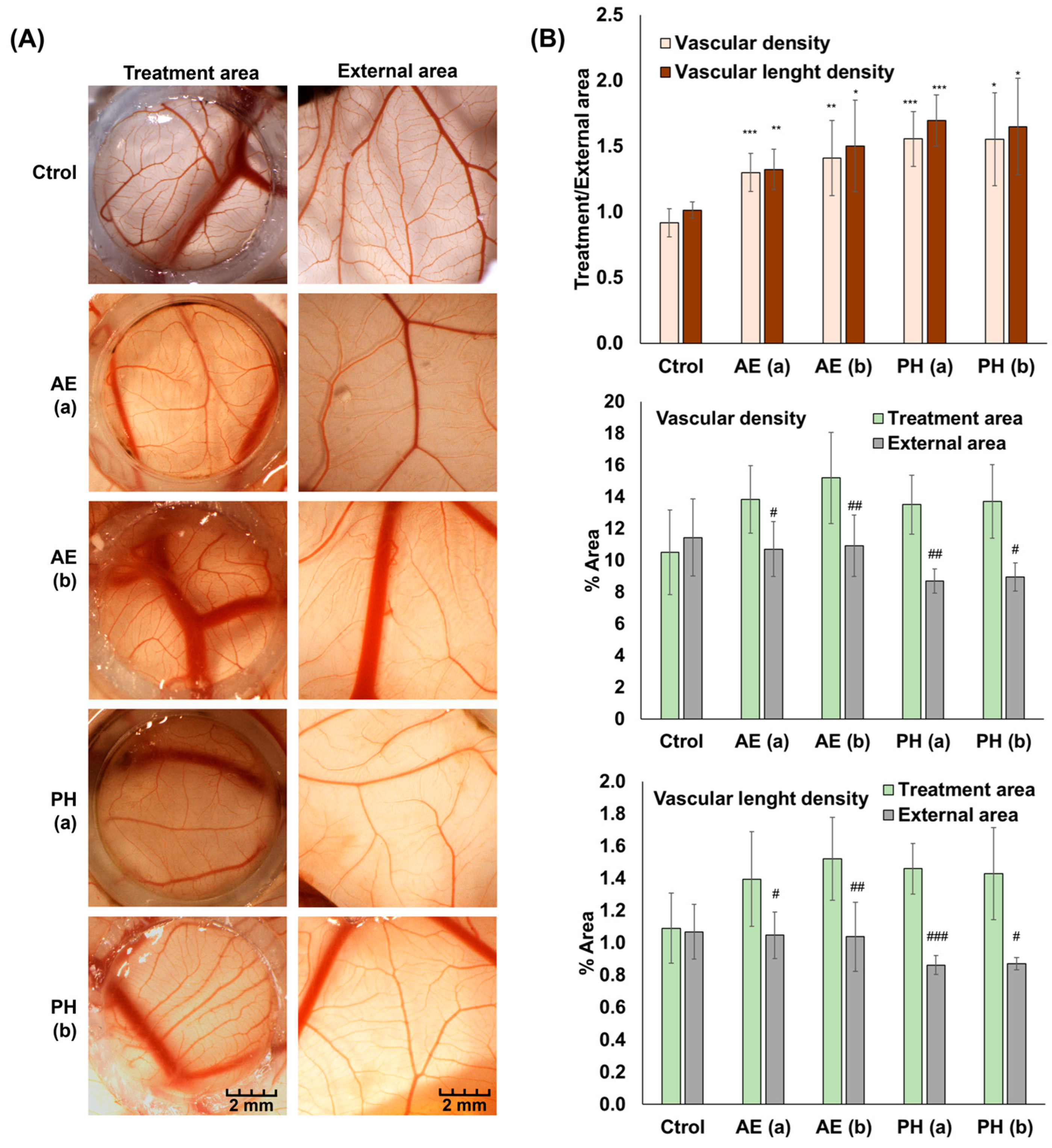
3.2.3. Angiogenesis Study
3.3. Antioxidant and Preventive Effect of Functional Extracts
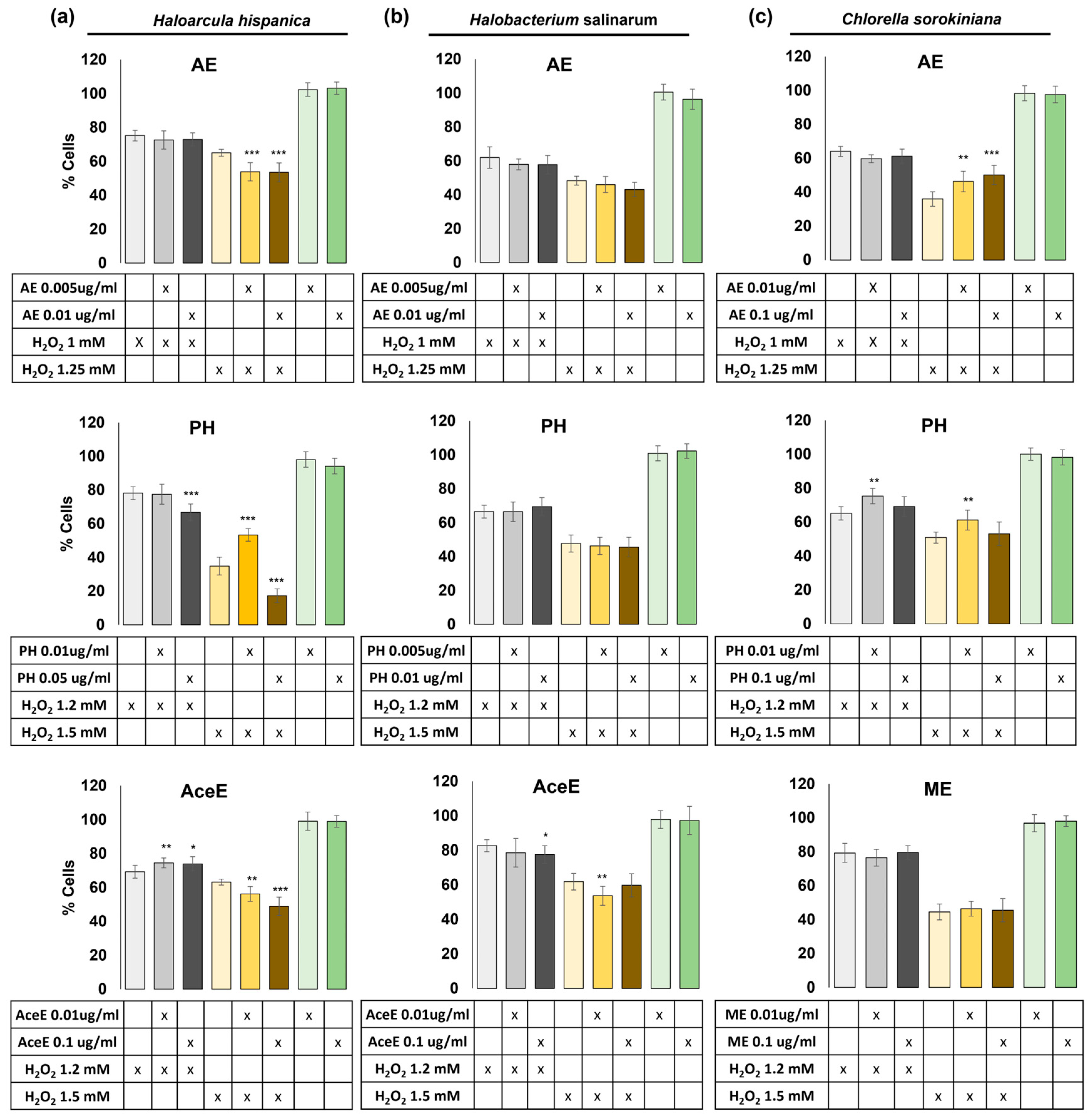
3.3.1. Protection Study against Reactive Oxygen Species
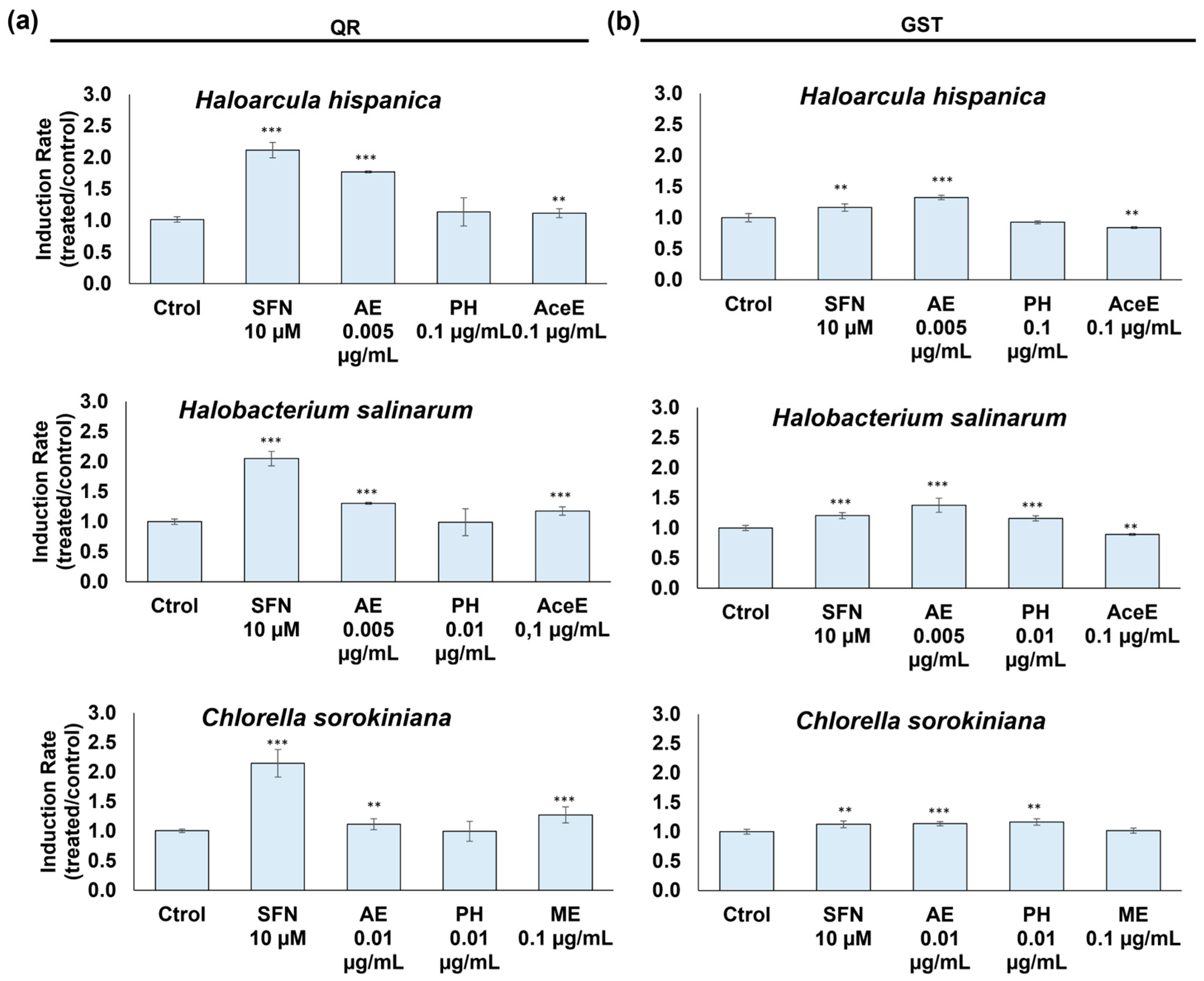
3.3.2. Detoxifying Enzyme Activity Enhancement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Shukla, Y.; George, J. Combinatorial Strategies Employing Nutraceuticals for Cancer Development. Ann. N. Y Acad. Sci. 2011, 1229, 162–175. [Google Scholar] [CrossRef]
- Martínez, C.; Mairet, F.; Bernard, O. Theory of Turbid Microalgae Cultures. J. Theor. Biol. 2018, 456, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal Prospects of Antioxidants From Algal Sources in Cancer Therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gu, X.; Yang, D.; Xu, S.; Wang, S.; Chen, X.; Wang, Z. Bioactive Substances and Potentiality of Marine Microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cui, H.-L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef]
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution UHPLC-Q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Bonete, M.-J.; León, R.; Kharroub, K. Characterization and Biological Activities of Carotenoids Produced by Three Haloarchaeal Strains Isolated from Algerian Salt Lakes. Arch. Microbiol. 2021, 204, 6. [Google Scholar] [CrossRef]
- Moghimipour, E.; Abedishirehjin, S.; Baghbadorani, M.A.; Handali, S. Bacteria and Archaea: A New Era of Cancer Therapy. J. Control. Release 2021, 338, 1–7. [Google Scholar] [CrossRef]
- Esposito, R.; Federico, S.; Glaviano, F.; Somma, E.; Zupo, V.; Costantini, M. Bioactive Compounds from Marine Sponges and Algae: Effects on Cancer Cell Metabolome and Chemical Structures. Int. J. Mol. Sci. 2022, 23, 10680. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, C.Y.; Tekarslan Şahin, H.; İnan, B.; Özçimen, D.; Erginer, Y.Ö. In Vitro Cytotoxic Activity of Microalgal Extracts Loaded Nano–Micro Particles Produced via Electrospraying and Microemulsion Methods. Biotechnol. Prog. 2019, 35, e2876. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Molina-Miras, A.; Aguilera-Sáez, L.M.; López-Rosales, L.; Cerón-García, M.d.C.; Sánchez-Mirón, A.; Olmo-García, L.; Carrasco-Pancorbo, A.; García-Camacho, F.; Molina-Grima, E.; et al. Production of Amphidinols and Other Bioproducts of Interest by the Marine Microalga Amphidinium Carterae Unraveled by Nuclear Magnetic Resonance Metabolomics Approach Coupled to Multivariate Data Analysis. J. Agric. Food Chem. 2019, 67, 9667–9682. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Barin-Le Guellec, C.; Lafay-Chebassier, C.; Ingrand, I.; Tournamille, J.-F.; Boudet, A.; Lanoue, M.-C.; Defossez, G.; Ingrand, P.; Perault-Pochat, M.-C.; Etienne-Grimaldi, M.-C. Toxicities Associated with Chemotherapy Regimens Containing a Fluoropyrimidine: A Real-Life Evaluation in France. Eur. J. Cancer 2020, 124, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, K.M.; Adams, E.J.; Loprinzi, C.L.; Lustberg, M.B. Recent Developments of Novel Pharmacologic Therapeutics for Prevention of Chemotherapy-Induced Peripheral Neuropathy. Clin. Cancer Res. 2019, 25, 6295–6301. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; González, K.; Mesta, F.; Couder, B.; Tavarez, Z.; Zavala, R.; Hernandez, I.; Garrido, G.; Rodeiro, I.; Vanden Berghe, W. Polyphenolic Fraction Obtained From Thalassia Testudinum Marine Plant and Thalassiolin B Exert Cytotoxic Effects in Colorectal Cancer Cells and Arrest Tumor Progression in a Xenograft Mouse Model. Front. Pharmacol. 2020, 11, 592985. [Google Scholar] [CrossRef]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New Drugs from the Sea: Pro-Apoptotic Activity of Sponges and Algae Derived Compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Abd El Latif, A.; Elkaw, E.M.; Alotaibi, A.S.; Alenzi, A.M.; Hamza, H.A. Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella Vulgaris and Its Blend with Different Vitamins. Molecules 2022, 27, 1602. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Jantakee, K.; Wathikthinnakon, M.; Panwong, S.; Pekkoh, J.; Duangjan, K.; Yenchitsomanus, P.; Panya, A. Microalga Chlorella sp. Extract Induced Apoptotic Cell Death of Cholangiocarcinoma via AKT/mTOR Signaling Pathway. Biomed. Pharmacother. 2023, 160, 114306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Johnson, E.J.; MacElroy, R.D.; Speer, H.L.; Bruff, B.S. Effects of Salts on the Halophilic Alga Dunaliella Viridis. J. Bacteriol. 1968, 95, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, M.; Sayago, A.; Ariza, J.; Barneto, A.G.; León, R. Characterization of a Bacterioruberin-Producing Haloarchaea Isolated from the Marshlands of the Odiel River in the Southwest of Spain. Biotechnol. Prog. 2016, 32, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Fawley, K.P.; Fawley, M.W. Observations on the Diversity and Ecology of Freshwater Nannochloropsis (Eustigmatophyceae), with Descriptions of New Taxa. Protist 2007, 158, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Gómez-Villegas, P.; Vigara, J.; Vila, M.; Varela, J.; Barreira, L.; Léon, R. Antioxidant, Antimicrobial, and Bioactive Potential of Two New Haloarchaeal Strains Isolated from Odiel Salterns (Southwest Spain). Biology 2020, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-J.; Ku, K.-L.; Lee, M.-H.; Su, N.-W. Influence of Nutritive Factors on C50 Carotenoids Production by Haloferax Mediterranei ATCC 33500 with Two-Stage Cultivation. Bioresour. Technol. 2010, 101, 6487–6493. [Google Scholar] [CrossRef] [PubMed]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Sánchez, C.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Cantarero, S.; Arrebola, F.; Fernández-Segura, E.; et al. Health Promoting Effects of Lupin (Lupinus albus Var. Multolupa) Protein Hydrolyzate and Insoluble Fiber in a Diet-Induced Animal Experimental Model of Hypercholesterolemia. Food Res. Int. 2013, 54, 1471–1481. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Fuel, M.; Mesas, C.; Martínez, R.; Ortiz, R.; Quiñonero, F.; Prados, J.; Porres, J.M.; Melguizo, C. Antioxidant and Antiproliferative Potential of Ethanolic Extracts from Moringa Oleifera, Tropaeolum Tuberosum and Annona Cherimola in Colorrectal Cancer Cells. Biomed. Pharmacother. 2021, 143, 112248. [Google Scholar] [CrossRef] [PubMed]
- Abolhasani, M.H.; Safavi, M.; Goodarzi, M.T.; Kassaee, S.M.; Azin, M. Identification and Anti-Cancer Activity in 2D and 3D Cell Culture Evaluation of an Iranian Isolated Marine Microalgae Picochlorum sp. RCC486. DARU J. Pharm. Sci. 2018, 26, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, F.; Baril, P.; Barkallah, M.; Perche, F.; Abdelkafi, S.; Fendri, I.; Pichon, C. Deciphering the Biological Activities of Dunaliella sp. Aqueous Extract from Stressed Conditions on Breast Cancer: From in Vitro to in Vivo Investigations. Int. J. Mol. Sci. 2020, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella Tertiolecta Extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- El-fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of Antioxidant and Anticancer Activity of Crude Extract and Different Fractions of Chlorella Vulgaris Axenic Culture Grown under Various Concentrations of Copper Ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological Properties of Carotenoids Extracted from Halobacterium Halobium Isolated from a Tunisian Solar Saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Zargar, M.; Zolfaghari, M.R.; Amoozegar, M.A. Carotenoid Pigment of Halophilic Archaeon Haloarcula sp. A15 Induces Apoptosis of Breast Cancer Cells. Cell Biochem. Funct. 2023, 41, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Alateyah, N.; Ahmad, S.M.S.; Gupta, I.; Fouzat, A.; Thaher, M.I.; Das, P.; Al Moustafa, A.-E.; Ouhtit, A. Haematococcus Pluvialis Microalgae Extract Inhibits Proliferation, Invasion, and Induces Apoptosis in Breast Cancer Cells. Front. Nutr. 2022, 9, 882956. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial Cells and VEGF in Vascular Development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer Effects of Marine Carotenoids, Fucoxanthin and Its Deacetylated Product, Fucoxanthinol, on Osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef]
- Ebrahimi Nigjeh, S.; Yusoff, F.M.; Mohamed Alitheen, N.B.; Rasoli, M.; Keong, Y.S.; bin Omar, A.R. Cytotoxic Effect of Ethanol Extract of Microalga, Chaetoceros Calcitrans, and Its Mechanisms in Inducing Apoptosis in Human Breast Cancer Cell Line. Biomed. Res. Int. 2013, 2013, 783690. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hong, J.-M.; Kim, E.J.; Jung, S.W.; Kim, S.-M.; Kim, J.E.; Kim, I.-C.; Kim, S. Anti-Inflammation and Anti-Cancer Activity of Ethanol Extract of Antarctic Freshwater Microalga, Micractinium sp. Int. J. Med. Sci. 2018, 15, 929–936. [Google Scholar] [CrossRef]
- Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I.; Anam, M.; Maharjan, S.; Amjad, Z.; Khan, S. A Systematic Review of Apoptosis in Correlation with Cancer: Should Apoptosis Be the Ultimate Target for Cancer Treatment? Cureus 2022, 14, e28496. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in Microalgae-Derived Phytosterols for Functional Food and Pharmaceutical Applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The Protective Effect of Dietary Phytosterols on Cancer Risk: A Systematic Meta-Analysis. J. Oncol. 2019, 2019, e7479518. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative Effects of Carotenoids Extracted from Chlorella ellipsoidea and Chlorella vulgaris on Human Colon Cancer Cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef]
- Adamczyk-Grochala, J.; Wnuk, M.; Duda, M.; Zuczek, J.; Lewinska, A. Treatment with Modified Extracts of the Microalga Planktochlorella Nurekis Attenuates the Development of Stress-Induced Senescence in Human Skin Cells. Nutrients 2020, 12, 1005. [Google Scholar] [CrossRef]
- Bari, E.; Arciola, C.R.; Vigani, B.; Crivelli, B.; Moro, P.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Bruni, G.; Chlapanidas, T.; et al. In Vitro Effectiveness of Microspheres Based on Silk Sericin and Chlorella vulgaris or Arthrospira platensis for Wound Healing Applications. Materials 2017, 10, 983. [Google Scholar] [CrossRef]
- Jarquín-Cordero, M.; Chávez, M.N.; Centeno-Cerdas, C.; Bohne, A.-V.; Hopfner, U.; Machens, H.-G.; Egaña, J.T.; Nickelsen, J. Towards a Biotechnological Platform for the Production of Human Pro-Angiogenic Growth Factors in the Green Alga Chlamydomonas Reinhardtii. Appl. Microbiol. Biotechnol. 2020, 104, 725–739. [Google Scholar] [CrossRef]
- Syarina, P.N.A.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound Healing Potential of Spirulina Platensis Extracts on Human Dermal Fibroblast Cells. EXCLI J. 2015, 14, 385–393. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella Vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Baati, H.; Siala, M.; Azri, C.; Ammar, E.; Trigui, M. Hydrolytic Enzyme Screening and Carotenoid Production Evaluation of Halophilic Archaea Isolated from Highly Heavy Metal-Enriched Solar Saltern Sediments. Braz. J. Microbiol. 2022, 53, 1893–1906. [Google Scholar] [CrossRef]
- Kesbiç, F.I.; Gültepe, N. Carotenoid Characterization, Fatty Acid Profiles, and Antioxidant Activities of Haloarchaeal Extracts. J. Basic. Microbiol. 2024, 64, 2300330. [Google Scholar] [CrossRef]
- Selim, S.; Akhtar, N.; Hagagy, N.; Alanazi, A.; Warrad, M.; El Azab, E.; Elamir, M.Y.M.; Al-Sanea, M.M.; Jaouni, S.K.A.; Abdel-Mawgoud, M.; et al. Selection of Newly Identified Growth-Promoting Archaea Haloferax Species with a Potential Action on Cobalt Resistance in Maize Plants. Front. Plant Sci. 2022, 13, 872654. [Google Scholar] [CrossRef]
- Castro, M.S.; Silva, J.C.; Machado, B.R.; Guimarães, P.S.; Lombardi, A.T.; Martins, C.D.M.G.; Zanette, J. Differential Effects of Atrazine on Chlorophyceae Species and Association with Morphology, Photosynthesis, Chlorophyll Content, and Glutathione-S-Transferase Activity. Environ. Toxicol. Chem. 2022, 41, 1675–1685. [Google Scholar] [CrossRef]
- Hamed, S.M.; Okla, M.K.; Al-Saadi, L.S.; Hozzein, W.N.; Mohamed, H.S.; Selim, S.; AbdElgawad, H. Evaluation of the Phycoremediation Potential of Microalgae for Captan Removal: Comprehensive Analysis on Toxicity, Detoxification and Antioxidants Modulation. J. Hazard. Mater. 2022, 427, 128177. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Chang, F.; Dang, C.; Fu, J. Combined Effects of Sulfamethoxazole and Erythromycin on a Freshwater Microalga, Raphidocelis Subcapitata: Toxicity and Oxidative Stress. Antibiotics 2021, 10, 576. [Google Scholar] [CrossRef]

| Microbial Species | EE IC50 (µg/mL) | EEM IC50 (µg/mL) | AE IC50 (µg/mL) | PH IC50 (µg/mL) |
|---|---|---|---|---|
| Dunaliella HM13 | >1000 | >1000 | 184.67 ± 15.71 | 477.98 ± 29.16 |
| Dunaliella HM5 | >1000 | >1000 | 137.56 ± 7.58 | 356.36 ± 11.22 |
| Picochlorum sp. | >600 | >600 | 194.47 ± 57.52 | 229.89 ± 14.84 |
| Chlorella sorokiniana | 523.20 ± 47.71 | 569.33 ± 88.59 | 54.05 ± 27.79 | 66.73 ± 7.64 |
| Chlamydomonas reinhardtii | >800 | 399.29 ± 9.42 | >150 | >400 |
| Haloarcula hispanica | >1000 | >1000 | 118.83 ± 57.52 | 153.56 ± 3.26 |
| Halobacterium salinarum | >1000 | >1000 | 138.47 ± 33.94 | 111.20 ± 22.20 |
| Microbial Species | EE IC50 (µg/mL) | EEM IC50 (µg/mL) | AE IC50 (µg/mL) | PH IC50 (µg/mL) |
|---|---|---|---|---|
| Dunaliella HM13 | 345.02 ± 33.10 | 198.75 ± 6.20 | 959.23 ± 24.53 | 718.24 ± 30.64 |
| Dunaliella HM5 | 346.46 ± 21.52 | 137.39 ± 8.91 | 593.04 ± 33.91 | 553.11 ± 57.24 |
| Picochlorum sp. | 575.85 ± 42.11 | 114.33 ± 8.91 | >600 | 749.90 ± 27.87 |
| Chlorella sorokiniana | 301.27 ± 22.61 | 59.21 ± 10.30 | 165.41 ± 7.34 | 242.99 ± 15.55 |
| Chlamydomonas reinhardtii | >800 | >300 | >400 | 316.25 ± 6.84 |
| Haloarcula hispanica | >1000 | 181.05 ± 7.69 | 160.44 ± 4.67 | >1000 |
| Halobacterium salinarum | >1000 | 171.29 ± 10.89 | 110.56 ± 17.12 | >1000 |
| Ref. | Microbial Species | Human Cancer Cell Line | Extract | IC50 Value (μg/mL) | Main Results |
|---|---|---|---|---|---|
| [21] | Chlorella vulgaris | PC-3 prostate Hep-G2 liver HCT-116 colorectal Hela cervical | Methanol | <100 | Supplementing the microalgae C. vulgaris with several vitamins showed an increase not only in its antioxidant and antitumor capacity but also an increase in its total proteins, biomass, and pigment content. |
| Methanol supplemented with Thiamine (vitamin B1) | <100 | ||||
| [22] | Chlorella sp. | A-549 lung Hela cervical MCF7 breast Huh7 hepatocellular CCA and KKU213A cholangiocarcinoma | Polysaccharide | >2000 | The antitumor effect of the ethanolic extract of Chlorella sp. was demonstrated by promoting cell death through the AKT/mTOR pathway. |
| Ethanol | >300 | ||||
| [34] | Dunaliella sp. | MDA-MB-231 and MCF-7 breast Hep-G2 liver A-549 lung | Methanol | >150 | Methanol and ethyl acetate extracts have a high content of phenolic compounds and carotenoids that are associated with an increase in apoptosis through the activation of caspase-3. |
| Ethyl acetate | >200 | ||||
| Chloroform | >500 | ||||
| Hexane | >500 | ||||
| [36] | Dunaliella tertiolecta | MCF-7 breast A-549 lung LNCaP prostate | Ethanol | >100 | The compound violaxanthin was identified as the molecule with the greatest antiproliferative potential present in the extract obtained with dichloromethane. |
| Dichloromethane | >100 | ||||
| [37] | Chlorella vulgaris | Hela cervical | Methanol | >125 | The methanolic extraction of C. vulgaris under copper-mediated stress conditions results in an antitumor effect on the Hela cell line. |
| [38] | Halobacterium halobium | Hep-G2 liver | Carotenoid extraction | >500 | Carotenoids obtained from Halobacterium halobium present an antitumor and antioxidant effect in the HepG2 line. |
| [39] | Haloarcula sp. | MCF-7 breast | Carotenoid extraction | >600 | The pigments obtained from the Haloarcula sp. archaea induce the expression of genes involved in apoptosis, thus having an antitumor effect in the breast cancer line MCF-7. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque, C.; Perazzoli, G.; Gómez-Villegas, P.; Vigara, J.; Martínez, R.; García-Beltrán, A.; Porres, J.M.; Prados, J.; León, R.; Melguizo, C. Extracts from Microalgae and Archaea from the Andalusian Coast: A Potential Source of Antiproliferative, Antioxidant, and Preventive Compounds. J. Mar. Sci. Eng. 2024, 12, 996. https://doi.org/10.3390/jmse12060996
Luque C, Perazzoli G, Gómez-Villegas P, Vigara J, Martínez R, García-Beltrán A, Porres JM, Prados J, León R, Melguizo C. Extracts from Microalgae and Archaea from the Andalusian Coast: A Potential Source of Antiproliferative, Antioxidant, and Preventive Compounds. Journal of Marine Science and Engineering. 2024; 12(6):996. https://doi.org/10.3390/jmse12060996
Chicago/Turabian StyleLuque, Cristina, Gloria Perazzoli, Patricia Gómez-Villegas, Javier Vigara, Rosario Martínez, Alejandro García-Beltrán, Jesús M. Porres, Jose Prados, Rosa León, and Consolación Melguizo. 2024. "Extracts from Microalgae and Archaea from the Andalusian Coast: A Potential Source of Antiproliferative, Antioxidant, and Preventive Compounds" Journal of Marine Science and Engineering 12, no. 6: 996. https://doi.org/10.3390/jmse12060996








