Mitigation of Vibrio-Induced Metabolic Perturbations in Argopecten purpuratus Scallop Larvae via Probiotic Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Argopecten purpuratus Scallop Larvae of Multiparent Origin
2.2. Scallop Larvae Pretreatment with Probiotic Candidate and Vibrio Infection
2.3. Sample Preparation for Metabolomic Analysis and Data Processing
2.4. Data Processing
2.5. Data Analysis
3. Results
3.1. Metabolome Profiling of Scallop Larvae Following Probiotic Pretreatment after Vibrio Infection
3.2. Metabolite Structural Classes and Key Enriched Pathways Identified in Scallop Larvae Following Probiotic Pretreatment after Vibrio Infection
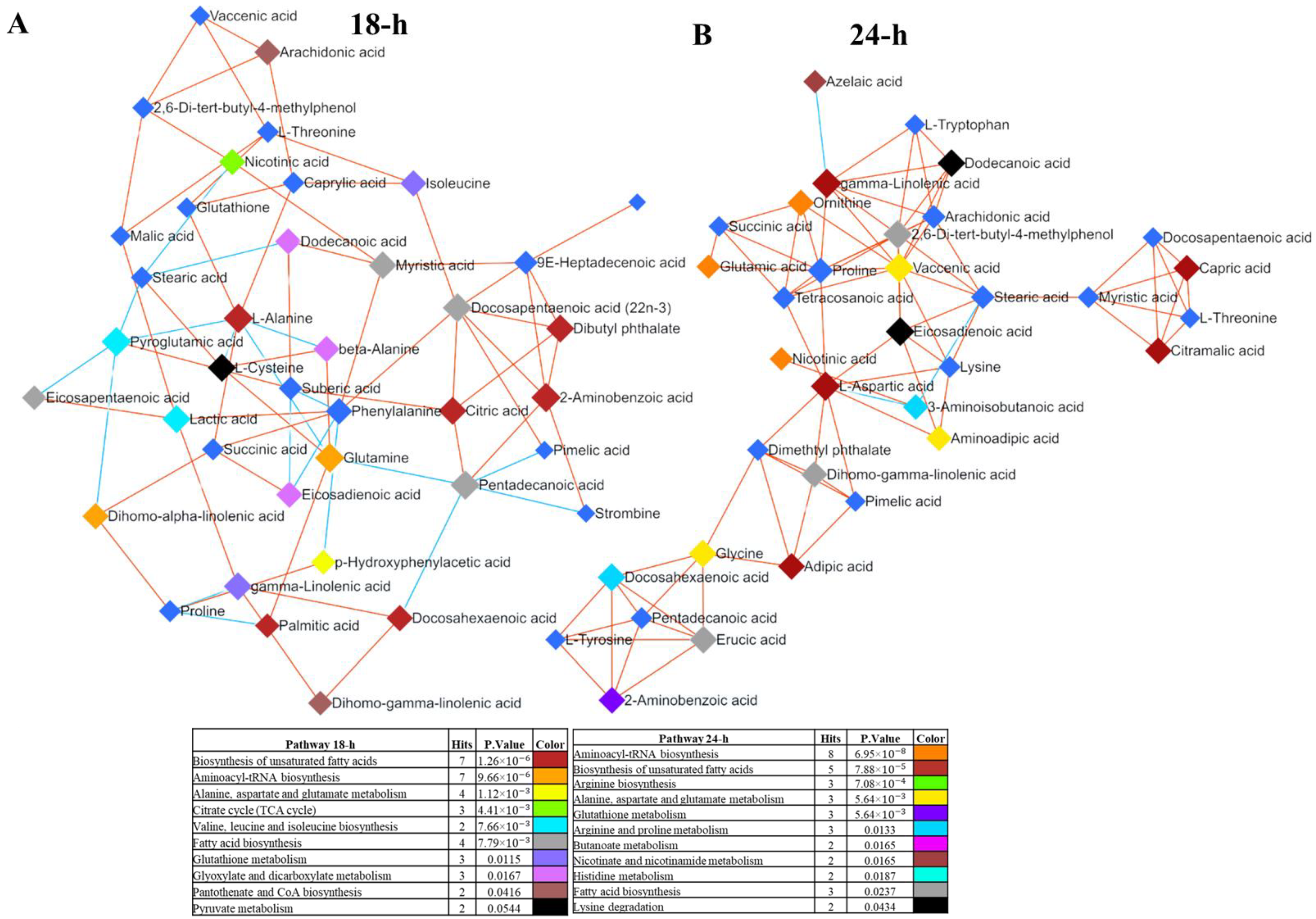
3.3. Network Analysis Reveals Interconnected Metabolites in Scallop Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Estado Mundial de la Pesca y la Acuicultura 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Bakit, J.; Álvarez, G.; Díaz, P.A.; Uribe, E.; Sfeir, R.; Villasante, S.; Bas, T.G.; Lira, G.; Pérez, H.; Hurtado, A.; et al. Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile. Fishes 2022, 7, 380. [Google Scholar] [CrossRef]
- Dubert, J.; Barja, J.L.; Romalde, J.L. New Insights into Pathogenic Vibrios Affecting Bivalves in Hatcheries: Present and Future Prospects. Front. Microbiol. 2017, 8, 762. [Google Scholar] [CrossRef]
- Rojas, R.; Miranda, C.D.; Romero, J.; Barja, J.L.; Dubert, J. Isolation and Pathogenic Characterization of Vibrio bivalvicida Associated With a Massive Larval Mortality Event in a Commercial Hatchery of Scallop Argopecten purpuratus in Chile. Front. Microbiol. 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzón, D.; Canesi, L.; Oyanedel, D.; Travers, M.; Charrière, G.M.; Pruzzo, C.; Vezzulli, L. Vibrio–bivalve interactions in health and disease. Environ. Microbiol. 2020, 22, 4323–4341. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Miranda, C.D.; Opazo, R.; Romero, J. Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck, 1819). J. Invertebr. Pathol. 2015, 124, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Dubert, J.; Romalde, J.L.; Prado, S.; Barja, J.L. Vibrio bivalvicida sp. nov., a novel larval pathogen for bivalve molluscs reared in a hatchery. Syst. Appl. Microbiol. 2016, 39, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Urtubia, R.; Miranda, C.D.; Rodríguez, S.; Dubert, J.; Barja, J.L.; Rojas, R. First Report, Characterization and Pathogenicity of Vibrio chagasii Isolated from Diseased Reared Larvae of Chilean Scallop, Argopecten purpuratus (Lamarck, 1819). Pathogens 2023, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Yue, F.; Shi, X.; Zhou, Z.; Wang, L.; Wang, M.; Yang, J.; Qiu, L.; Song, L. The expression of immune-related genes during the ontogenesis of scallop Chlamys farreri and their response to bacterial challenge. Fish Shellfish. Immunol. 2013, 34, 855–864. [Google Scholar] [CrossRef]
- Rojas, I.; Cárcamo, C.; Stambuk, F.; Mercado, L.; Rojas, R.; Schmitt, P.; Brokordt, K. Expression of immune-related genes during early development of the scallop Argopecten purpuratus after Vibrio splendidus challenge. Aquaculture 2021, 533, 736132. [Google Scholar] [CrossRef]
- Rojas, I.; Cárcamo, C.B.; Defranchi, Y.; Jeno, K.; Rengel, J.; Araya, M.; Tarnok, M.E.; Aguilar, L.; Álvarez, G.; Schmitt, P.; et al. A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus. Animals 2023, 13, 1416. [Google Scholar] [CrossRef]
- Jeria, E.; Oyanedel, D.; Rojas, R.; Farlora, R.; Lira, G.; Mercado, A.; Muñoz, K.; Destoumieux-Garzón, D.; Brokordt, K.; Schmitt, P. Resistance of Argopecten purpuratus scallop larvae to Vibriosis is associated with the front-loading of immune genes and enhanced antimicrobial response. Front. Immunol. 2023, 14, 1150280. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Balcázar, J.L. Biological Approaches for Disease Control in Aquaculture: Advantages, Limitations and Challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Paillard, C.; Gueguen, Y.; Wegner, K.M.; Bass, D.; Pallavicini, A.; Vezzulli, L.; Arzul, I. Recent advances in bivalve-microbiota interactions for disease prevention in aquaculture. Curr. Opin. Biotechnol. 2022, 73, 225–232. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut Microbiota and its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Fallet, M.; Montagnani, C.; Petton, B.; Dantan, L.; de Lorgeril, J.; Comarmond, S.; Chaparro, C.; Toulza, E.; Boitard, S.; Escoubas, J.-M.; et al. Early life microbial exposures shape the Crassostrea gigas immune system for lifelong and intergenerational disease protection. Microbiome 2022, 10, 85. [Google Scholar] [CrossRef]
- Lorgen-Ritchie, M.; Webster, T.U.; McMurtrie, J.; Bass, D.; Tyler, C.R.; Rowley, A.; Martin, S.A.M. Microbiomes in the context of developing sustainable intensified aquaculture. Front. Microbiol. 2023, 14, 1200997. [Google Scholar] [CrossRef]
- Lee, S.-J.; Noh, D.-I.; Lee, Y.-S.; Hasan, T.; Hur, S.W.; Lee, S.; Jeong, S.-M.; Lee, J.M.; Lee, E.-W.; Kim, K.-W.; et al. Effects of host-associated low-temperature probiotics in olive flounder (Paralichthys olivaceus) aquaculture. Sci. Rep. 2024, 14, 2134. [Google Scholar] [CrossRef]
- Muñoz-Cerro, K.; González, R.; Mercado, A.; Lira, G.; Rojas, R.; Yáñez, C.; Cuadros, F.; Oyanedel, D.; Brokordt, K.; Schmitt, P. Scallop larvae resistant to a pathogenic Vibrio harbor host-associated bacteria with probiotic potential. Aquaculture 2024, 579, 740217. [Google Scholar] [CrossRef]
- Hossain, M.K.; Ishak, S.D.; Iehata, S.; Noordiyana, M.N.; Kader, A.; Abol-Munafi, A.B. Growth performance, fatty acid profile, gut, and muscle histo-morphology of Malaysian mahseer, Tor tambroides post larvae fed short-term host associated probiotics. Aquac. Fish. 2024, 9, 35–45. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Esteban, M.Á.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-Associated Probiotics: A Key Factor in Sustainable Aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Frizzo, R.; Bortoletto, E.; Riello, T.; Leanza, L.; Schievano, E.; Venier, P.; Mammi, S. NMR Metabolite profiles of the bivalve mollusc Mytilus galloprovincialis before and after immune stimulation with Vibrio splendidus. Front. Mol. Biosci. 2021, 8, 686770. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.A.; Venter, L.; Welford, M.R.; Kumanan, K.; Alfaro, A.C.; Ragg, N.L. Effects of seawater temperature and acute Vibrio sp. challenge on the haemolymph immune and metabolic responses of adult mussels (Perna canaliculus). Fish Shellfish. Immunol. 2022, 128, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nie, H.; Yan, X. Metabolomic analysis provides new insights into the heat-hardening response of Manila clam (Ruditapes philippinarum) to high temperature stress. Sci. Total. Environ. 2023, 857, 159430. [Google Scholar] [CrossRef] [PubMed]
- Azizan, A.; Carter, J.; Venter, L.; Young, T.; Sharma, S.S.; Chen, T.; Alfaro, A.C. Investigating the effect of bacterial coinfections on juvenile and adult green-lipped mussels (Perna canaliculus). J. World Aquac. Soc. 2024, 55, 386–403. [Google Scholar] [CrossRef]
- Grandiosa, R.; Young, T.; Van Nguyen, T.; Mérien, F.; Alfaro, A.C. Immune response in probiotic-fed New Zealand black-footed abalone (Haliotis iris) under Vibrio splendidus challenge. Fish Shellfish. Immunol. 2020, 104, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Grandiosa, R.; Mérien, F.; Young, T.; Van Nguyen, T.; Gutierrez, N.; Kitundu, E.; Alfaro, A.C. Multi-strain probiotics enhance immune responsiveness and alters metabolic profiles in the New Zealand black-footed abalone (Haliotis iris). Fish Shellfish. Immunol. 2018, 82, 330–338. [Google Scholar] [CrossRef]
- Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2020, 159, i–xi. [CrossRef]
- Aggio, R.; Villas−Bôas, S.G.; Ruggiero, K. Metab: An R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics 2011, 27, 2316–2318. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; E MacDonald, P.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Goodacre, R. Multiblock principal component analysis: An efficient tool for analyzing metabolomics data which contain two influential factors. Metabolomics 2012, 8, 37–51. [Google Scholar] [CrossRef]
- Janková, J.; van de Geer, S. Confidence intervals for high-dimensional inverse covariance estimation. Electron. J. Stat. 2015, 9, 1205–1229. [Google Scholar] [CrossRef]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Dandekar, T.; Heesemann, J.; Goebel, W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat. Rev. Microbiol. 2010, 8, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D. Amino acid metabolism and signalling pathways: Potential targets in the control of infection and immunity. Eur. J. Clin. Nutr. 2021, 75, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Tremblay, R. Effect of varying levels of dietary essential fatty acid during early ontogeny of the sea scallop Placopecten magellanicus. J. Exp. Mar. Biol. Ecol. 2005, 310, 73–86. [Google Scholar] [CrossRef]
- Gagné, R.; Tremblay, R.; Pernet, F.; Miner, P.; Samain, J.-F.; Olivier, F. Lipid requirements of the scallop Pecten maximus (L.) during larval and post-larval development in relation to addition of Rhodomonas salina in diet. Aquaculture 2010, 309, 212–221. [Google Scholar] [CrossRef]
- Telahigue, K.; Rabeh, I.; Mhadhbi, L.; Nechi, S.; Chelbi, E.; Ben Ali, M.; Hedfi, A.; Al-Harbi, M.S.; Hajji, T. Glyphosate exposure modulates lipid composition, histo-architecture and oxidative stress status and induces neurotoxicity in the smooth scallop Flexopecten glaber. Pestic. Biochem. Physiol. 2022, 184, 105099. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhijun, T.; Zheng, G.; Dong, C.; Yang, Y.; Wu, H. The role of Arachidonic acid regulatory network in the metabolism of paralytic shellfish toxins in Mytilus galloprovincialis-Based on combined transcriptome and metabolome analysis. Haiyang Xuebao 2023, 45, 142–152. [Google Scholar] [CrossRef]
- Yin, Z.; Nie, H.; Jiang, K.; Yan, X. Molecular Mechanisms Underlying Vibrio Tolerance in Ruditapes philippinarum Revealed by Comparative Transcriptome Profiling. Front. Immunol. 2022, 13, 879337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, N.; Zhang, C.; Wang, J.; Ma, H.; Li, S.; Zheng, H. Pathogenesis of black shell disease and its effects on survival and growth in the noble scallop Chlamys nobilis. Aquaculture 2024, 578, 740044. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Nguyen, T.V.; Rodríguez, J.A.; Bayot, B.; Domínguez-Borbor, C.; Sonnenholzner, S.; Azizan, A.; Venter, L. Evaluation of immune stimulatory products for whiteleg shrimp (Penaeus vannamei) by a metabolomics approach. Fish Shellfish. Immunol. 2021, 120, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite Profiles of Lactic Acid Bacteria in Grass Silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, A.; Dawid, C.; Gisch, N.; Schmid, C.; Raasch, L.; Gerster, T.; Schäffer, M.; Smakowska-Luzan, E.; Belkhadir, Y.; Vlot, A.C.; et al. Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science 2019, 364, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, S.; Shruthi, B.; Vanitha, P.; Sreenivasa, M. Probiotics and their postbiotics for the control of opportunistic fungal pathogens: A review. Biotechnol. Rep. 2023, 38, e00800. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Li, J.; Mao, T.; Feng, S.; Li, J.; Lai, M. 2 Hydroxybutyric Acid-Producing Bacteria in Gut Microbiome and Fusobacterium nucleatum Regulates 2 Hydroxybutyric Acid Level In Vivo. Metabolites 2023, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Gu, Y.; Hong, Y.; Sheng, L.; Chen, L.; Zhang, F.; Hou, J.; Zhang, W.; Zhang, Z.; Jia, W.; et al. Vancomycin pretreatment attenuates acetaminophen-induced liver injury through 2-hydroxybutyric acid. J. Pharm. Anal. 2020, 10, 560–570. [Google Scholar] [CrossRef]
- Kong, W.; Wu, Z.; Liu, Y.; Yan, C.; Zhang, J.; Sun, Y. RNA-seq analysis revealing the immune response of Neocaridina denticulata sinensis gill to Vibrio parahaemolyticus infection. Fish Shellfish. Immunol. 2022, 130, 409–417. [Google Scholar] [CrossRef]
- Corporeau, C.; Petton, S.; Vilaça, R.; Delisle, L.; Quéré, C.; Le Roy, V.; Dubreuil, C.; Lacas-Gervais, S.; Guitton, Y.; Artigaud, S.; et al. Harsh intertidal environment enhances metabolism and immunity in oyster (Crassostrea gigas) spat. Mar. Environ. Res. 2022, 180, 105709. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, J.; Shi, X.; Liu, Q.; Shao, Y.; Li, C.; Zhao, X. Energy metabolism pathways control the fate of Sinonovacula constricta and induction of immune response under Vibrio parahaemolyticus challenge. Aquaculture 2023, 569, 739364. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Yue, X.; Liu, B. Dynamic immune and metabolism response of clam Meretrix petechialis to Vibrio challenge revealed by a time series of transcriptome analysis. Fish Shellfish. Immunol. 2019, 94, 17–26. [Google Scholar] [CrossRef] [PubMed]
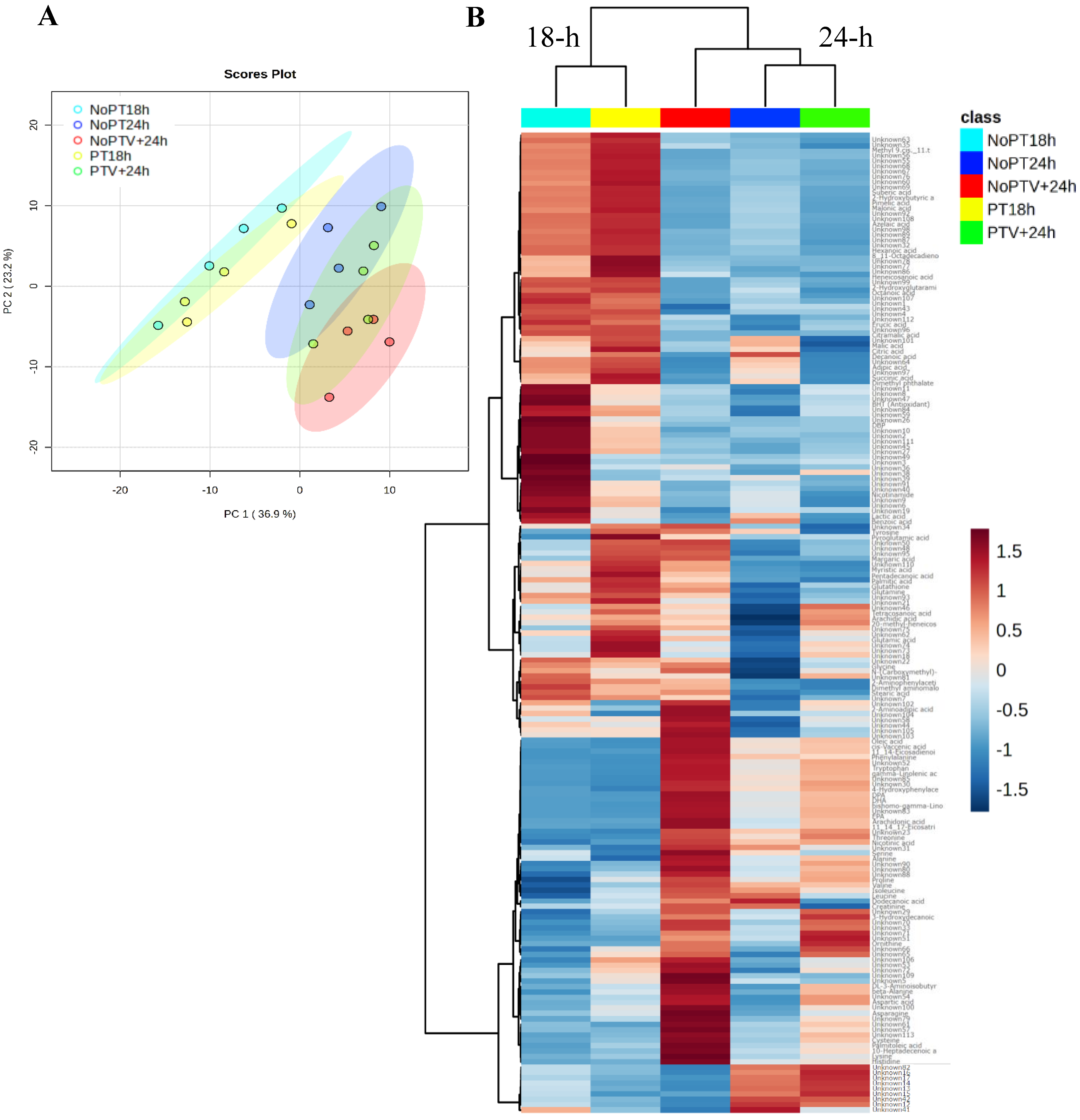

| Structural Class | Significant Known Metabolites | FDR | ID | NoPT18h | PT18h | NoPT24h | PTV+24h | NoPTV+24h |
|---|---|---|---|---|---|---|---|---|
| Fatty acyls | Arachidonic acid | 1.67 × 10−5 | C06425 | ↓ | ↓ | ↓ | ↑ | ↑↑ |
| 11,14,17-Eicosatrienoic acid | 1.93 × 10−5 | C16522 | ↓ | ↓ | ↓ | ↑ | ↑↑ | |
| gamma-Linolenic acid | 2.82 × 10−5 | C06426 | ↓ | ↓↓ | ↓ | ↑ | ↑↑ | |
| bishomo-gamma-Linolenic acid | 5.97 × 10−5 | C03242 | ↓ | ↓ | ↑ | ↑ | ↑↑ | |
| Azelaic acid | 1.18 × 10−4 | C08261 | ↑ | ↑↑ | ↓ | ↓ | ↓ | |
| Suberic acid | 1.36 × 10−4 | C08278 | ↑ | ↑↑ | ↓ | ↓ | ↓ | |
| 11,14-Eicosadienoic | 2.49 × 10−4 | C16525 | ↓ | ↓ | ↑ | ↑ | ↑↑ | |
| Pimelic acid | 2.49 × 10−4 | C02656 | ↑ | ↑↑ | ↓ | ↓ | ↓ | |
| cis-Vaccenic acid | 1.01 × 10−3 | C08367 | ↓ | ↑↑ | ↑ | ↑ | ↓↓ | |
| Oleic acid | 1.46 × 10−3 | C00712 | ↓ | ↑↑ | ↑ | ↑ | ↓↓ | |
| 8,11-Octadecadienoic acid | 4.33 × 10−3 | C04056 | ↑ | ↓ | ↓ | ↓ | ↑↑ | |
| Adipic acid | 4.59 × 10−3 | C06104 | ↑ | ↑ | ↑ | ↓ | ↓ | |
| Citramalic acid | 6.92 × 10−3 | C00815 | ↑↑ | ↑↑ | ↓ | ↓ | ↓ | |
| Hexanoic acid | 8.27 × 10−3 | C01585 | ↑ | ↑ | ↓ | ↓ | ↓ | |
| Erucic acid | 8.46 × 10−3 | C08316 | ↑ | ↑ | ↓ | ↓ | ↓ | |
| Heneicosanoic acid | 2.16 × 10−2 | 0002345 | ↑ | ↑ | ↓ | ↓ | ↓ | |
| 10-Heptadecenoic acid | 2.51 × 10−2 | C16536 | ↓ | ↓ | ↑ | ↑ | ↑↑ | |
| Eicosapentaenoic acid (EPA) | 1.33 × 10−5 | C06428 | ↓ | ↓ | ↓ | ↑ | ↑↑ | |
| Docosahexaenoic acid (DHA) | 1.67 × 10−5 | C06429 | ↓ | ↓ | ↓ | ↑ | ↑↑ | |
| Docosapentaenoic acid (DPA) | 1.67 × 10−5 | C16513 | ↓ | ↓ | ↓ | ↑ | ↑↑ | |
| Octanoic acid | 3.16 × 10−2 | C06423 | ↑ | ↑↑ | ↓ | ↓ | ↓ | |
| Palmitoleic acid | 3.80 × 10−2 | C08362 | ↓ | ↓ | ↑ | ↑ | ↑↑ | |
| Carboxylic acid and derivatives | Threonine | 1.33 × 10−5 | C00188 | ↓↓ | ↓↓ | ↑ | ↑ | ↑↑ |
| Malonic acid | 8.79 × 10−4 | C00383 | ↑ | ↓ | ↓ | ↓ | ↑↑ | |
| Phenylalanine | 9.42 × 10−4 | C00079 | ↓↓ | ↑↑ | ↑ | ↑ | ↓ | |
| Succinic acid | 9.75 × 10−3 | C00042 | ↑ | ↑↑ | ↓ | ↓ | ↓ | |
| Cysteine | 4.43 × 10−2 | C00097 | ↓ | ↓ | ↓ | ↑ | ↑↑ | |
| Hydroxy acid and derivatives | 2-Hydroxybutyric acid | 5.78 × 10−4 | C05984 | ↑ | ↑↑ | ↓ | ↓ | ↓ |
| Lactic acid | 1.03 × 10−2 | C00186 | ↑↑ | ↑ | ↑ | ↓ | ↓ | |
| 3-Hydroxydecanoic acid | 1.32 × 10−2 | 0010725 | ↓ | ↓ | ↓ | ↑ | ↑↑ | |
| Benzene and derivatives | Dibutyl phthalate (DBP) | 2.67 × 10−3 | C14214 | ↑↑ | ↓ | ↓ | ↓ | ↓ |
| Butylated hydroxytoluene (BHT) | 7.61 × 10−3 | C14693 | ↑↑ | ↓ | ↓ | ↓ | ↓ | |
| Pyridines and derivatives | Nicotinic acid | 1.59 × 10−4 | C00253 | ↓↓ | ↓ | ↑ | ↑ | ↑↑ |
| Indoles and derivatives | Tryptophan | 1.93 × 10−5 | C00078 | ↓ | ↓↓ | ↑ | ↑ | ↑↑ |
| Hydroxy dicarboxylic acids | 2-Hydroxyglutaramic acid | 8.47 × 10−4 | - | ↑↑ | ↑↑ | ↓ | ↓ | ↓ |
| Structural Classes | Significant Known Metabolites | Non-Significant Known Metabolites |
|---|---|---|
| Fatty Acyls | Arachidonic acid | 20-methyl heneicosanoic acid |
| 11,14,17-Eicosatrienoic acid | Arachidic acid | |
| gamma-Linolenic acid | Decanoic acid | |
| bishomo-gamma-Linolenic acid | Dodecanoic acid | |
| Azelaic acid | Margaric acid | |
| Suberic acid | Methyl (9Z,11E,13E) Octadecatrienoic acid | |
| 11,14-Eicosadienoic | Myristic acid | |
| Pimelic acid | Palmitic acid | |
| cis-Vaccenic acid | Pentadecanoic acid | |
| Oleic acid | Stearic acid | |
| 8,11-Octadecadienoic acid | Tetracosanoic acid | |
| Adipic acid | ||
| Citramalic acid | ||
| Hexanoic acid | ||
| Erucic acid | ||
| Heneicosanoic acid | ||
| 10-Heptadecenoic acid | ||
| EPA (Eicosapentaenoic acid) | ||
| DHA (Docosahexaenoic acid) | ||
| DPA (Docosapentaenoic acid) | ||
| Octanoic acid | ||
| Palmitoleic acid | ||
| Carboxylic acid and derivatives | Threonine | Dimethyl aminomalonic acid |
| Malonic acid | N-Carboxymethyl-L-alanine | |
| Phenylalanine | 2-Aminoadipic acid | |
| Succinic acid | Alanine | |
| Cysteine | Asparagine | |
| Aspartic acid | ||
| beta-Alanine | ||
| Citric acid | ||
| Creatinine | ||
| DL-3-Aminoisobutyric acid | ||
| Glutamic acid | ||
| Glutamine | ||
| Glutathione | ||
| Glycine | ||
| Histidine | ||
| Isoleucine | ||
| Leucine | ||
| Lysine | ||
| Ornithine | ||
| Proline | ||
| Pyroglutamic acid | ||
| Serine | ||
| Tyrosine | ||
| Valine | ||
| Hydroxy acid and derivatives | 2-Hydroxybutyric acid | Malic acid |
| Lactic acid | ||
| 3-Hydroxydecanoic acid | ||
| Benzene and substituted derivatives | DBP (Dibutyl phthalate) | 2-Aminophenylacetic acid |
| BHT (Antioxidant) | Benzoic acid | |
| Dimethyl phthalate | ||
| Pyridines and derivatives | Nicotinic acid | Nicotinamide |
| Indoles and derivatives, phenols | Tryptophan | 4-Hydroxyphenylacetic acid |
| Hydroxydicarboxylic acids | 2-Hydroxyglutaramic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Cerro, K.; Venter, L.; Young, T.; Alfaro, A.C.; Brokordt, K.; Schmitt, P. Mitigation of Vibrio-Induced Metabolic Perturbations in Argopecten purpuratus Scallop Larvae via Probiotic Pretreatment. J. Mar. Sci. Eng. 2024, 12, 1138. https://doi.org/10.3390/jmse12071138
Muñoz-Cerro K, Venter L, Young T, Alfaro AC, Brokordt K, Schmitt P. Mitigation of Vibrio-Induced Metabolic Perturbations in Argopecten purpuratus Scallop Larvae via Probiotic Pretreatment. Journal of Marine Science and Engineering. 2024; 12(7):1138. https://doi.org/10.3390/jmse12071138
Chicago/Turabian StyleMuñoz-Cerro, Katherine, Leonie Venter, Tim Young, Andrea C. Alfaro, Katherina Brokordt, and Paulina Schmitt. 2024. "Mitigation of Vibrio-Induced Metabolic Perturbations in Argopecten purpuratus Scallop Larvae via Probiotic Pretreatment" Journal of Marine Science and Engineering 12, no. 7: 1138. https://doi.org/10.3390/jmse12071138





