Understanding Carbon Footprint in Sustainable Land-Based Marine Aquaculture: Exploring Production Techniques
Abstract
1. Introduction
2. Mitigation Strategies
2.1. Diversification through Low-Trophic-Level Species
2.2. Preservation of High-Carbon Sequestration Sites
2.3. Polyculture
2.4. Nutrition and Feeding Efficiency
2.5. Implementing Waste Management Technologies
2.6. Energy Efficiency
2.7. Organic Aquaculture Standards
- System design and location are related to contamination from outside sources, the introduction of exotic species, escapes, contamination with effluent discharges, the use and reuse of water and maintaining water quality.
- Sources of stock, breeds and breeding concern the preference for local species and the prohibition of polyploidy, the use of hormones and the handling of the daylight period.
- The feeding and nutrition of aquaculture animals concern the efficient use of food to minimize loss and the use of organic feed ingredients sourced from certified organic sources whenever possible.
- Health and welfare are related to measures designed to provide adequate space, shelter and environmental enrichment, with the aim of promoting natural behaviors and minimizing stress. Practices such as overcrowding, confinement and the use of stressful handling techniques are prohibited (including during harvest and transportation).
- Processing and labeling operations must maintain detailed records of all inputs, practices and activities related to production. This includes the documentation of feed ingredients, water quality monitoring results, stocking densities and health management practices.
3. Land-Based Aquaculture Farming Systems: In the Race for an Eco-Friendly Status
3.1. Recirculating Aquaculture Systems (RASs)
3.2. Integrated Multi-Trophic Aquaculture Systems (IMTAs)
3.3. Biofloc Technology (BFT) Systems
3.4. Extensive Aquaculture: Earthen Ponds and Intertidal Aquaculture
4. Conclusions, Challenges and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Blanchard, J.L.; Watson, R.A.; Fulton, E.A.; Cottrell, R.S.; Nash, K.L.; Bryndum-Buchholz, A.; Büchner, M.; Carozza, D.A.; Cheung, W.W.; Elliott, J. Linked Sustainability Challenges and Trade-Offs among Fisheries, Aquaculture and Agriculture. Nat. Ecol. Evol. 2017, 1, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Mission Restore Our Ocean and Waters Implementation Plan; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Action Plan for a Maritime Strategy in the Atlantic Area. Delivering Smart, Sustainable and Inclusive Growth; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- European Commission Blue Growth. Supporting Sustainable Growth of the Marine and Maritime Sectors. Available online: https://s3platform.jrc.ec.europa.eu (accessed on 17 October 2023).
- United Nations. Goal 13: Climate Action. Available online: https://www.un.org/sustainabledevelopment/climate-change/ (accessed on 14 February 2024).
- MacLeod, M.J.; Hasan, M.R.; Robb, D.H.F.; Mamun-Ur-Rashid, M. Quantifying Greenhouse Gas Emissions from Global Aquaculture. Sci. Rep. 2020, 10, 11679. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.M.; Olhoff, A. Lessons from a Decade of Emissions Gap Assessments; DTU Library: Delhi, India, 2019. [Google Scholar]
- Raul, C.; Pattanaik, S.S.; Prakash, S.; Sreedharan, K.; Bharti, S. Greenhouse Gas Emissions from Aquaculture Systems. World Aquac. 2020, 57, 57–61. [Google Scholar]
- Troell, M.; Naylor, R.L.; Metian, M.; Beveridge, M.; Tyedmers, P.H.; Folke, C.; Arrow, K.J.; Barrett, S.; Crépin, A.-S.; Ehrlich, P.R. Does Aquaculture Add Resilience to the Global Food System? Proc. Natl. Acad. Sci. USA 2014, 111, 13257–13263. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Aas, T.S.; Åsgård, T. Utilisation of Feed Resources in Production of Atlantic Salmon (Salmo salar) in Norway. Aquaculture 2015, 448, 365–374. [Google Scholar] [CrossRef]
- Adamidou, S.; Nengas, I.; Henry, M.; Ioakei Midoy, N.; Rigos, G.; Bell, G.J.; Jauncey, K. Effects of Dietary Inclusion of Peas, Chickpeas and Faba Beans on Growth, Feed Utilization and Health of Gilthead Seabream (Sparus aurata). Aquac. Nutr. 2011, 17, e288–e296. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Mente, E.; Karapanagiotidis, I.T.; Vlontzos, G.; Athanassiou, C.G. Insect-Based Feed Ingredients for Aquaculture: A Case Study for Their Acceptance in Greece. Insects 2021, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Bujas, T.; Koričan, M.; Vukić, M.; Soldo, V.; Vladimir, N.; Fan, A. Review of Energy Consumption by the Fish Farming and Processing Industry in Croatia and the Potential for Zero-Emissions Aquaculture. Energies 2022, 15, 8197. [Google Scholar] [CrossRef]
- Troell, M.; Tyedmers, P.; Kautsky, N.; Rönnbäck, P. Aquaculture and Energy Use. Encycl. Energy 2004, 1, 97–108. [Google Scholar]
- Vo, T.T.E.; Ko, H.; Huh, J.-H.; Park, N. Overview of Solar Energy for Aquaculture: The Potential and Future Trends. Energies 2021, 14, 6923. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, S.; Wang, Z.; Wang, F.; Gao, Q.; Tian, X.; Xiong, Y. Variations in CO2 Fluxes from Grass Carp Ctenopharyngodon idella Aquaculture Polyculture Ponds. Aquac. Environ. Interact. 2015, 8, 31–40. [Google Scholar] [CrossRef]
- Yuan, J.; Xiang, J.; Liu, D.; Kang, H.; He, T.; Kim, S.; Lin, Y.; Freeman, C.; Ding, W. Rapid Growth in Greenhouse Gas Emissions from the Adoption of Industrial-Scale Aquaculture. Nat. Clim. Chang. 2019, 9, 318–322. [Google Scholar] [CrossRef]
- Tan, J.; Lichtfouse, E.; Luo, M.; Liu, Y.; Tan, F.; Zhang, C.; Chen, X.; Huang, J.; Xiao, L. Aquaculture Drastically Increases Methane Production by Favoring Acetoclastic Rather than Hydrogenotrophic Methanogenesis in Shrimp Pond Sediments. Aquaculture 2023, 563, 738999. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Khanal, S.K. Nitrous Oxide (N2O) Emission from Aquaculture: A Review. Environ. Sci. Technol. 2012, 46, 6470–6480. [Google Scholar] [CrossRef] [PubMed]
- Green, B.M. Fertilizer Use in Aquaculture. In Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 29–63. [Google Scholar]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture Wastewater Treatment Technologies and Their Sustainability: A Review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, S.; Ji, C.; Zou, J.; Zhou, Q.; Liu, S. A Comparison of Methane Emissions Following Rice Paddies Conversion to Crab-Fish Farming Wetlands in Southeast China. Environ. Sci. Pollut. Res. 2016, 23, 1505–1515. [Google Scholar] [CrossRef]
- Hynes, R.K.; Knowles, R. Production of Nitrous Oxide by Nitrosomonas europaea: Effects of Acetylene, pH, and Oxygen. Can. J. Microbiol. 1984, 30, 1397–1404. [Google Scholar] [CrossRef]
- Hanaki, K.; Hong, Z.; Matsuo, T. Production of Nitrous Oxide Gas during Denitrification of Wastewater. Water Sci. Technol. 1992, 26, 1027–1036. [Google Scholar] [CrossRef]
- Elvy, J.E.; Symonds, J.E.; Hilton, Z.; Walker, S.P.; Tremblay, L.A.; Casanovas, P.; Herbert, N.A. The Relationship of Feed Intake, Growth, Nutrient Retention, and Oxygen Consumption to Feed Conversion Ratio of Farmed Saltwater Chinook Salmon (Oncorhynchus tshawytscha). Aquaculture 2022, 554, 738184. [Google Scholar] [CrossRef]
- Plouviez, M.; Shilton, A.; Packer, M.A.; Guieysse, B. Nitrous Oxide Emissions from Microalgae: Potential Pathways and Significance. J. Appl. Phycol. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Blancheton, J.-P.; Aubin, J. Towards Environmentally Sustainable Aquaculture: Comparison between Two Trout Farming Systems Using Life Cycle Assessment. Aquac. Eng. 2009, 40, 113–119. [Google Scholar] [CrossRef]
- Cao, L.; Diana, J.S.; Keoleian, G.A. Role of Life Cycle Assessment in Sustainable Aquaculture. Rev. Aquac. 2013, 5, 61–71. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, K.-C.; Lin, W.-C.; Wu, M.-H. Carbon Footprint Analysis in the Aquaculture Industry: Assessment of an Ecological Shrimp Farm. J. Clean. Prod. 2017, 168, 1101–1107. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in Aquafeeds for a Sustainable Aquaculture Industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Metian, M.; Froehlich, H.E.; Blanchard, J.L.; Sand Jacobsen, N.; McIntyre, P.B.; Nash, K.L.; Williams, D.R.; Bouwman, L.; Gephart, J.A. Time to Rethink Trophic Levels in Aquaculture Policy. Rev. Aquac. 2021, 13, 1583–1593. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Jacobsen, N.S.; Essington, T.E.; Clavelle, T.; Halpern, B.S. Avoiding the Ecological Limits of Forage Fish for Fed Aquaculture. Nat. Sustain. 2018, 1, 298–303. [Google Scholar] [CrossRef]
- Bandara, T. Alternative Feed Ingredients in Aquaculture: Opportunities and Challenges. J. Entomol. Zool. Stud. 2018, 6, 3087–3094. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K. Feeding Aquaculture in an Era of Finite Resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Le Vay, L.; Buck, B.H.; Costa-Pierce, B.A.; Dewhurst, T.; Heasman, K.G.; Nevejan, N.; Nielsen, P.; Nielsen, K.N.; Park, K.; et al. Prospects of Low Trophic Marine Aquaculture Contributing to Food Security in a Net Zero-Carbon World. Front. Sustain. Food Syst. 2022, 6, 875509. [Google Scholar] [CrossRef]
- Nijdam, D.; Rood, T.; Westhoek, H. The Price of Protein: Review of Land Use and Carbon Footprints from Life Cycle Assessments of Animal Food Products and Their Substitutes. Food Policy 2012, 37, 760–770. [Google Scholar] [CrossRef]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The Environmental Cost of Animal Source Foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Hachero-Cruzado, I.; González-Romero, P.; Jiménez-Prada, P.; Cassell, C.; Ros, M. Towards Integrated Multi-Trophic Aquaculture: Lessons from Caprellids (Crustacea: Amphipoda). PLoS ONE 2016, 11, e0154776. [Google Scholar] [CrossRef] [PubMed]
- Gephart, J.A.; Henriksson, P.J.; Parker, R.W.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S. Environmental Performance of Blue Foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef]
- Jones, A.R.; Alleway, H.K.; McAfee, D.; Reis-Santos, P.; Theuerkauf, S.J.; Jones, R.C. Climate-Friendly Seafood: The Potential for Emissions Reduction and Carbon Capture in Marine Aquaculture. BioScience 2022, 72, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional Value and Food Safety of Bivalve Molluscan Shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Duarte, C.M.; Delgado-Huertas, A.; Marti, E.; Gasser, B.; Martin, I.S.; Cousteau, A.; Neumeyer, F.; Reilly-Cayten, M.; Boyce, J.; Kuwae, T.; et al. Carbon Burial in Sediments below Seaweed Farms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Feng, J.-C.; Sun, L.; Yan, J. Carbon Sequestration via Shellfish Farming: A Potential Negative Emissions Technology. Renew. Sustain. Energy Rev. 2023, 171, 113018. [Google Scholar] [CrossRef]
- Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem Services Provided by Seaweeds. Hydrobiology 2023, 2, 75–96. [Google Scholar] [CrossRef]
- Jiménez-Prada, P.; Hachero-Cruzado, I.; Guerra-García, J. Aquaculture Waste as Food for Amphipods: The Case of Gammarus insensibilis in Marsh Ponds from Southern Spain. Aquac. Int. 2020, 29, 139–153. [Google Scholar] [CrossRef]
- Castilla-Gavilán, M.; Guerra-García, J.; Moreno-Oliva, J.; Hachero-Cruzado, I. How Much Waste Can the Amphipod Gammarus insensibilis Remove from Aquaculture Effluents? A First Step toward IMTA. Aquaculture 2023, 573, 739552. [Google Scholar] [CrossRef]
- Ribes-Navarro, A.; Navarro, J.C.; Hontoria, F.; Kabeya, N.; Standal, I.B.; Evjemo, J.O.; Monroig, Ó. Biosynthesis of Long-Chain Polyunsaturated Fatty Acids in Marine Gammarids: Molecular Cloning and Functional Characterisation of Three Fatty Acyl Elongases. Mar. Drugs 2021, 19, 226. [Google Scholar] [CrossRef]
- Ahmed, N.; Cheung, W.W.; Thompson, S.; Glaser, M. Solutions to Blue Carbon Emissions: Shrimp Cultivation, Mangrove Deforestation and Climate Change in Coastal Bangladesh. Mar. Policy 2017, 82, 68–75. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Glaser, M. Integrated Mangrove-Shrimp Cultivation: Potential for Blue Carbon Sequestration. Ambio 2018, 47, 441–452. [Google Scholar] [CrossRef]
- Maldonado, C.; Cuzon, G.; Guzmán, E.; Brito, R.; Soto, L.; Arena, L.; Gaxiola, G. Effect of an Herbivorous Diet on Energy Balance of Litopenaeus vannamei at Selected Ontogenetic Stages. Aquaculture 2009, 296, 123–128. [Google Scholar] [CrossRef][Green Version]
- Tolentino-Pablico, G.; Bailly, N.; Froese, R.; Elloran, C. Seaweeds Preferred by Herbivorous Fishes. In Nineteenth International Seaweed Symposium, Proceedings of the 19th International Seaweed Symposium, Kobe, Japan, 26–31 March 2007; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Developments in Applied Phycology; Springer: Dordrecht, The Netherlands, 2009; pp. 483–488. ISBN 978-1-4020-9619-8. [Google Scholar]
- Li, Y.; Zhang, Q.; Liu, Y. Rabbitfish—An Emerging Herbivorous Marine Aquaculture Species. In Aquaculture in China; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 329–334. ISBN 978-1-119-12075-9. [Google Scholar]
- Lozano-Muñoz, I.; Castellaro, G.; Bueno, G.; Wacyk, J. Herbivorous Fish (Medialuna ancietae) as a Sustainable Alternative for Nutrition Security in Northern Chile. Sci. Rep. 2022, 12, 1619. [Google Scholar] [CrossRef]
- Jose, D.M.; Divya, P. A Review on Aquaculture Important Fish Chanos chanos, Forsskal 1775, the Milkfish. J. Aquac. Trop. 2022, 37, 1–26. [Google Scholar] [CrossRef]
- Putra, D.; Muhsinah, M.; Arisa, I. The Substitution of Soybean Meal by Fermented Tofu Dregs in the Milkfish (Chanos chanos) Diet; IOP Publishing: Bristol, UK, 2021; Volume 674, p. 012102. [Google Scholar]
- Herawati, V.E.; Pinandoyo, P.; Windarto, S.; Hariyadi, P.; Hutabarat, J.; Darmanto, Y.; Rismaningsih, N.; Prayitno, S.B.; Radjasa, O.K. Maggot Meal (Hermetia illucens) Substitution on Fish Meal to Growth Performance, and Nutrient Content of Milkfish (Chanos chanos). HAYATI J. Biosci. 2020, 27, 154. [Google Scholar] [CrossRef]
- Aslamyah, S.; Umam, M.K.; Lestari, A.D. The Effect of Protein Levels in Fermentation Feed Supplemented Lumbricus sp. Extract as Feed Additive on Growth Performance and Body Chemical Composition of Milkfish, Chanos chanos Forskal 1775; IOP Publishing: Bristol, UK, 2020; Volume 575, p. 012036. [Google Scholar]
- Nurfadillah, N.; Ningsih, H.; Rahimi, S.; Dewiyanti, I.; Mellisa, S.; Syahril, A. The Effect of Ethanolic Extracts Ulva lactuca on Growth Performance and Survival Rate of Milk Fish (Chanos chanos); IOP Publishing: Bristol, UK, 2021; Volume 674, p. 012049. [Google Scholar]
- Martínez, F.P.; Bermúdez, L.; Aznar, M.J.; Moyano, F.J. Evaluation of Enzyme Additives on the Nutritional Use of Feeds with a High Content of Plant Ingredients for Mugil cephalus. Fishes 2019, 4, 56. [Google Scholar] [CrossRef]
- Biswas, G.; De, D.; Thirunavukkarasu, A.; Natarajan, M.; Sundaray, J.; Kailasam, M.; Kumar, P.; Ghoshal, T.; Ponniah, A.; Sarkar, A. Effects of Stocking Density, Feeding, Fertilization and Combined Fertilization-Feeding on the Performances of Striped Grey Mullet (Mugil cephalus L.) Fingerlings in Brackishwater Pond Rearing Systems. Aquaculture 2012, 338, 284–292. [Google Scholar] [CrossRef]
- Jana, S.N.; Sudesh; Garg, S.K.; Sabhlok, V.P.; Bhatnagar, A. Nutritive Evaluation of Lysine-and Methionine-Supplemented Raw vs Heat-Processed Soybean to Replace Fishmeal as a Dietary Protein Source for Grey Mullet, Mugil cephalus, and Milkfish, Chanos chanos. J. Appl. Aquac. 2012, 24, 69–80. [Google Scholar] [CrossRef]
- Gisbert, E.; Mozanzadeh, M.T.; Kotzamanis, Y.; Estévez, A. Weaning Wild Flathead Grey Mullet (Mugil cephalus) Fry with Diets with Different Levels of Fish Meal Substitution. Aquaculture 2016, 462, 92–100. [Google Scholar] [CrossRef]
- Lal, R. Carbon Sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 815–830. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, Carbon, and Climate Change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Arifanti, V.B.; Kauffman, J.B.; Hadriyanto, D.; Murdiyarso, D.; Diana, R. Carbon Dynamics and Land Use Carbon Footprints in Mangrove-Converted Aquaculture: The Case of the Mahakam Delta, Indonesia. For. Ecol. Manag. 2019, 432, 17–29. [Google Scholar] [CrossRef]
- Yang, P.; Lai, D.Y.; Yang, H.; Lin, Y.; Tong, C.; Hong, Y.; Tian, Y.; Tang, C.; Tang, K.W. Large Increase in CH4 Emission Following Conversion of Coastal Marsh to Aquaculture Ponds Caused by Changing Gas Transport Pathways. Water Res. 2022, 222, 118882. [Google Scholar] [CrossRef]
- Yang, P.; Bastviken, D.; Lai, D.; Jin, B.; Mou, X.; Tong, C.; Yao, Y. Effects of Coastal Marsh Conversion to Shrimp Aquaculture Ponds on CH4 and N2O Emissions. Estuar. Coast. Shelf Sci. 2017, 199, 125–131. [Google Scholar] [CrossRef]
- Ferrón, S.; Ortega, T.; Forja, J.M. Benthic Fluxes in a Tidal Salt Marsh Creek Affected by Fish Farm Activities: Río San Pedro (Bay of Cádiz, SW Spain). Mar. Chem. 2009, 113, 50–62. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Morris, E.P.; Flecha, S.; Figuerola, J.; Costas, E.; Navarro, G.; Ruiz, J.; Rodriguez, P.; Huertas, E. Contribution of Doñana Wetlands to Carbon Sequestration. PLoS ONE 2013, 8, e71456. [Google Scholar] [CrossRef] [PubMed]
- Friess, D.A.; Yando, E.S.; Alemu, J.B.; Wong, L.-W.; Soto, S.D.; Bhatia, N. Ecosystem Services and Disservices of Mangrove Forests and Salt Marshes. In Oceanography and Marine Biology; Taylor & Francis: Abingdon, UK, 2020. [Google Scholar]
- Walton, M.; Vilas, C.; Cañavate, J.P.; González-Ortegón, E.; Prieto, A.; Van Bergeijk, S.; Green, A.J.; Librero, M.; Mazuelos, N.; Le Vay, L. A Model for the Future: Ecosystem Services Provided by the Aquaculture Activities of Veta La Palma, Southern Spain. Aquaculture 2015, 448, 382–390. [Google Scholar] [CrossRef]
- Erwin, K.L. Wetlands and Global Climate Change: The Role of Wetland Restoration in a Changing World. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Čížková, H.; Květ, J.; Comin, F.A.; Laiho, R.; Pokorný, J.; Pithart, D. Actual State of European Wetlands and Their Possible Future in the Context of Global Climate Change. Aquat. Sci. 2013, 75, 3–26. [Google Scholar] [CrossRef]
- Green, A.J.; Alcorlo, P.; Peeters, E.T.; Morris, E.P.; Espinar, J.L.; Bravo-Utrera, M.A.; Bustamante, J.; Díaz-Delgado, R.; Koelmans, A.A.; Mateo, R. Creating a Safe Operating Space for Wetlands in a Changing Climate. Front. Ecol. Environ. 2017, 15, 99–107. [Google Scholar] [CrossRef]
- Han, T.; Shi, R.; Qi, Z.; Huang, H.; Liang, Q.; Liu, H. Interactive Effects of Oyster and Seaweed on Seawater Dissolved Inorganic Carbon Systems: Implications for Integrated Multi-Trophic Aquaculture. Aquac. Environ. Interact. 2017, 9, 469–478. [Google Scholar] [CrossRef]
- Thomas, M.; Pasquet, A.; Aubin, J.; Nahon, S.; Lecocq, T. When More Is More: Taking Advantage of Species Diversity to Move towards Sustainable Aquaculture. Biol. Rev. 2021, 96, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Gavilán, M.; Muñoz-Martínez, M.; Zuasti, E.; Canoura-Baldonado, J.; Mondoñedo, R.; Hachero-Cruzado, I. Yield, Nutrients Uptake and Lipid Profile of the Halophyte Salicornia ramosissima Cultivated in Two Different Integrated Multi-Trophic Aquaculture Systems (IMTA). Aquaculture 2024, 583, 740547. [Google Scholar] [CrossRef]
- Wang, X.; Broch, O.J.; Forbord, S.; Handå, A.; Skjermo, J.; Reitan, K.I.; Vadstein, O.; Olsen, Y. Assimilation of Inorganic Nutrients from Salmon (Salmo salar) Farming by the Macroalgae (Saccharina latissima) in an Exposed Coastal Environment: Implications for Integrated Multi-Trophic Aquaculture. J. Appl. Phycol. 2014, 26, 1869–1878. [Google Scholar] [CrossRef]
- Strand, Ø.; Jansen, H.M.; Jiang, Z.; Robinson, S.M. Perspectives on Bivalves Providing Regulating Services in Integrated Multi-Trophic Aquaculture. In Goods and Services of Marine Bivalves; Springer: Berlin/Heidelberg, Germany, 2019; pp. 209–230. [Google Scholar]
- Zhang, D.; Tian, X.; Dong, S.; Chen, Y.; Feng, J.; He, R.-P.; Zhang, K. Carbon Budgets of Two Typical Polyculture Pond Systems in Coastal China and Their Potential Roles in the Global Carbon Cycle. Aquac. Environ. Interact. 2020, 12, 105–115. [Google Scholar] [CrossRef]
- Davis, D.A. Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Sawston, UK, 2022; ISBN 0-12-822992-6. [Google Scholar]
- Hardy, R.W. Utilization of Plant Proteins in Fish Diets: Effects of Global Demand and Supplies of Fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Moñino, A.V.; Tomás Vidal, A.; Salvador, V.J.M.; Pla Torres, M.; Jover Cerdá, M. Soybean Meal as a Protein Source in Gilthead Sea Bream (Sparus aurata L.) Diets: Effects on Growth and Nutrient Utilization. Aquac. Res. 2007, 38, 82–90. [Google Scholar] [CrossRef]
- Pereira, T.; Oliva-Teles, A. Evaluation of Corn Gluten Meal as a Protein Source in Diets for Gilthead Sea Bream (Sparus aurata L.) Juveniles. Aquac. Res. 2003, 34, 1111–1117. [Google Scholar] [CrossRef]
- Gómez-Requeni, P.; Mingarro, M.; Kirchner, S.; Calduch-Giner, J.; Médale, F.; Corraze, G.; Panserat, S.; Martin, S.; Houlihan, D.; Kaushik, S. Effects of Dietary Amino Acid Profile on Growth Performance, Key Metabolic Enzymes and Somatotropic Axis Responsiveness of Gilthead Sea Bream (Sparus aurata). Aquaculture 2003, 220, 749–767. [Google Scholar] [CrossRef]
- Alarcón, F.J.; Moyano, F.J.; Díaz, M. Effect of Inhibitors Present in Protein Sources on Digestive Proteases of Juvenile Sea Bream (Sparus aurata). Aquat. Living Resour. 1999, 12, 233–238. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional Factors Present in Plant-Derived Alternate Fish Feed Ingredients and Their Effects in Fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; Van Huis, A. Can Diets Containing Insects Promote Animal Health? J. Insects Food Feed. 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Hachero-Cruzado, I.; Betancor, M.B.; Coronel-Dominguez, A.J.; Manchado, M.; Alarcón-López, F.J. Assessment of Full-Fat Tenebrio molitor as Feed Ingredient for Solea senegalensis: Effects on Growth Performance and Lipid Profile. Animals 2024, 14, 595. [Google Scholar] [CrossRef]
- Sogari, G.; Bellezza Oddon, S.; Gasco, L.; van Huis, A.; Spranghers, T.; Mancini, S. Review: Recent Advances in Insect-Based Feeds: From Animal Farming to the Acceptance of Consumers and Stakeholders. Animals 2023, 17, 100904. [Google Scholar] [CrossRef]
- Quang Tran, H.; Van Doan, H.; Stejskal, V. Environmental Consequences of Using Insect Meal as an Ingredient in Aquafeeds: A Systematic View. Rev. Aquac. 2022, 14, 237–251. [Google Scholar] [CrossRef]
- Ogunkalu, O. Effects of Feed Additives in Fish Feed for Improvement of Aquaculture. Eurasian J. Food Sci. Technol. 2019, 3, 49–57. [Google Scholar]
- Encarnação, P. 5—Functional Feed Additives in Aquaculture Feeds. In Aquafeed Formulation; Nates, S.F., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 217–237. ISBN 978-0-12-800873-7. [Google Scholar]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Otero, P.; Soria-Lopez, A.; Cassani, L.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods 2022, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The Application of Single-Cell Ingredients in Aquaculture Feeds—A Review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Ponce, M.; Anguís, V.; Fernández-Díaz, C. Assessing the Role of Ulvan as Immunonutrient in Solea senegalensis. Fish Shellfish Immunol. 2024, 146, 109399. [Google Scholar] [CrossRef]
- Liang, Q.; Yuan, M.; Xu, L.; Lio, E.; Zhang, F.; Mou, H.; Secundo, F. Application of Enzymes as a Feed Additive in Aquaculture. Mar. Life Sci. Technol. 2022, 4, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Kalhoro, H.; Zhou, J.; Hua, Y.; Ng, W.-K.; Ye, L.; Zhang, J.; Shao, Q. Soy Protein Concentrate as a Substitute for Fish Meal in Diets for Juvenile Acanthopagrus schlegelii: Effects on Growth, Phosphorus Discharge and Digestive Enzyme Activity. Aquac. Res. 2018, 49, 1896–1906. [Google Scholar] [CrossRef]
- González-Riopedre, M.; Márquez, L.; Sieiro, M.; Vázquez, U.; Maroto, J.; Barcia, R.; Moyano, F. Use of Purified Extracts from Fish Viscera as an Enzyme Additive in Feeds for Juvenile Marine Fish. In New Additives and Ingredients in the Formulation of Aquafeeds; Centro Tecnologico del Mar-Fundacion (CETMAR): Vigo, Spain, 2013. [Google Scholar]
- Robb, D.H.; Crampton, V.O.; Robb, D.; Crampton, V. On-Farm Feeding and Feed Management: Perspectives from the Fish Feed Industry. On-Farm Feed. Feed Manag. Aquac. 2013, 489, 518. [Google Scholar]
- Farmer, A. Phosphate Pollution: A Global Overview of the Problem. In Phosphorus: Polluter and Resource of the Future—Removal and Recovery from Wastewater; Schaum, C., Ed.; IWA Publishing: London, UK, 2018; pp. 35–55. [Google Scholar]
- Chen, S.; Timmons, M.B.; Aneshansley, D.J.; Bisogni, J.J., Jr. Suspended Solids Characteristics from Recirculating Aquacultural Systems and Design Implications. Aquaculture 1993, 112, 143–155. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) Analysis: Main Issues on Management and Future Challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Martins, C.; Eding, E.H.; Verdegem, M.C.; Heinsbroek, L.T.; Schneider, O.; Blancheton, J.-P.; d’Orbcastel, E.R.; Verreth, J. New Developments in Recirculating Aquaculture Systems in Europe: A Perspective on Environmental Sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate Toxicity to Aquatic Animals: A Review with New Data for Freshwater Invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Hussain, T. Chapter 7—Water and Wastewater Treatment through Ozone-Based Technologies. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–172. ISBN 978-0-323-85583-9. [Google Scholar]
- Kurniawan, S.; Novarini; Yuliwati, E.; Ariyanto, E.; Morsin, M.; Sanudin, R.; Nafisah, S. Greywater Treatment Technologies for Aquaculture Safety: Review. J. King Saud. Univ.-Eng. Sci. 2023, 35, 327–334. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Ahmad, A.; Mohd Said, N.S.; Mohd Rahim, N.F.; Mohammad Alnawajha, M.; Abu Hasan, H.; Othman, A.R.; Purwanti, I.F. Potential of Valuable Materials Recovery from Aquaculture Wastewater: An Introduction to Resource Reclamation. Aquac. Res. 2021, 52, 2954–2962. [Google Scholar] [CrossRef]
- Ezziddine, M.; Liltved, H. Nutrients Recovery from Aquaculture Waste for Use as Fertilizer in Soilless Growth Systems. Int. Symp. Grow. Media Compost. Substrate Anal. 2019, 1305, 399–406. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Santiesteban-Romero, B.; Poss, G.; Sosa-Hernández, J.E.; Iqbal, H.M.; Parra-Saldívar, R.; Bonaccorso, A.D.; Melchor-Martínez, E.M. Sustainable Production of Biofuels and Bioderivatives from Aquaculture and Marine Waste. Front. Chem. Eng. 2023, 4, 1072761. [Google Scholar] [CrossRef]
- Mirzoyan, N.; Tal, Y.; Gross, A. Anaerobic Digestion of Sludge from Intensive Recirculating Aquaculture Systems: Review. Aquaculture 2010, 306, 1–6. [Google Scholar] [CrossRef]
- Del Campo, L.M.; Ibarra, P.; Gutiérrez, X.; Takle, H.R. Utilization of Sludge from Recirculation Aquaculture Systems. Nofima Rapp. 2010, 9. [Google Scholar]
- Dumay, J.; Clément, N.; Morançais, M.; Fleurence, J. Optimization of Hydrolysis Conditions of Palmaria palmata to Enhance R-Phycoerythrin Extraction. Bioresour. Technol. 2013, 131, 21–27. [Google Scholar] [CrossRef]
- Le Strat, Y.; Ruiz, N.; Fleurence, J.; Pouchus, Y.-F.; Déléris, P.; Dumay, J. Marine Fungal Abilities to Enzymatically Degrade Algal Polysaccharides, Proteins and Lipids: A Review. J. Appl. Phycol. 2022, 34, 1131–1162. [Google Scholar] [CrossRef]
- Le Guillard, C.; Bergé, J.-P.; Donnay-Moreno, C.; Cornet, J.; Ragon, J.-Y.; Fleurence, J.; Dumay, J. Optimization of R-Phycoerythrin Extraction by Ultrasound-Assisted Enzymatic Hydrolysis: A Comprehensive Study on the Wet Seaweed Grateloupia turuturu. Mar. Drugs 2023, 21, 213. [Google Scholar] [CrossRef] [PubMed]
- Perčić, M.; Vladimir, N.; Fan, A. Techno-Economic Assessment of Alternative Marine Fuels for Inland Shipping in Croatia. Renew. Sustain. Energy Rev. 2021, 148, 111363. [Google Scholar] [CrossRef]
- Folke, C.; Kautsky, N. Aquaculture with Its Environment: Prospects for Sustainability. Ocean Coast. Manag. 1992, 17, 5–24. [Google Scholar] [CrossRef]
- Ion, I.V.; Popescu, F.; Coman, G.; Frătița, M. Heat Requirement in an Indoor Recirculating Aquaculture System. Energy Rep. 2022, 8, 11707–11714. [Google Scholar] [CrossRef]
- Kuyumcu, M.E.; Tutumlu, H.; Yumrutaş, R. Performance of a Swimming Pool Heating System by Utilizing Waste Energy Rejected from an Ice Rink with an Energy Storage Tank. Energy Convers. Manag. 2016, 121, 349–357. [Google Scholar] [CrossRef]
- Via-Aqua Conclusion Du Programme AQUAGRINERGIE. Available online: https://www.via-aqua.fr/ (accessed on 3 June 2024).
- Bergman, K.; Henriksson, P.J.G.; Hornborg, S.; Troell, M.; Borthwick, L.; Jonell, M.; Philis, G.; Ziegler, F. Recirculating Aquaculture Is Possible without Major Energy Tradeoff: Life Cycle Assessment of Warmwater Fish Farming in Sweden. Environ. Sci. Technol. 2020, 54, 16062–16070. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Davy, D.; Sciortino, J.; Beveridge, M.C.; Arnason, R.; Gudmundsson, A. Countering Climate Change: Measures and Tools to Reduce Energy Use and Greenhouse Gas Emission in Fisheries and Aquaculture. In Impacts of Climate Change on Fisheries and Aquaculture; FAO: Rome, Italy, 2018; p. 585. [Google Scholar]
- Perdikaris, C.; Paschos, I. Organic Aquaculture in Greece: A Brief Review. Rev. Aquac. 2010, 2, 102–105. [Google Scholar] [CrossRef]
- Lagutkina, L.Y.; Ponomarev, S. Organic Aquaculture as Promising Trend of the Fish Industry Development. Agric. Biol. 2018, 53, 326–336. [Google Scholar] [CrossRef]
- IFOAM (International Federation of Organic Agriculture Movements). IFOAM General Assembly; IFOAM: Vignola, Italy, 2008. [Google Scholar]
- Angel, D.; Jokumsen, A.; Lembo, G. Aquaculture Production Systems and Environmental Interactions. In Organic Aquaculture: Impacts and Future Developments; Lembo, G., Mente, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 103–118. ISBN 978-3-030-05603-2. [Google Scholar]
- Sicuro, B. An Overview of Organic Aquaculture in Italy. Aquaculture 2019, 509, 134–139. [Google Scholar] [CrossRef]
- Labatut, R.A.; Olivares, J.F. Culture of Turbot (Scophthalmus maximus) Juveniles Using Shallow Raceways Tanks and Recirculation. Aquac. Eng. 2004, 32, 113–127. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Lemarié, G.; Breuil, G.; Petochi, T.; Marino, G.; Triplet, S.; Dutto, G.; Fivelstad, S.; Coeurdacier, J.-L.; Blancheton, J.-P. Effects of Rearing Density on Sea Bass (Dicentrarchus labrax) Biological Performance, Blood Parameters and Disease Resistance in a Flow through System. Aquat. Living Resour. 2010, 23, 109–117. [Google Scholar] [CrossRef]
- Salas-Leiton, E.; Anguis, V.; Manchado, M.; Cañavate, J.P. Growth, Feeding and Oxygen Consumption of Senegalese Sole (Solea senegalensis) Juveniles Stocked at Different Densities. Aquaculture 2008, 285, 84–89. [Google Scholar] [CrossRef]
- Murray, F.; Bostock, J.; Fletcher, D. Review of Recirculation Aquaculture System Technologies and Their Commercial Application; University of Stirling Aquaculture: Scotland, UK, 2014. [Google Scholar]
- Foysal, M.; Fotedar, R.; Gupta, S.; Chaklader, M. Biological Ball Filters Regulate Bacterial Communities in Marron (Cherax cainii) Culture System. Lett. Appl. Microbiol. 2019, 68, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kovács, B.D.; de Jesus Gregersen, K.J.; Rüppel, F.; von Danwitz, A.; Pedersen, L.-F. Evaluating Protein Skimmer Performance in a Commercial Seawater Recirculating Aquaculture System (RAS). Aquac. Eng. 2023, 103, 102369. [Google Scholar] [CrossRef]
- de Jesus Gregersen, K.J.; Pedersen, P.B.; Pedersen, L.-F.; Liu, D.; Dalsgaard, J. UV Irradiation and Micro Filtration Effects on Micro Particle Development and Microbial Water Quality in Recirculation Aquaculture Systems. Aquaculture 2020, 518, 734785. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture Systems. In Aquaculture Production Systems; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 245–277. [Google Scholar]
- Engle, C.R.; Kumar, G.; van Senten, J. Resource-Use Efficiency in US Aquaculture: Farm-Level Comparisons across Fish Species and Production Systems. Aquac. Environ. Interact. 2021, 13, 259–275. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating Aquaculture Systems (RAS): Environmental Solution and Climate Change Adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Ranjan, R.; Megarajan, S.; Xavier, B.; Raju, S.S.; Ghosh, S.; Gopalakrishnan, A. Design and Performance of Recirculating Aquaculture System for Marine Finfish Broodstock Development. Aquac. Eng. 2019, 85, 90–97. [Google Scholar] [CrossRef]
- Godoy-Olmos, S.; Jauralde, I.; Monge-Ortiz, R.; Milián-Sorribes, M.C.; Jover-Cerdá, M.; Tomás-Vidal, A.; Martínez-Llorens, S. Influence of Diet and Feeding Strategy on the Performance of Nitrifying Trickling Filter, Oxygen Consumption and Ammonia Excretion of Gilthead Sea Bream (Sparus aurata) Raised in Recirculating Aquaculture Systems. Aquac. Int. 2022, 30, 581–606. [Google Scholar] [CrossRef]
- Almeida, D.B.; Magalhães, C.; Sousa, Z.; Borges, M.T.; Silva, E.; Blanquet, I.; Mucha, A.P. Microbial Community Dynamics in a Hatchery Recirculating Aquaculture System (RAS) of Sole (Solea senegalensis). Aquaculture 2021, 539, 736592. [Google Scholar] [CrossRef]
- Meriç, İ. Mineral Element and Nutrient Composition of Two Newly-Introduced Fish Species (Dentex dentex and Seriola dumerili) in Recirculating Aquaculture System (RAS). GIDA/J. Food 2017, 42, 163–168. [Google Scholar] [CrossRef]
- Yang, J.; Ni, Q.; Zhang, Y.; Xu, B. Construction Technology on RAS for Shrimp Culture. Trans. Chin. Soc. Agric. Eng. 2010, 26, 136–140. [Google Scholar]
- Suantika, G.; Situmorang, M.L.; Kurniawan, J.B.; Pratiwi, S.A.; Aditiawati, P.; Astuti, D.I.; Azizah, F.F.N.; Djohan, Y.A.; Zuhri, U.; Simatupang, T.M. Development of a Zero Water Discharge (ZWD)—Recirculating Aquaculture System (RAS) Hybrid System for Super Intensive White Shrimp (Litopenaeus vannamei) Culture under Low Salinity Conditions and Its Industrial Trial in Commercial Shrimp Urban Farming in Gresik, East Java, Indonesia. Aquac. Eng. 2018, 82, 12–24. [Google Scholar]
- Fauzi, R.L.; Pamungkas, A.P.; Purwadi, D. White Shrimp Litopenaeus vannamei Based Agroindustry through Recirculating Aquaculture System to Increase Competitiveness; EDP Sciences: Les Ulis, France, 2020; Volume 147, p. 01002. [Google Scholar]
- Grosso, L.; Rakaj, A.; Fianchini, A.; Morroni, L.; Cataudella, S.; Scardi, M. Integrated Multi-Trophic Aquaculture (IMTA) System Combining the Sea Urchin Paracentrotus lividus, as Primary Species, and the Sea Cucumber Holothuria tubulosa as Extractive Species. Aquaculture 2021, 534, 736268. [Google Scholar] [CrossRef]
- Sartori, D.; Scuderi, A.; Sansone, G.; Gaion, A. Echinoculture: The Rearing of Paracentrotus lividus in a Recirculating Aquaculture System—Experiments of Artificial Diets for the Maintenance of Sexual Maturation. Aquac. Int. 2015, 23, 111–125. [Google Scholar] [CrossRef]
- Sartori, D.; Gaion, A. Can Sea Urchins Benefit from an Artificial Diet? Physiological and Histological Assessment for Echinoculture Feasibility Evaluation. Aquac. Nutr. 2016, 22, 1214–1221. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. A Review of Influencing Factors on a Recirculating Aquaculture System: Environmental Conditions, Feeding Strategies, and Disinfection Methods. J. World Aquac. Soc. 2023, 54, 566–602. [Google Scholar] [CrossRef]
- Chang, C.; Fang, W.; Jao, R.-C.; Shyu, C.; Liao, I.-C. Development of an Intelligent Feeding Controller for Indoor Intensive Culturing of Eel. Aquac. Eng. 2005, 32, 343–353. [Google Scholar] [CrossRef]
- Steinberg, K.; Zimmermann, J.; Meyer, S.; Schulz, C. Start-up of Recirculating Aquaculture Systems: How Do Water Exchange Rates Influence Pikeperch (Sander lucioperca) and Water Composition? Aquac. Eng. 2018, 83, 151–159. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S.T. Abnormal Swimming Behavior and Increased Deformities in Rainbow Trout Oncorhynchus mykiss Cultured in Low Exchange Water Recirculating Aquaculture Systems. Aquac. Eng. 2011, 45, 109–117. [Google Scholar] [CrossRef]
- Pedersen, P.B.; von Ahnen, M.; Fernandes, P.; Naas, C.; Pedersen, L.-F.; Dalsgaard, J. Particle Surface Area and Bacterial Activity in Recirculating Aquaculture Systems. Aquac. Eng. 2017, 78, 18–23. [Google Scholar] [CrossRef]
- Davidson, J.; Barrows, F.T.; Kenney, P.B.; Good, C.; Schroyer, K.; Summerfelt, S.T. Effects of Feeding a Fishmeal-Free versus a Fishmeal-Based Diet on Post-Smolt Atlantic Salmon Salmo salar Performance, Water Quality, and Waste Production in Recirculation Aquaculture Systems. Aquac. Eng. 2016, 74, 38–51. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Barrows, F.T.; Welsh, C.; Kenney, P.B.; Summerfelt, S.T. Comparing the Effects of Feeding a Grain-or a Fish Meal-Based Diet on Water Quality, Waste Production, and Rainbow Trout Oncorhynchus mykiss Performance within Low Exchange Water Recirculating Aquaculture Systems. Aquac. Eng. 2013, 52, 45–57. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. (Eds.) Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy Use in Recirculating Aquaculture Systems (RAS): A Review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- Badiola, M.; Basurko, O.C.; Gabiña, G.; Mendiola, D. Integration of Energy Audits in the Life Cycle Assessment Methodology to Improve the Environmental Performance Assessment of Recirculating Aquaculture Systems. J. Clean. Prod. 2017, 157, 155–166. [Google Scholar] [CrossRef]
- Kucuk, H.; Midilli, A.; Özdemir, A.; Çakmak, E.; Dincer, I. Exergetic Performance Analysis of a Recirculating Aquaculture System. Energy Convers. Manag. 2010, 51, 1033–1043. [Google Scholar] [CrossRef]
- Badiola, M.; Cabezas, O.; Curtin, R.; García, M.; Gartzia, I.; Mendiola, D. Land Based On-Growing of Atlantic Cod (Gadus morhua) to Marketable Size–a Feasibility Study from the Basque Country (Northern Spain); AZTI: San Sebastian, Spain, 2014. [Google Scholar]
- MacLeod, M.; Hasan, M.R.; Robb, D.H.; Mamun-Ur-Rashid, M. Quantifying and Mitigating Greenhouse Gas Emissions from Global Aquaculture; FAO Fisheries and Aquaculture Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; ISBN 978-92-5-131992-5. [Google Scholar]
- Kodama, M. Overview and History of IMTA, from Ancient to Modern Times; Aquaculture Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2022; pp. 2–5. [Google Scholar]
- Ryther, J.H.; Goldman, J.C.; Gifford, C.E.; Huguenin, J.E.; Wing, A.S.; Clarner, J.P.; Williams, L.D.; Lapointe, B.E. Physical Models of Integrated Waste Recycling- Marine Polyculture Systems. Aquaculture 1975, 5, 163–177. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Chopin, T.; Yarish, C.; Wilkes, R.; Belyea, E.; Lu, S.; Mathieson, A. Developing Porphyra/Salmon Integrated Aquaculture for Bioremediation and Diversification of the Aquaculture Industry. J. Appl. Phycol. 1999, 11, 463–472. [Google Scholar] [CrossRef]
- Chopin, T. Integrated Multi-Trophic Aquaculture. N. Aquac. 2006, 12, 4. [Google Scholar]
- Dunbar, M.B.; Malta, E.; Brunner, L.; Hughes, A.; Ratcliff, J.; Johnson, M.; Jacquemin, B.; Michel, R.; Cunha, M.; Oliveira, G.; et al. Defining Integrated Multi-Trophic Aquaculture: A Consensus. Aquac. Eur. 2020, 45, 22–27. [Google Scholar]
- United Nations Goal 12: Ensure Sustainable Consumption and Production Patterns. Available online: https://sdgs.un.org/goals/goal12 (accessed on 17 October 2023).
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the Art and Challenges for Offshore Integrated Multi-Trophic Aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and Nutrient Removal Performance of the Seaweed in a Land-Based Pilot Scale System. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Shpigel, M. Mariculture Systems, Integrated Land-Based. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 6309–6318. ISBN 978-1-4419-0851-3. [Google Scholar]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ashkenazi, N.; Ben-Ezra, D. Ulva lactuca Biofilter from a Land-Based Integrated Multi Trophic Aquaculture (IMTA) System as a Sole Food Source for the Tropical Sea Urchin Tripneustes gratilla elatensis. Aquaculture 2018, 496, 221–231. [Google Scholar] [CrossRef]
- Pereira, R.; Yarish, C.; Critchley, A.T. Seaweed Aquaculture for Human Foods in Land Based and IMTA Systems. In Applications of Seaweeds in Food and Nutrition; Elsevier: Amsterdam, The Netherlands, 2024; pp. 77–99. [Google Scholar]
- Guerra-García, J.M.; Martínez-Pita, I.; Šegvić-Bubić, T.; Manchado, M.; Arechavala-Lopez, P.; Calado, R.; Marchio, E.; Gentry, R.; Tlusty, M.F.; Rhyne, A.; et al. Chapter 5—Aquaculture and Conservation. In Coastal Habitat Conservation; Espinosa, F., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 111–146. ISBN 978-0-323-85613-3. [Google Scholar]
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The Economics of Integrated Multi-Trophic Aquaculture: Where Are We Now and Where Do We Need to Go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Nederlof, M.A.J.; Verdegem, M.C.J.; Smaal, A.C.; Jansen, H.M. Nutrient Retention Efficiencies in Integrated Multi-Trophic Aquaculture. Rev. Aquac. 2022, 14, 1194–1212. [Google Scholar] [CrossRef]
- Chang, B.-V.; Liao, C.-S.; Chang, Y.-T.; Chao, W.-L.; Yeh, S.-L.; Kuo, D.-L.; Yang, C.-W. Investigation of a Farm-Scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture. Sustainability 2019, 11, 1880. [Google Scholar] [CrossRef]
- Jerónimo, D.; Lillebø, A.I.; Santos, A.; Cremades, J.; Calado, R. Performance of Polychaete Assisted Sand Filters under Contrasting Nutrient Loads in an Integrated Multi-Trophic Aquaculture (IMTA) System. Sci. Rep. 2020, 10, 20871. [Google Scholar] [CrossRef]
- Ramli, N.M.; Verreth, J.A.J.; Yusoff, F.M.; Nurulhuda, K.; Nagao, N.; Verdegem, M.C.J. Integration of Algae to Improve Nitrogenous Waste Management in Recirculating Aquaculture Systems: A Review. Front. Bioeng. Biotechnol. 2020, 8, 1004. [Google Scholar] [CrossRef]
- Checa, D.; Macey, B.M.; Bolton, J.J.; Brink-Hull, M.; O’Donohoe, P.; Cardozo, A.; Poersch, L.H.; Sánchez, I. Circularity Assessment in Aquaculture: The Case of Integrated Multi-Trophic Aquaculture (IMTA) Systems. Fishes 2024, 9, 165. [Google Scholar] [CrossRef]
- Cunha, M.E.; Quental-Ferreira, H.; Parejo, A.; Gamito, S.; Ribeiro, L.; Moreira, M.; Monteiro, I.; Soares, F.; Pousão-Ferreira, P. Understanding the Individual Role of Fish, Oyster, Phytoplankton and Macroalgae in the Ecology of Integrated Production in Earthen Ponds. Aquaculture 2019, 512, 734297. [Google Scholar] [CrossRef]
- Aubin, J.; Hussenot, J. Integrated Aquaculture-Agriculture and Agroecology in Aquaculture: Views from Europe-Advancing Integrated Agriculture-Aquaculture through Agroecology; FAO Workshop: Montpellier, France, 2018. [Google Scholar]
- Hossain, A.; Senff, P.; Glaser, M. Lessons for Coastal Applications of IMTA as a Way towards Sustainable Development: A Review. Appl. Sci. 2022, 12, 11920. [Google Scholar] [CrossRef]
- Ahmed, N.; Bunting, S.W.; Glaser, M.; Flaherty, M.S.; Diana, J.S. Can Greening of Aquaculture Sequester Blue Carbon? Ambio 2017, 46, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Huang, L.; Peng, Y.; Tan, K.; Tan, K. The Effects of Bivalve Aquaculture on Carbon Storage in the Water Column and Sediment of Aquaculture Areas. Sci. Total Environ. 2024, 937, 173538. [Google Scholar] [CrossRef]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A.; Ralph, P.J. Can Macroalgae Contribute to Blue Carbon? An Australian Perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using Marine Macroalgae for Carbon Sequestration: A Critical Appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Summary for Policymakers: Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Liu, Y.; Zhang, J.; Wu, W.; Zhong, Y.; Li, H.; Wang, X.; Yang, J.; Zhang, Y. Effects of Shellfish and Macro-Algae IMTA in North China on the Environment, Inorganic Carbon System, Organic Carbon System, and Sea–Air CO2 Fluxes. Front. Mar. Sci. 2022, 9, 864306. [Google Scholar] [CrossRef]
- Holdt, S.L.; Edwards, M.D. Cost-Effective IMTA: A Comparison of the Production Efficiencies of Mussels and Seaweed. J. Appl. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Nobre, A.M.; Robertson-Andersson, D.; Neori, A.; Sankar, K. Ecological–Economic Assessment of Aquaculture Options: Comparison between Abalone Monoculture and Integrated Multi-Trophic Aquaculture of Abalone and Seaweeds. Aquaculture 2010, 306, 116–126. [Google Scholar] [CrossRef]
- Chary, K.; Aubin, J.; Sadoul, B.; Fiandrino, A.; Covès, D.; Callier, M.D. Integrated Multi-Trophic Aquaculture of Red Drum (Sciaenops Ocellatus) and Sea Cucumber (Holothuria scabra): Assessing Bioremediation and Life-Cycle Impacts. Aquaculture 2020, 516, 734621. [Google Scholar] [CrossRef]
- Chopin, T.; Lively, A.; Wiper, J.; Totten, L. Canadian IMTA Kelps Get Organic Certification and Are Ready to Hit the Marketplace. Aquac. Eur. 2014, 39, 14–15. [Google Scholar]
- Khanjani, M.H.; Zahedi, S.; Mohammadi, A. Integrated Multitrophic Aquaculture (IMTA) as an Environmentally Friendly System for Sustainable Aquaculture: Functionality, Species, and Application of Biofloc Technology (BFT). Environ. Sci. Pollut. Res. 2022, 29, 67513–67531. [Google Scholar] [CrossRef] [PubMed]
- Emerenciano, M.; Gaxiola, G.; Cuzon, G. Biofloc Technology (BFT): A Review for Aquaculture Application and Animal Food Industry. Biomass Now-Cultiv. Util. 2013, 12, 301–328. [Google Scholar]
- Samocha, T.M. Sustainable Biofloc Systems for Marine Shrimp; Academic Press: Cambridge, MA, USA, 2019; ISBN 0-12-818239-3. [Google Scholar]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; World Aquaculture Society: Baton Rouge, LA, USA, 2009; ISBN 978-1-888807-16-5. [Google Scholar]
- Crab, R. Bioflocs Technology: An Integrated System for the Removal of Nutrients and Simultaneous Production of Feed in Aquaculture. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2010. [Google Scholar]
- Zimmermann, S.; Kiessling, A.; Zhang, J. The Future of Intensive Tilapia Production and the Circular Bioeconomy without Effluents: Biofloc Technology, Recirculation Aquaculture Systems, Bio-RAS, Partitioned Aquaculture Systems and Integrated Multitrophic Aquaculture. Rev. Aquac. 2023, 15, 22–31. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Biofloc Systems for Sustainable Production of Economically Important Aquatic Species: A Review. Sustainability 2021, 13, 7255. [Google Scholar] [CrossRef]
- Lauderdale, C.V.; Aldrich, H.C.; Lindner, A.S. Isolation and Characterization of a Bacterium Capable of Removing Taste- and Odor-Causing 2-Methylisoborneol from Water. Water Res. 2004, 38, 4135–4142. [Google Scholar] [CrossRef]
- Guttman, L.; van Rijn, J. Isolation of Bacteria Capable of Growth with 2-Methylisoborneol and Geosmin as the Sole Carbon and Energy Sources. Appl. Environ. Microbiol. 2012, 78, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2013; Volume 4503. [Google Scholar]
- Zhao, D.; Pan, L.; Huang, F.; Wang, C.; Xu, W. Effects of Different Carbon Sources on Bioactive Compound Production of Biofloc, Immune Response, Antioxidant Level, and Growth Performance of Litopenaeus vannamei in Zero-water Exchange Culture Tanks. J. World Aquac. Soc. 2016, 47, 566–576. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J. Engineering Analysis of the Stoichiometry of Photoautotrophic, Autotrophic, and Heterotrophic Removal of Ammonia–Nitrogen in Aquaculture Systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Burford, M.A.; Thompson, P.J.; McIntosh, R.P.; Bauman, R.H.; Pearson, D.C. The Contribution of Flocculated Material to Shrimp (Litopenaeus vannamei) Nutrition in a High-Intensity, Zero-Exchange System. Aquaculture 2004, 232, 525–537. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The Basics of Bio-Flocs Technology: The Added Value for Aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Ulloa Walker, D.A.; Morales Suazo, M.C.; Emerenciano, M.G.C. Biofloc Technology: Principles Focused on Potential Species and the Case Study of Chilean River Shrimp Cryphiops Caementarius. Rev. Aquac. 2020, 12, 1759–1782. [Google Scholar] [CrossRef]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc Technology in Aquaculture: Beneficial Effects and Future Challenges. Aquaculture 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Crab, R.; Lambert, A.; Defoirdt, T.; Bossier, P.; Verstraete, W. The Application of Bioflocs Technology to Protect Brine Shrimp (Artemia franciscana) from Pathogenic Vibrio Harveyi. J. Appl. Microbiol. 2010, 109, 1643–1649. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Use of Biofloc Technology in Shrimp Aquaculture: A Comprehensive Review, with Emphasis on the Last Decade. Rev. Aquac. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Emerenciano, M.G.; Miranda-Baeza, A.; Martínez-Porchas, M.; Poli, M.A.; do Vieira, F.N. Biofloc Technology (BFT) in Shrimp Farming: Past and Present Shaping the Future. Front. Mar. Sci. 2021, 8, 813091. [Google Scholar] [CrossRef]
- Bossier, P.; Ekasari, J. Biofloc Technology Application in Aquaculture to Support Sustainable Development Goals. Microb. Biotechnol. 2017, 10, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Holanda, M.; Ravagnan, E.; Lara, G.; Santana, G.; Furtado, P.; Cardozo, A.; Wasielesky, W., Jr.; Poersch, L.H. Integrated Multitrophic Culture of Shrimp Litopenaeus vannamei and Tilapia Oreochromis niloticus in Biofloc System: A Pilot Scale Study. Front. Mar. Sci. 2023, 10, 1060846. [Google Scholar] [CrossRef]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; de Lorenzo, M.A.; Hayashi, L.; do Nascimento Vieira, F. Sea Lettuce Integrated with Pacific White Shrimp and Mullet Cultivation in Biofloc Impact System Performance and the Sea Lettuce Nutritional Composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- Carvalho, A.; Costa, L.C.d.O.; Holanda, M.; Poersch, L.H.; Turan, G. Influence of Total Suspended Solids on the Growth of the Sea Lettuce Ulva lactuca Integrated with the Pacific White Shrimp Litopenaeus vannamei in a Biofloc System. Fishes 2023, 8, 163. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated Multitrophic Aquaculture Applied to Shrimp Rearing in a Biofloc System. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Xu, W.-J.; Pan, L.-Q. Enhancement of Immune Response and Antioxidant Status of Litopenaeus vannamei Juvenile in Biofloc-Based Culture Tanks Manipulating High C/N Ratio of Feed Input. Aquaculture 2013, 412, 117–124. [Google Scholar] [CrossRef]
- Crab, R.; Kochva, M.; Verstraete, W.; Avnimelech, Y. Bio-Flocs Technology Application in over-Wintering of Tilapia. Aquac. Eng. 2009, 40, 105–112. [Google Scholar] [CrossRef]
- Choudhury, A.; Lepine, C.; Witarsa, F.; Good, C. Anaerobic Digestion Challenges and Resource Recovery Opportunities from Land-Based Aquaculture Waste and Seafood Processing Byproducts: A Review. Bioresour. Technol. 2022, 354, 127144. [Google Scholar] [CrossRef] [PubMed]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The Prospects of Biofloc Technology (BFT) for Sustainable Aquaculture Development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- Manan, H.; Kasan, N.A.; Ikhwanuddin, M.; Kamaruzzan, A.S.; Jalilah, M.; Fauzan, F.; Suloma, A.; Amin-Safwan, A. Biofloc Technology in Improving Shellfish Aquaculture Production—A Review. Ann. Anim. Sci. 2023. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, Y.; Tian, H.; Pan, S.; Yang, X.; Gao, Q.; Dong, S. Rapidly Increased Greenhouse Gas Emissions by Pacific White Shrimp Aquacultural Intensification and Potential Solutions for Mitigation in China. Aquaculture 2024, 587, 740825. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Sharma, K.; Brotto, A.C.; Khanal, S.K. Nitrogen Transformations in Intensive Aquaculture System and Its Implication to Climate Change through Nitrous Oxide Emission. Bioresour. Technol. 2013, 130, 314–320. [Google Scholar] [CrossRef]
- Soares, D.C.; Henry-Silva, G.G. Emission and Absorption of Greenhouse Gases Generated from Marine Shrimp Production (Litopeneaus vannamei) in High Salinity. J. Clean. Prod. 2019, 218, 367–376. [Google Scholar] [CrossRef]
- Izquierdo, C.; Usero, J.; Gracia, I. Speciation of Heavy Metals in Sediments from Salt Marshes on the Southern Atlantic Coast of Spain. Mar. Pollut. Bull. 1997, 34, 123–128. [Google Scholar] [CrossRef]
- Yúfera, M.; Arias, A.M. Traditional Polyculture in “Esteros” in the Bay of Cádiz (Spain). Hopes and Expectancies for the Prevalence of a Unique Activity in Europe; European Aquaculture Society: Stavanger, Norway, 2010. [Google Scholar]
- Guerra-García, J.; Calero-Cano, S.; Donázar-Aramendía, I.; Giráldez, I.; Morales, E.; Arechavala-Lopez, P.; Cervera-Currado, J. Farming Sparus aurata (Teleostei: Sparidae) in Marsh Ponds: Trophic Characterization and Trace Metal Accumulation. Mar. Environ. Res. 2023, 188, 106007. [Google Scholar] [CrossRef] [PubMed]
- Forrest, B.M.; Keeley, N.B.; Hopkins, G.A.; Webb, S.C.; Clement, D.M. Bivalve Aquaculture in Estuaries: Review and Synthesis of Oyster Cultivation Effects. Aquaculture 2009, 298, 1–15. [Google Scholar] [CrossRef]
- Costa, L.C.D.O.; Xavier, J.A.A.; Neves, L.F.D.M.; De Azambuja, A.M.V.; Junior, W.W.; Figueiredo, M.R.C. Polyculture of Litopenaeus vannamei Shrimp and Mugil platanus Mullet in Earthen Ponds. Rev. Bras. de Zootec. 2013, 42, 605–611. [Google Scholar] [CrossRef][Green Version]
- Sipaúba-Tavares, L.; Donadon, A.; Milan, R. Water Quality and Plankton Populations in an Earthen Polyculture Pond. Braz. J. Biol. 2011, 71, 845–855. [Google Scholar] [CrossRef]
- Cunha, M.E.; Quental-Ferreira, H.; Leão, A.C.; Matias, A.; Matias, D.; Joaquim, S.; Soares, F.; Pousão-Ferreira, P. Integrated Multi-Trophic Aquaculture in Earthen Ponds; Proceddings of the AQUA 2012; Global Aquaculture: Securing our Future: Prague, Czech Republic, 2012. [Google Scholar]
- Jiménez-Prada, P.; Hachero-Cruzado, I.; Giráldez, I.; Fernández-Diaz, C.; Vilas, C.; Cañavate, J.P.; Guerra-García, J.M. Crustacean Amphipods from Marsh Ponds: A Nutritious Feed Resource with Potential for Application in Integrated Multi-Trophic Aquaculture. PeerJ 2018, 6, e4194. [Google Scholar] [CrossRef]
- Miller, D.; Semmens, K. Waste Management in Aquaculture. West Va. Univ. Ext. Serv. Publ. No. AQ02-1. USA 2002, 8. Available online: https://freshwater-aquaculture.extension.org/wp-content/uploads/2019/08/WasteManagemetninAquaculture.pdf (accessed on 1 June 2024).
- Mitchell, I.M. In Situ Biodeposition Rates of Pacific Oysters (Crassostrea gigas) on a Marine Farm in Southern Tasmania (Australia). Aquaculture 2006, 257, 194–203. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Fang, J.G.; Han, T.T.; Mao, Y.Z.; Li, J.Q.; Du, M.R. The Role of Gracilaria lemaneiformis in Eliminating the Dissolved Inorganic Carbon Released from Calcification and Respiration Process of Chlamys farreri. J. Appl. Phycol. 2014, 26, 545–550. [Google Scholar] [CrossRef]
- Willer, D.F.; Aldridge, D.C. Sustainable Bivalve Farming Can Deliver Food Security in the Tropics. Nat. Food 2020, 1, 384–388. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, K.W.; Yang, P.; Yang, H.; Tong, C.; Song, C.; Tan, L.; Zhao, G.; Zhou, X.; Sun, D. Assessing Carbon Greenhouse Gas Emissions from Aquaculture in China Based on Aquaculture System Types, Species, Environmental Conditions and Management Practices. Agric. Ecosyst. Environ. 2022, 338, 108110. [Google Scholar] [CrossRef]
- Terrados, J.; Otero, M.; Bacci, T.; Didderen, K.; La Porta, B.; Teunis, M.; Bouma, T.J. Blue Carbon Ecosystem Restoration. In Manual for the Creation of Blue Carbon Projects in Europe and the Mediterranean; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2021. [Google Scholar]
- Kosten, S.; Almeida, R.M.; Barbosa, I.; Mendonça, R.; Santos Muzitano, I.; Sobreira Oliveira-Junior, E.; Vroom, R.J.E.; Wang, H.-J.; Barros, N. Better Assessments of Greenhouse Gas Emissions from Global Fish Ponds Needed to Adequately Evaluate Aquaculture Footprint. Sci. Total Environ. 2020, 748, 141247. [Google Scholar] [CrossRef]
- Vasanth, M.; Muralidhar, M.; Saraswathy, R.; Nagavel, A.; Dayal, J.S.; Jayanthi, M.; Lalitha, N.; Kumararaja, P.; Vijayan, K.K. Methodological Approach for the Collection and Simultaneous Estimation of Greenhouse Gases Emission from Aquaculture Ponds. Env. Monit. Assess. 2016, 188, 671. [Google Scholar] [CrossRef]
- Chen, G.; Bai, J.; Bi, C.; Wang, Y.; Cui, B. Global Greenhouse Gas Emissions from Aquaculture: A Bibliometric Analysis. Agric. Ecosyst. Environ. 2023, 348, 108405. [Google Scholar] [CrossRef]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D. Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Edwards, P. Aquaculture, Poverty Impacts and Livelihoods; ODI: London, UK, 2000. [Google Scholar]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I. Aquaculture: Global Status and Trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated Multi-Trophic Aquaculture (IMTA) in Marine Temperate Waters. Integr. Maric. Glob. Rev. FAO Fish. Aquac. Tech. Pap. 2009, 529, 7–46. [Google Scholar]
- Rosa, J.; Lemos, M.F.L.; Crespo, D.; Nunes, M.; Freitas, A.; Ramos, F.; Pardal, M.Â.; Leston, S. Integrated Multitrophic Aquaculture Systems—Potential Risks for Food Safety. Trends Food Sci. Technol. 2020, 96, 79–90. [Google Scholar] [CrossRef]
- Gunning, D.; Maguire, J.; Burnell, G. The Development of Sustainable Saltwater-Based Food Production Systems: A Review of Established and Novel Concepts. Water 2016, 8, 598. [Google Scholar] [CrossRef]
- Chopin, T.; MacDonald, B.; Robinson, S.; Cross, S.; Pearce, C.; Knowler, D.; Noce, A.; Reid, G.; Cooper, A.; Speare, D.; et al. The Canadian Integrated Multi-Trophic Aquaculture Network (CIMTAN)—A Network for a New Era of Ecosystem Responsible Aquaculture. Fisheries 2013, 38, 297–308. [Google Scholar] [CrossRef]
- Sidey, M. Aquaculture Regulation in Canada: A Case for Modernization, Standardization and Collaboration. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2018. [Google Scholar]
- Bueno, P.B. Widening the Horizon of Asian Mariculture with IMTA. J. Indian Soc. Coast. Agric. Res. 2021, 39, 239–248. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Cabello, F.; Young, K.; Carvajal, J.; Varela, D.A.; Henríquez, L. Salmon Aquaculture and Coastal Ecosystem Health in Chile: Analysis of Regulations, Environmental Impacts and Bioremediation Systems. Ocean Coast. Manag. 2009, 52, 243–249. [Google Scholar] [CrossRef]
- Camelo-Guarin, S.; Molinet, C.; Soto, D. Recommendations for Implementing Integrated Multitrophic Aquaculture in Commercial Farms at the Landscape Scale in Southern Chile. Aquaculture 2021, 544, 737116. [Google Scholar] [CrossRef]
- Stenton-Dozey, J.M.E.; Heath, P.; Ren, J.S.; Zamora, L.N. New Zealand Aquaculture Industry: Research, Opportunities and Constraints for Integrative Multitrophic Farming. N. Z. J. Mar. Freshw. Res. 2021, 55, 265–285. [Google Scholar] [CrossRef]

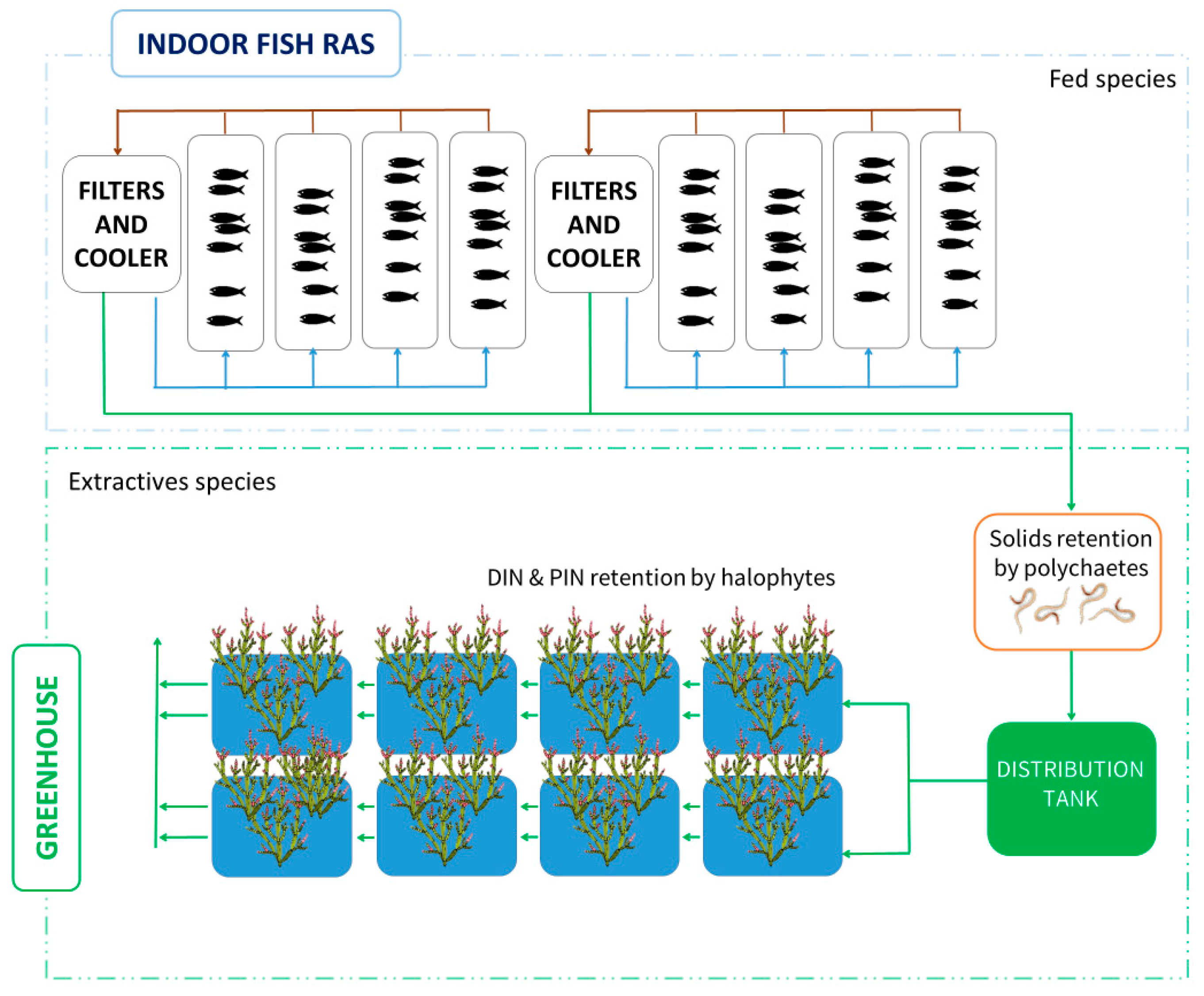
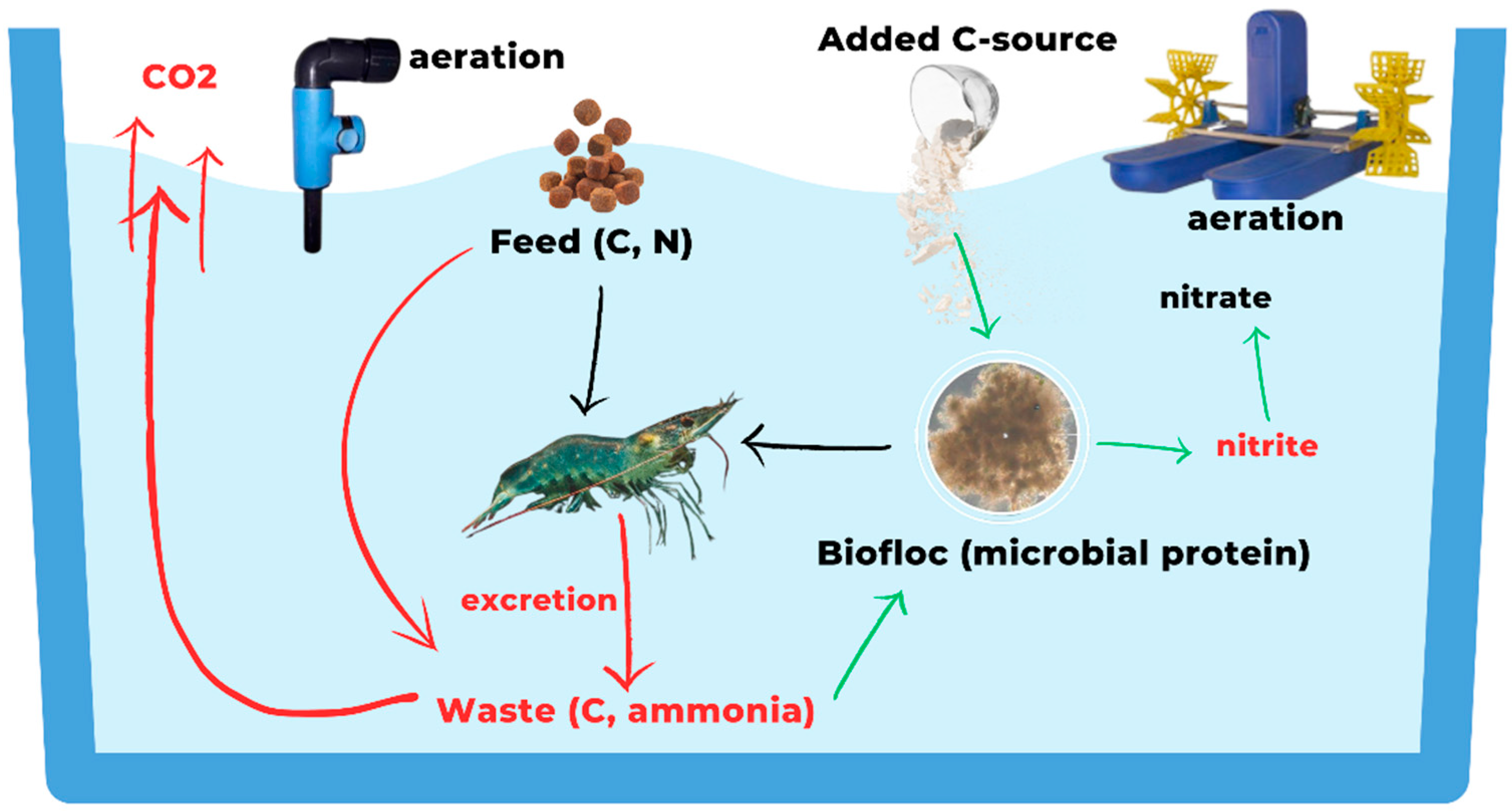

| Farming System | Low-Trophic-Level Species | Polyculture | Feeding Efficiency Strategies | Waste Management | Energy Efficiency | Carbon Sequestration | Carbon Emissions |
|---|---|---|---|---|---|---|---|
| RAS | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | 6109 kg CO2e t−1 WW [42] |
| IMTA | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | no data |
| BFT | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | 5945 kg CO2e t−1 of shrimp [164] |
| Extensive systems | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | ★★★★ | 392 kg CO2e t−1 of bivalves WW [42] ~65 t CO2e ha−1 y−1 [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castilla-Gavilán, M.; Guerra-García, J.M.; Hachero-Cruzado, I.; Herrera, M. Understanding Carbon Footprint in Sustainable Land-Based Marine Aquaculture: Exploring Production Techniques. J. Mar. Sci. Eng. 2024, 12, 1192. https://doi.org/10.3390/jmse12071192
Castilla-Gavilán M, Guerra-García JM, Hachero-Cruzado I, Herrera M. Understanding Carbon Footprint in Sustainable Land-Based Marine Aquaculture: Exploring Production Techniques. Journal of Marine Science and Engineering. 2024; 12(7):1192. https://doi.org/10.3390/jmse12071192
Chicago/Turabian StyleCastilla-Gavilán, Marta, José Manuel Guerra-García, Ismael Hachero-Cruzado, and Marcelino Herrera. 2024. "Understanding Carbon Footprint in Sustainable Land-Based Marine Aquaculture: Exploring Production Techniques" Journal of Marine Science and Engineering 12, no. 7: 1192. https://doi.org/10.3390/jmse12071192
APA StyleCastilla-Gavilán, M., Guerra-García, J. M., Hachero-Cruzado, I., & Herrera, M. (2024). Understanding Carbon Footprint in Sustainable Land-Based Marine Aquaculture: Exploring Production Techniques. Journal of Marine Science and Engineering, 12(7), 1192. https://doi.org/10.3390/jmse12071192







