Express Diagnosis and Prediction of Remote Mass Mortality of Scallop Mizuhopecten yessoensis in Mariculture Farms Using Biomarkers
Abstract
1. Introduction
2. Materials and Methods
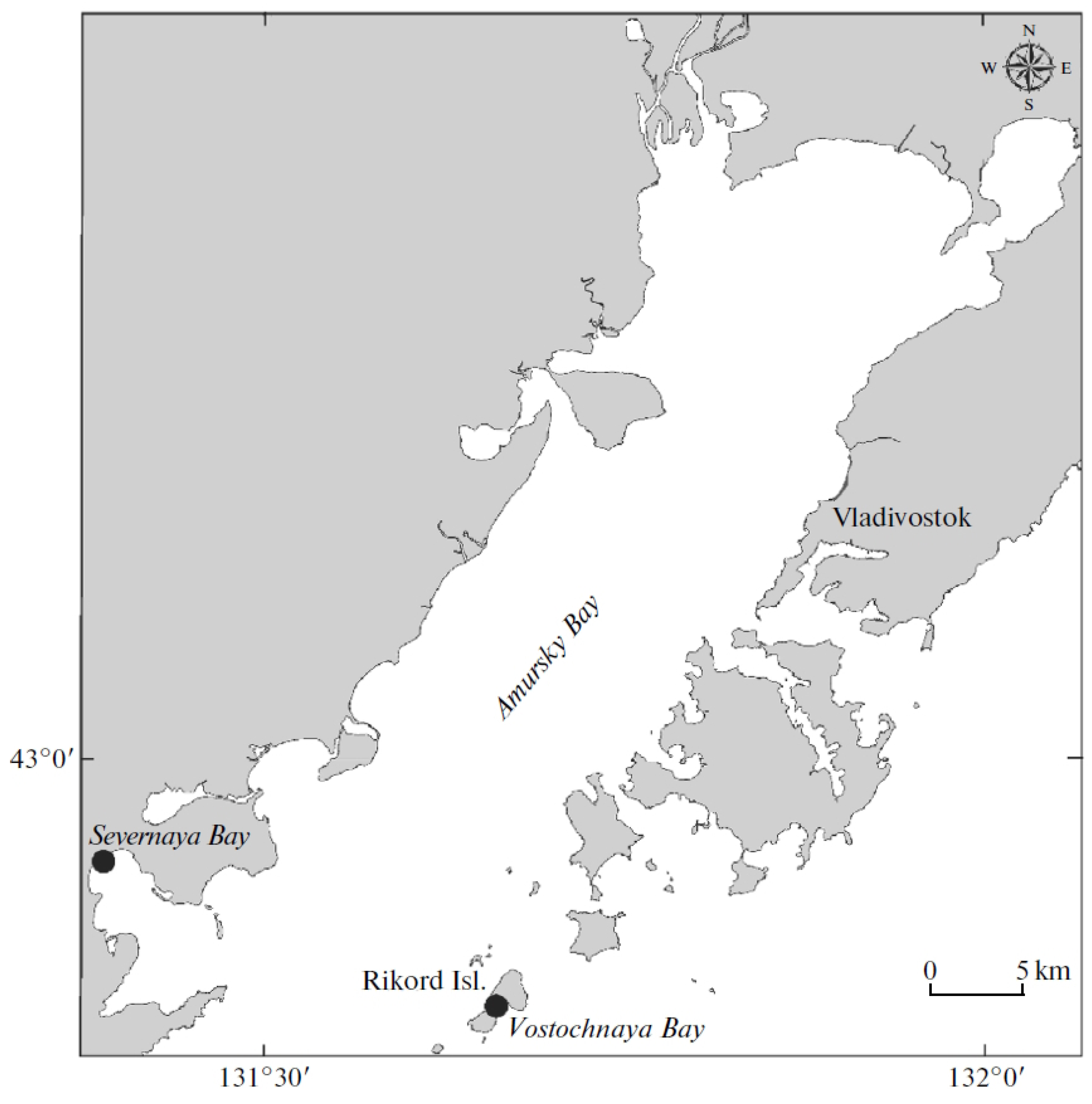
2.1. Site of Bivalve Collection and Materials
2.2. Determination of the Damage to DNA Molecules
2.3. Determination of Malondialdehyde (MDA) Content
2.4. Statistics
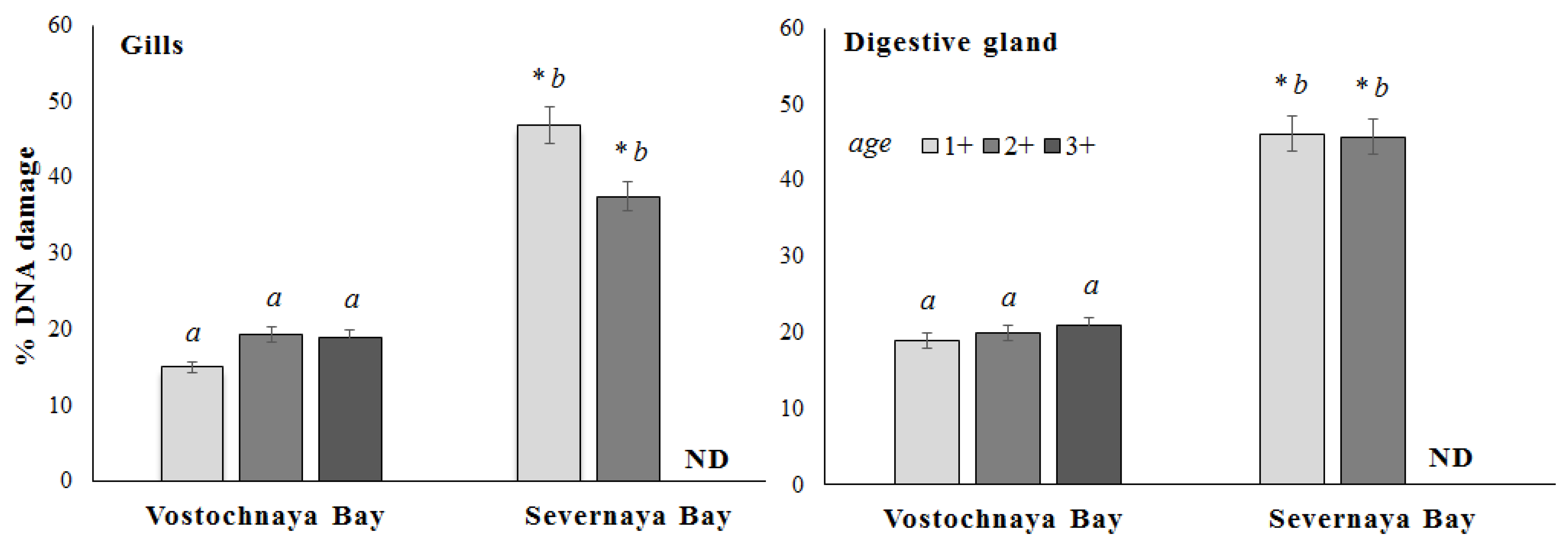
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018. In Meeting the Sustainable Development Goals; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024. In Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Xiao, J.; Ford, S.E.; Yang, H.; Zhang, G.; Zhang, F.; Guo, X. Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- Lan, Y.; Ye, T.; Xue, Y.; Liu, H.; Zhang, H.; Cheng, D.; Zhao, M.; Zhang, Y.; Li, S.; Ma, H.; et al. Phyiological and immunological responses to mass mortality in noble scallop Chlamys nobilis cultured in Nan’ao waters of Shantou, China. Fish Shellfish Immunol. 2018, 82, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Read, P.A.; Fernandes, T.F. Management of environmental impacts of marine aquaculture in Europe. Aquaculture 2003, 226, 139–163. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Li, R.; Wang, Z.; Xia, B.; Zhang, L. Density-dependent mortality of the scallop Chlamys farreri in grow-out cuture. Aquac Res. 2006, 37, 842–844. [Google Scholar] [CrossRef]
- Hingant, M.; Mallarino, S.; Conforto, E.; Dubillot, E.; Barbier, P.; Bringer, A.; Thomas, H. Artificial weathering of plastics used in oyster farming. Sci. Total Environ. 2023, 868, 161638. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Lin, C.; Tu, C.; Zhang, C.; Xu, S.; Yang, H. Association of heavy metals with plastics used in aquaculture. Mar. Poll. Bull. 2022, 174, 113312. [Google Scholar] [CrossRef] [PubMed]
- Koval’, I.V.; Ovchinnikova, I.A. Sostoyanie i tendencii razvitiya akvakul’tury v Primorskom krae. Vestnik TGU 2013, 1, 36–47. [Google Scholar]
- Collins, A.R. Investigating oxidative DNA damage and its pepair using the comet assay. Mutat. Res. 2009, 681, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic properties of polystyrene (PS) microspheres in the filter-feeder mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Kwon, B.G.; Amamiya, K.; Sato, H.; Chung, S.Y.; Kodera, Y.; Kim, S.K.; Lee, E.J.; Saido, K. Monitoring of styrene oligomers as indicators of polystyrene plastic pollution in the North-West Pacific Ocean. Chemosphere 2017, 180, 500–505. [Google Scholar] [CrossRef]
- Lee, S.; Alam, M.B.; Lee, S.; Jung, M.; Shim, W.J.; Kim, S. Identification and quantification of photodegradation products of disposed expanded polystyrene buoy used in aquaculture. Mar. Poll. Bull. 2023, 192, 114998. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Releasing of hexabromocyclododecanes from expanded polystyrenes in seawater -field and laboratory experiments. Chemosphere 2017, 185, 798–805. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Han, G.M.; Joon, S.W. Rapid Production of Micro- and Nanoplastics by Fragmentation of Expanded Polystyrene Exposed to Sunlight. Environ. Sci. Technol. 2020, 54, 11191–11200. [Google Scholar] [CrossRef]
- Mattsson, K.; Björkroth, F.; Karlsson, T.; Hassellöv, M. Nanofragmentation of Expanded Polystyrene under Simulated Environmental Weathering (Thermooxidative Degradation and Hydrodynamic Turbulence). Front. Mar. Sci. 2021, 7, 578178. [Google Scholar] [CrossRef]
- Dvoretsky, A.; Dvoretsky, V. Biological Aspects, Fisheries, and Aquaculture of Yesso Scallops in Russian Waters of the Sea of Japan. Diversity 2022, 14, 399. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Bramini, M.; Rotini, A.; Manfra, L.; Migliore, L.; Piazza, V.; Garaventa, F.; Faimali, M. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Mar. Environ. Res. 2018, 141, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Prokic, M.D.; Radovanovic, T.B.; Gavric, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Dovzhenko, N.V.; Chelomin, V.P.; Mazur, A.A.; Kukla, S.P.; Slobodskova, V.V.; Istomina, A.A.; Zhukovskaya, A.F. Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs). J. Mar. Sci. Eng. 2022, 10, 707. [Google Scholar] [CrossRef]
- Zacchi, F.L.; Flores-Nunes, F.; Mattos, J.J.; Lima, D.; Lüchmann, K.H.; Sasaki, S.T.; Bícego, M.C.; Taniguchi, S.; Montone, R.C.; Almeida, E.A.; et al. Biochemical and molecular responses in oysters Crassostrea brasiliana collected from estuarine aquaculture areas in Southern Brazil. Mar. Poll. Bull. 2018, 135, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the Monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodskova, V.V.; Dovzhenko, N.V.; Kukla, S.P.; Chelomin, V.P.; Mazur, A.A. Express Diagnosis and Prediction of Remote Mass Mortality of Scallop Mizuhopecten yessoensis in Mariculture Farms Using Biomarkers. J. Mar. Sci. Eng. 2024, 12, 1151. https://doi.org/10.3390/jmse12071151
Slobodskova VV, Dovzhenko NV, Kukla SP, Chelomin VP, Mazur AA. Express Diagnosis and Prediction of Remote Mass Mortality of Scallop Mizuhopecten yessoensis in Mariculture Farms Using Biomarkers. Journal of Marine Science and Engineering. 2024; 12(7):1151. https://doi.org/10.3390/jmse12071151
Chicago/Turabian StyleSlobodskova, Valentina Vladimirovna, Nadezhda Vladimirovna Dovzhenko, Sergey Petrovich Kukla, Victor Pavlovich Chelomin, and Andrey Alexandrovich Mazur. 2024. "Express Diagnosis and Prediction of Remote Mass Mortality of Scallop Mizuhopecten yessoensis in Mariculture Farms Using Biomarkers" Journal of Marine Science and Engineering 12, no. 7: 1151. https://doi.org/10.3390/jmse12071151
APA StyleSlobodskova, V. V., Dovzhenko, N. V., Kukla, S. P., Chelomin, V. P., & Mazur, A. A. (2024). Express Diagnosis and Prediction of Remote Mass Mortality of Scallop Mizuhopecten yessoensis in Mariculture Farms Using Biomarkers. Journal of Marine Science and Engineering, 12(7), 1151. https://doi.org/10.3390/jmse12071151





