Ecology of Intertidal Rocky Shores Related to Examples of Coastal Geology across Phanerozoic Time
Abstract
:1. Introduction
2. Background on Coastal Geography and Geology
3. Operational Definitions and Study Methods
4. Results
4.1. Review of Granite Rocky Shores and Biotas with Different Climates by Latitude
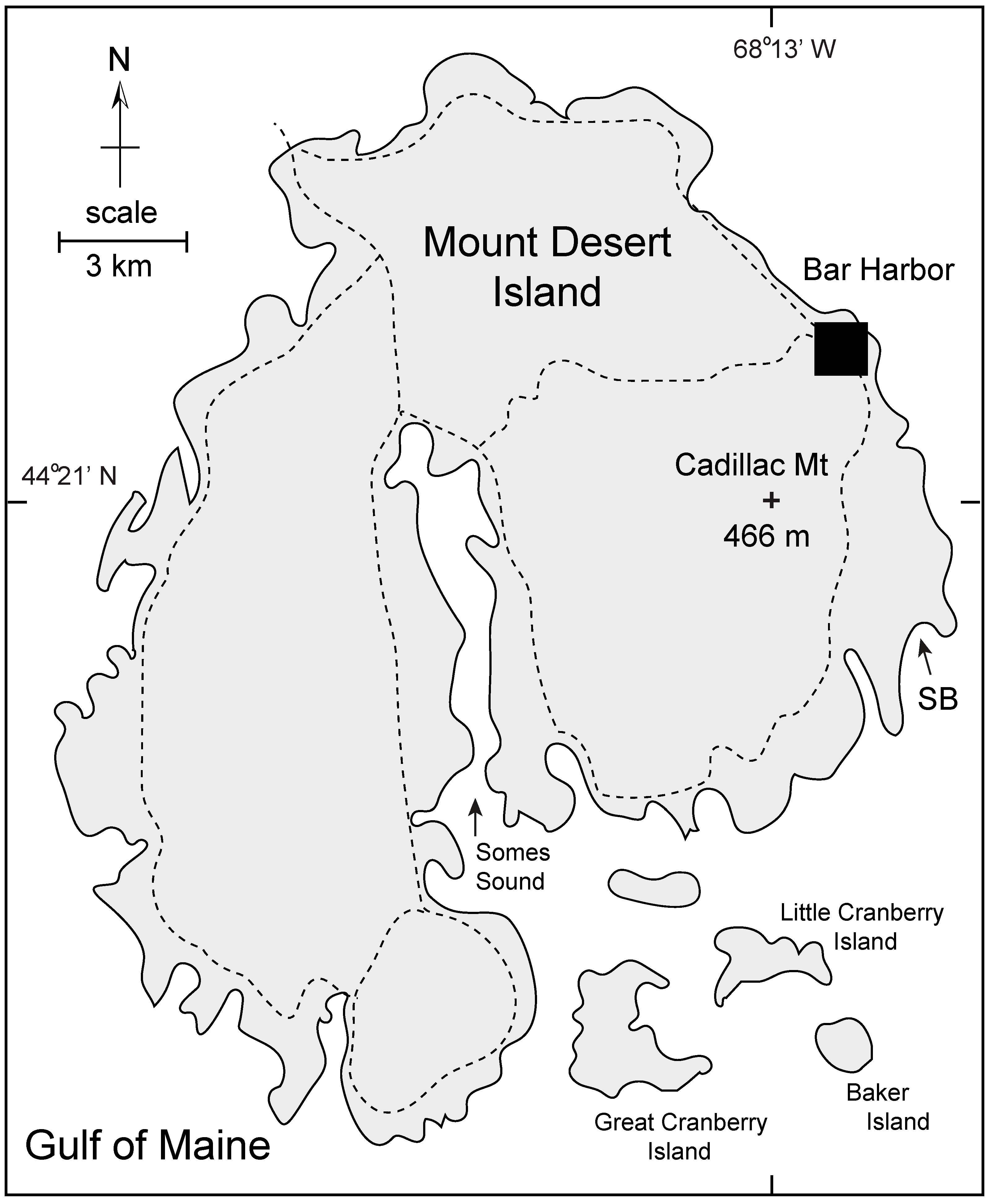
4.1.1. Mount Desert Island (Maine, USA)
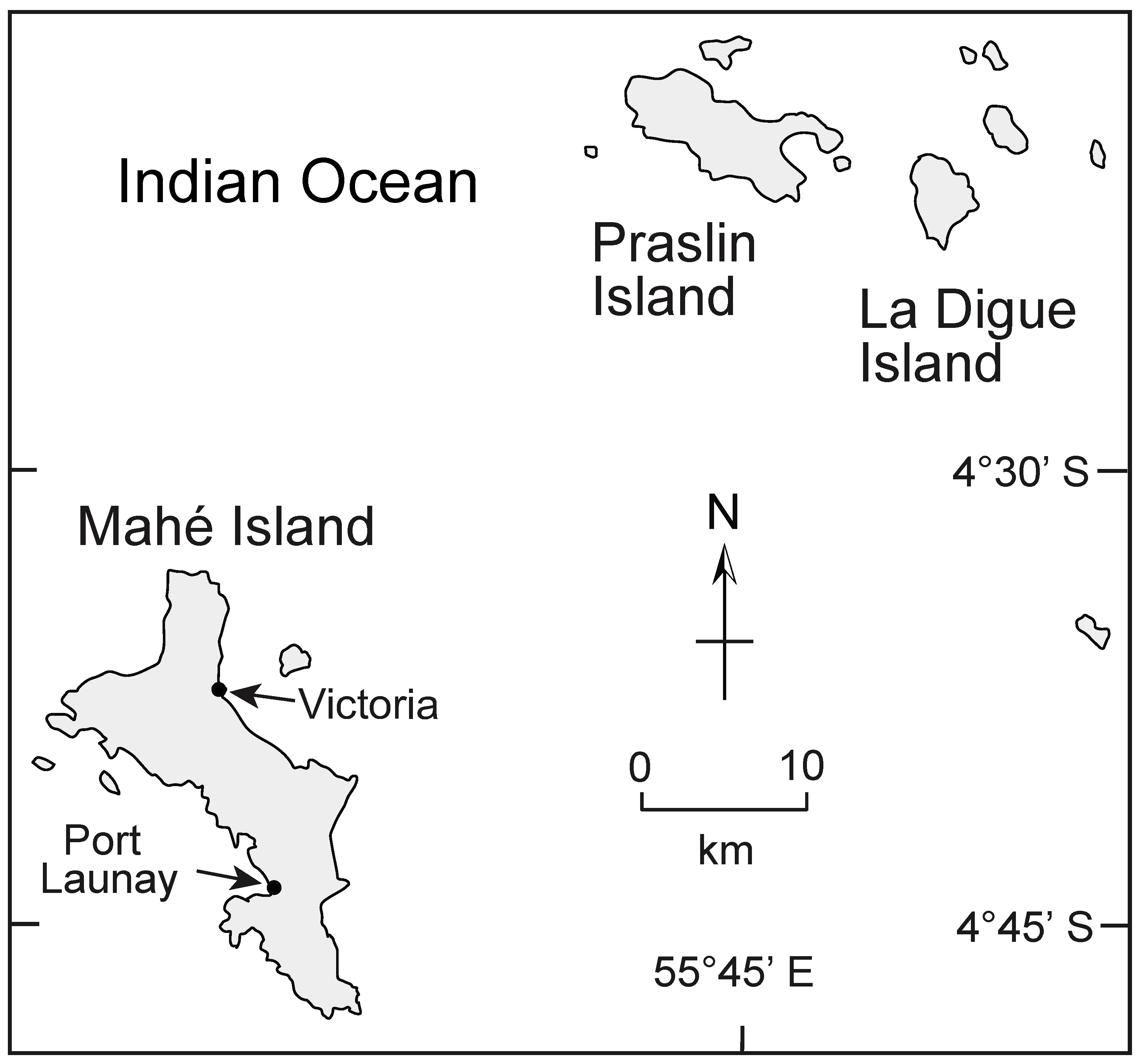
4.1.2. Mahé and Praslin Islands in the Republic of Seychelles
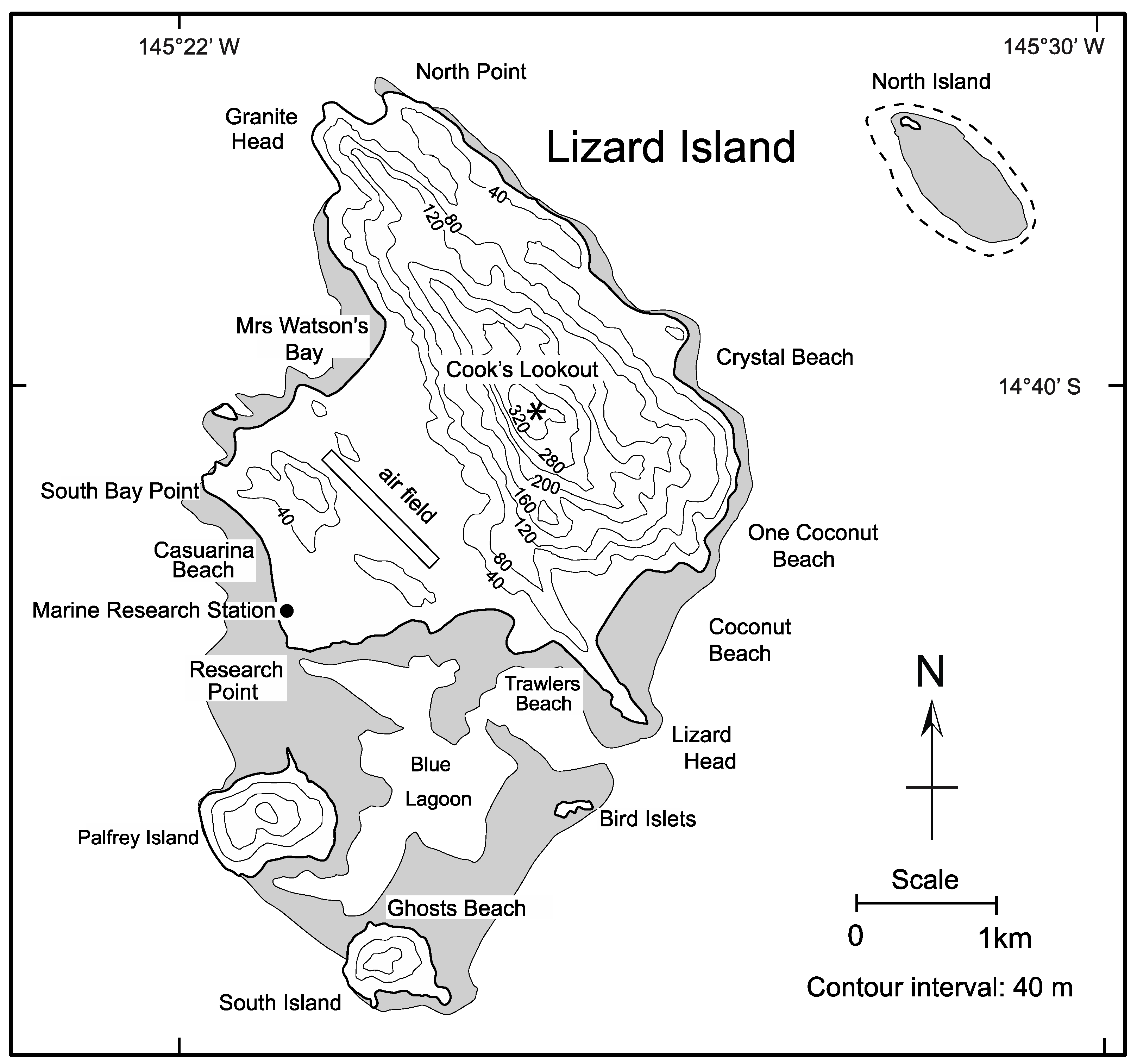
4.1.3. Lizard Island in the Great Barrier Reef off Queensland, Australia
4.2. Clues to Potential Fossilization from Beach Deposits
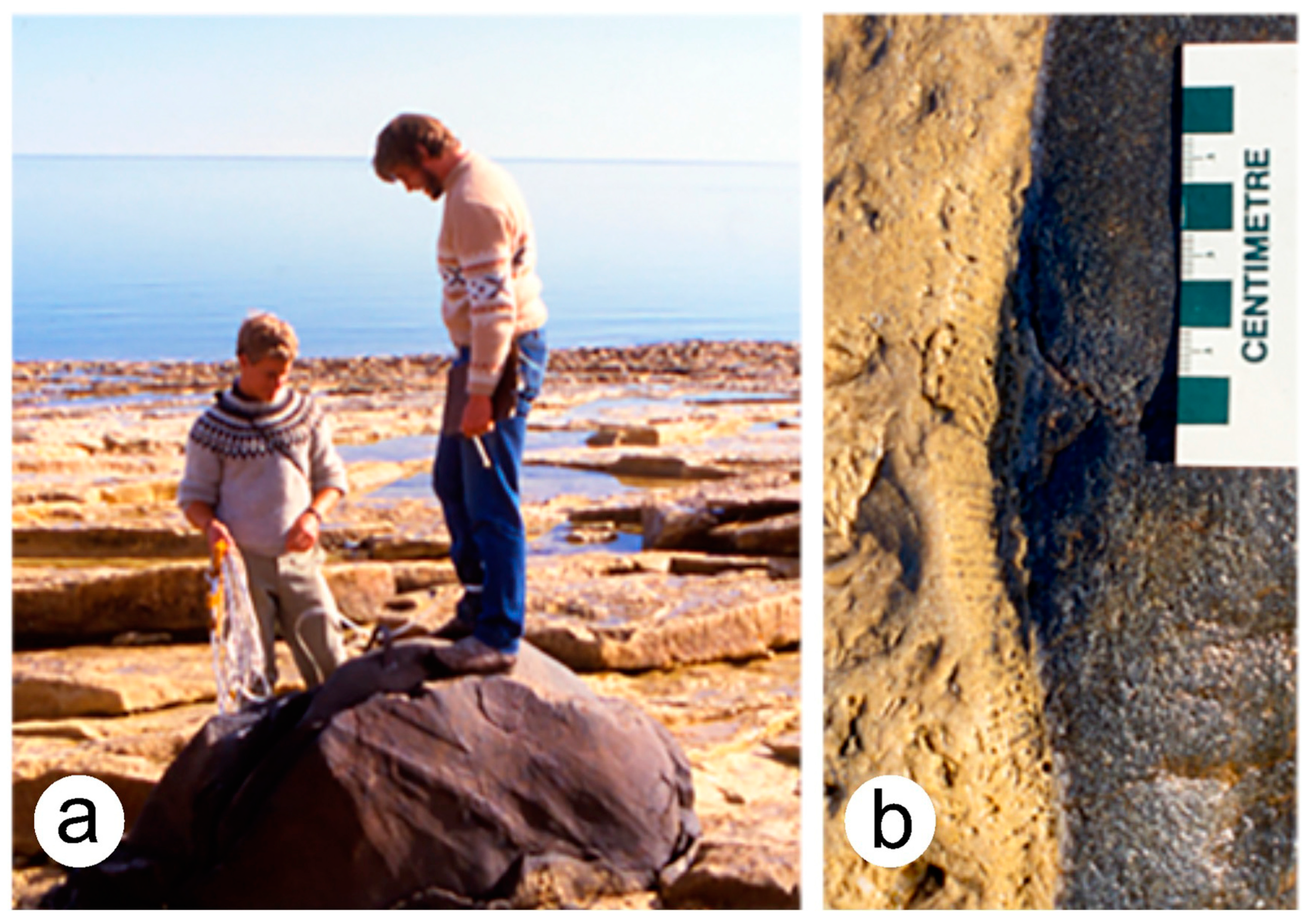
4.2.1. Analysis of Sand Beach on Mount Desert Island, Maine
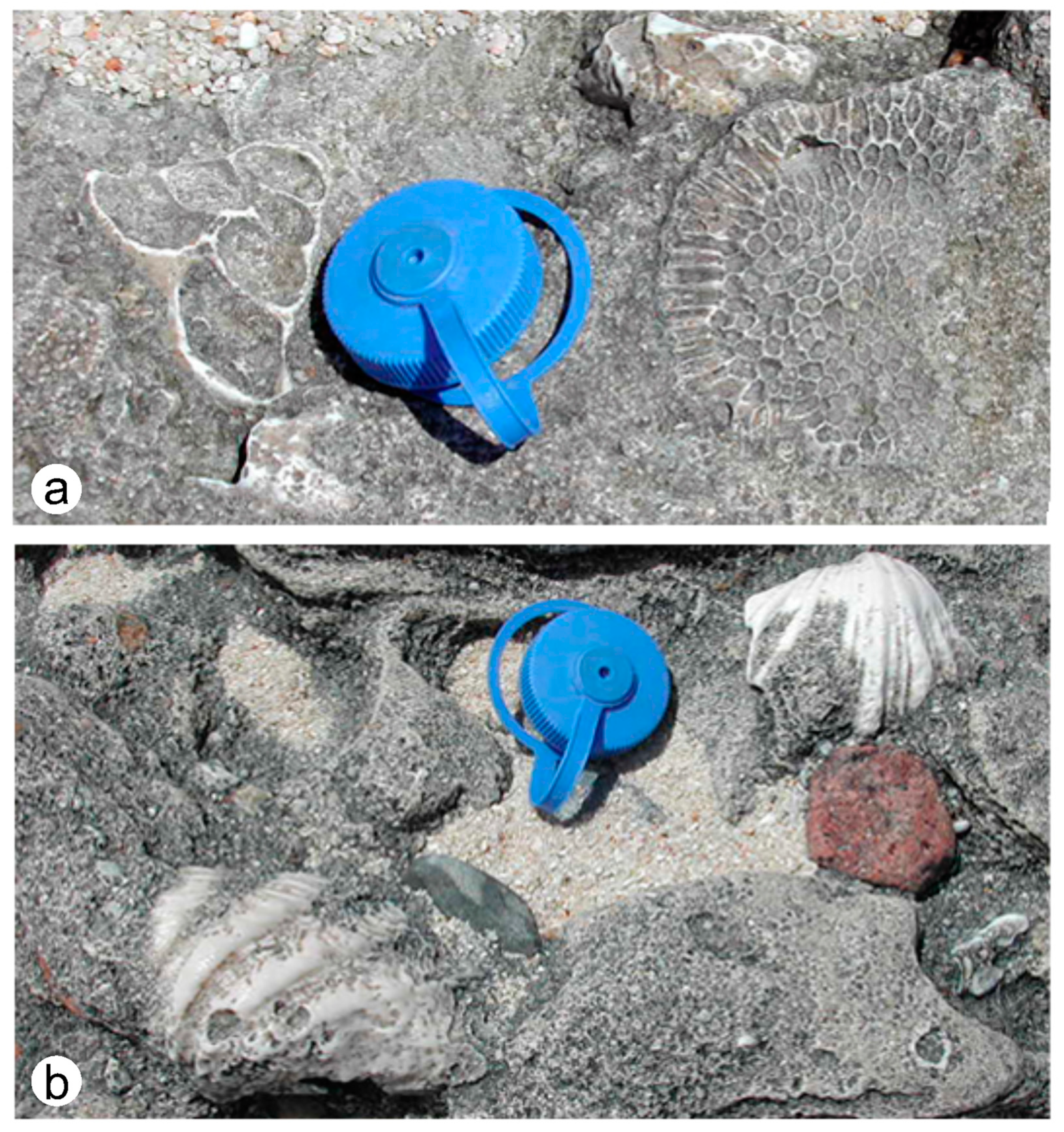
4.2.2. Beachrock on Palfrey Island in a Subtropical Setting
4.3. Review of Pre-Historic Rocky-Shore Biotas from the Fossil Record
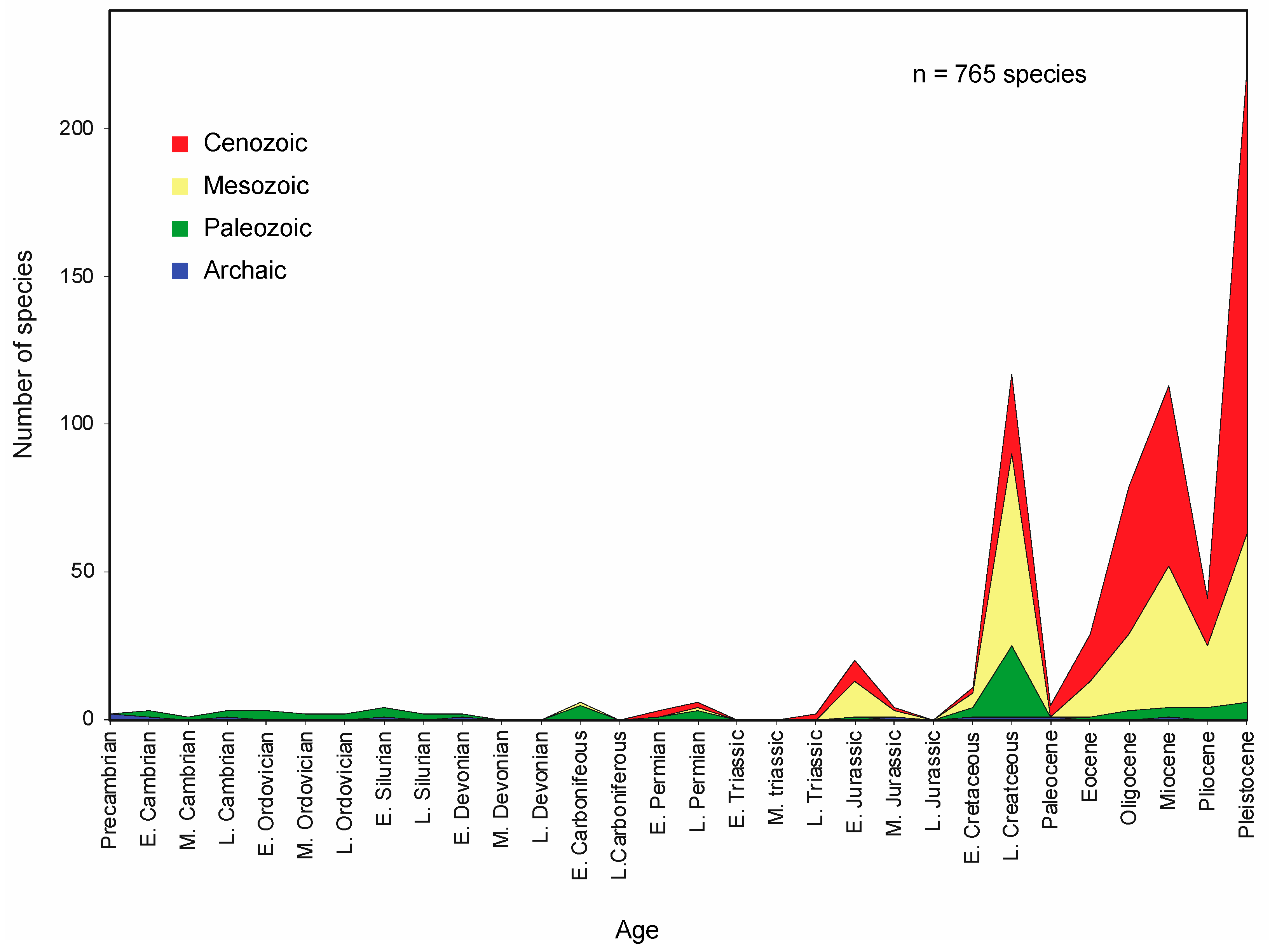
4.3.1. Analysis of Phanerozoic Rocky Shore Publications
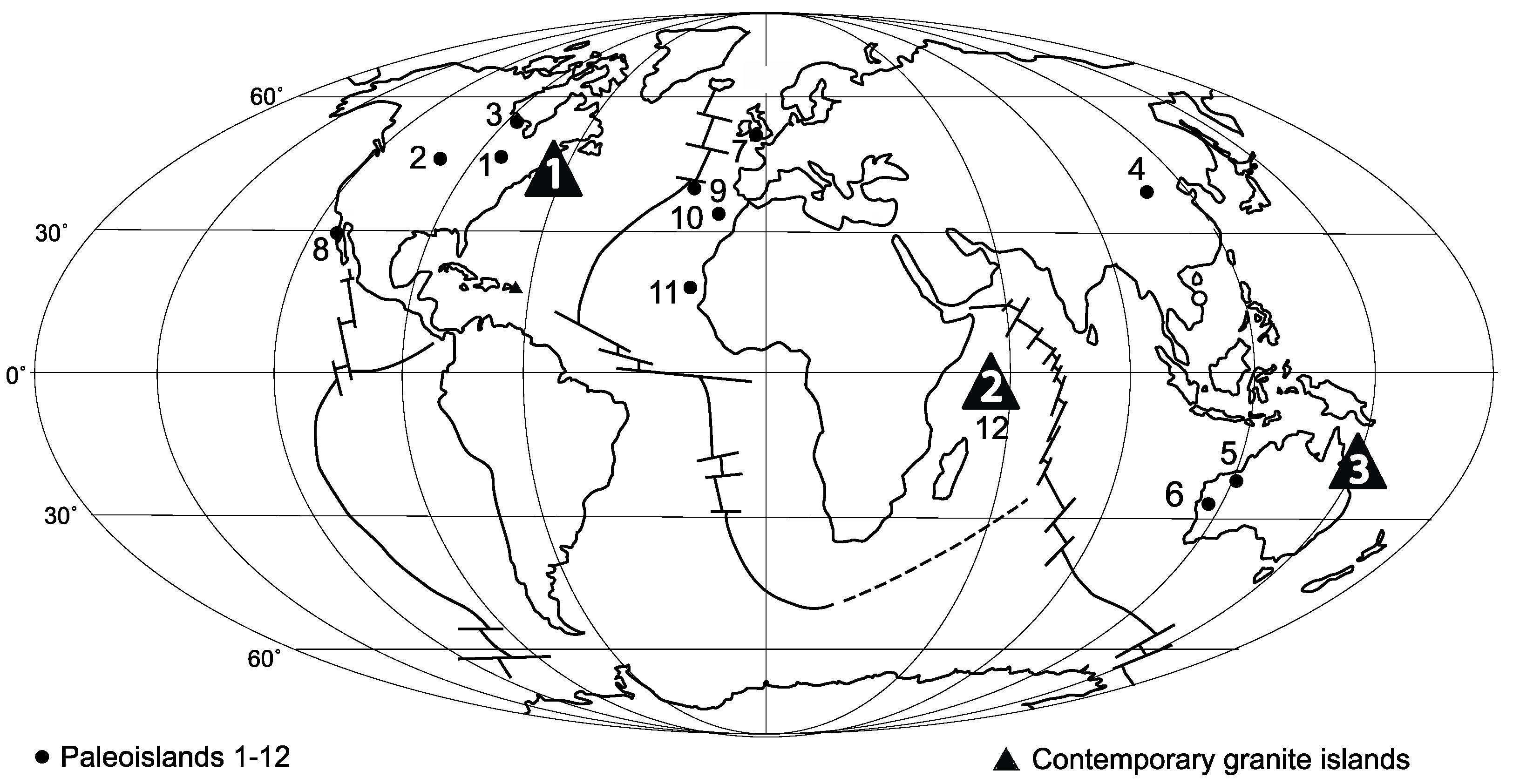
4.3.2. Review of Phanerozoic Rocky Shore Studies on Paleoislands
5. Discussion
Role of External Factors in the Physical Environment
6. Conclusions
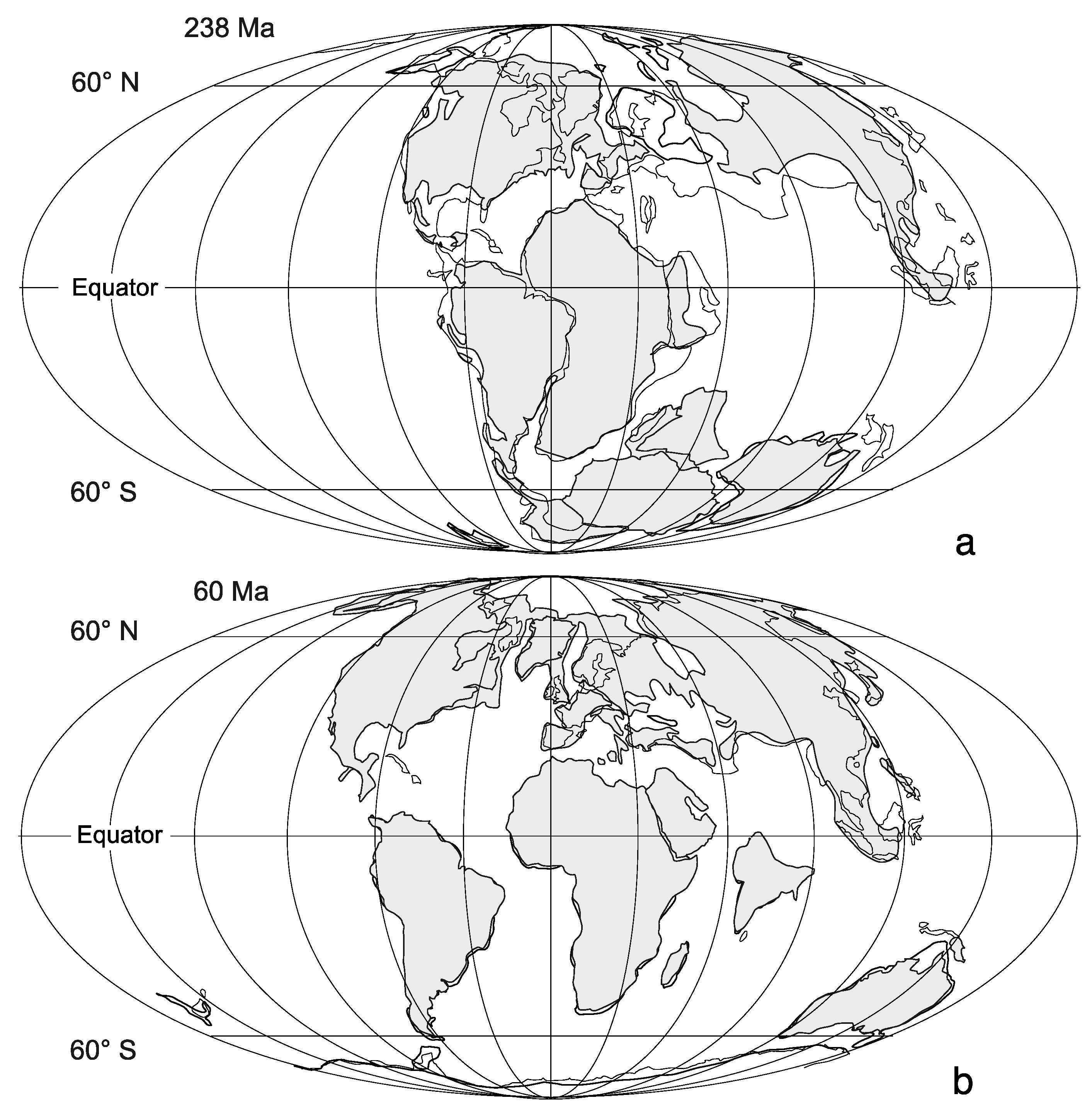
- The available habitat of rocky shores accessible by intertidal life has varied through geologic time as moderated by the mechanics of plate tectonics. A single large continent like Pangaea 250 million years ago offered a reduced shoreline compared with the wide dispersal of many and much smaller continents as today. The paucity of Triassic rocky shores that remain to be detected in the geologic record is related, in part, to this scenario in plate tectonics.
- The hard substrate of a rocky shoreline is foundational on any kind of igneous, metamorphic, or sedimentary rock. However, igneous rocks like granite or basalt with greater hardness and higher density wear better against erosion and make a more stable home for colonizing organisms, which are able to colonize and recolonize at a faster rate than rock recession.
- Changes in global sea level through geologic time have influenced the location of rocky shores, often most prominent along continental margins but also appearing in epicontinental settings far inland during intervals of high sea level.
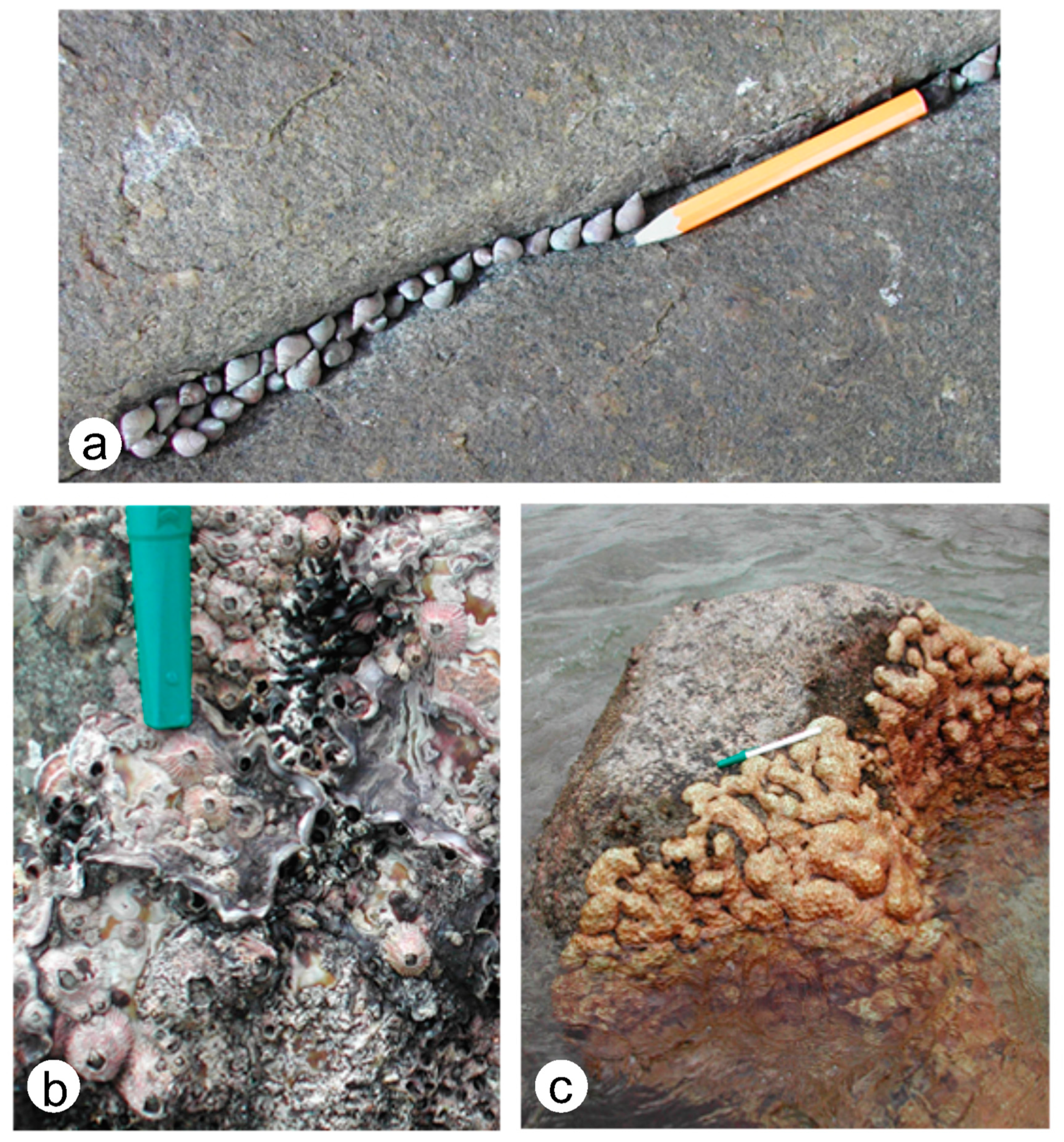
- Marine invertebrates and many algae are capable of attachment to a rocky shore by direct cementation or other means, whereas mobile forms adhere by suction of a strong muscular “foot” or by wedging into crevices or other irregularities in the rock surface. Some bivalves and sponges are capable of rock boring. All such groups have a distinct paleontological record.
- Available data show a steady increase in the number of such marine invertebrates and certain marine algae across geologic time, although membership was modified by the intervention of mass extinctions that affected life in other ecosystems, as well. Latitudinal temperature gradients appear to have varied through geologic time, meaning that analogous diversity gradients from tropical to more temperate settings may have been less pronounced than today.
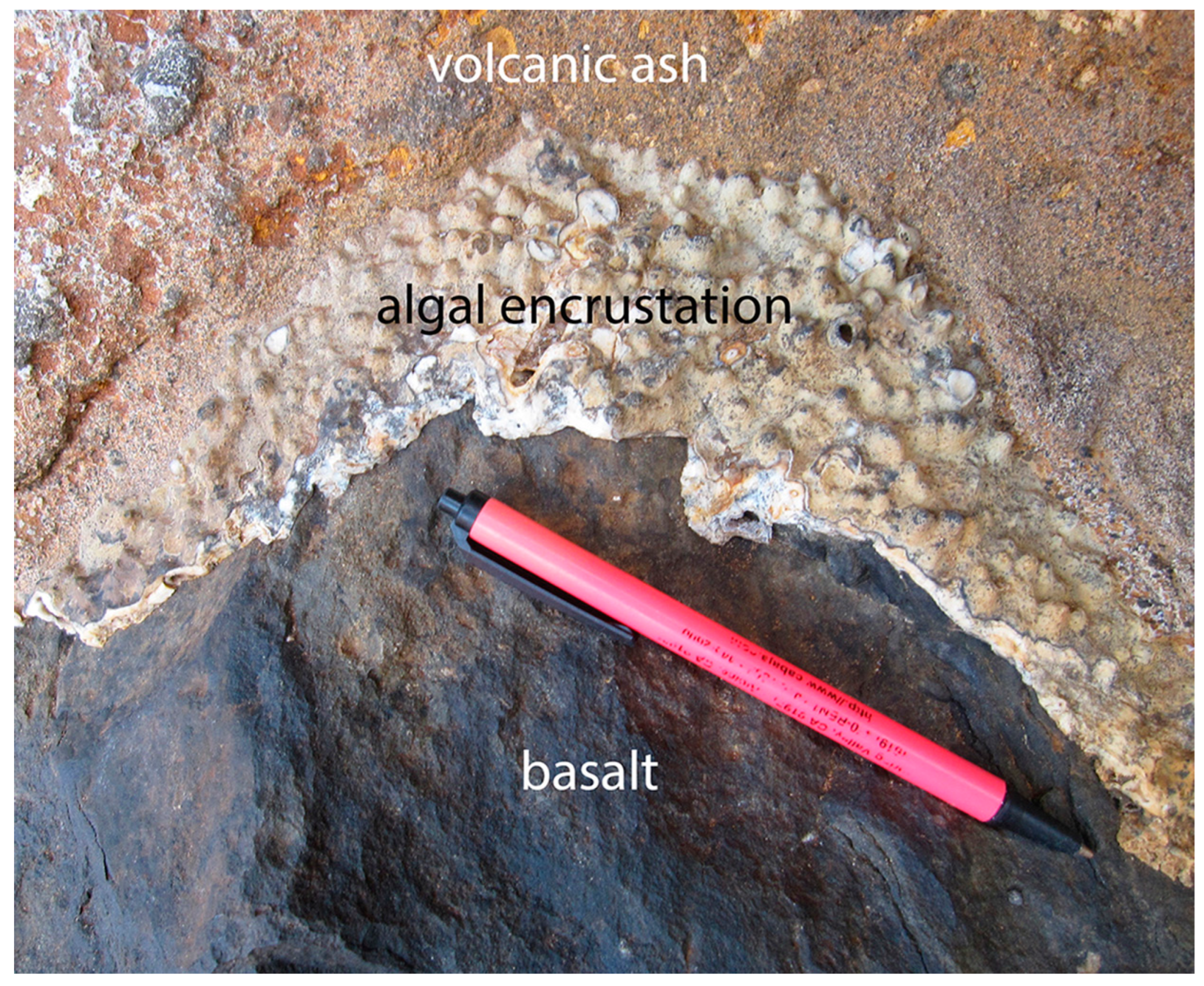
- The preservation of rocky-shore species as fossils in their original growth position on a rock substrate is more commonly encountered than once assumed and was typically mediated by sudden catastrophic events ending in burial by rapid rates of rising sea level, storm deposits, or volcanic ejecta such as ash or coarser tephra.
Funding
Acknowledgments
Conflicts of Interest
References
- National Geographic Atlas of the World, 11th ed.; National Geographic Society: Washington, DC, USA, 2019; 448p.
- Bird, C.F.; Schwartz, M.L. The World’s Coastline; Van Nostrand Reinhold: New York, NY, USA, 1985; 1071p. [Google Scholar]
- Carson, R. The Edge of the Sea; Houghton Mifflin Co.: Boston, MA, USA, 1955; 276p. [Google Scholar]
- Hecker, R.F. Introduction to Paleoecology; American Elsevier Publishing Co.: New York, NY, USA, 1966; 166p. [Google Scholar]
- Selden, P.; Nudds, J. Evolution of Fossil Ecosystems; University of Chicago Press: Chicago, IL, USA, 2004; 160p. [Google Scholar]
- Zakharov, D.O.; Bindeman, I.N. Triple oxygen and hydrogen isotopic study of hydrothermally altered rocks from the 2.43–2.41 Ga Vetreny belt, Russia: An insight into the early Paleoprterozoic seawater. Geochem. Colsmochim. Acta 2019, 248, 185–209. [Google Scholar] [CrossRef]
- Bindeman, I.N.; O’Neil, J. Earth’s earliest hydrosphere recorded by the oldest hydrothermally-altered oceanic crust: Triple oxygen and hydrogen isotopes in the 4.3–3.8 Ga Nuvvuagittuq belt, Canada. Earth Planet. Sci. Lett. 2022, 586, 117539. [Google Scholar] [CrossRef]
- Byerly, G.R.; Lower, D.R.; Walsh, M.M. Stromatolites from the 3300–3500-Myr Swaziland Supergroup, Barberton Mountain Land, South Africa. Nature 1986, 319, 489–491. [Google Scholar] [CrossRef]
- Logan, B.W. Cryptozoon and associated stromatolites from the recent, Shark Bay, Western Australia. J. Geol. 1961, 69, 517–533. [Google Scholar] [CrossRef]
- Johnson, M.E.; Baarli, B.G. Development of intertidal biotas through Phanerozoic time, pp. 63–128. In Earth and Life; Talent, J.A., Ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 2012; 1100p. [Google Scholar]
- Emery, K.O.; Kuhn, G.G. Sea cliffs: Their processes, profiles, and classification. Geol. Soc. Am. Bull. 1982, 93, 644–654. [Google Scholar] [CrossRef]
- Bird, E.C.F. Coastal Geomorphology: An Introduction; John Wiley & Sons: Chichester, UK, 2008; 448p. [Google Scholar]
- Johnson, M.E. Why are ancient rocky shores so uncommon? J. Geol. 1988, 96, 469–480. [Google Scholar] [CrossRef]
- Deny, M.W. Biology and the Mechanics of the Wave-Swept Environment; Princeton University Press: Princeton, NJ, USA, 1998; 329p. [Google Scholar]
- Stephenson, T.A.; Stephenson, A. The universal features of zonation between tide-markes on rocky coasts. J. Ecol. 1949, 37, 289–305. [Google Scholar] [CrossRef]
- Sunamura, T. Geomorphologyu of Rocky Coasts; John Wiley & Sons: Chichester, UK, 1992; 302p. [Google Scholar]
- Johnson, M.E. Islands in Deep Time: Ancient Landscapes Lost and Found; Columbia University Press: New York, NY, USA, 2023; 312p. [Google Scholar]
- Twidale, R. Granite Landforms; Elsevier: Amsterdam, The Netherlands, 1982; 372p. [Google Scholar]
- Twidale, R. The research frontier and beyond: Granitic terrains. Geomorphology 1993, 7, 187–223. [Google Scholar] [CrossRef]
- Brinkhurst, R.O.; Linkletter, L.E.; Lord, E.I.; Connors, S.A.; Dadswell, M.J. A Preliminary Guide to the Littoral and Sublittoral Marine Invertebrates of Passamaquddy Bay; Huntsman Marine Laboratory: St. Andrews, NB, Canada, 1976; 166p. [Google Scholar]
- Johnson, M.E.; Baarli, B.G. Erosion and burial of granite rocky shores in the Recent and Late Pleistocene of the Seychelles Islands: Physical and biological perspectives. J. Coast. Res. 2005, 21, 867–879. [Google Scholar] [CrossRef]
- Braithwaite, C.J.R. Geology of the Seychelles. In Biogeography and Ecology of the Seychelles Islands; Stoddart, D.R., Ed.; W. Junk Publishers: The Hague, The Netherlands, 1984; pp. 17–38. [Google Scholar]
- Suwa, K.; Tokieda, K.; Hoshino, M. Palaeomagnetic and petrological reconstruction of the Seychelles. Precambrian Res. 1994, 69, 281–292. [Google Scholar] [CrossRef]
- Taylor, J.D. Coral reef and associated invertebrate communities (mainly molluscan) around Mahé, Seychelles. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1968, 254, 129–206. [Google Scholar]
- Jerrett, A.G. Marine Shells of the Seychelles; Carole Green Publishing: Cambridge, UK, 2000; 149p. [Google Scholar]
- Philipps, C.J.; Bellwood, D.R. The hydrodynamics of Lizard Island lagoon, Great Barrier Reef. Coral Reefs 2024, 43, 881–897. [Google Scholar] [CrossRef]
- Davey, K. Seashore Life of Australia; New Holland Publishers: Sydney, Australia, 1998; 144p. [Google Scholar]
- Friedman, G.M. Breachrocks record Holocene events, including natural disasters. Carbonates Evaporites 2011, 26, 97–109. [Google Scholar] [CrossRef]
- Leonard, J.E.; Cameron, B.W. Origin of a high-latitude carbonate beach: Mt. Desert Island, Maine. Northeast. Geol. 1981, 28, 1–28. [Google Scholar]
- Barndhardt, W.W.; Kellley, J.T. Carbonate accumulation on the inner continental shelf of Maine; a modern consequence of late Quaternary glaciation and sea-level change. J. Sediment. Res. 1995, 65, 195–207. [Google Scholar]
- Dott, R.H., Jr. Cambrian tropical storm waves in Wisconsin. Geology 1974, 2, 243–246. [Google Scholar] [CrossRef]
- Eoff, J.D. Sequence stratigraphy of the Upper Cambrian (Furongian, Jiangshanian and Sunwaptan) Tunnel City Group, Upper Mississippi Valley: Transgressing assumptions of cratonic flooding. Sediment. Geol. 2014, 302, 87–101. [Google Scholar] [CrossRef]
- Johnson, M.E.; Wilson, M.A.; Redden, J.A. Borings in quartzite surf boulders from the Upper Cambrian basal Deadwood Formation, Black Hills of South Dakota. Ichnos 2010, 17, 48–55. [Google Scholar] [CrossRef]
- Johnson, M.E.; Baarli, B.G. Encrusting corals on a latest Ordovician to earliest Silurian rocky shore, southwest Hudson Bay, Manitoba, Canada. Geology 1987, 15, 15–17. [Google Scholar] [CrossRef]
- Nelson, S.J.; Johnson, M.E. Jens Munk Archipelago: Ordovician-Silurian islands in the Churchill area of the Hudson Bay Lowlands, Northern Manitoba. J. Geol. 2002, 110, 577–589. [Google Scholar] [CrossRef]
- Johnson, M.E.; Rong, J.Y.; Wang, C.Y.; Wang, P. Continental island from the Upper Silurian (Ludfordian Stage) of Inner Mongolia: Implications for eustasy and paleogeography. Geology 2001, 29, 955–958. [Google Scholar] [CrossRef]
- Rong, J.; Johnson, M.E.; Deng, Z.; Dong, D.; Yaosong, S.; Baarli, B.G.; Wang, G. Coral-stromatoporoid faunas from the shores of a late Silurian island, Inner Mongolia, North China. Mem. Assoc. Australas. Palaeontol. 2013, 44, 95–105. [Google Scholar]
- Johnson, M.E.; Webb, G.E. Outer rocky shores of the Mowanbini Archipelago, Devonian Reef Complex, Canning Basin, Western Australia. J. Geol. 2007, 115, 583–600. [Google Scholar] [CrossRef]
- Baarli, B.G.; Webb, G.; Johnson, M.E.; Cook, A.G.; Walsh, D.R. Shoal-water dynamics and coastal biozones in a sheltered-island setting: Upper Devonian Pillara Limestone (Western Australia). Lethaia 2016, 49, 507–523. [Google Scholar] [CrossRef]
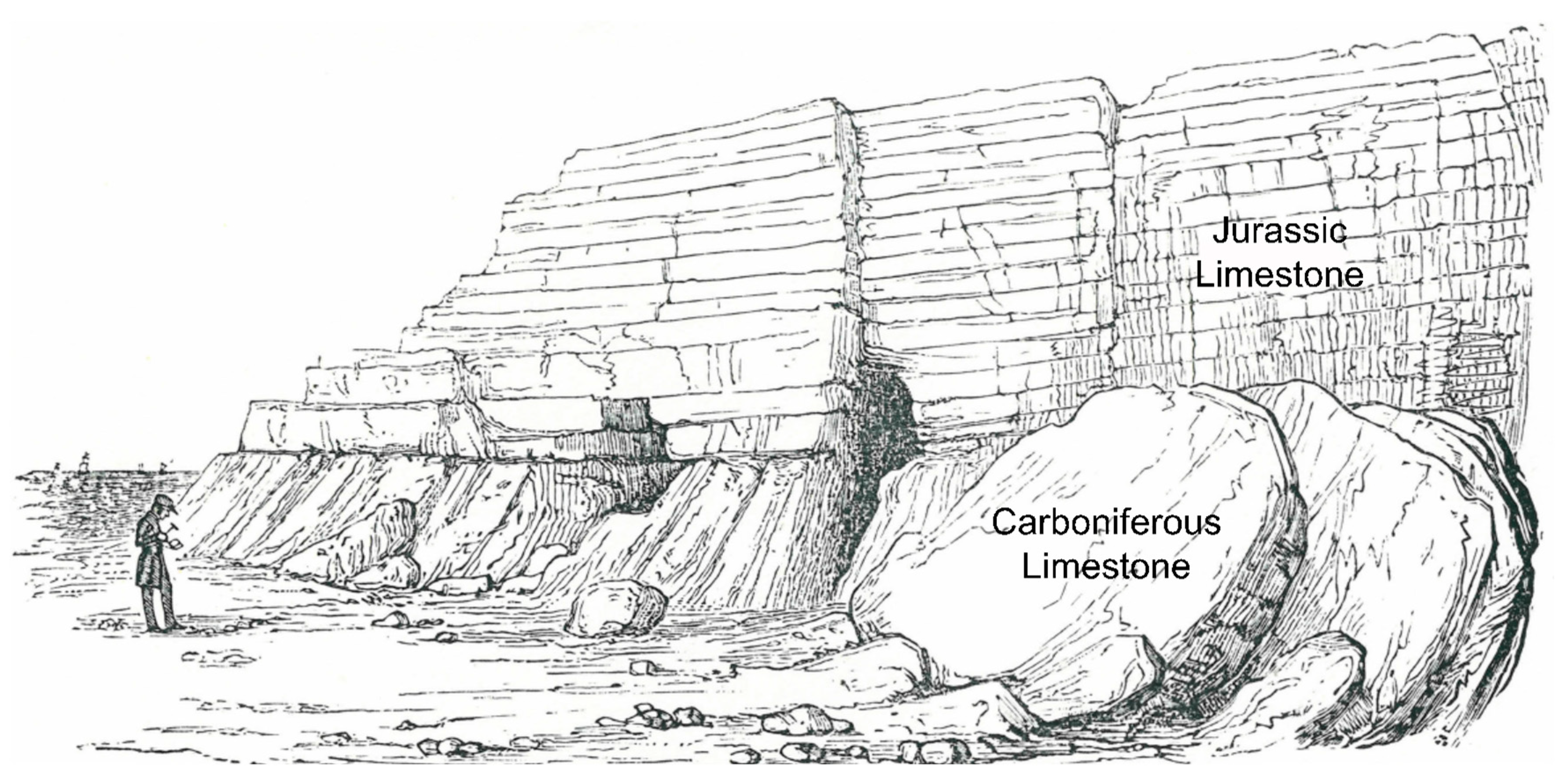
- De la Beche, H.T. On the Formation of the Rocks of South Wales and South Western England; Memoirs of the Geological Survey of Great Britain; Geological Survey: London, England, 1846; pp. 1–2167. [Google Scholar]
- Johnson, M.E.; McKerrow, W.S. The Sutton Stone: An early Jurassic rocky shore deposit in South Wales. Palaeontology 1995, 38, 529–541. [Google Scholar]
- Johnson, M.E.; Ledesma-Vázquez, J.; Clark, H.C.; Zwiebel, J.A. Coastal evolution of Late Cretaceous and Pleistocene rocky shores: Pacific rim of northern Baja California, Mexico. Geol. Soc. Am. Bull. 1996, 108, 708–721. [Google Scholar] [CrossRef]
- Johnson, M.E.; Hayes, M.L. Dichotomous facies on a Late Cretaceous rocky island as related to wind and wave patterns (Baja California, Mexico). Palaios 1993, 8, 385–395. [Google Scholar] [CrossRef]
- Santos, A.; Mayoral, E.; da Silva, C.M.; Cahão, M.; Johnson, M.E.; Baarli, B.G. Miocene intertidal zonation on a volcanically active shoreline: Porto Santo in the Madeira Archipelago, Portugal. Lethaia 2011, 44, 26–32. [Google Scholar] [CrossRef]
- Rebelo, A.C.; Rasser, M.W.; Kroh, A.; Johnson, M.E.; Ramalho, R.S.; Melo, C.; Uchman, A.; Berning, B.; Silva, L.; Zanon, V.; et al. Rocking around a volcanic island shelf: Pliocene rhodolith beds from Malbusca, Santa Maria Island (Azores, NE Atlantic). Facies 2016, 62, 22. [Google Scholar] [CrossRef]
- Johnson, M.E.; Uchman, A.; Costa, P.J.M.; Ramalho, R.S.; Ávila, S.P. Intense hurricane transports sand onshore: Example from the Pliocene Malbusca section on Santa Maria Isalnd (Azores, Portugal). Mar. Geol. 2017, 385, 244–249. [Google Scholar] [CrossRef]
- Darwin, C. Geological Observations on the Volcanic Islands Visited during the Voyage of the H.M.S. Beagle., Together with Some Brief Notices on the Geology of Australia and the Cape of Good Hope. Being the Second Part of the Geology of the Voyage of the Beagle, under the Command of Capt. FitzRoy, RN.N. during the Years 1832 to 1836; Smith, Elder & Co.: London, UK, 1844; 175p. [Google Scholar]
- Baarli, B.G.; Santos, A.G.; Mayoral, E.J.; Ledesma-Vázquez, J.; Johnson, M.E.; da Silva, C.M.; Cachão, M. What darwin did not see: Pleistocene fossil assemblages on a high-energy coast at Ponta das Bicudas, Santiago, Cape Verde islands. Geol. Mag. 2013, 150, 83–189. [Google Scholar] [CrossRef]
- Wegener, A. The Origin of Continents and Oceans; (first English edition translated from the third German edition); Methuen & Co., Ltd.: London, UK, 1924; 246p. [Google Scholar]
- Johnson, M.E. Uniformitarianism as a guide to rocky-shore ecosystems in the geological record. Can. J. Earth Sci. 2006, 43, 1119–1147. [Google Scholar] [CrossRef]
- Husson, J.M.; Peters, S.E. Nature of the sedimentary rock record and its implications for Earth System evolution. Emerg. Top. Life Sci. 2018, 2, 125–136. [Google Scholar] [PubMed]
- Marsaglia, K.M.; Klein, G.D. The paleogeography of Paleozoic and Mesozoic storm depositional systems. J. Geol. 1983, 91, 117–142. [Google Scholar] [CrossRef]
- Pietzsch, K. Geologie von Sahzen; Veb Deutcher Verlag der Wissenschaften: Berlin, Germany, 1962; pp. 17–22. [Google Scholar]
- Surlyk, F.; Sørensen, A.M. An early Campanian rocky shore at Ivö Klack, southern Sweden. Cretac. Res. 2010, 31, 567–576. [Google Scholar] [CrossRef]
- Gale, A.S.; Sørensen, A.M. Taxonomy and palaeoecology of thoracican cirripedes (Crustacea) from a Campanian rocky shoreline at Ivö Klack, southern Sweden. Cretac. Res. 2015, 54, 212–242. [Google Scholar] [CrossRef]
- Wilson, M.; Taylor, P.D. Palaeoecology of hard substrate faunas from the Cretaceous Qahlah Formation of the Oman Mountains. Palaeontology 2001, 44, 21–41. [Google Scholar] [CrossRef]
- Zhang, L.; Hay, W.W.; Wang, C.; Gu, X. The evolution of latitudinal temperature gradients from the latest Cretaceous through the Presnt. Earth-Sci. Rev. 2019, 189, 147–158. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, M.E. Ecology of Intertidal Rocky Shores Related to Examples of Coastal Geology across Phanerozoic Time. J. Mar. Sci. Eng. 2024, 12, 1399. https://doi.org/10.3390/jmse12081399
Johnson ME. Ecology of Intertidal Rocky Shores Related to Examples of Coastal Geology across Phanerozoic Time. Journal of Marine Science and Engineering. 2024; 12(8):1399. https://doi.org/10.3390/jmse12081399
Chicago/Turabian StyleJohnson, Markes E. 2024. "Ecology of Intertidal Rocky Shores Related to Examples of Coastal Geology across Phanerozoic Time" Journal of Marine Science and Engineering 12, no. 8: 1399. https://doi.org/10.3390/jmse12081399
APA StyleJohnson, M. E. (2024). Ecology of Intertidal Rocky Shores Related to Examples of Coastal Geology across Phanerozoic Time. Journal of Marine Science and Engineering, 12(8), 1399. https://doi.org/10.3390/jmse12081399







