Discrete Element Study on Mechanical Properties of MICP-Treated Sand under Triaxial Compression
Abstract
1. Introduction
2. DEM Model
2.1. Contact Model for DEM Simulations
2.2. Numerical Specimen Preparation of MICP-Treated Sand
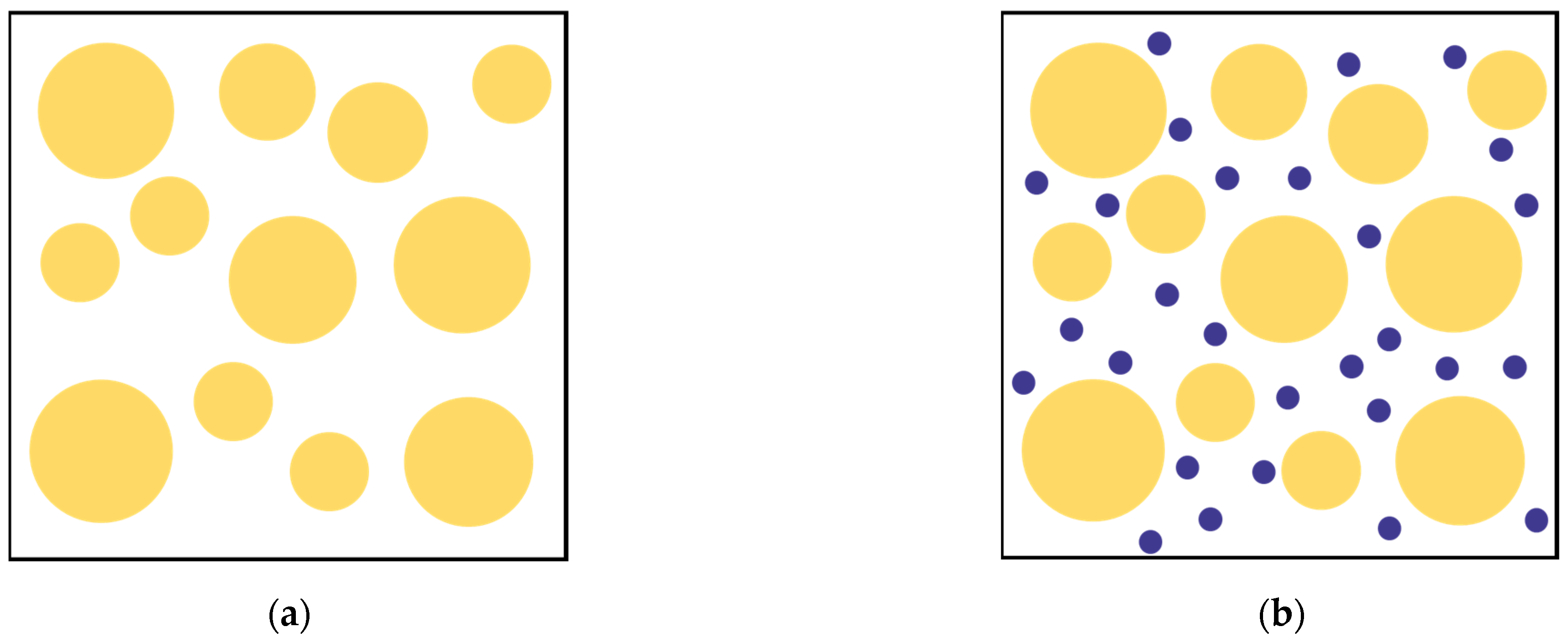
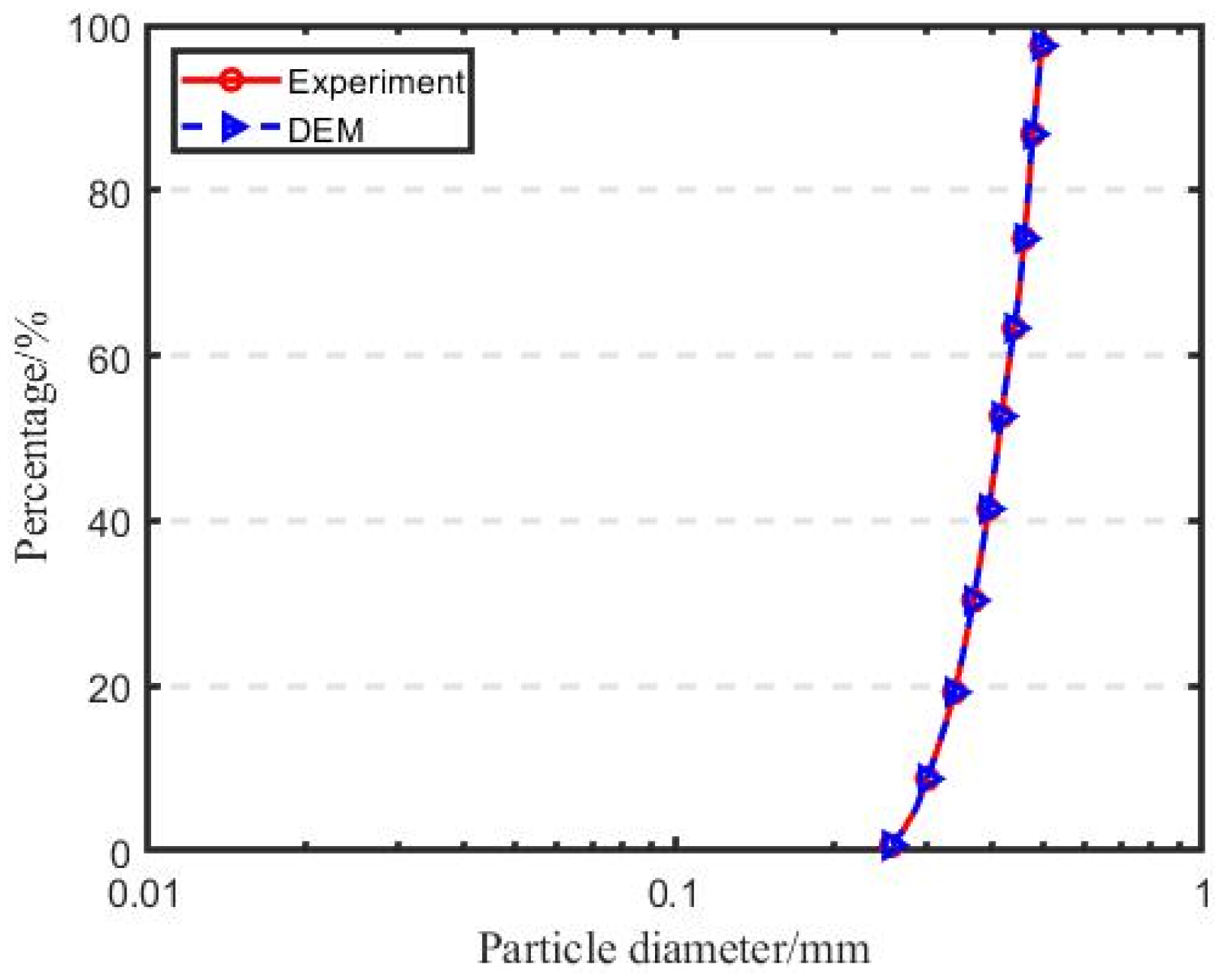
- Sand sample preparation involves generating 7007 nonoverlapping particles, representing sand particles, with half diameter using gradation within the space enclosed by rigid walls, and then expanding to the desired size to simulate the sand’s particle size distribution curve, as shown in Figure 3. All contacts between the sand particles are assigned to the rolling resistance linear contact model. During this phase, the friction coefficient (μ) and the rolling resistance coefficient (μr) are both set to zero. Subsequently, the sand particles are subjected to compression until reaching an isotropic stress state of 10 kPa is reached. The porosity (e) is correlated with the values of μ and μr, and adjustments to these coefficients are made to attain the corresponding relative density [27].
- Calcium carbonate particles are generated while the fixed wall remains stationary. The target diameter of the calcium carbonate particles is set at one-eighth of the median particle size of the sand. It should be noted that the number of calcium carbonate particles is determined by the calcium carbonate content measured in the laboratory pickling test, and the specific calculation formula is detailed as follows.
- 3.
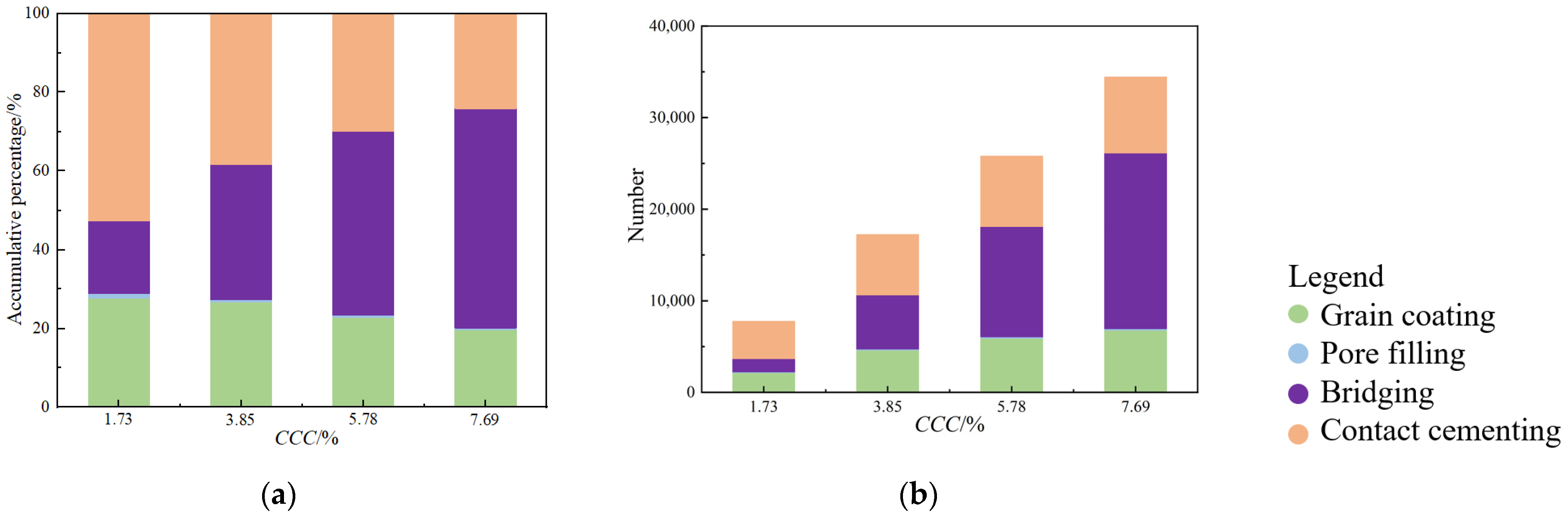
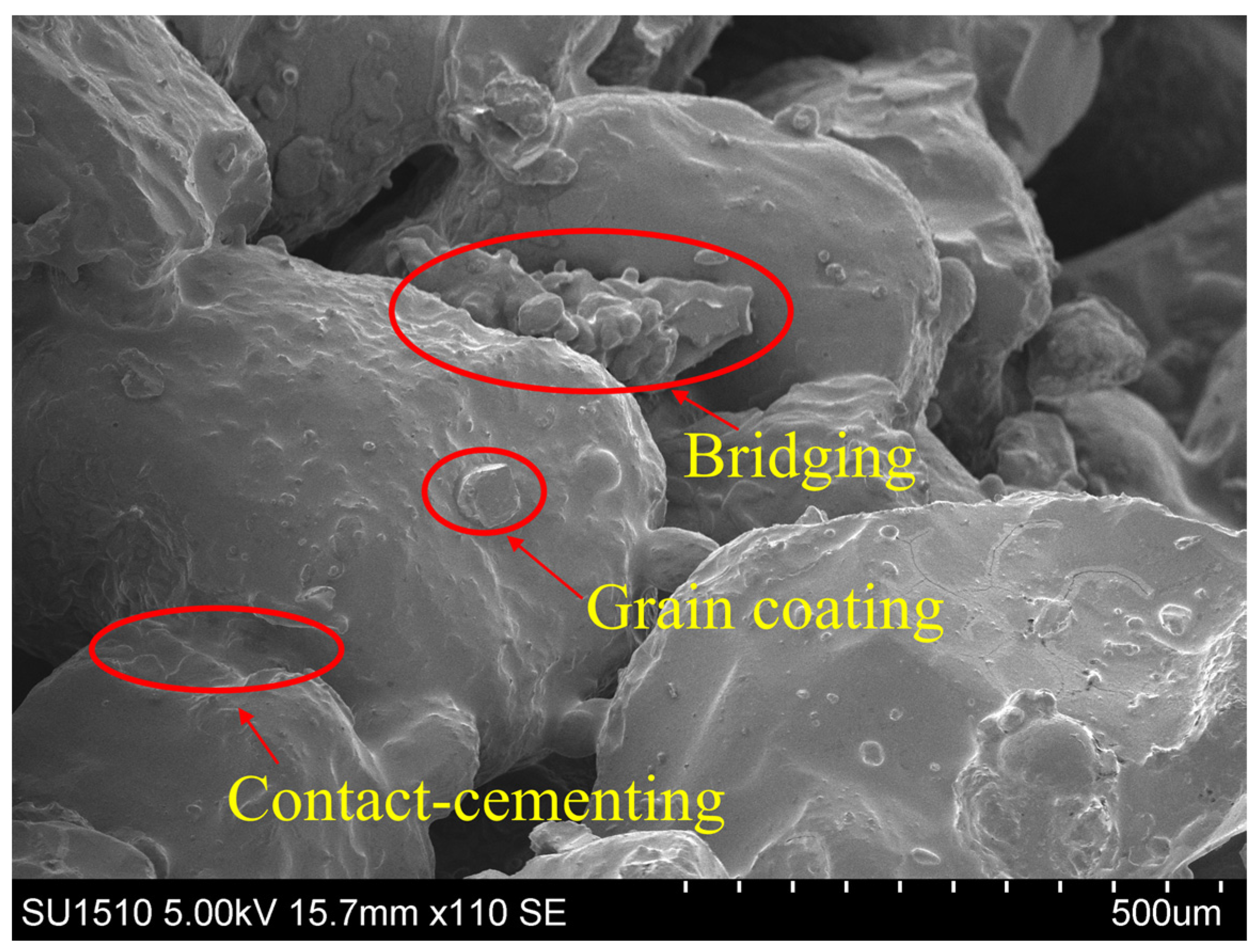
- A driving force, denoted as F, is applied in a random direction to all calcium carbonate particles. The cohesion at particle contact and the normal and shear forces between the particles are utilized to counterbalance this driving force. Figure 2f reveals that, based on the relative positions of small and large particles, calcium carbonate can be categorized into four precipitation modes: contact cementing, bridging, grain coating, and pore filling. This demonstrates that the numerical sample preparation method for MICP-treated sand, as presented in this study, is capable of reflecting the microstructure of physical samples.
2.3. Triaxial Compression Test
2.4. Contact Model Parameter
3. Results and Discussion
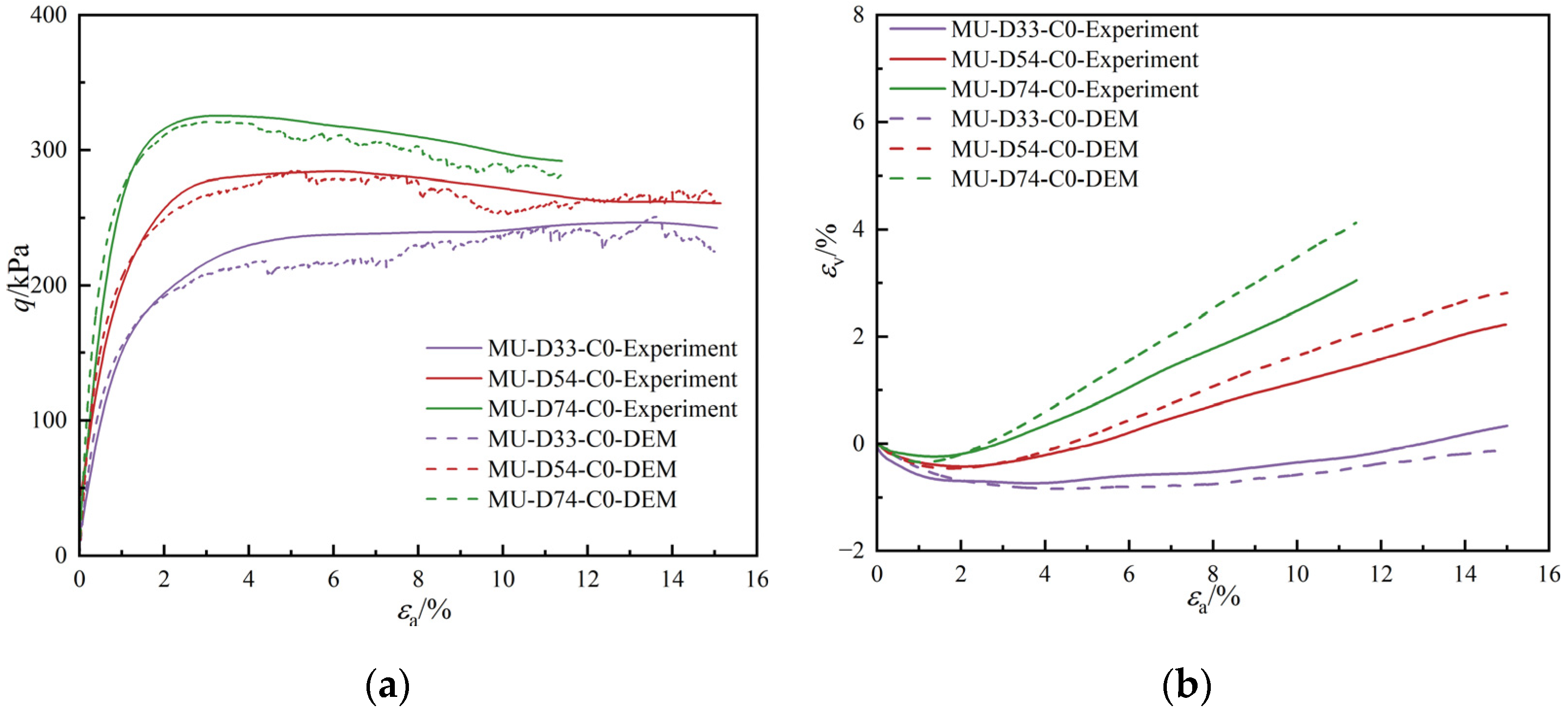
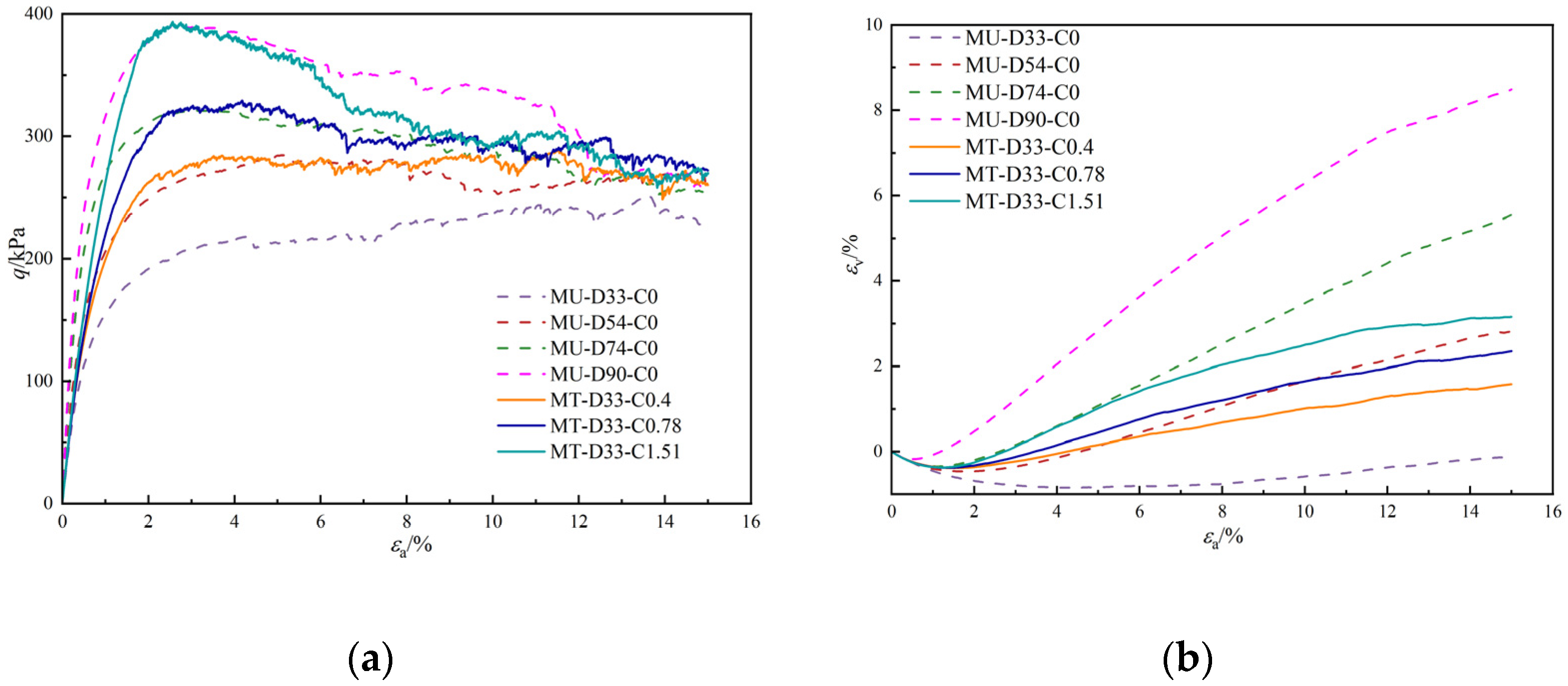
3.1. Macroresponse
3.2. Microresponse
3.2.1. Microstructure Characteristics
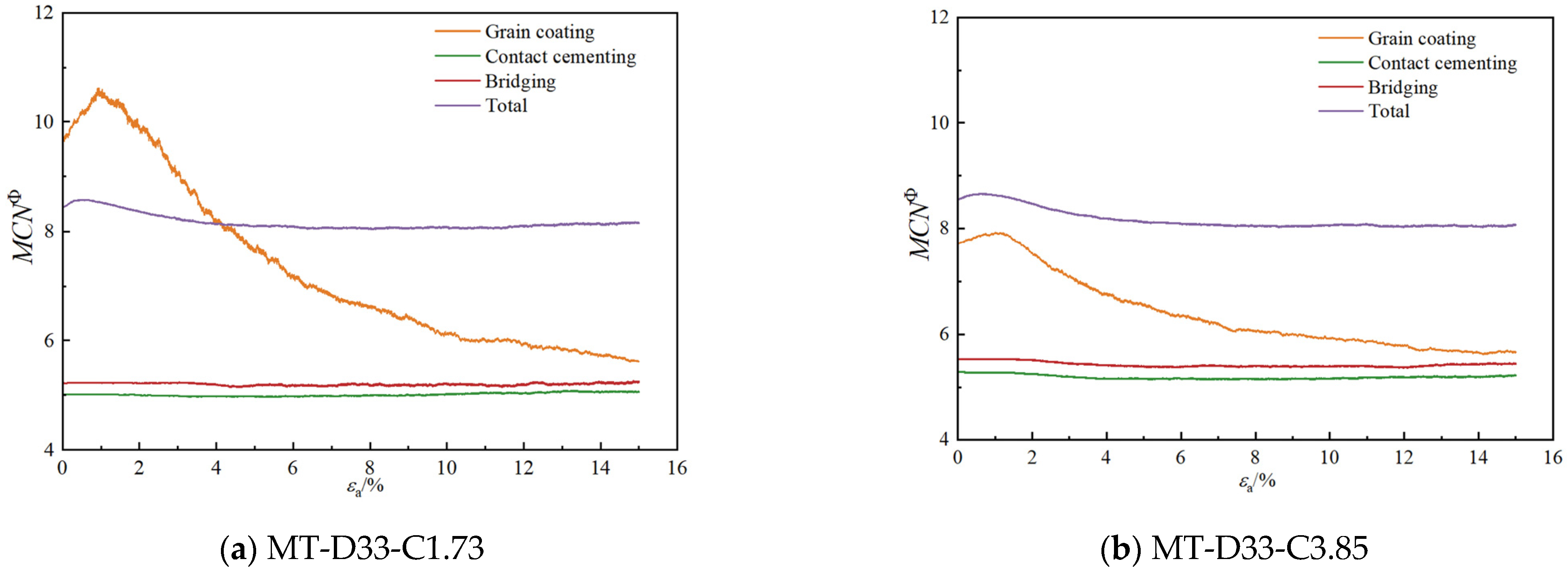
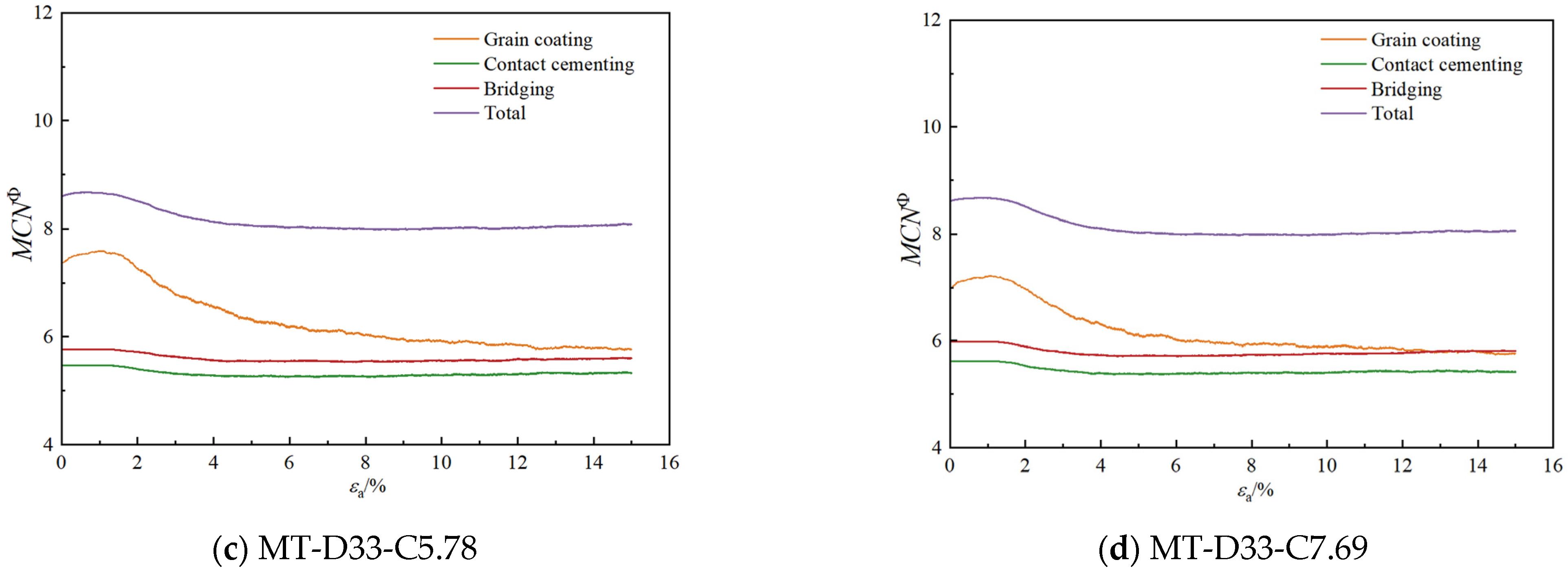
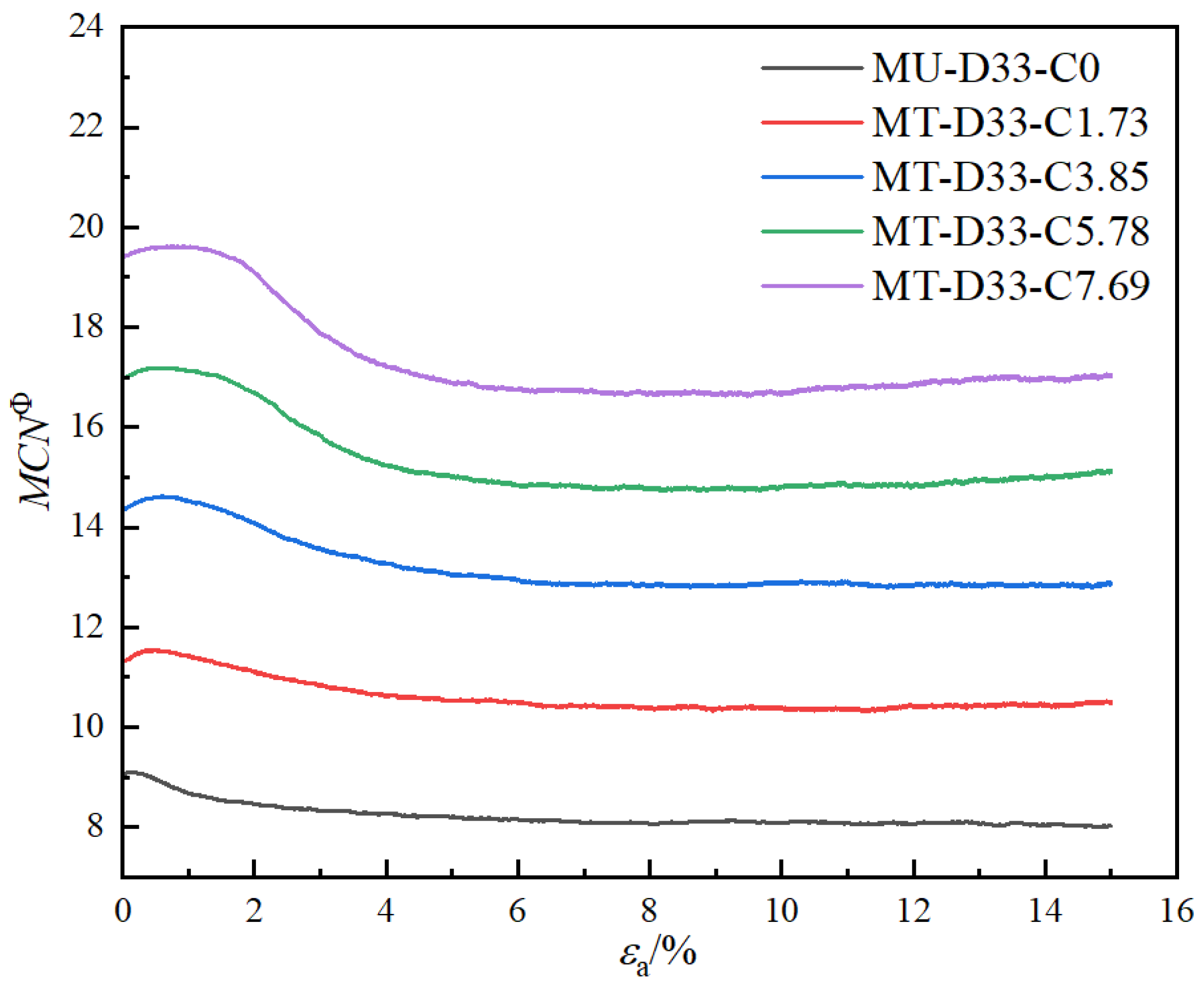
3.2.2. Coordination Numbers
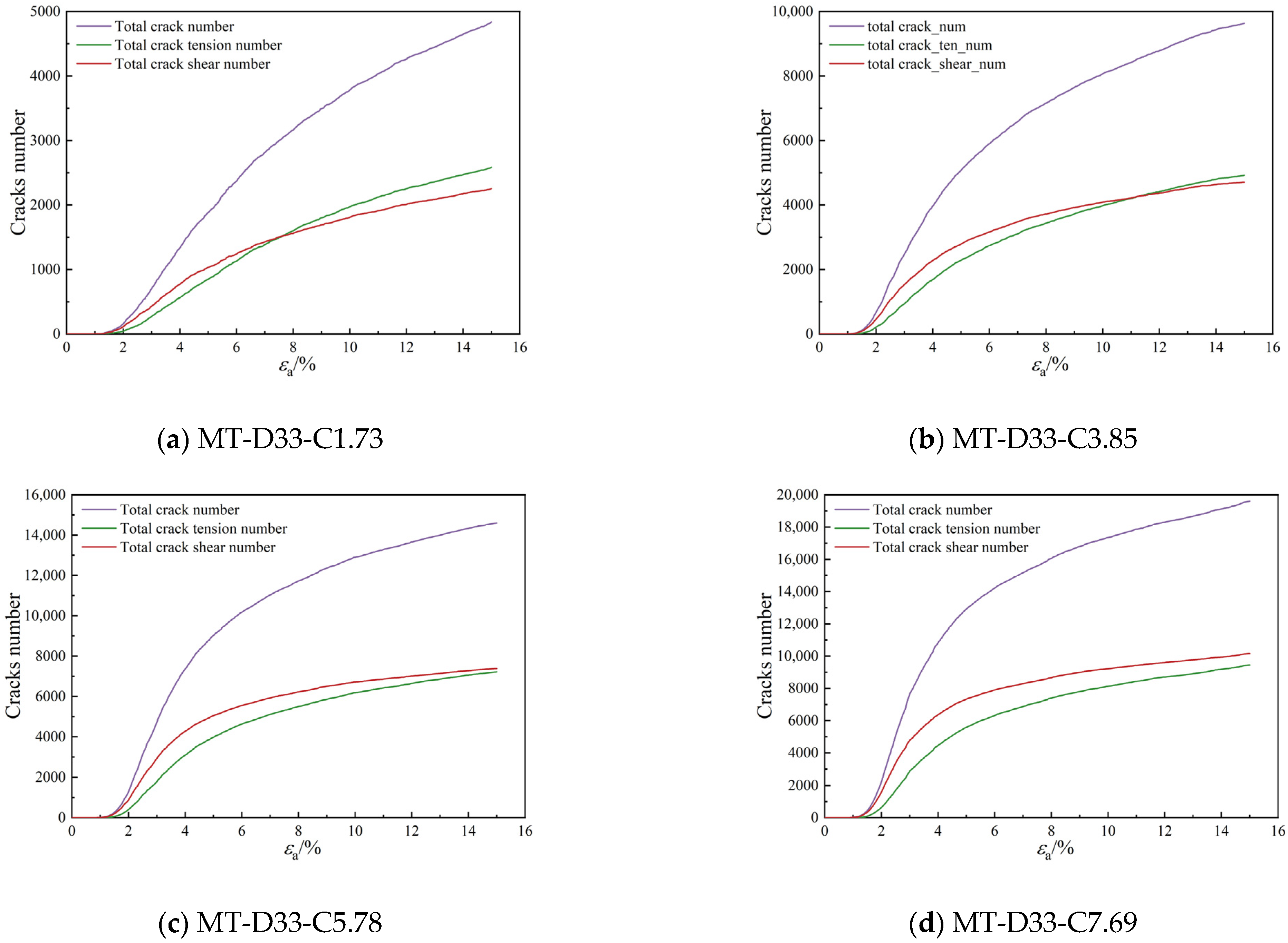
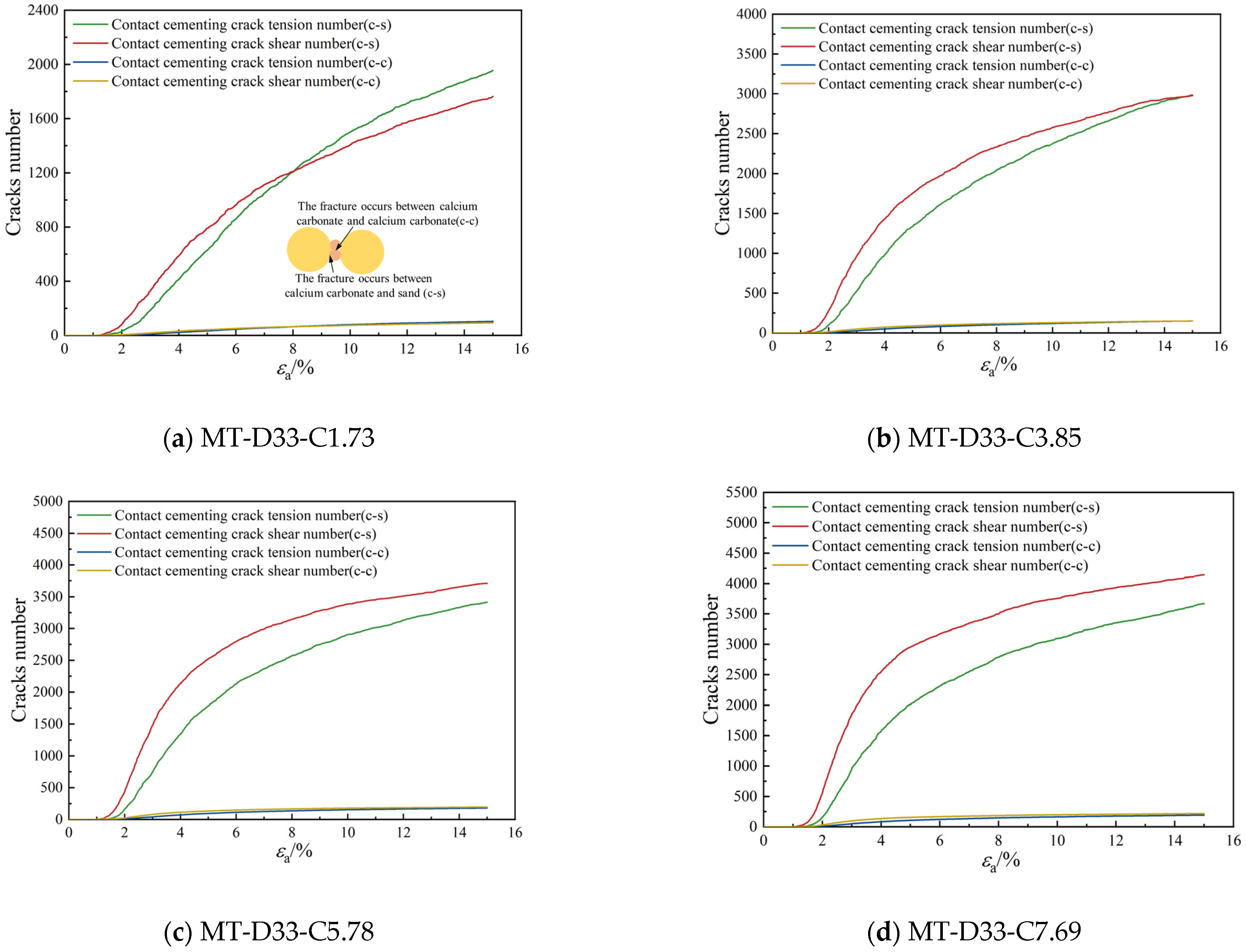
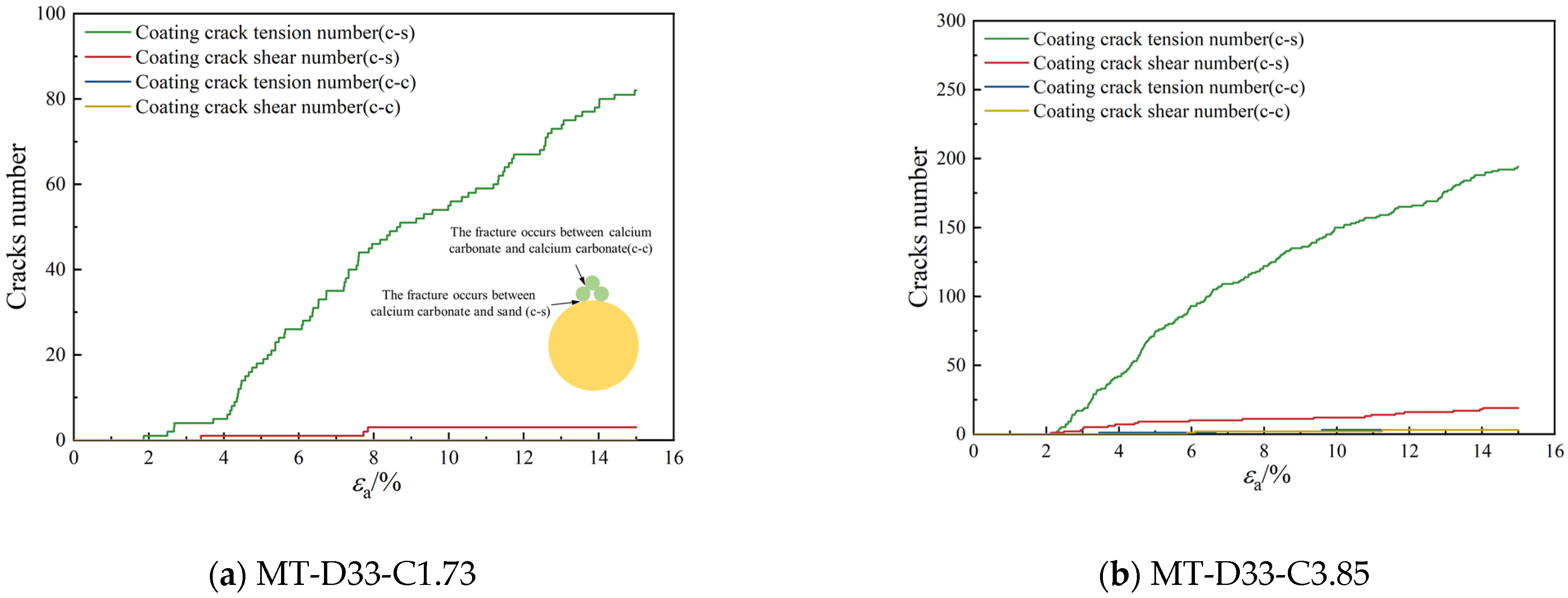
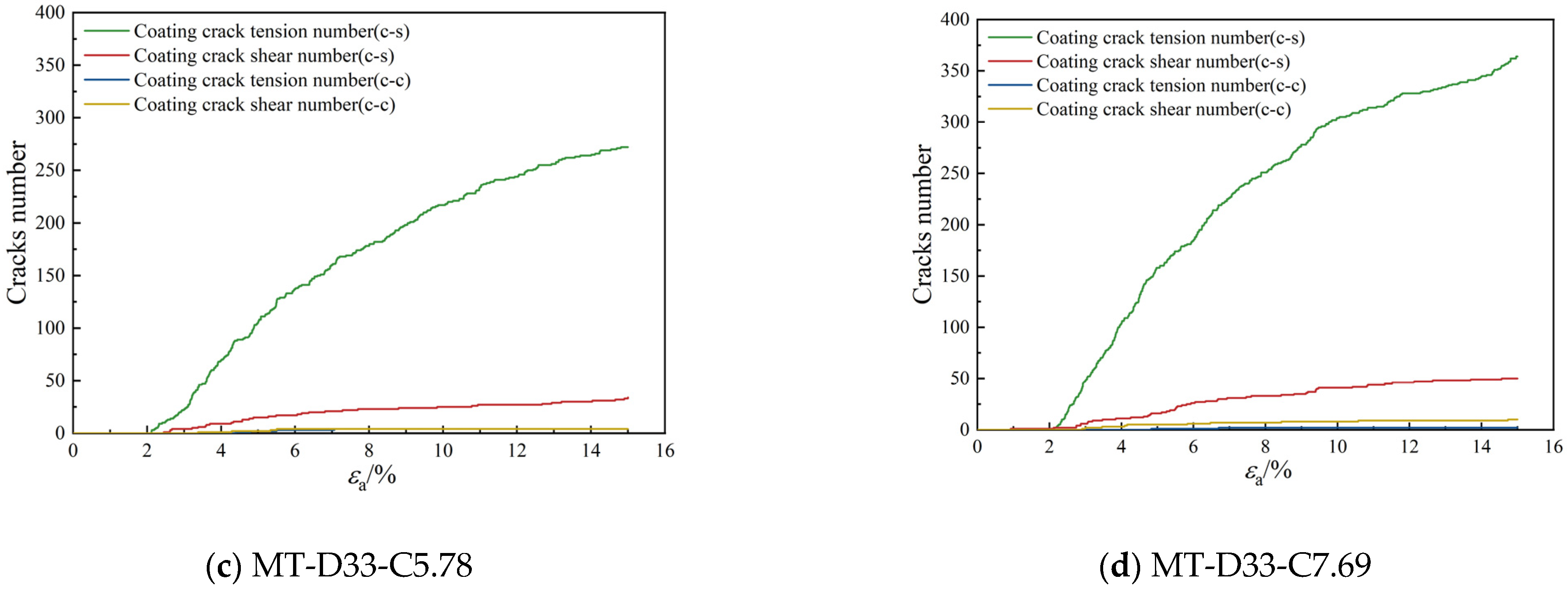
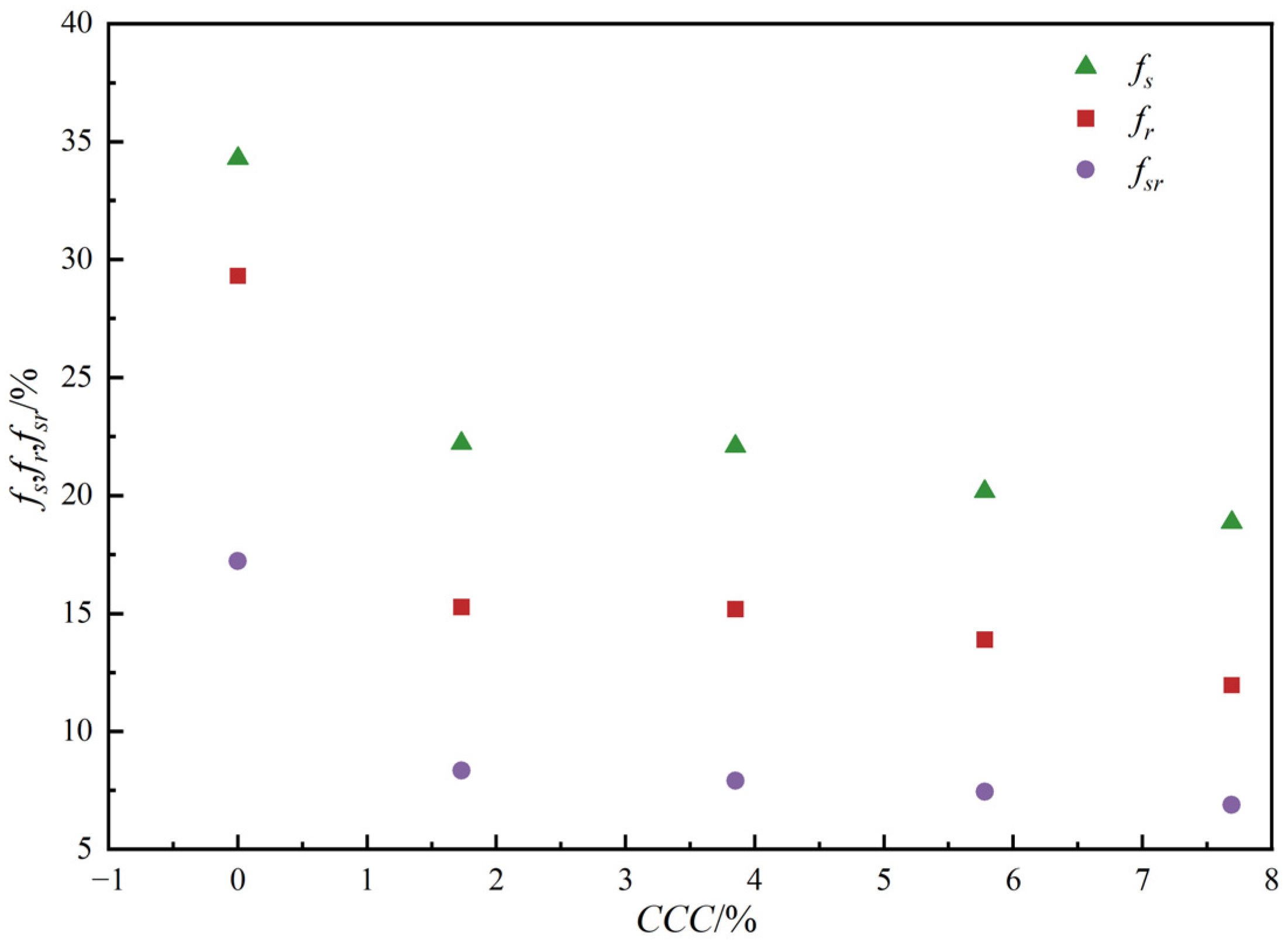
3.2.3. Bond Breakage Behavior
3.2.4. Rolling and Sliding Contacts
4. Conclusions
- The peak strength of MICP-treated sand with a 7.69% calcium carbonate content is 4.5 times higher than that of untreated sand. This reveals that the calcium carbonate content can significantly enhance soil strength. In addition, the discrete element model provides a possibility to prepare MICP-treated sand with a very small calcium carbonate content. Loose sand with just a 1.51% calcium carbonate content demonstrates nearly the same peak strength as when the relative density is increased to 90%; the volume change of MICP-treated sand is smaller than that observed with increased relative density. The MICP technique emerges as a more efficient strategy than simply increasing relative density for enhancing strength.
- The SEM images show that calcium carbonate tends to form between two sand particles rather than on the sand surface. This observation aligns with the proportion results of the calcium carbonate precipitation mode in the MICP-treated sand numerical model, further proving the accuracy of the MICP numerical model. The fact that there is a higher proportion of effective calcium carbonate crystals explains why even low amounts of calcium carbonate can significantly improve the mechanical properties of sand.
- As the calcium carbonate content increases, so does the mechanical coordination number of the sand, signifying a more stable microstructure of the sand phase. The development of cracks involves varied fracture patterns corresponding to the different precipitation modes of calcium carbonate. In the case of contact cementing and bridging modes, shear cracks are the first to occur, followed by tensile cracks. In contrast, in the grain coating mode, tensile cracks appear first and are later followed by shear cracks. In both contact cementing and grain coating modes, the initial fractures occur between sand and calcium carbonate. However, in the bridging mode, fractures initially manifest between calcium carbonate particles themselves. Calcium carbonate contributes to a reduction in both sliding and rolling movements among sand particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-Cementation of Sandy Soil Using Microbially Induced Carbonate Precipitation for Marine Environments. Géotechnique 2014, 64, 1010–1013. [Google Scholar] [CrossRef]
- Kannan, K.; Bindu, J.; Vinod, P. Engineering Behaviour of MICP Treated Marine Clays. Mar. Georesources Geotechnol. 2020, 38, 761–769. [Google Scholar] [CrossRef]
- Yujie, L.; Zhen, G.; Zuqiang, Y.; Yilong, L.; MengTao, X. Experimental Study on the Reaction Process and Engineering Characteristics of Marine Calcareous Sand Reinforced by Eco-Friendly Methods. Appl. Ocean. Res. 2023, 138, 103641. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, W.; Liu, Z.; Wang, Q.; Chen, Y. Influence of Injection Methods on Calcareous Sand Cementation by EICP Technique. Constr. Build. Mater. 2023, 363, 129724. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, L.; Xiao, J.; Zheng, Y.; Ma, S.; Pan, T. Mechanical Properties and Acoustic Emission Characteristics of Microbial Cemented Backfill with Various Particle Size Distributions of Recycled Aggregates. Constr. Build. Mater. 2024, 417, 135269. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial Method for Construction of an Aquaculture Pond in Sand. Géotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.-L. Optimization of Calcium-Based Bioclogging and Biocementation of Sand. Acta Geotech 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Konstantinou, C.; Wang, Y.; Biscontin, G. A Systematic Study on the Influence of Grain Characteristics on Hydraulic and Mechanical Performance of MICP-Treated Porous Media. Transp. Porous Media 2023, 147, 305–330. [Google Scholar] [CrossRef]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical Processes and Geotechnical Applications: Progress, Opportunities and Challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental Optimization of Microbial-Induced Carbonate Precipitation for Soil Improvement. J. Geotech. Geoenvironmental Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Qabany, A.A.; Soga, K. Effect of Chemical Treatment Used in MICP on Engineering Properties of Cemented Soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Yang, H.; Li, Y.; Zhu, J. An Innovative Eco-Friendly Method for Scour Protection around Monopile Foundation. Appl. Ocean Res. 2022, 123, 103177. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Chen, X.; Cao, P.; Cao, L.; Chen, W. Mechanical Properties and Constitutive Model of Calcareous Sand Strengthened by MICP. J. Mar. Sci. Eng. 2023, 11, 819. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Tong, Z.; Wu, W.; Chen, X.; Deng, X.; Xie, Y. Research on the Effect of Natural Seawater in Domesticating Bacillus Pasteurii and Reinforcing Calcareous Sand. J. Mar. Sci. Eng. 2024, 12, 542. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Ye, Z.; Shen, C.; Zhou, W. Interface Shear Behavior between MICP-Treated Calcareous Sand and Steel. J. Mater. Civ. Eng. 2021, 33, 04020455. [Google Scholar] [CrossRef]
- Li, J.; Webb, C.; Pandiella, S.S.; Campbell, G.M. Discrete Particle Motion on Sieves—A Numerical Study Using the DEM Simulation. Powder Technol. 2003, 133, 190–202. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, R.; Wang, H.; Wang, J. DEM Investigation of Particle Anti-Rotation Effects on the Micromechanical Response of Granular Materials. Granul. Matter 2013, 15, 315–326. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, M.; Soga, K.; Luding, S. Micro Origins for Macro Behavior in Granular Media. Granul. Matter 2016, 18, 59. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Chen, Y.; Zhou, B.; Xue, S.; Zhu, X. Deformation Mechanisms of Coexistence Type Methane Hydrate-Bearing Sands: A Particulate-Scale Investigation. J. Nat. Gas Sci. Eng. 2022, 102, 104604. [Google Scholar] [CrossRef]
- Feng, K.; Montoya, B.M.; Evans, T.M. Discrete Element Method Simulations of Bio-Cemented Sands. Comput. Geotech. 2017, 85, 139–150. [Google Scholar] [CrossRef]
- Khoubani, A.; Evans, T.M.; Montoya, B.M. Particulate Simulations of Triaxial Tests on Bio-Cemented Sand Using a New Cementation Model. In Proceedings of the Geo-Chicago 2016: Sustainability, Energy, and the Geoenvironment, Chicago, IL, USA, 8 August 2016; pp. 84–93. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.T.; Brown, D.G. Investigation of Pore-Scale CaCO3 Distributions and Their Effects on Stiffness and Permeability of Sands Treated by Microbially Induced Carbonate Precipitation (MICP). Soils Found. 2020, 60, 944–961. [Google Scholar] [CrossRef]
- Gao, Y.; Hang, L.; He, J.; Chu, J. Mechanical Behaviour of Biocemented Sands at Various Treatment Levels and Relative Densities. Acta Geotech. 2019, 14, 697–707. [Google Scholar] [CrossRef]
- Zhang, A.; Dieudonné, A.-C. Effects of Carbonate Distribution Pattern on the Mechanical Behaviour of Bio-Cemented Sands: A DEM Study. Comput. Geotech. 2023, 154, 105152. [Google Scholar] [CrossRef]
- Wu, H.; Wu, W.; Liang, W.; Dai, F.; Liu, H.; Xiao, Y. 3D DEM Modeling of Biocemented Sand with Fines as Cementing Agents. Int. J. Numer. Anal. Methods Geomech. 2023, 47, 212–240. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Huang, X. DEM Analysis of Monotonic and Cyclic Behaviors of Sand Based on Critical State Soil Mechanics Framework. Comput. Geotech. 2020, 128, 103787. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, C.; Sun, Y.; Wang, S.; Wu, H.; Chen, H.; Liu, H. Compression Behavior of MICP-Treated Sand with Various Gradations. Acta Geotech. 2021, 16, 1391–1400. [Google Scholar] [CrossRef]
- Thornton, C. Numerical Simulations of Deviatoric Shear Deformation of Granular Media. Géotechnique 2000, 50, 43–53. [Google Scholar] [CrossRef]
- Huang, X.; O’Sullivan, C.; Hanley, K.J.; Kwok, C.Y. Discrete-Element Method Analysis of the State Parameter. Géotechnique 2014, 64, 954–965. [Google Scholar] [CrossRef]
- Huang, X.; Kwok, C.; Hanley, K.J.; Zhang, Z. DEM Analysis of the Onset of Flow Deformation of Sands: Linking Monotonic and Cyclic Undrained Behaviours. Acta Geotech. 2018, 13, 1061–1074. [Google Scholar] [CrossRef]




| ID | Initial Relative Density of Sand (%) | CCC (%) | CD Experiment |
|---|---|---|---|
| MU-D33-C0 | 33.1% | √ | |
| MU-D54-C0 | 54.1% | √ | |
| MU-D74-C0 | 73.9% | √ | |
| MU-D90-C0 | 89.3% | ||
| MT-D33-C0.4 | 33.1% | 0.40 | |
| MT-D33-C0.78 | 33.1% | 0.78 | |
| MT-D33-C1.51 | 33.1% | 1.51 | |
| MT-D33-C1.73 | 33.1% | 1.73 | √ |
| MT-D33-C3.85 | 33.1% | 3.85 | √ |
| MT-D33-C5.78 | 33.1% | 5.78 | √ |
| MT-D33-C7.69 | 33.1% | 7.69 | √ |
| Parameters | Value |
|---|---|
| Density of sand | 2650 kg/m3 |
| Normal stiffness of sand particles (kn) | kn = k0 × r |
| Shear stiffness of sand particles (ks) Normal-to-shear stiffness ratio of sand particles (α) | ks = α × kn |
| Rolling resistance coefficient of sand particles (μr) | 0.5 |
| Density of carbonate | 2444 kg/m3 |
| Sand–carbonate contact effective modulus | 12 × 108 Pa |
| Normal-to-shear stiffness ratio of sand–carbonate contacts | 2.0 |
| Sand–carbonate bond effective modulus | 12 × 108 Pa |
| Normal-to-shear stiffness ratio of sand–carbonate contacts | 2.0 |
| Sand–carbonate tensile strength | 24 × 106 Pa |
| Sand–carbonate cohesion | 12 × 108 Pa |
| Sand–carbonate friction angle | 26° |
| Carbonate–carbonate effective modulus | 12 × 108 Pa |
| Normal-to-shear stiffness ratio of carbonate–carbonate contacts | 2.0 |
| Carbonate–carbonate bond effective modulus | 12 × 108 Pa |
| Normal-to-shear stiffness ratio of carbonate–carbonate contacts | 2.0 |
| Carbonate–carbonate tensile strength | 24 × 106 Pa |
| Carbonate–carbonate cohesion | 12 × 108 Pa |
| Carbonate–carbonate friction angle | 26° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Zhou, J.; Shen, L.; Ji, Y.; Li, W.; Cheng, Y. Discrete Element Study on Mechanical Properties of MICP-Treated Sand under Triaxial Compression. J. Mar. Sci. Eng. 2024, 12, 1503. https://doi.org/10.3390/jmse12091503
Xie L, Zhou J, Shen L, Ji Y, Li W, Cheng Y. Discrete Element Study on Mechanical Properties of MICP-Treated Sand under Triaxial Compression. Journal of Marine Science and Engineering. 2024; 12(9):1503. https://doi.org/10.3390/jmse12091503
Chicago/Turabian StyleXie, Liquan, Jingsong Zhou, Li Shen, Yifan Ji, Wenlin Li, and Yaofei Cheng. 2024. "Discrete Element Study on Mechanical Properties of MICP-Treated Sand under Triaxial Compression" Journal of Marine Science and Engineering 12, no. 9: 1503. https://doi.org/10.3390/jmse12091503
APA StyleXie, L., Zhou, J., Shen, L., Ji, Y., Li, W., & Cheng, Y. (2024). Discrete Element Study on Mechanical Properties of MICP-Treated Sand under Triaxial Compression. Journal of Marine Science and Engineering, 12(9), 1503. https://doi.org/10.3390/jmse12091503






