Tidal Impacts on Zooplankton Dynamics in a Major Ocean-Lagoon Channel: Insights from a 25-Hour Intensive Survey in the Cotonou Channel, Benin
Abstract
:1. Introduction
2. Materials and Methods
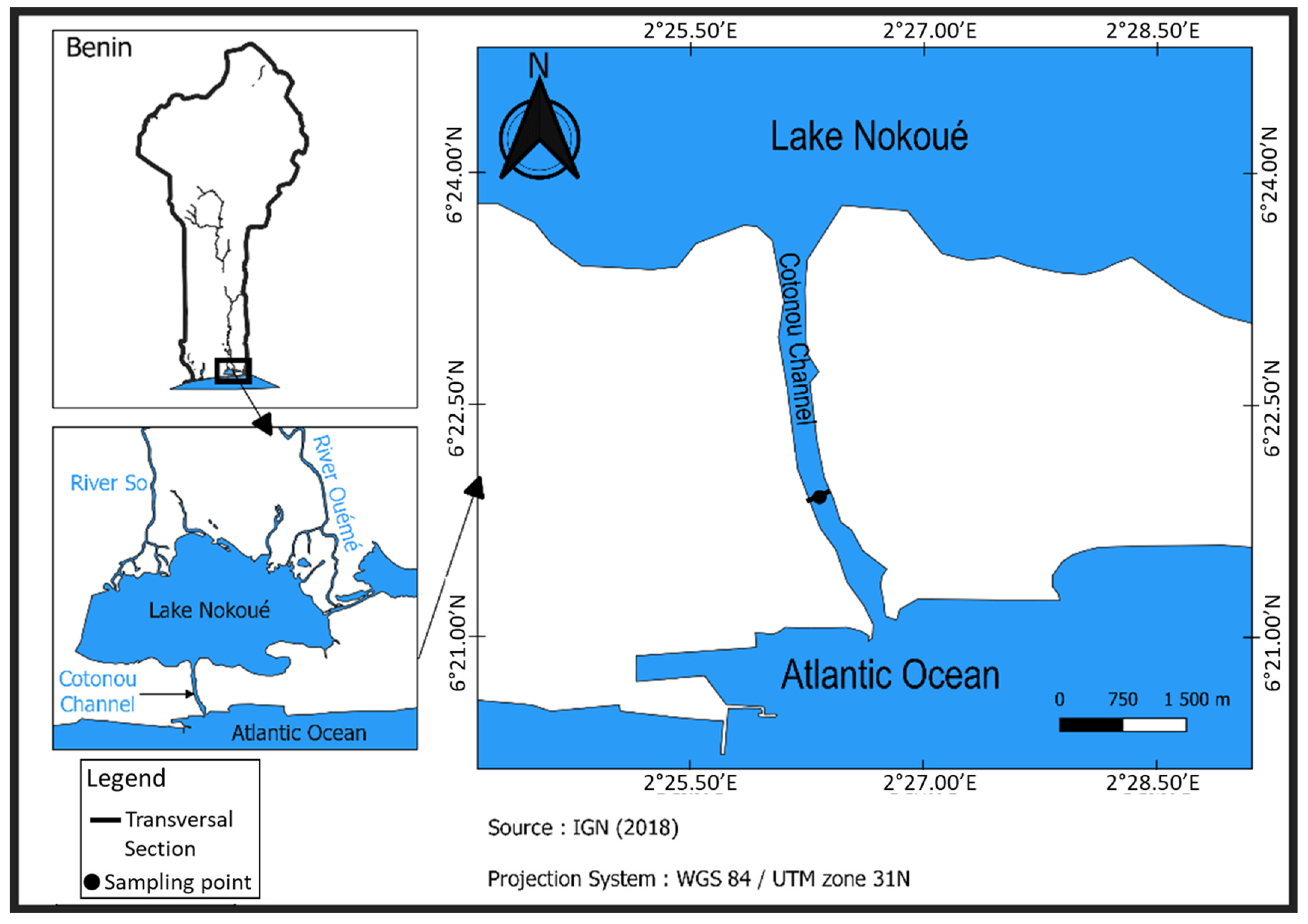
2.1. Study Area and Tide Characteristics
2.2. Sample Collection and Analysis Techniques
2.2.1. Sampling Location and Duration
2.2.2. Physicochemical Parameters
2.2.3. Water Exchange Assessment
2.2.4. Zooplankton Collection, Preservation and Identification
2.3. Statistical Analysis
2.3.1. Diversity Indices
- Species richness, which corresponds to the number of species present S.
- Shannon–Wiener Diversity Index (H′, in bits), which evaluates the extent of species diversity in the environment using the formula:where ni is the number of individuals for species i, and N is the total number of individuals in the environment.
- Piélou’s evenness index (J), which measures the evenness (or equitable distribution) of species in a population relative to a theoretical equal distribution among all species. It is calculated using the formula:
2.3.2. Univariate and Multivariate Analysis
3. Results
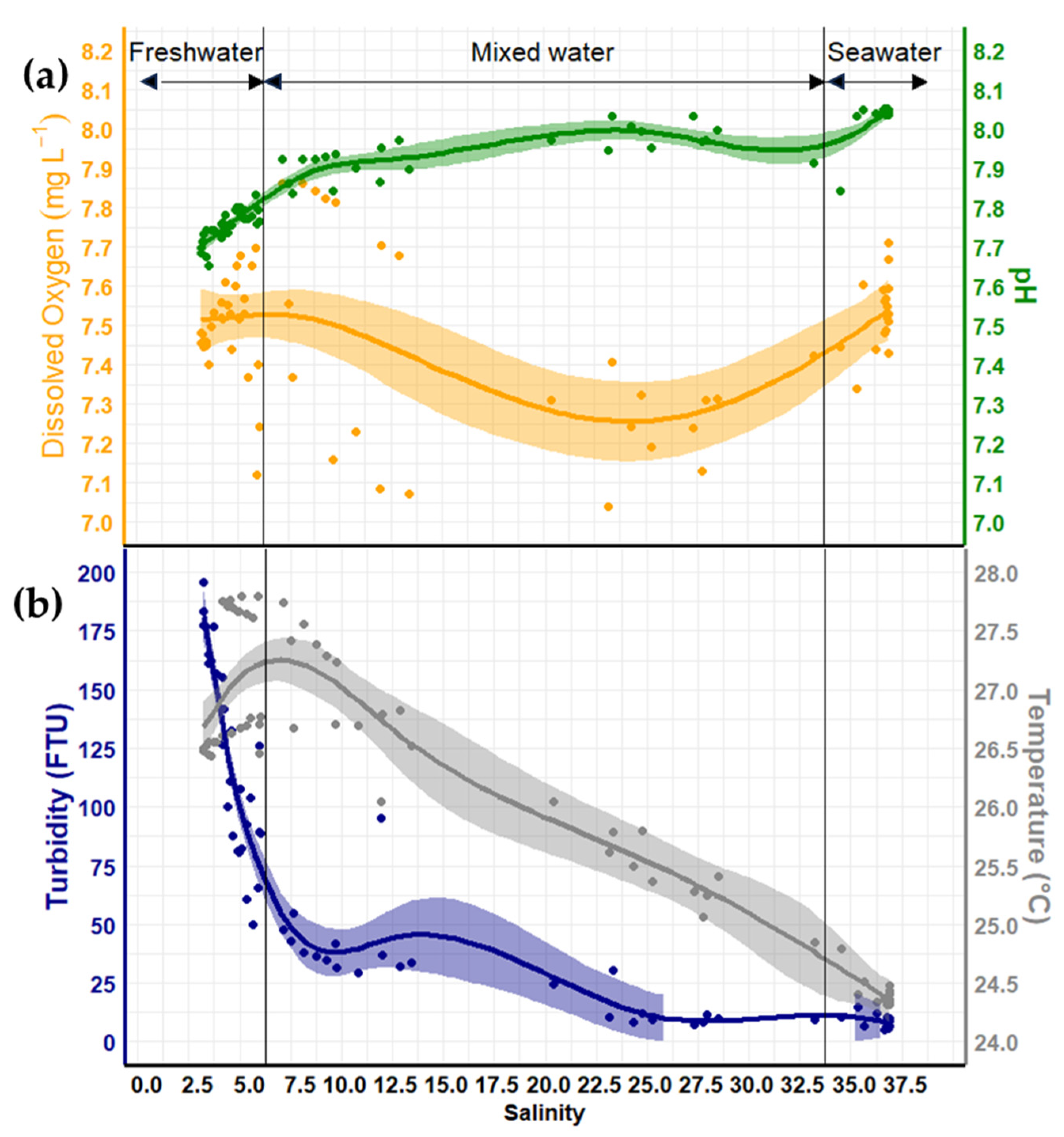
3.1. Variation of Physicochemical Parameters
3.2. Water Mass Characteristics
3.3. Taxonomic Composition, Diversity, and Abundance of Zooplankton
3.4. Occurrence of Zooplankton Taxa
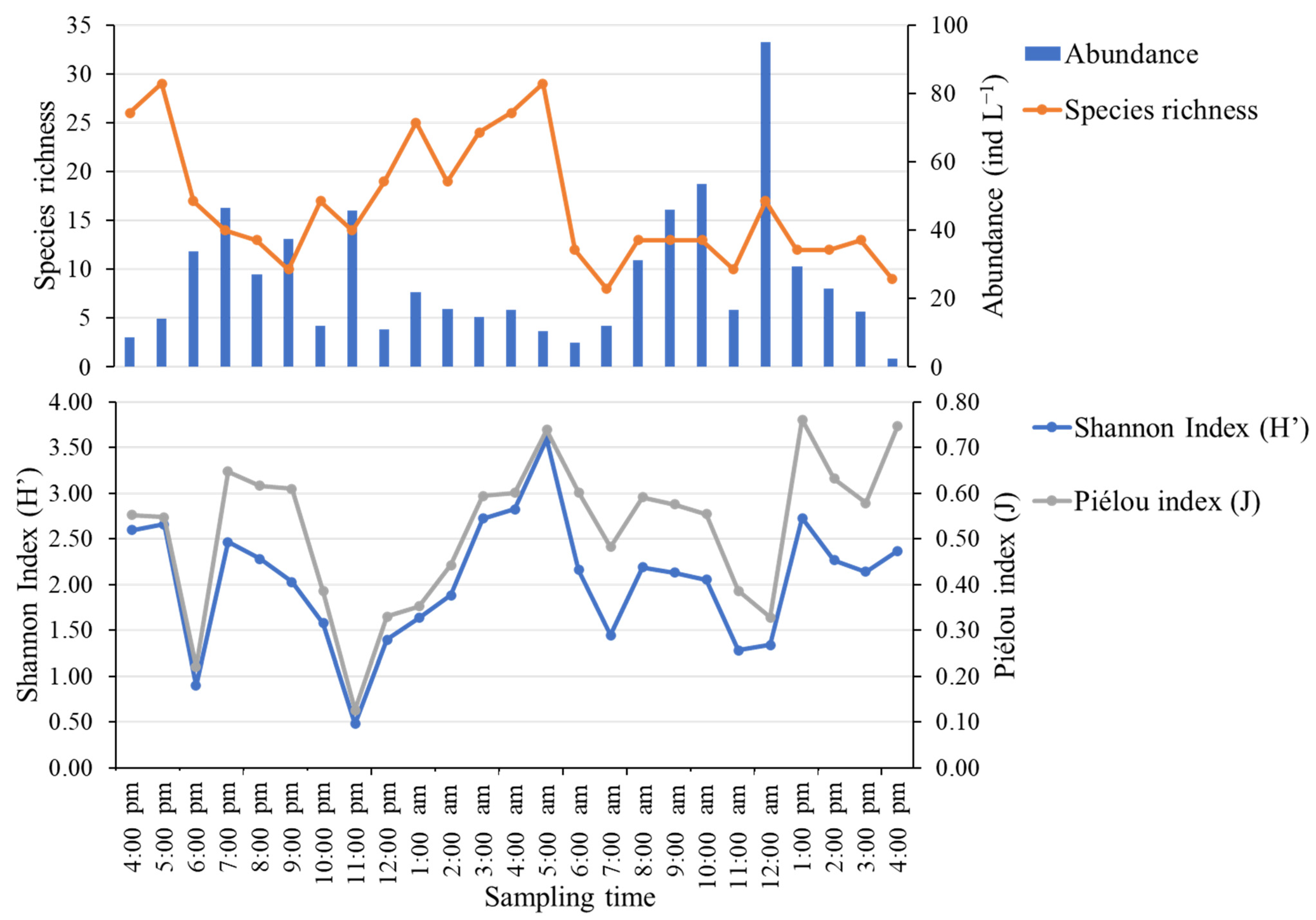
3.5. Temporal Variation in Zooplankton Diversity and Abundance
3.6. Relationship between Zooplankton and Environmental Parameters
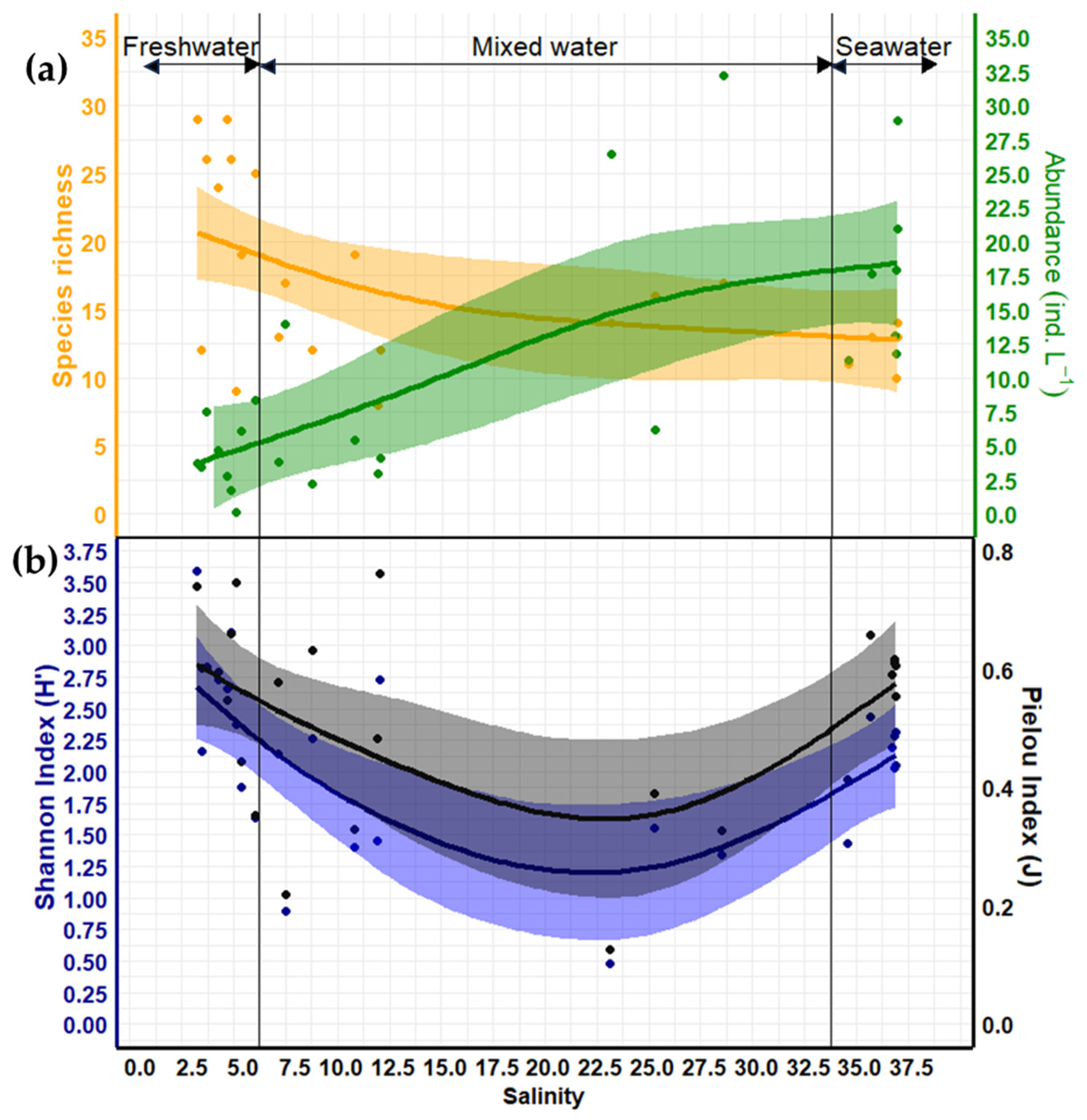
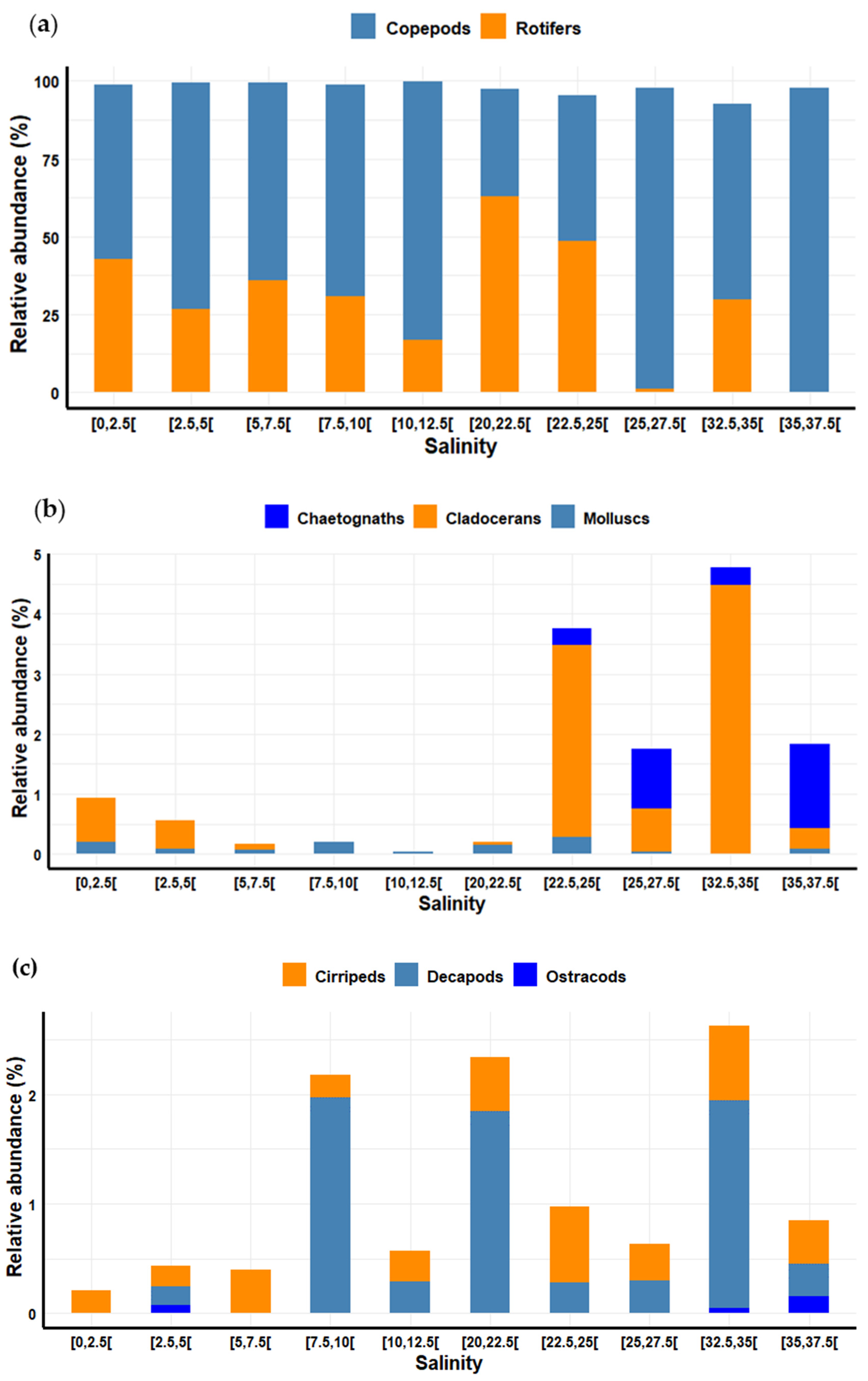
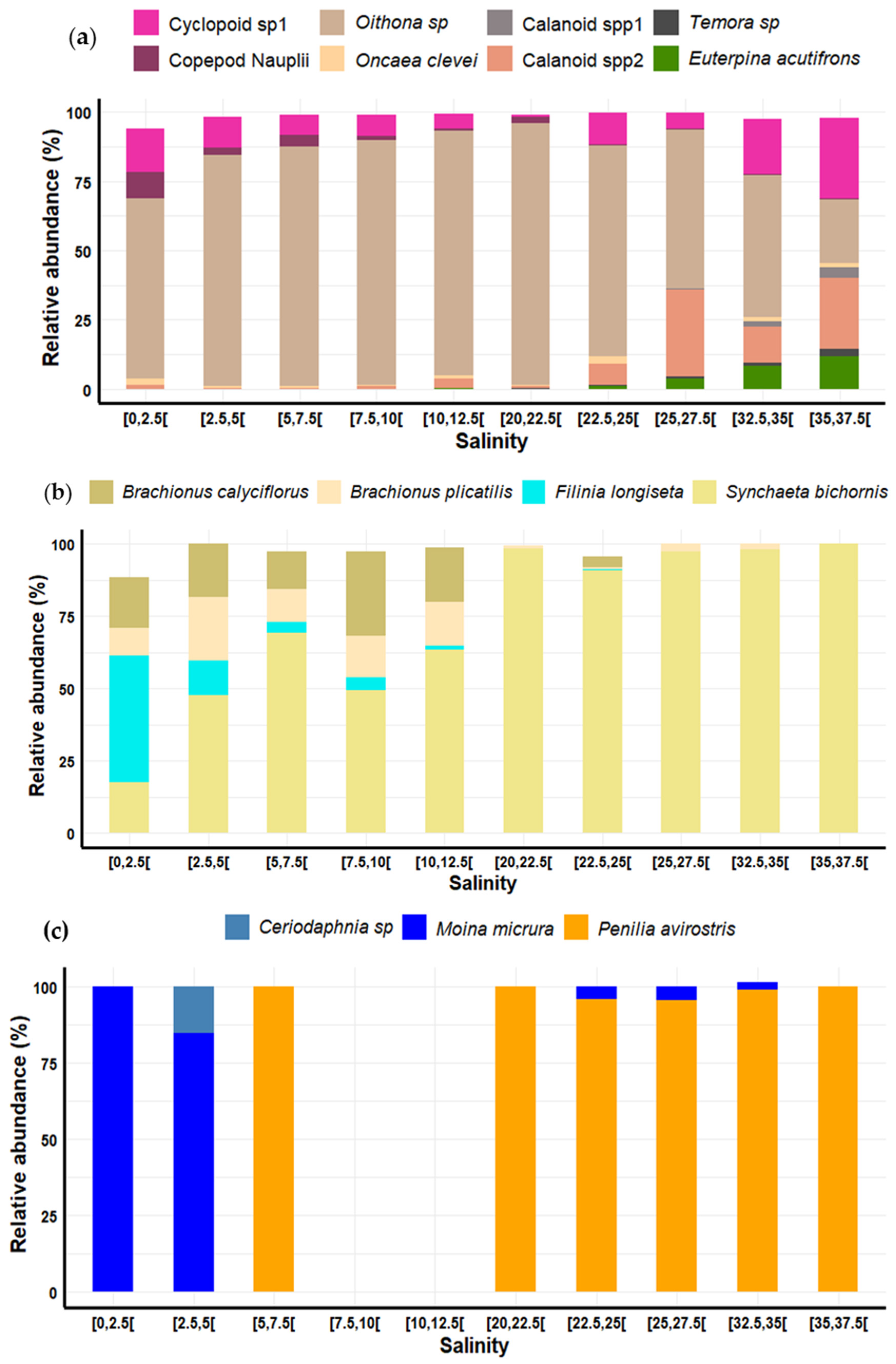
3.7. Variation in Zooplankton Diversity and Abundance Relative to Salinity
4. Discussion
4.1. On the Physicochemical Parameter Variations
4.2. On the Zooplankton Community
4.2.1. Taxonomic Composition and Abundance
4.2.2. Species-Specific Observations
4.2.3. Salinity Effects
4.3. On the Limitations of Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etilé, R.N.; Kouassi, M.; Pagano, M.; Yao, S.; Aka, M.N.; Douba, V.N. Diel Variation of Zooplankton Community Composition, Abundance and Biomass in a West African Tropical Coastal Lagoon (Grand-Lahou, Côte d’Ivoire). Int. J. Agric. Innov. Res. 2015, 3, 1641–1655. [Google Scholar]
- Zandagba, J.; Moussa, M.; Obada, E.; Afouda, A. Hydrodynamic Modeling of Nokoué Lake in Benin. Hydrology 2016, 3, 44. [Google Scholar] [CrossRef]
- Gnohossou, P.; Lalèyè, P.; Atachi, P.; Magali, G.; Villanueva, M.C.; Moreau, J. Temporal Variations in the Food Habits of Some Fish Species in Lake Nokoué, Benin. Afr. J. Aquat. Sci. 2013, 38, 43–47. [Google Scholar] [CrossRef]
- Laleye, P.; Villanueva, C.; Entsua-Mensah, C.M.; Moreau, J. A Review of the Aquatic Living Resources in Gulf of Guinea Lagoons, with Particular Emphasis on Fisheries Management Issues. J. Afrotropical. Zool. 2007, 10, 123–136. [Google Scholar]
- Lederoun, D.; Amoussou, G.; Baglo, I.S.; Adjibogoun, H.; Vodougnon, H.; Moreau, J.; Lalèyè, P.A. Growth, Mortality and Yield of Sarotherodon melanotheron melanotheron (Rüppell, 1852) in the Lake Nokoué and Porto-Novo Lagoon Complex Benin, West Africa. Aquat. Living Resour. 2020, 33, 18. [Google Scholar] [CrossRef]
- Ntangyong, I.L.; Chaigneau, A.; Morel, Y.; Assogba, A.; Okpeitcha, V.O.; Duhaut, T.; Stieglitz, T.; Van Beek, P.; Baloitcha, E.; Sohou, Z.; et al. Seasonal and Interannual Variations of Suspended Particulate Matter in a West-African Lagoon (Nokoué Lagoon, Benin): Impact of Rivers and Wind. Estuar. Coast. Shelf Sci. 2024, 304, 108821. [Google Scholar] [CrossRef]
- Adandedjan, D.; Makponse, E.; Hinvi, L.C.; Laleye, P. Données Préliminaires Sur La Diversité Du Zooplancton Du Lac Nokoué (Sud-Bénin). J. Appl. Biosci. 2017, 115, 11476–11489. [Google Scholar] [CrossRef]
- Chaigneau, A.; Ouinsou, F.T.; Akodogbo, H.H.; Dobigny, G.; Avocegan, T.T.; Dossou-Sognon, F.U.; Okpeitcha, V.O.; Djihouessi, M.B.; Azémar, F. Physicochemical Drivers of Zooplankton Seasonal Variability in a West African Lagoon (Nokoué Lagoon, Benin). J. Mar. Sci. Eng. 2023, 11, 556. [Google Scholar] [CrossRef]
- Dorak, Z.; Gaygusuz, Ö.; Tarkan, A.S.; Aydin, H. Diurnal Vertical Distribution of Zooplankton in a Newly Formed Reservoir (Tahtalı Reservoir, Kocaeli): The Role of Abiotic Factors and Chlorophyll a. Turk. J. Zool. 2013, 37, 218–227. [Google Scholar] [CrossRef]
- Bubu-Davies, O.A.; Ugwumba, O.A. Effects of Tide on Zooplankton Community of a Tributary of Upper Bonny Estuary, Niger Delta, Nigeria. Int. J. Sci. Res. Knowl. 2013, 1, 325–342. [Google Scholar] [CrossRef]
- Mofu, L.; Woodford, D.J.; Wasserman, R.J.; Dalu, T.; Weyl, O.L.F. Diet of Glossogobius callidus (Teleostei: Gobiidae) in Freshwater Impoundments in the Sundays River Valley of the Eastern Cape, South Africa. Afr. J. Aquat. Sci. 2019, 44, 415–420. [Google Scholar] [CrossRef]
- Chaigneau, A.; Okpeitcha, O.V.; Morel, Y.; Stieglitz, T.; Assogba, A.; Benoist, M.; Allamel, P.; Honfo, J.; Awoulmbang Sakpak, T.D.; Rétif, F.; et al. From Seasonal Flood Pulse to Seiche: Multi-Frequency Water-Level Fluctuations in a Large Shallow Tropical Lagoon (Nokoué Lagoon, Benin). Estuar. Coast. Shelf Sci. 2022, 267, 107767. [Google Scholar] [CrossRef]
- Morel, Y.; Chaigneau, A.; Okpeitcha, V.O.; Stieglitz, T.; Assogba, A.; Duhaut, T.; Rétif, F.; Peugeot, C.; Sohou, Z. Terrestrial or Oceanic Forcing? Water Level Variations in Coastal Lagoons Constrained by River Inflow and Ocean Tides. Adv. Water Resour. 2022, 169, 104309. [Google Scholar] [CrossRef]
- Okpeitcha, O.V.; Chaigneau, A.; Morel, Y.; Stieglitz, T.; Pomalegni, Y.; Sohou, Z.; Mama, D. Seasonal and Interannual Variability of Salinity in a Large West-African Lagoon (Nokoué Lagoon, Benin). Estuar. Coast. Shelf Sci. 2022, 264, 107689. [Google Scholar] [CrossRef]
- Okpeitcha, O.V.; Chaigneau, A.; Morel, Y.; Duhaut, T.; Marsaleix, P.; Rétif, F.; Honfo, J.; Stieglitz, T.; Sohou, Z.; Sintondji, L.O.; et al. Modeling Seasonal Salinity Variations in a Large West African Lagoon (Nokoué, Benin): Major Drivers and Mechanisms. Reg. Stud. Mar. Sci. 2024, 69, 103330. [Google Scholar] [CrossRef]
- Menéndez, M.C.; Piccolo, M.C.; Hoffmeyer, M.S. Short-Term Variability on Mesozooplankton Community in a Shallow Mixed Estuary (Bahía Blanca, Argentina): Influence of Tidal Cycles and Local Winds. Estuar. Coast. Shelf Sci. 2012, 112, 11–22. [Google Scholar] [CrossRef]
- da Costa, K.G.; Bezerra, T.R.; Monteiro, M.C.; Vallinoto, M.; Berrêdo, J.F.; Pereira, L.C.C.; Costa, R.M. da Tidal-Induced Changes in the Zooplankton Community of an Amazon Estuary. J. Coast. Res. 2013, 29, 756–765. [Google Scholar] [CrossRef]
- Chazarreta, J.; Dutto, S.; Berasategui, A.A.; Paniagua, G.F.; Fritz, L.J.; Cuadrado, D.G.; Hoffmeyer, M.S. Zooplankton Community Modulated by Spatial and Tidal Changes in the Bahía Blanca Estuary, Argentina. Reg. Stud. Mar. Sci. 2020, 36, 101277. [Google Scholar] [CrossRef]
- Krumme, U.; Liang, T.-H. Tidal-Induced Changes in a Copepod-Dominated Zooplankton Community in a Macrotidal Mangrove Channel in Northern Brazil. Zool. Stud. 2004, 43, 404–414. [Google Scholar]
- Badahoui, A.; Fiogbe, E.; Boko, M. Les Causes de La Dégradation Du Lac Ahémé et Ses Chenaux. Int. J. Biol. Chem. Sci. 2011, 4, 882–897. [Google Scholar] [CrossRef]
- Honfo, K.J.; Chaigneau, A.; Morel, Y.; Duhaut, T.; Marsaleix, P.; Okpeitcha, O.V.; Stieglitz, T.; Ouillon, S.; Baloitcha, E.; Rétif, F. Water Mass Circulation and Residence Time Using Eulerian Approach in a Large Coastal Lagoon (Nokoué Lagoon, Benin, West Africa). Ocean. Model. 2024, 190, 102388. [Google Scholar] [CrossRef]
- Ahlstrom, E.H. A Revision of the Rotatorian Genera Brachionus et Platyias with Descriptions New Species et Two New Varieties. Bull. Am. Mus. Nat. Hist. 1940, 44, 148–184. [Google Scholar]
- Dussart, B.H. Copépodes. In Flore et Faune Aquatiques de l’Afrique Sahélo-Soudanienne: Tome 1; ORSTOM: Paris, France, 1980; pp. 333–356. [Google Scholar]
- Pourriot, R. Rotifères. In Flore et Faune Aquatiques de l’Afrique Sahélo-Soudanienne; Société Linnéenne de Lyon: Paris, France, 1980; pp. 219–244. [Google Scholar]
- Conway, D.V.P.; White, R.G.; Hugues-Dit-Ciles, J.; Gallienne, C.P.; Robins, D.B. Guide to the Coastal et Surface Zooplankton of the South-Western Indian Ocean; Marine Biological Association of the United Kingdom: Plymouth, UK, 2003. [Google Scholar]
- Fontaneto, D.; De Smet, W.H.; Melone, G. Identification Key to the Genera of Marine Rotifers Worldwide. Meiofauna Mar. 2008, 16, 75–99. [Google Scholar]
- Al-Yamani, F.Y.; Skryabin, V.; Gubanova, A.; Khvorov, S.; Prusova, I. Marine Zooplankton: Practical Guide for the Northwestern Arabian Gulf; Kuwait Institute for Scientific Research: Safat, Kuwait, 2011; Volume 1, 208p. [Google Scholar]
- Conway, D.V.P. Marine Zooplankton of Southern Britain. In Part 1: Radiolaria, Heliozoa, Foraminifera, Ciliophora, Cnidaria, Ctenophora, Platyhelminthes, Nemertea, Rotifera et Mollusca; Marine Biological Association of the United Kingdom: Plymouth, UK, 2012. [Google Scholar]
- Conway, D.V.P. Marine Zooplankton of Southern Britain. In Part 2: Arachnida, Pycnogonida, Cladocera, Facetotecta, Cirripedia et Copepoda; Marine Biological Association of the United Kingdom: Plymouth, UK, 2012. [Google Scholar]
- Conway, D.V.P. Marine Zooplankton of Southern Britain. In Part 3: Ostracoda, Stomatopoda, Nebaliacea, Mysida, Amphipoda, Isopoda, Cumacea, Euphausiacea, Decapoda, Annelida, Tardigrada, Nematoda, Phoronida, Bryozoa, Entoprocta, Brachiopoda, Echinodermata, Chaetognatha, Hemichordata and Chordata; Marine Biological Association of the United Kingdom: Plymouth, UK, 2015. [Google Scholar]
- Haney, J.F.; Aliberti, M.A.; Allan, E.; Allard, S.; Bauers, D.J.; Beagen, W.; Bradtr, S.R.; Carlson, B.; Carlson, S.C.; Doan, U.M.; et al. An Image-Based Key to the Zooplankton of North America “Version 5.0”. Available online: http://cfb.unh.edu/cfbkey/html/index.html (accessed on 10 July 2021).
- LaMay, M.; Hayes-Pontius, E.; Ater, I.; Mihuc, T. A Revised Key to the Zooplankton of Lake Champlain. Sci. Discipulorum 2013, 6, 1–41. [Google Scholar]
- Swadling, K.M.; Slotwinski, A.; Davies, C.; Beard, J.; McKinnon, A.D.; Coman, F.; Murphy, N.; Tonks, M.; Rochester, W.; Conway, D.V.P.; et al. Australian Marine Zooplankton: A Taxonomic Guide and Atlas. Version 1.0 February 2013. Available online: https://www.imas.utas.edu.au/zooplankton/image-key (accessed on 8 July 2021).
- Aboul Ezz, S.M.; Abdel Aziz, N.E.; Abou Zaid, M.M.; El Raey, M.; Abo-Taleb, H.A. Environmental Assessment of El-Mex Bay, Southeastern Mediterranean by Using Rotifera as a Plankton Bio-Indicator. Egypt. J. Aquat. Res. 2014, 40, 43–57. [Google Scholar] [CrossRef]
- Glime, J.M. Invertebrates: Rotifer Taxa—Monogononta Chapt. 4-7 a-b-c. Bryophyt. Ecol. 2017, 2, 7–37. [Google Scholar]
- Glime, J.M. Arthropods: Crustacea—Copepoda et Cladocera. Chapt. 10-1. In Bryophyte Ecology; Michigan Technological University: Houghton, MI, USA, 2017; pp. 10-1-1–10-1-20. [Google Scholar]
- Wilke, T.; Ahlrichs, W.H.; Bininda-Emonds, O.R.P. A Weighted Taxonomic Matrix Key for Species of the Rotifer Genus Synchaeta (Rotifera, Monogononta, Synchaetidae). ZooKeys 2019, 871, 1. [Google Scholar] [CrossRef]
- Magurran, A.E. Diversity Indices and Species Abundance Models. In Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; pp. 7–45. ISBN 978-94-015-7360-3. [Google Scholar]
- Marcon, E. Mesures de La Biodiversité. Ph.D. Thesis, AgroParisTech, Kourou, France, 2015. [Google Scholar]
- Dajoz, R. Précis D’écologie, 5th ed.; Dunod Université; Dunod: Paris, France, 1985; ISBN 978-2-04-016439-3. [Google Scholar]
- R Core Team _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-Project.Org (accessed on 8 February 2024).
- Odountan, O.; de Bisthoven, L.J.; Koudenoukpo, C.; Abou, Y. Spatio-Temporal Variation of Environmental Variables and Aquatic Macroinvertebrate Assemblages in Lake Nokoué, a RAMSAR Site of Benin. Afr. J. Aquat. Sci. 2019, 44, 219–231. [Google Scholar] [CrossRef]
- Lalèyè, P.; Niyonkuru, C.; Moreau, J.; Teugels, G.G. Spatial and Seasonal Distribution of the Ichthyofauna of Lake Nokoué, Bénin, West Africa. Afr. J. Aquat. Sci. 2003, 28, 151–161. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research 2023. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 26 August 2024).
- Gao, X.; Song, J.; Li, X. Zooplankton Spatial and Diurnal Variations in the Changjiang River Estuary before Operation of the Three Gorges Dam. Chin. J. Oceanol. Limnol. 2011, 29, 591–602. [Google Scholar] [CrossRef]
- Ara, K. Temporal Variability and Production of the Planktonic Copepod Community in the Cananéia Lagoon Estuarine System, São Paulo, Brazil. Zool. Stud. 2004, 43, 179–186. [Google Scholar]
- Zhang, H.; Ekvall, M.K.; Xu, J.; Hansson, L. Counteracting Effects of Recruitment and Predation Shape Establishment of Rotifer Communities under Climate Change. Limnol. Oceanogr. 2015, 60, 1577–1587. [Google Scholar] [CrossRef]
- Attayde, J.L.; Bozelli, R.L. Assessing the Indicator Properties of Zooplankton Assemblages to Disturbance Gradients by Canonical Correspondence Analysis. Can. J. Fish. Aquat. Sci. 1998, 55, 1789–1797. [Google Scholar] [CrossRef]
- Kozlowsky-Suzuki, B.; Bozelli, R.L. Resilience of a Zooplankton Community Subjected to Marine Intrusion in a Tropical Coastal Lagoon. Hydrobiologia 2004, 522, 165–177. [Google Scholar] [CrossRef]
- Branco, C.W.C.; Kozlowsky-Suzuki, B.; Esteves, F.A. Environmental Changes and Zooplankton Temporal and Spatial Variation in a Disturbed Brazilian Coastal Lagoon. Braz. J. Biol. 2007, 67, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Schallenberg, M.; Hall, C.J.; Burns, C.W. Consequences of Climate-Induced Salinity Increases on Zooplankton Abundance and Diversity in Coastal Lakes. Mar. Ecol. Prog. Ser. 2003, 251, 181–189. [Google Scholar] [CrossRef]
- Donadel, L.; Cardoso, L.D.S.; Torgan, L.C. Plankton Community Dynamics in a Subtropical Lagoonal System and Related Factors. An. Acad. Bras. Ciênc. 2016, 88, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Wiafe, G.; Yaqub, H.B.; Mensah, M.A.; Frid, C.L.J. Impact of Climate Change on Long-Term Zooplankton Biomass in the Upwelling Region of the Gulf of Guinea. ICES J. Mar. Sci. 2008, 65, 318–324. [Google Scholar] [CrossRef]
- Aka, N.M.; Etile, R.N.; Joany, T.; N’Da, K. Peuplement Zooplanctonique Du Plateau Continental Ivoirien: Diversité, Abondance et Biomasse. Int. J. Biol. Chem. Sci. 2018, 12, 129–140. [Google Scholar] [CrossRef]



| H Test | Mean Value | ||||
|---|---|---|---|---|---|
| Variables | X2 (df = 2) | p-Values | Freshwater | Mixed Water | Seawater |
| Salinity (PSU) | 21.26 *** | 2.41 × 10−5 | 3.3 a | 13.8 b | 35.1 c |
| Temperature (°C) | 14.91 *** | 5.79 × 10−4 | 27.0 a | 26.5 a | 24.4 b |
| Turbidity (FTU) | 18.24 *** | 1.09 × 10−4 | 123 a | 35 b | 8c |
| ANOVA | Mean value | ||||
| F–Value (df = 2) | p-values | F | M | S | |
| Dissolved oxygen (mg L−1) | 0.56 n.s. | 5.79 × 10−1 | 7.5a | 7.4 a | 7.5 a |
| pH | 48.14 *** | 9.23 × 10−9 | 7.8a | 7.9 b | 8.0 c |
| Phylum | Groups | Species | Total Relative Abundance | Group-Specific Abundance | Focc (%) |
|---|---|---|---|---|---|
| Rotifera | Rotifers | Anuraeopsis navicula Rousselet, 1911 | 0.060 | 0.237 | 16 |
| Anuraeopsis fissa (Gosse, 1851) | 0.017 | 0.069 | 20 | ||
| Brachionus angularis Gosse, 1851 | 0.108 | 0.428 | 32 | ||
| Brachionus bidentatus Anderson, 1889 | 0.012 | 0.048 | 4 | ||
| Brachionus calyciflorus Pallas, 1766 | 2.128 | 8.477 | 52 | ||
| Brachionus caudatus Barrois and Daday, 1894 | 0.154 | 0.612 | 44 | ||
| Brachionus falcatus Zacharias, 1898 | 0.036 | 0.144 | 24 | ||
| Brachionus mirabilis Daday, 1897 | 0.004 | 0.017 | 4 | ||
| Brachionus plicatilis Müller, 1786 | 2.905 | 11.573 | 76 | ||
| Brachionus quadridentatus Hermann, 1783 | 0.009 | 0.035 | 8 | ||
| Keratella cochlearis (Gosse, 1851) | 0.004 | 0.016 | 4 | ||
| Keratella lenzi Hauer, 1953 | 0.016 | 0.065 | 4 | ||
| Keratella sp. | 0.031 | 0.122 | 16 | ||
| Keratella tropica Apstein, 1907) | 0.121 | 0.481 | 40 | ||
| Plationus patulus (Müller, 1786) | 0.026 | 0.103 | 12 | ||
| Trichotria sp. | 0.004 | 0.016 | 4 | ||
| Filinia longiseta (Ehrenberg, 1834) | 3.277 | 13.057 | 64 | ||
| Filinia opoliensis (Zacharias, 1898) | 0.152 | 0.604 | 32 | ||
| Filinia terminalis (Plate, 1886) | 0.018 | 0.072 | 16 | ||
| Lecane leontina (Turner, 1892) | 0.024 | 0.094 | 24 | ||
| Monostyla closterocerca (Schmarda, 1859) | 0.004 | 0.015 | 4 | ||
| Collurella uncinata (Müller, 1773) | 0.010 | 0.038 | 4 | ||
| Lepadella sp. | 0.016 | 0.063 | 12 | ||
| Cephallodella sp. | 0.001 | 0.003 | 4 | ||
| Resticula melandocus (Gosse, 1887) | 0.010 | 0.038 | 8 | ||
| Rotaria neptunia (Ehrenberg, 1830) | 0.004 | 0.017 | 4 | ||
| Philodina sp. | 0.033 | 0.131 | 24 | ||
| Philodina sp2 | 0.001 | 0.003 | 4 | ||
| Polyarthra sp. | 0.040 | 0.160 | 24 | ||
| Synchaeta bicornis Smith, 1904 | 15.478 | 61.664 | 80 | ||
| Synchaeta pectinata Ehrenberg, 1832 | 0.001 | 0.004 | 4 | ||
| Synchaeta grandis Zacharias, 1893 | 0.222 | 0.886 | 44 | ||
| Synchaeta sp. | 0.161 | 0.643 | 52 | ||
| Testudinella patina (Hermann, 1783) | 0.012 | 0.050 | 8 | ||
| Trichocerca brachyura (Gosse, 1851) | 0.004 | 0.016 | 4 | ||
| Athropoda | Copepods | Acanthocyclops sp. | 0.075 | 0.103 | 32 |
| Cyclopoid sp1 | 0.836 | 1.145 | 92 | ||
| Cyclopoid sp2 | 0.075 | 0.103 | 28 | ||
| Ectocyclops sp. | 0.327 | 0.447 | 76 | ||
| Corycaeus sp. | 0.107 | 0.147 | 20 | ||
| Corycaeus sp1 | 0.001 | 0.001 | 4 | ||
| Oithona sp. | 7.536 | 10.324 | 88 | ||
| Oithona plumifera Baird, 1843 | 0.069 | 0.095 | 28 | ||
| Oncaea clevei Früchtl, 1923 | 0.637 | 0.873 | 44 | ||
| Calanoid sp1 | 0.013 | 0.018 | 8 | ||
| Temora turbinata (Dana, 1849) | 0.228 | 0.313 | 28 | ||
| Temora sp. | 3.092 | 4.237 | 52 | ||
| Harpacticoid sp. | 0.062 | 0.086 | 44 | ||
| Microsetella norvegica (Boeck, 1865) | 0.374 | 0.513 | 44 | ||
| Microsetella rosea (Dana, 1847) | 0.035 | 0.048 | 32 | ||
| Macrosetella gracilis (Dana, 1846) | 0.149 | 0.204 | 52 | ||
| Euterpina acutifrons (Dana, 1847) | 0.906 | 1.242 | 56 | ||
| Cladocerans | Moina micrura Kurz, 1875 | 0.184 | 23.792 | 44 | |
| Penilia avirostris Dana, 1849 | 0.575 | 74.481 | 44 | ||
| Ceriodaphnia sp. | 0.013 | 1.726 | 8 | ||
| Cirripeds | Cirripede larva | 0.001 | 0.463 | 4 | |
| Decapod | Decapoda sp. | 0.402 | 100 | 52 | |
| Ostracod | Ostracoda sp. | 0.019 | 100 | 16 | |
| Mollusca | Mollusc | Gastropoda sp. | 0.081 | 100 | 64 |
| Chaetognatha | Chaetognaths | Sagitta sp1 | 0.211 | 61.554 | 20 |
| Sagitta sp2 | 0.132 | 38.446 | 16 | ||
| Phylum | Groups | Other zooplankton | Total Relative abundance | Group-specific abundance | Focc (%) |
| Athropoda | Copepods | Cyclopoid spp. | 0.035 | 0.047 | 20 |
| Calanoid spp1 | 11.534 | 15.800 | 100 | ||
| Calanoid spp2 | 1.481 | 2.029 | 84 | ||
| Copepod Nauplii | 45.423 | 62.227 | 100 | ||
| Cirripeds | Cirriped Nauplii | 0.284 | 99.537 | 80 |
| H test | Mean Value | ||||
|---|---|---|---|---|---|
| Variables | X2 (df = 2) | p-Values | Freshwater | Mixed Water | Seawater |
| Abundance (ind L−1) | 10.34 *** | 5.7 × 10−3 | 12.52 a | 30.92 a | 36.88 b |
| ANOVA | Mean value | ||||
| F-Value (df = 2) | p-values | Freshwater | Mixed water | Seawater | |
| Shannon index (H′) | 5.54 * | 1.1 × 10−2 | 2.49 a | 2.06 ab | 1.59 b |
| Piélou index (J) | 2.67 n.s. | 9.2 × 10−2 | 0.58 a | 0.43 a | 0.57 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akodogbo, H.H.; Dossou-Sognon, F.U.; Ouinsou, F.T.; Avocegan, T.T.; Kouglo, J.P.; Okpeitcha, O.V.; Assogba, A.; Sohou, Z.; Morel, Y.; Chaigneau, A. Tidal Impacts on Zooplankton Dynamics in a Major Ocean-Lagoon Channel: Insights from a 25-Hour Intensive Survey in the Cotonou Channel, Benin. J. Mar. Sci. Eng. 2024, 12, 1519. https://doi.org/10.3390/jmse12091519
Akodogbo HH, Dossou-Sognon FU, Ouinsou FT, Avocegan TT, Kouglo JP, Okpeitcha OV, Assogba A, Sohou Z, Morel Y, Chaigneau A. Tidal Impacts on Zooplankton Dynamics in a Major Ocean-Lagoon Channel: Insights from a 25-Hour Intensive Survey in the Cotonou Channel, Benin. Journal of Marine Science and Engineering. 2024; 12(9):1519. https://doi.org/10.3390/jmse12091519
Chicago/Turabian StyleAkodogbo, Hervé Hotèkpo, Fridolin Ubald Dossou-Sognon, François Talomonwo Ouinsou, Thalasse Tchémangnihodé Avocegan, Junior Patric Kouglo, Olaègbè Victor Okpeitcha, Arnaud Assogba, Zacharie Sohou, Yves Morel, and Alexis Chaigneau. 2024. "Tidal Impacts on Zooplankton Dynamics in a Major Ocean-Lagoon Channel: Insights from a 25-Hour Intensive Survey in the Cotonou Channel, Benin" Journal of Marine Science and Engineering 12, no. 9: 1519. https://doi.org/10.3390/jmse12091519





