A Method for Estimating the Distribution of Trachinotus ovatus in Marine Cages Based on Omnidirectional Scanning Sonar
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Collection
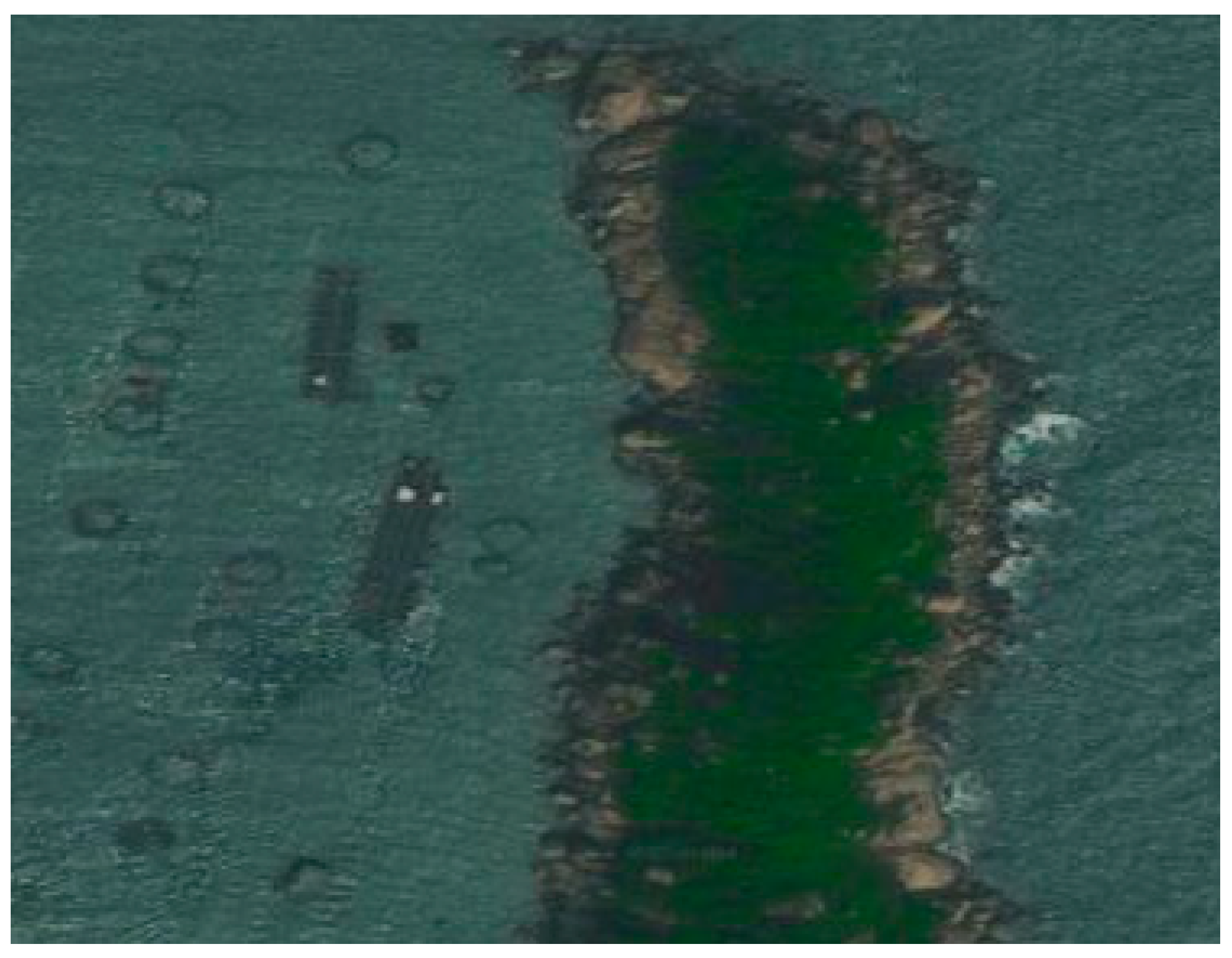
2.1.1. Experimental Site and Biological Samples
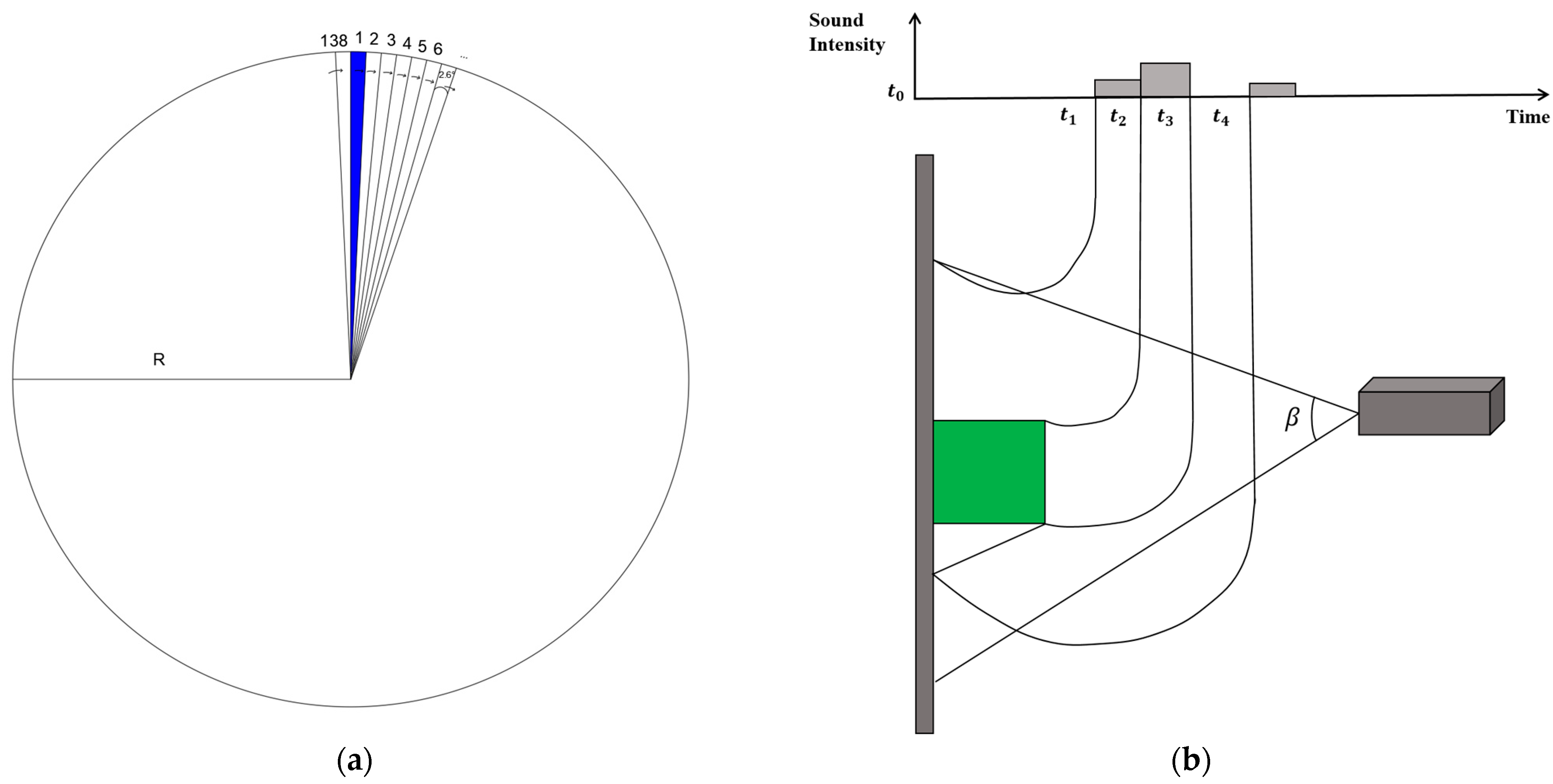
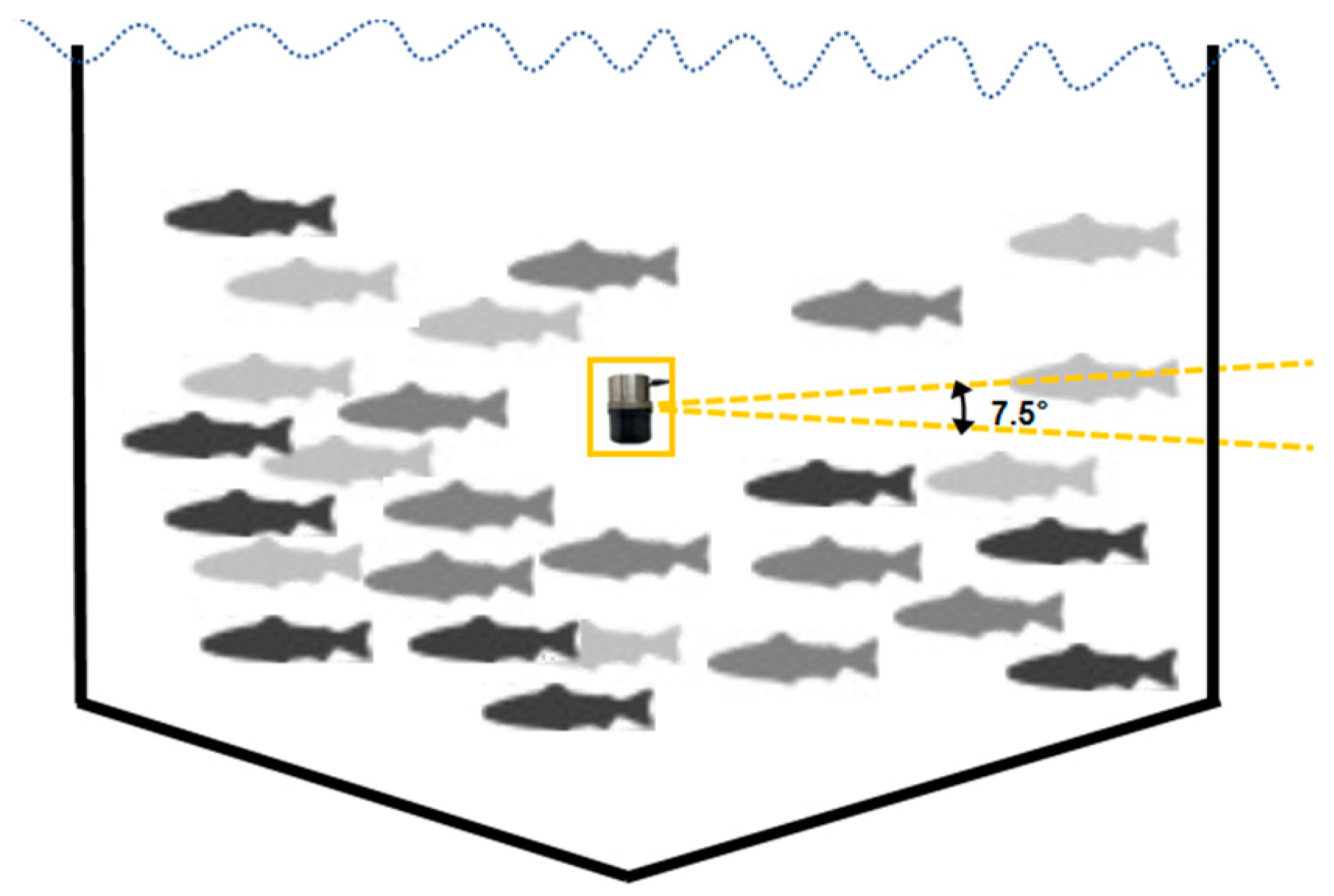
2.1.2. Device and Operating Mode
2.1.3. Data Acquisition
2.2. Data Saving Method
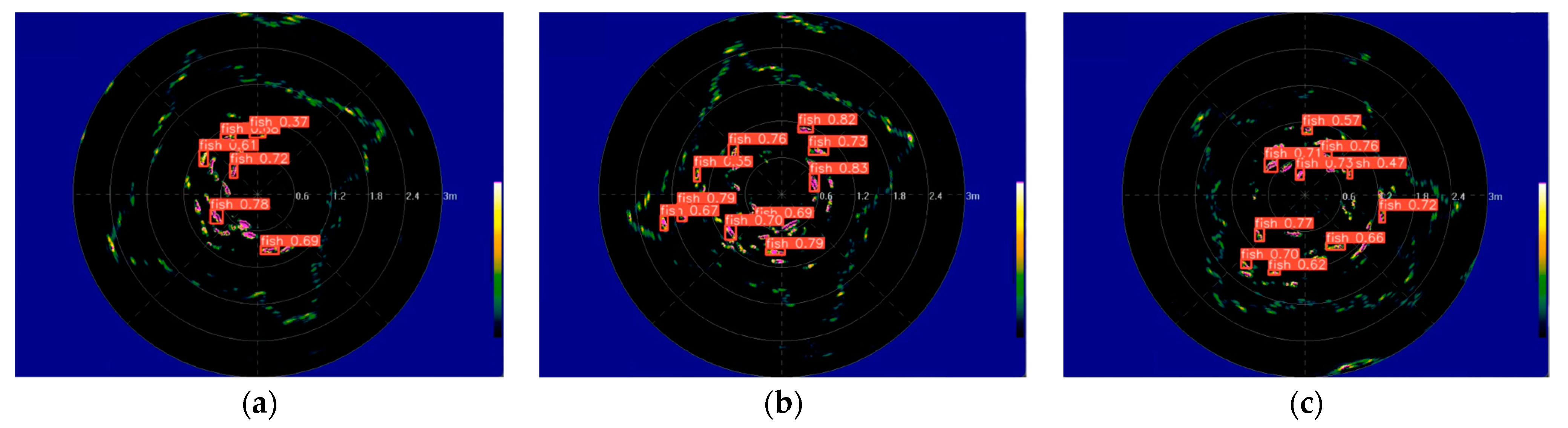
2.3. Object Identification Module
2.3.1. Identification Model CS-YOLOv8s
2.3.2. Dataset Creation
2.3.3. Evaluation Indexes
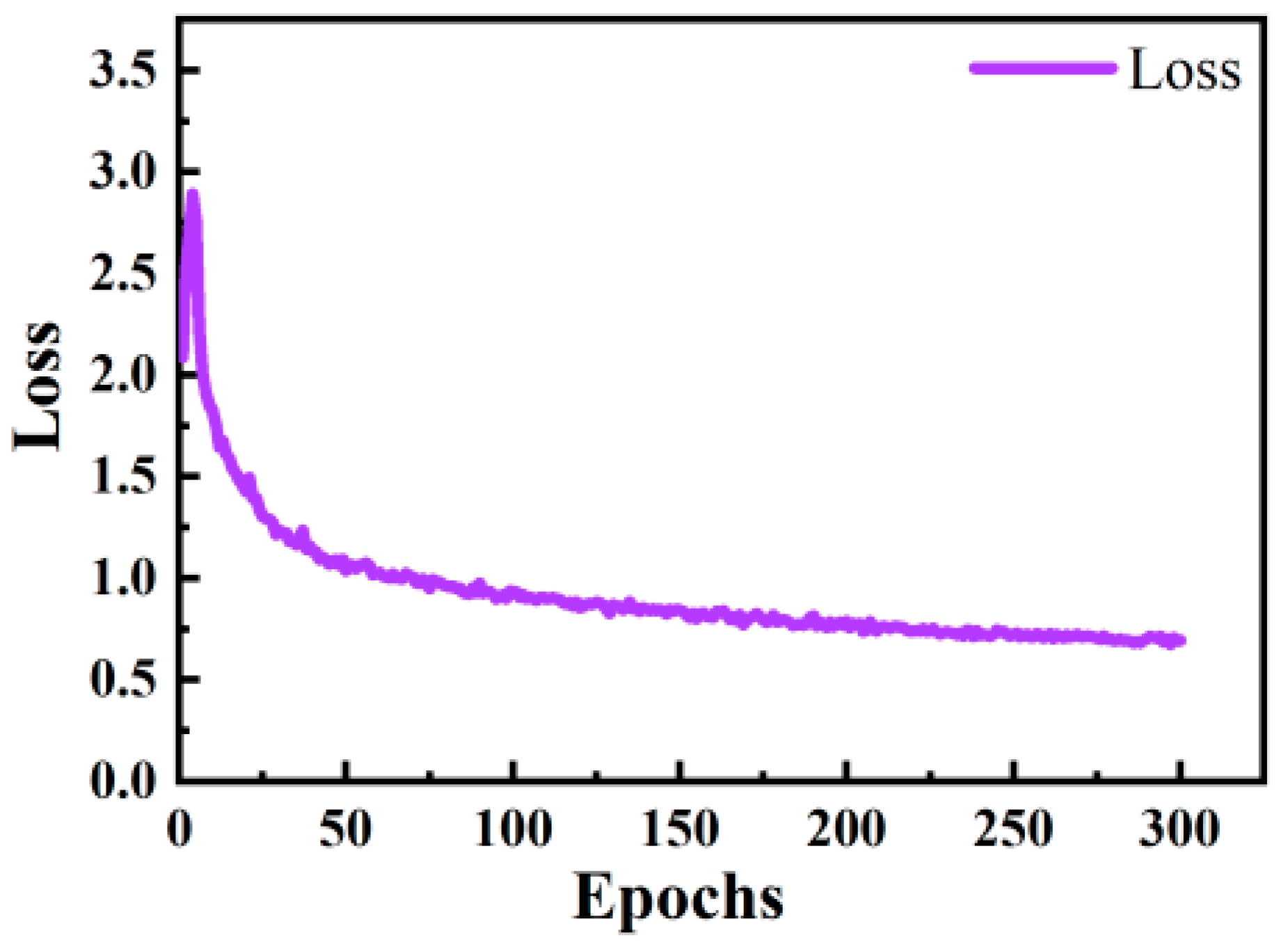
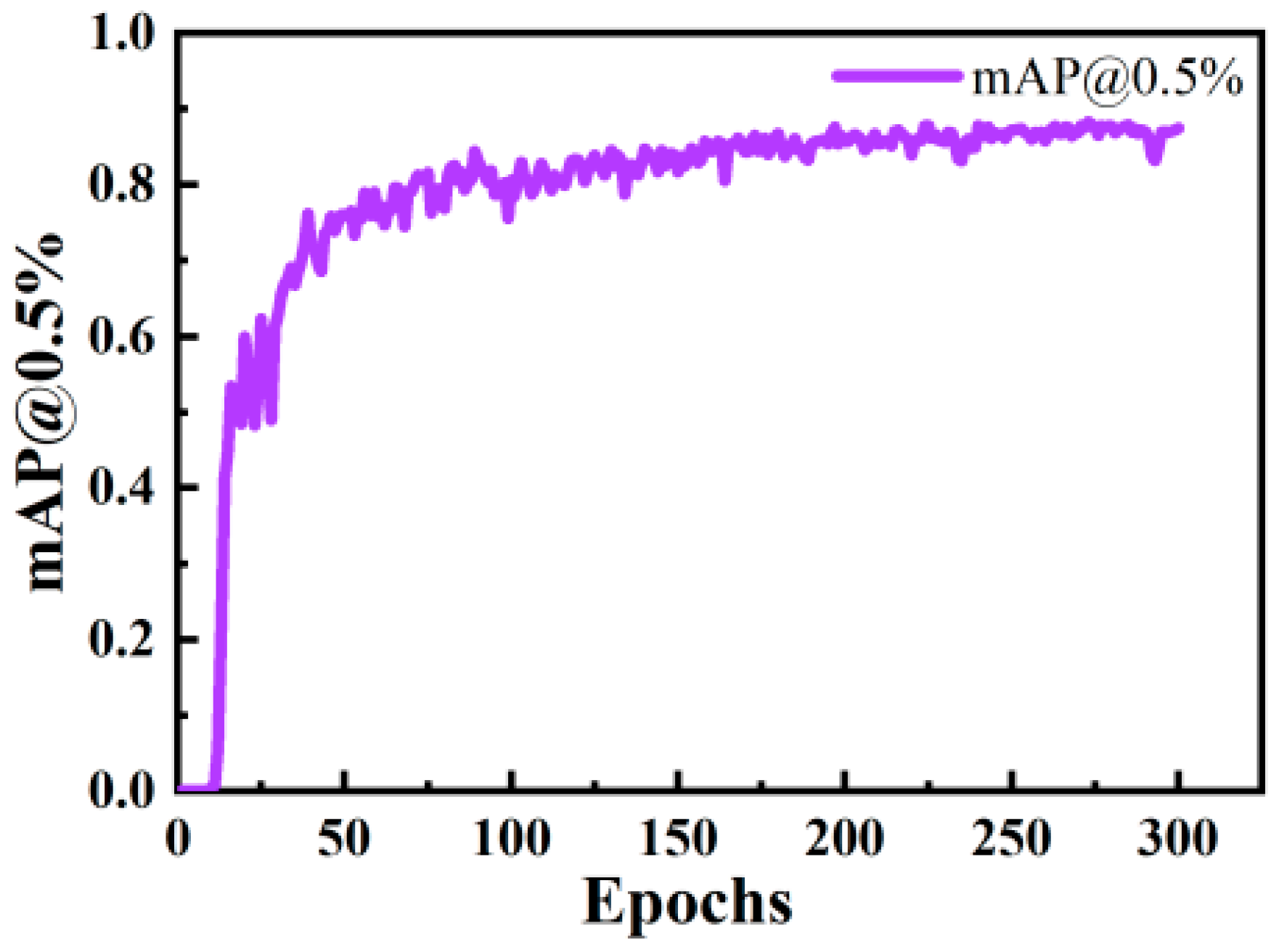
2.3.4. Training Process
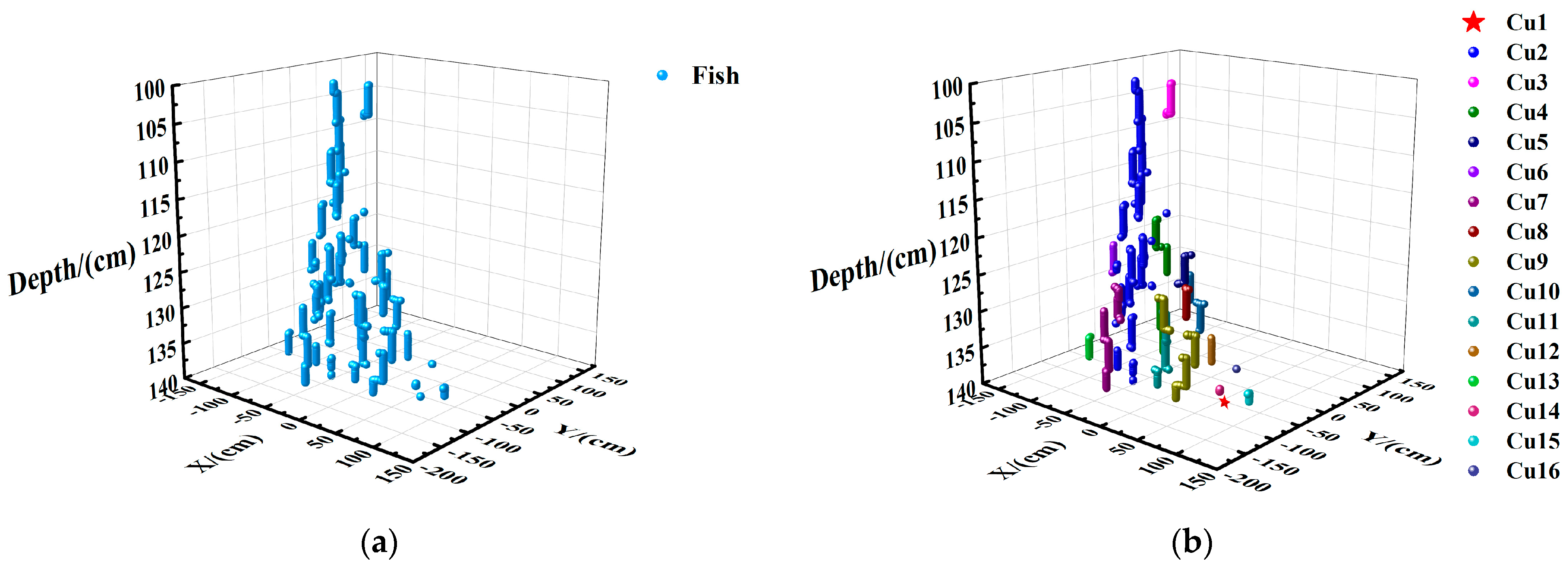
2.4. DBSCAN Clustering Module
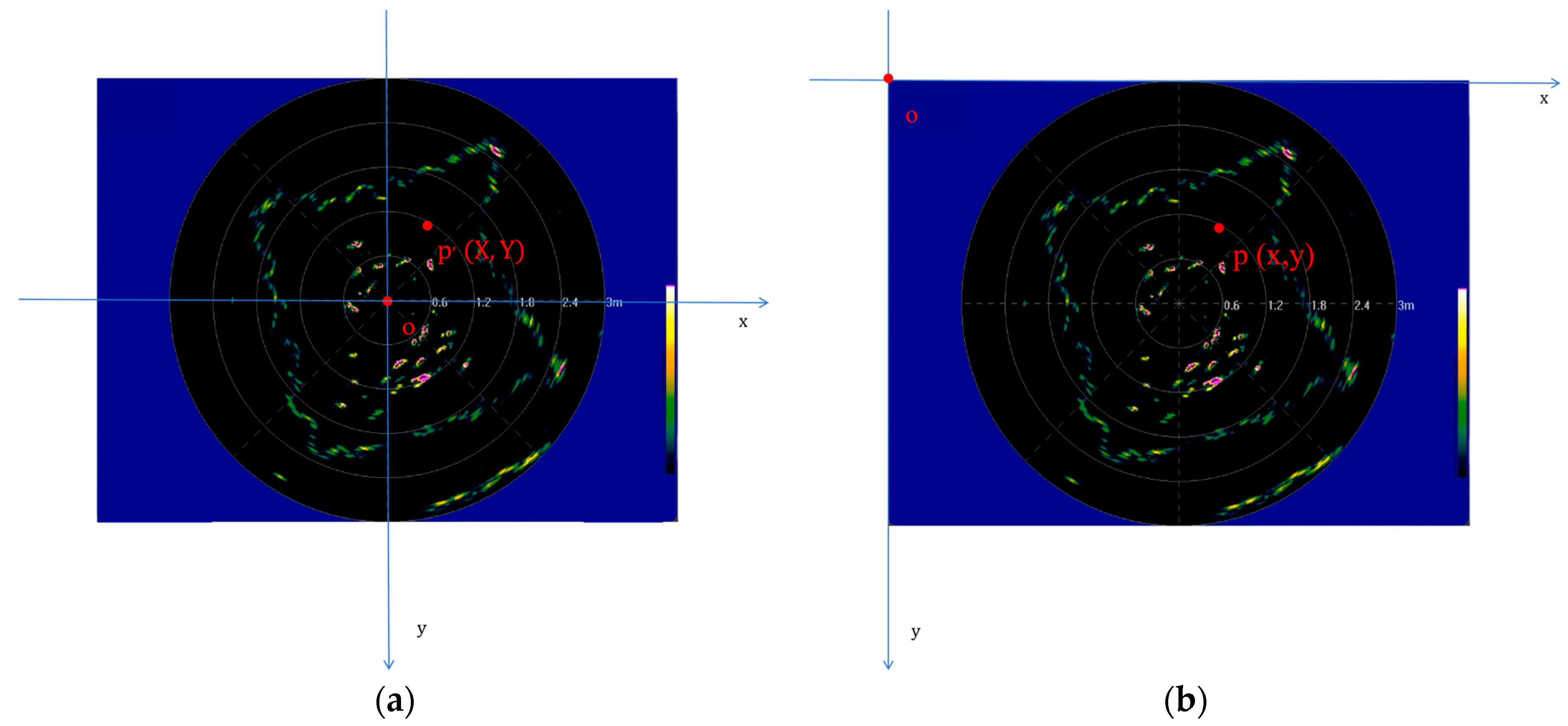
2.4.1. Pre-Processing of Clustering Data
2.4.2. DBSCAN Clustering
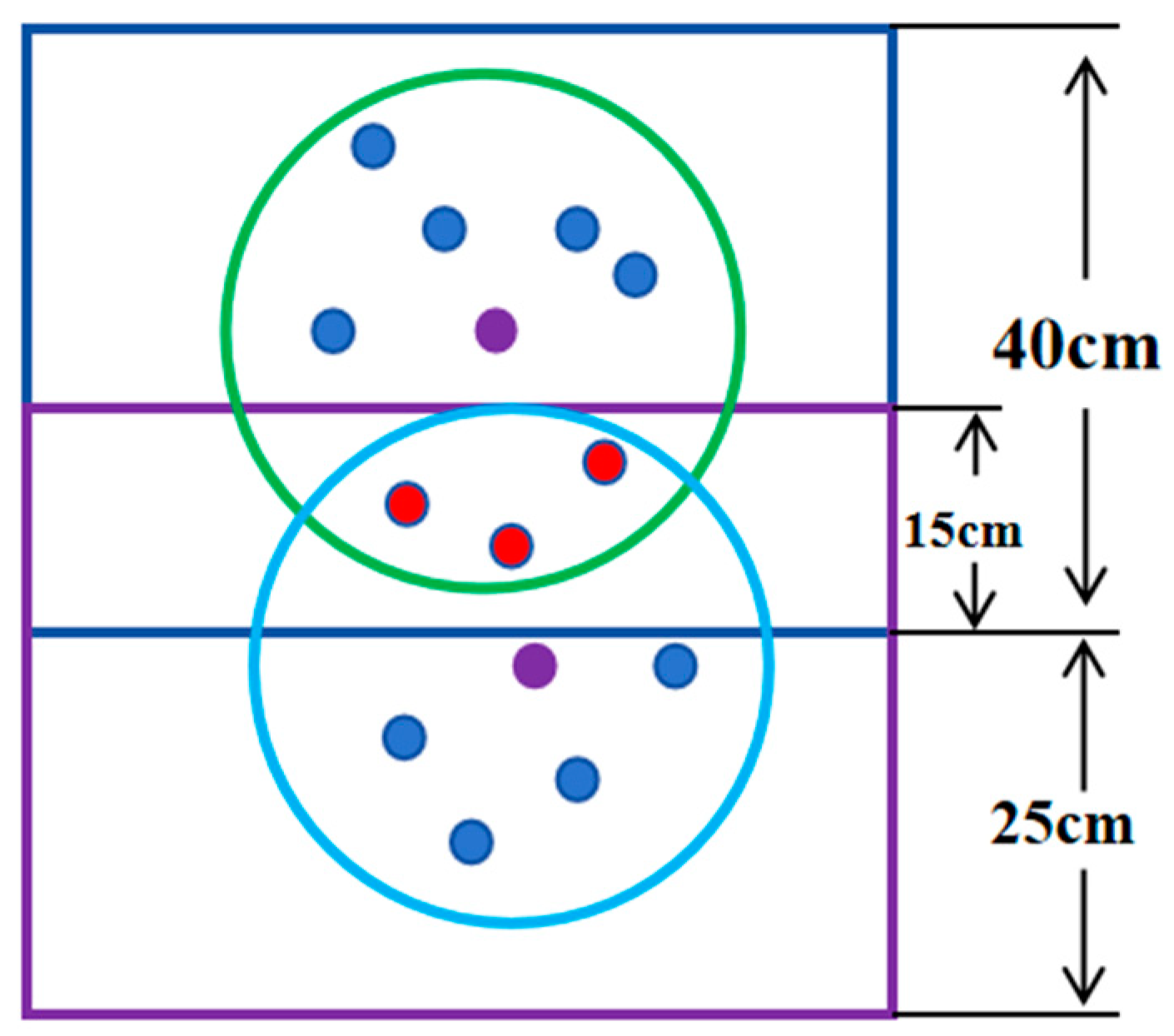
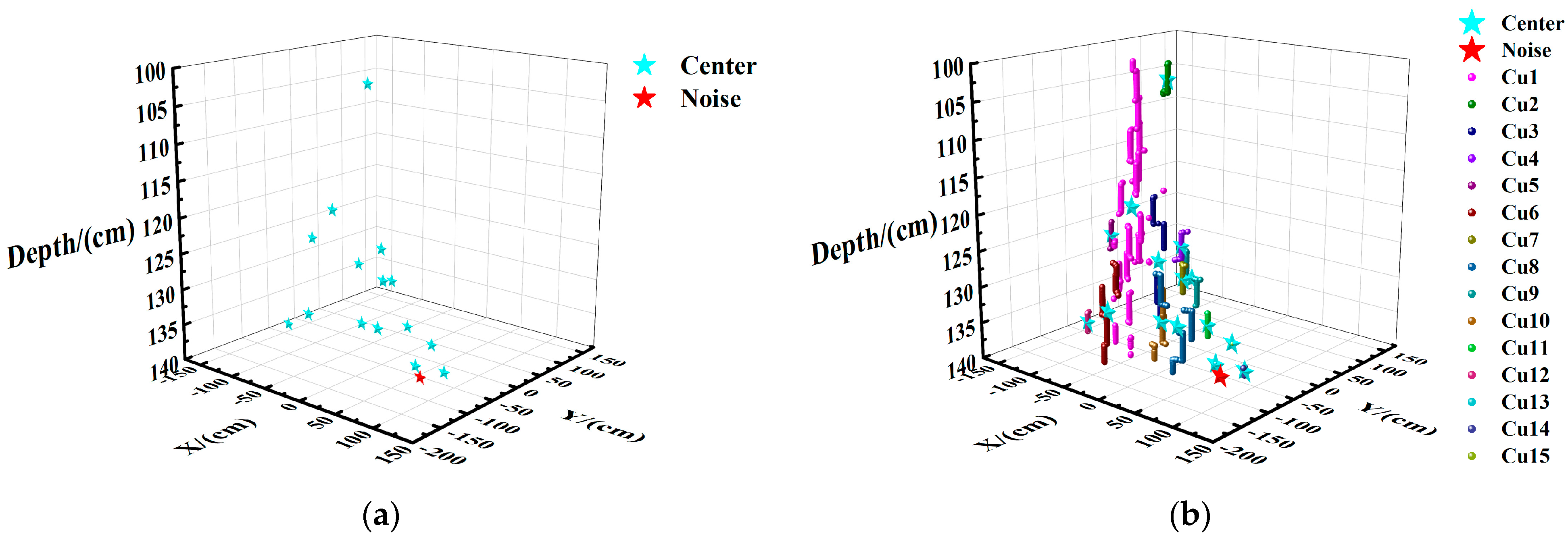
2.4.3. Reduction and Display of Noise Point
2.4.4. Reduction and Experiment Display of Center
2.4.5. Data Statistics of Water Layer
3. Results
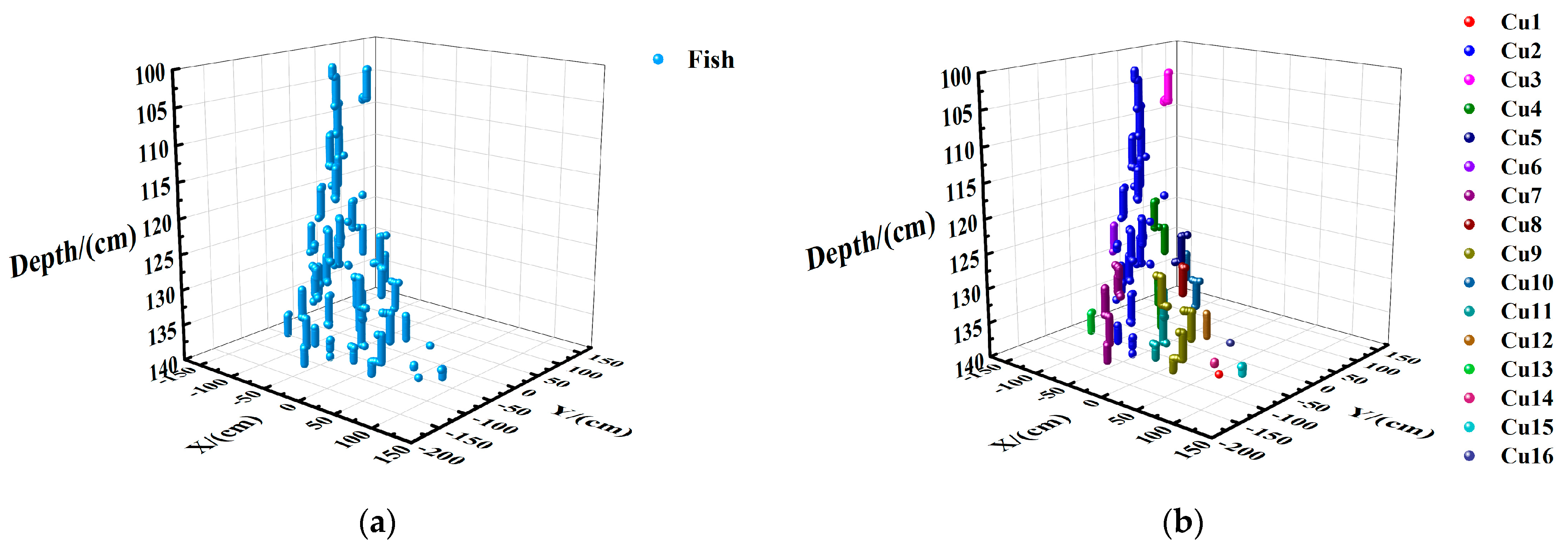
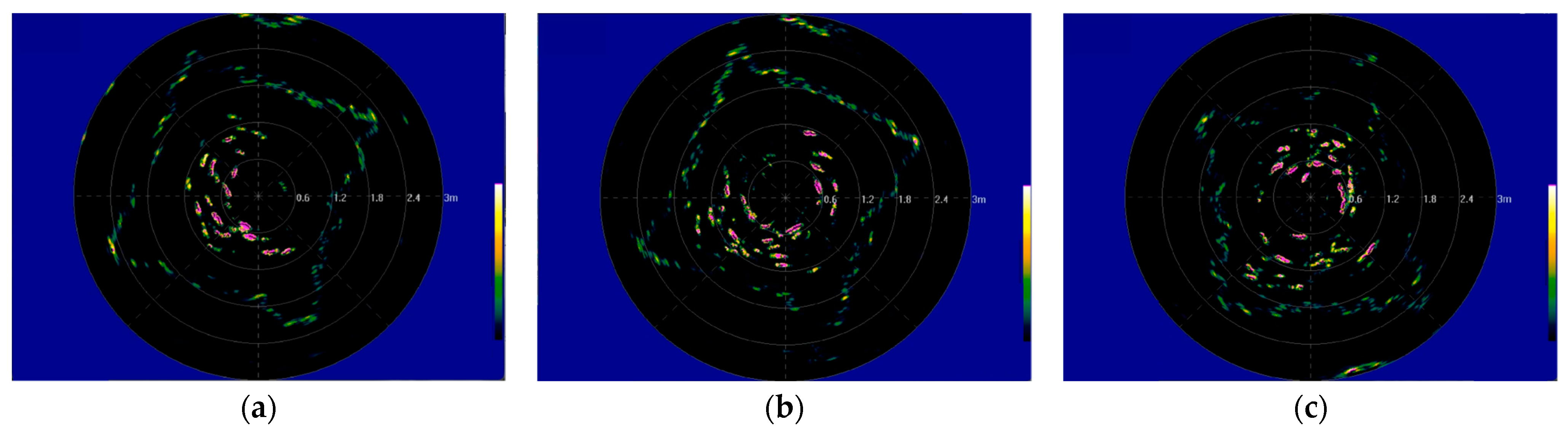
3.1. Result of Cage Data Acquisition
3.2. Data Processing
3.3. Result Analysis
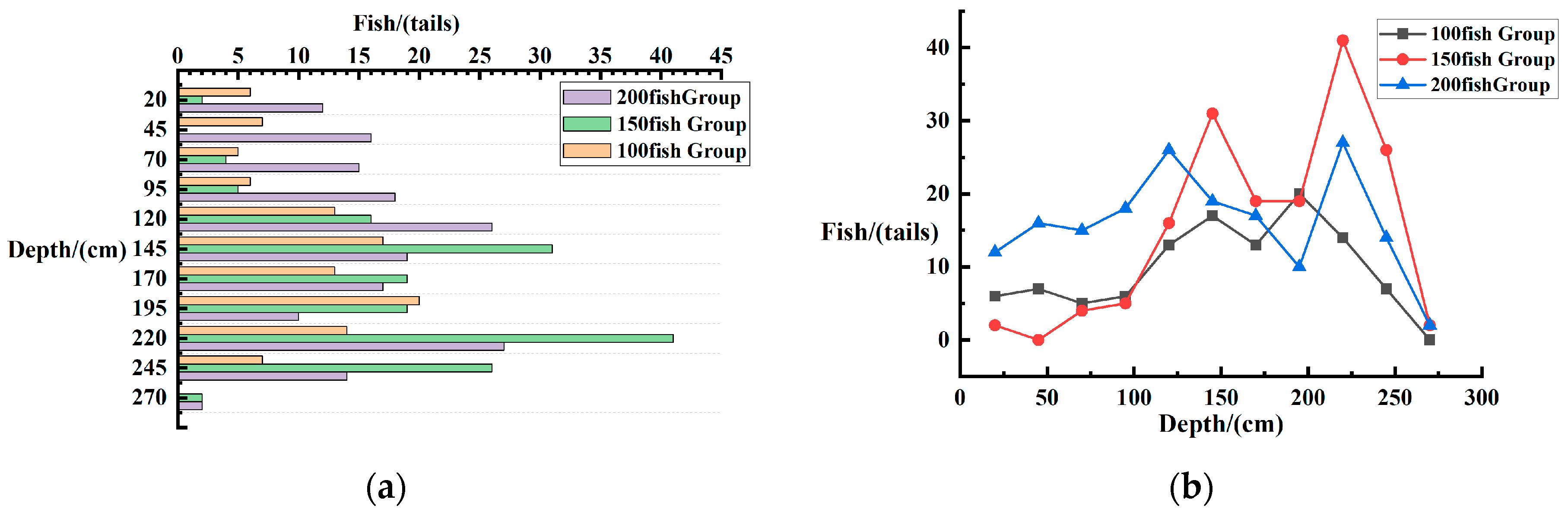
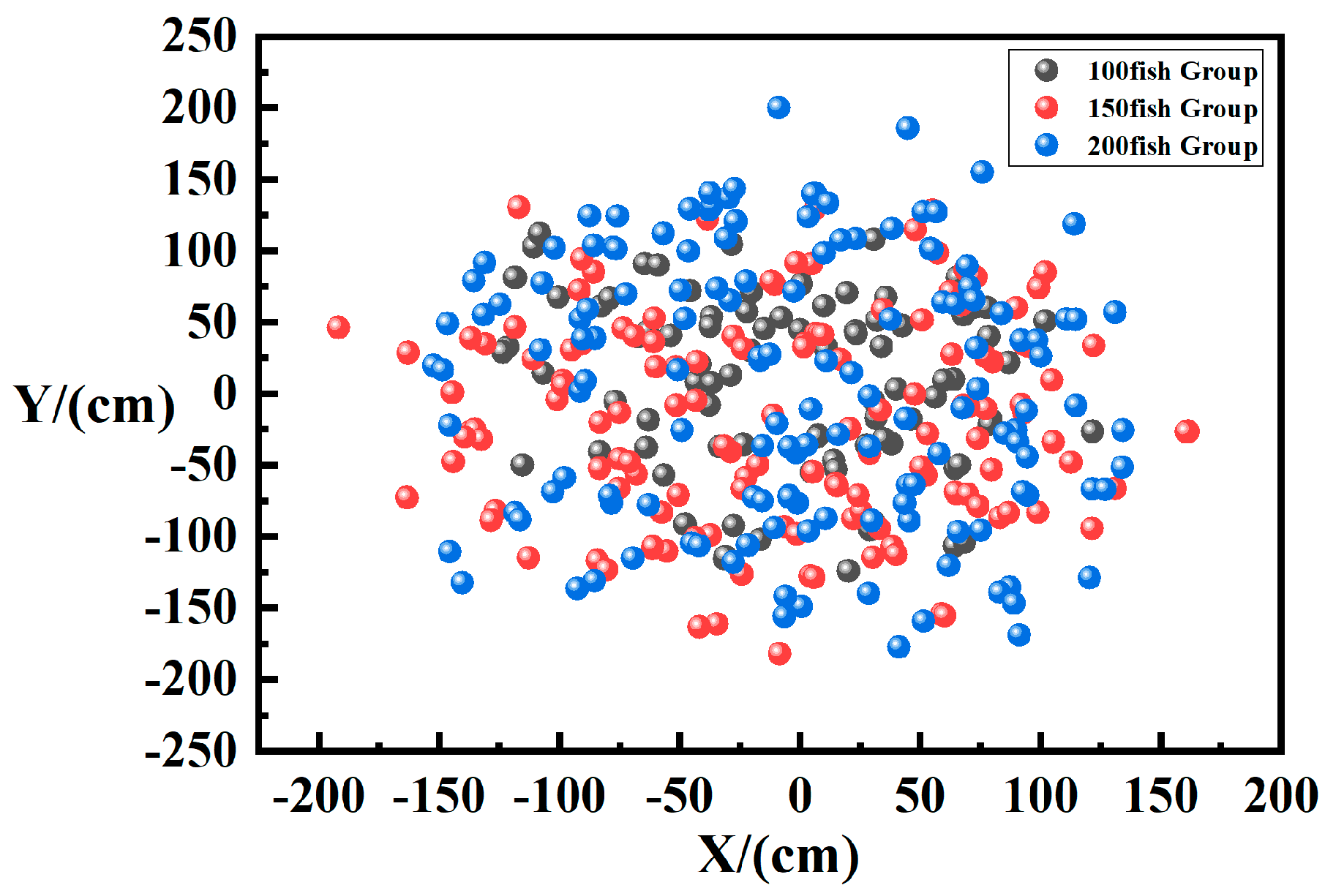
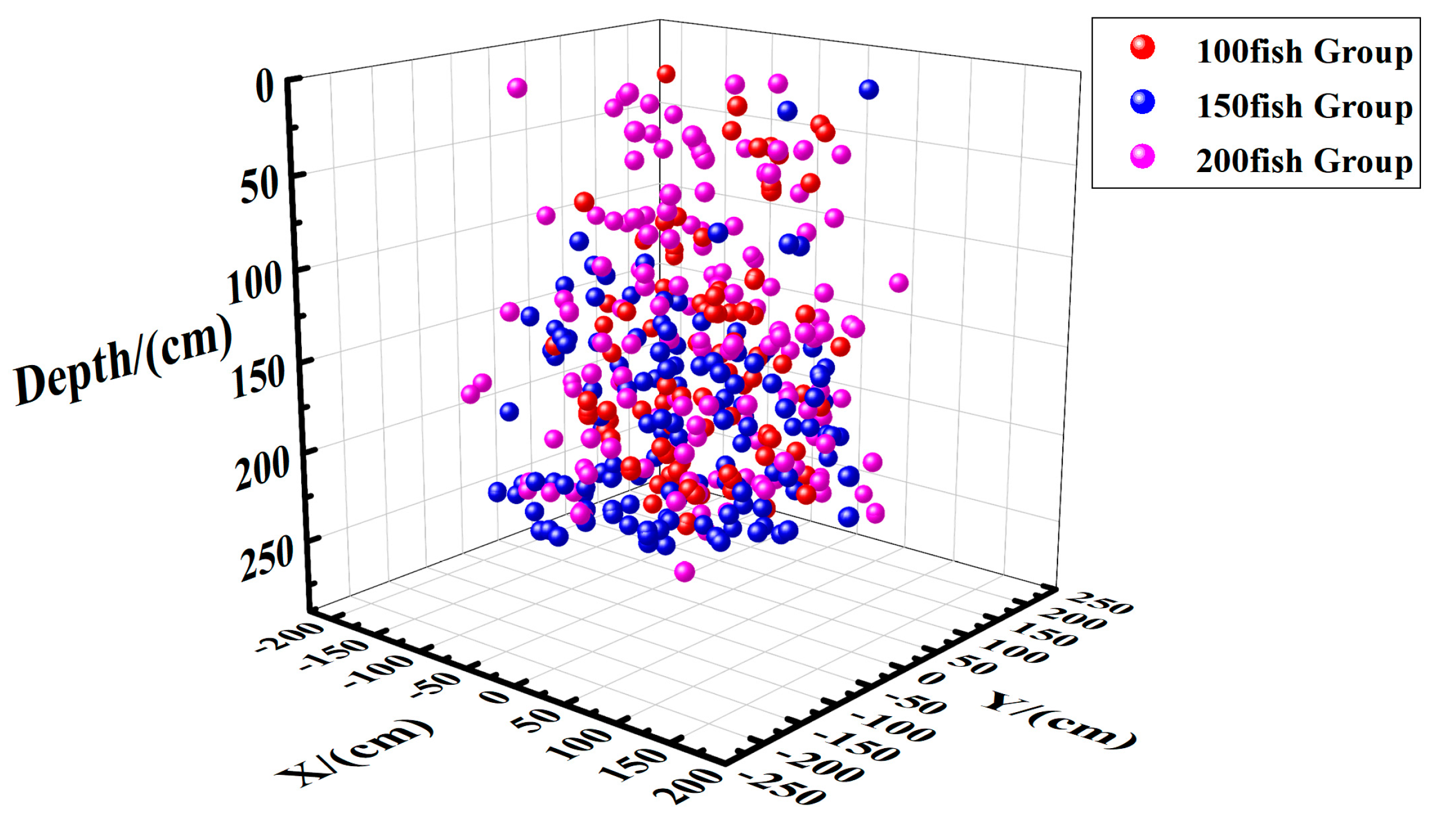
3.3.1. Effects of Quantity on Fish Distribution
Horizontal Spatial Distribution of Fish
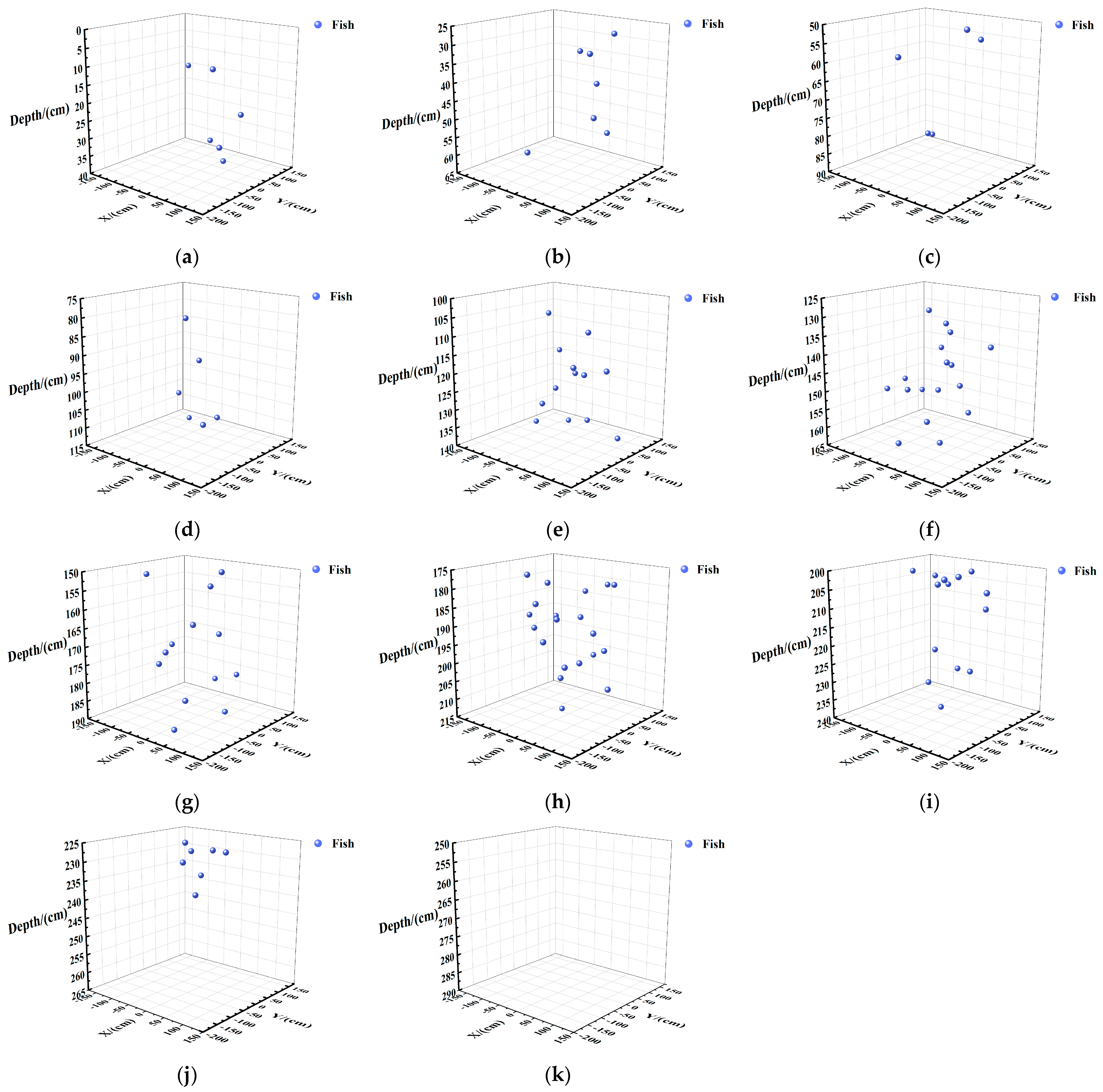
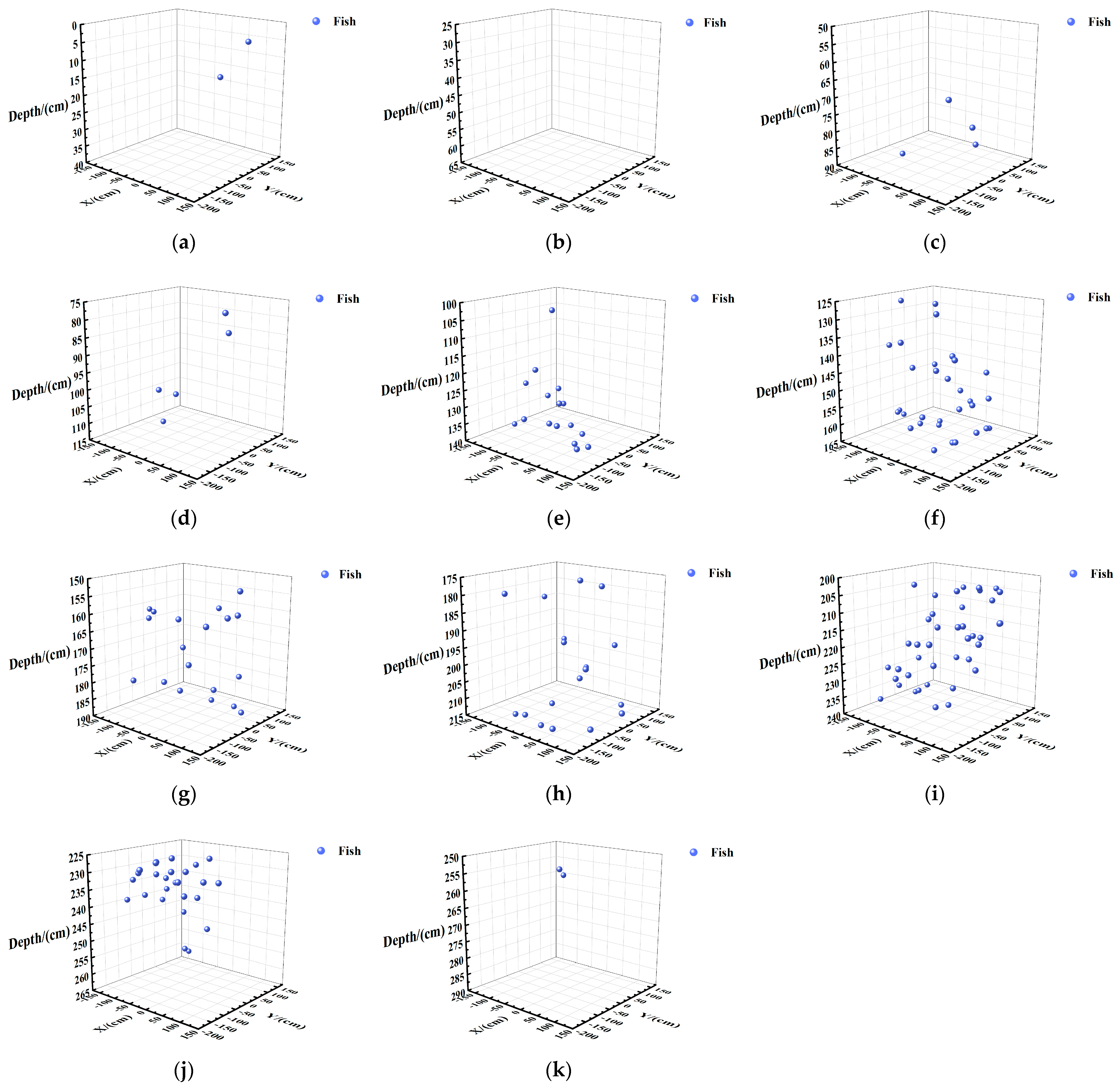
Vertical Spatial Distribution of Fish in Each Layer
Vertical Spatial Distribution of Fish
3.3.2. Effects of Sea Water Temperature on Fish Distribution
4. Discussion
5. Conclusions
- (1)
- The fish sonar image object detection model was enhanced and trained by incorporating an attention mechanism into the YOLOv8s backbone network. This modification aimed to improve the model’s capability to detect small objects, making it more suitable for detecting fish in sonar images. Subsequently, ablation studies and model comparison experiments were conducted. The results demonstrated that the improved CS-YOLOv8s network achieved a mAP@0.5 score that was 1.4% higher than that of the original YOLOv8s algorithm, as illustrated by the experimental results.
- (2)
- The quantity of fish was estimated layer by layer using the DBSCAN clustering algorithm, which provided the estimated positional coordinates of fish within each layer. This approach effectively addressed the issue of significant statistical errors associated with existing methods for tracking fish swimming, resulting in more accurate estimations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Z.X.; Hong, G.Q.; Li, Q.B.; Liu, S.H.; Wang, S.; Ma, Y. Seasonal dynamics of coastal pollution migration in open waters with intensive marine ranching. Mar. Environ. Res. 2023, 190, 106101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Gui, F.; Zhou, Q.; Cai, H.W.; Xu, K.D.; Zhao, S. Carbon Footprint of a Large Yellow Croaker Mariculture Models Based on Life-Cycle Assessment. Sustainability 2023, 15, 6658. [Google Scholar] [CrossRef]
- Jeeva, J.C.; Ghosh, S.; Raju, S.S.; Megarajan, S.; Vipinkumar, V.P.; Edward, L.; Narayanakumar, R. Success of cage farming of marine finfishes in doubling farmers’ income: A techno-social impact analysis. Curr. Sci 2022, 123, 1031–1037. [Google Scholar] [CrossRef]
- Banno, K.; Gao, S.; Anichini, M.; Stolz, C.; Tuene, S.A.; Gansel, L.C. Expanded vision for the spatial distribution of Atlantic salmon in sea cages. Aquaculture 2024, 588, 740879. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Zrini, Z.A.; Sandrelli, R.M. Atlantic salmon (Salmo salar) cage-site distribution, behavior, and physiology during a Newfoundland heat wave. Front. Physiol. 2021, 12, 719594. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, Á.; Patursson, Ø.; Kristmundsson, J.; Dam, S.P.; Muleid, M.; Klebert, P. Waves and currents decrease the available space in a salmon cage. PLoS ONE 2022, 17, e0263850. [Google Scholar] [CrossRef] [PubMed]
- Ndashe, K.; Hang’ombe, B.M.; Changula, K.; Yabe, J.; Samutela, M.T.; Songe, M.M.; Kefi, A.S.; Chilufya, L.N.; Sukkel, M. An Assessment of the risk factors associated with disease outbreaks across tilapia farms in Central and Southern Zambia. Fishes 2023, 8, 49. [Google Scholar] [CrossRef]
- Muñoz, L.; Aspillaga, E.; Palmer, M.; Saraiva, J.L.; Arechavala-Lopez, P. Acoustic telemetry: A tool to monitor fish swimming behavior in sea-cage aquaculture. Front. Mar. Sci. 2020, 7, 645. [Google Scholar] [CrossRef]
- Proud, R.; Kloser, R.J.; Handegard, N.O.; Cox, M.J.; Brierley, A.S. Mapping the global prey-field: Combining acoustics, optics and net samples to reduce uncertainty in estimates of mesopelagic biomass. J. Acoust. Soc. Am. 2019, 146, 2898. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, Y.H.; Ji, Y.Q.; Feng, A.W.; Zhao, S.J.; Wang, C.H. Compact multi-spectral-resolution Wynne-Offner imaging spectrometer with a long slit. Appl. Opt. 2024, 63, 1577–1582. [Google Scholar] [CrossRef]
- Duan, Z.; Yuan, Y.; Lu, J.C.; Wang, J.L.; Li, Y.; Svanberg, S.; Zhao, G.Y. Underwater spatially, spectrally, and temporally resolved optical monitoring of aquatic fauna. Opt. Express 2020, 28, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Pagniello, C.M.L.S.; Butler, J.; Rosen, A.; Sherwood, A.; Roberts, P.L.D.; Parnell, P.E.; Jaffe, J.S.; Širović, A. An Optical Imaging System for Capturing Images in Low-Light Aquatic Habitats Using Only Ambient Light. Oceanography 2021, 34, 71–77. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.Y.; Shen, T.; Yu, H.H.; Li, D.L. A BlendMask-VoVNetV2 method for quantifying fish school feeding behavior in industrial aquaculture. Comput. Electron. Agric. 2023, 211, 108005. [Google Scholar] [CrossRef]
- Helminen, J.; Dauphin, G.J.; Linnansaari, T. Length measurement accuracy of adaptive resolution imaging sonar and a predictive model to assess adult Atlantic salmon (Salmo salar) into two size categories with long-range data in a river. J. Fish Biol. 2020, 97, 1009–1026. [Google Scholar] [CrossRef]
- Le, Z.Y. Exploration and suggestion on effective protection of offshore fishery resources based on acoustic technology. ChinaAquatic Prod. 2022, 5, 58–60. [Google Scholar]
- Shen, W.; Peng, Z.F.; Zhang, J. Identification and counting of fish targets using adaptive resolution imaging sonar. J. Fish Biol. 2024, 104, 422–432. [Google Scholar] [CrossRef]
- Helminen, J.; O’Sullivan, A.M.; Linnansaari, T. Measuring tailbeat frequencies of three fish species from adaptive resolution imaging sonar data. Trans. Am. Fish. Soc. 2021, 150, 627–636. [Google Scholar] [CrossRef]
- Wei, Y.G.; Duan, Y.H.; An, D. Monitoring fish using imaging sonar: Capacity, challenges and future perspective. Fish Fish. 2022, 23, 1347–1370. [Google Scholar] [CrossRef]
- Hobbs, D.; Bigot, M.; Smith, R.E.W. Rio doce acoustic surveys of fish biomass and aquatic habitat. Integr. Environ. Assess. Manag. 2020, 16, 615–621. [Google Scholar] [CrossRef]
- Feng, Y.H.; Wei, Y.G.; Sun, S.; Liu, C.; An, D.; Wang, J. Fish abundance estimation from multi-beam sonar by improved MCNN. Aquat. Ecol. 2023, 57, 895–911. [Google Scholar] [CrossRef]
- Stacy-Duffy, W.L.; Redman, R.A.; Roswell, C.R.; Peterson, S.D.; Czesny, S.J. Evaluation of fish spawning habitat at offshore reefs in southwest Lake Michigan using side-scan sonar and underwater video. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4092. [Google Scholar] [CrossRef]
- Jing, D.X.; Zhou, H.Y.; Han, J.; Zhang, J. Fish abundance estimation based on an imaging sonar. Appl. Acoust. 2019, 38, 705–711. [Google Scholar] [CrossRef]
- Chevallay, M.; Du Dot, T.J.; Goulet, P.; Fonvieille, N.; Craig, C.; Picard, B.; Guinet, C. Spies of the deep: An animal-borne active sonar and bioluminescence tag to characterise mesopelagic prey size and behaviour in distinct oceanographic domains. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2024, 203, 104214. [Google Scholar] [CrossRef]
- Cui, Z.; Zhu, H.H.; Chen, C.; Liu, W.; Wang, Q.; Chai, Z.G. Study on Fish Resource Assessment Method Based on Imaging Sonar. In Proceedings of the 2022 3rd International Conference on Geology, Map** and Remote Sensing (ICGMRS), Zhoushan, China, 22–24 April 2022; IEEE: New York, NY, USA, 2022; pp. 327–331. [Google Scholar] [CrossRef]
- Talaat, F.M.; Hanaa, Z.E. An improved fire detection approach based on YOLO-v8 for smart cities. Neural Comput. Appl. 2023, 35, 20939–20954. [Google Scholar] [CrossRef]
- Li, Y.H.; Yao, T.; Pan, Y.W.; Mei, T. Contextual Transformer Networks for Visual Recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiao, H.W.; Zhang, C.; Li, M.Y.; Zhao, B.; Liu, G.W.; Ji, J.T. Hydroponic lettuce defective leaves identification based on improved YOLOv5s. Front. Plant Sci. 2023, 14, 1242337. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhu, Z.Y.; Zhou, M.; Li, D.; Zhang, J.T.; Qin, M.Y.; Liu, B.L. Extraction of Tooth Cusps based on DBSCAN Density Clustering and Neighborhood Search Algorithm. Crit. Rev. Biomed. Eng. 2024, 52, 27–37. [Google Scholar] [CrossRef]
- Cong, T.T.; Tong, C.F.; Zhao, C.J.; Chen, Z.T.; He, Z.F.; Liu, M.Y. Community composition and distribution characteristics of the fish assemblages in the rivers of Chongming Island in summer. Acta Ecol. Sin. 2021, 41, 2067–2076. [Google Scholar] [CrossRef]
- Mennad, M.; Bachari, N.E.I.; Ferhani, K.; Chabet Dis, C.; Bennoui, A.; Neghli, L.; Ben Smail, S. Spatiotemporal Distribution of Small Pelagic Fish Schools in the Western Algerian Coast. Thalass. Int. J. Mar. Sci. 2023, 39, 923–930. [Google Scholar] [CrossRef]
- Wu, Y.D.; Yu, X.K.; Suo, N.; Bai, H.Q.; Ke, Q.Z.; Chen, J.; Pan, Y.; Zheng, W.Q.; Xu, P. Thermal tolerance, safety margins and acclimation capacity assessments reveal the climate vulnerability of large yellow croaker aquaculture. Aquaculture 2022, 561, 738665. [Google Scholar] [CrossRef]
- Rioja, R.A.; Palomar-Abesamis, N.; Juinio-Meñez, M.A. Correction to: Associated effects of shading on the behavior, growth, and survival of Stichopus cf. horrens juveniles. Aquac. Int. 2021, 30, 1087–1088. [Google Scholar] [CrossRef]
- Li, Y.; Gao, C.X.; Chen, J.H.; Wang, Q.; Zhao, J. Spatial–temporal distribution characteristics of Harpadon nehereus in the Yangtze River Estuary and its relationship with environmental factors. Front. Mar. Sci. 2024, 11, 1340522. [Google Scholar] [CrossRef]
- Kurbanov, Y.K.; Vinogradskaya, A.V. Distribution, Ecology and Size Composition of the White-Blotched Skate Bathyraja maculata (Arhynchobatidae) in the Northeastern Sea of Okhotsk during the Hydrological Summer. J. Ichthyol. 2023, 63, 1092–1101. [Google Scholar] [CrossRef]
- Li, R.; Amenyogbe, E.; Lu, Y.; Jin, J.; Xie, R.; Huang, J. Effects of low-temperature stress on intestinal structure, enzyme activities and metabolomic analysis of juvenile golden pompano (Trachinotus ovatus). Front. Mar. Sci. 2023, 10, 1114120. [Google Scholar] [CrossRef]
- Tamim, A.T.; Begum, H.; Shachcho, S.A.; Khan, M.M.; Yeboah-Akowuah, B.; Masud, M.; AI-Amri, J.F. Development of IoT Based Fish Monitoring System for Aquaculture. Intell. Autom. Soft Comput. 2022, 32, 56–71. [Google Scholar] [CrossRef]
- Tseng, C.H.; Kuo, Y.F. Detecting and counting harvested fish and identifying fish types in electronic monitoring system videos using deep convolutional neural networks. ICES J. Mar. Sci. 2020, 77, 1367–1378. [Google Scholar] [CrossRef]
- Xing, B.W.; Sun, M.; Liu, Z.C.; Guan, L.W.; Han, J.T.; Yan, C.X.; Han, C. Sonar Fish School Detection and Counting Method Based on Improved YOLOv8 and BoT-SORT. J. Mar. Sci. Eng. 2024, 12, 964. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Hu, G.K. Utilization of species checklist data in revealing the spatial distribution of fish diversity. J. Fish Biol. 2020, 97, 817–826. [Google Scholar] [CrossRef]









| Item | Technical Index |
|---|---|
| Operating frequency | 667 kHz |
| Range of action | 3 m |
| Beam opening angle(Vertical × Horizontal) | |
| Distance pixel/precision | 5 cm |
| Scanning area (polar coordinates) | 360° |
| Maximum working depth | 3 m |
| Pan tilt rotation frequency | 2.5 s/circle |
| Cylinder size | 57 mm × 99 mm |
| Cluster Center | X/cm | Y/cm | Depth/cm |
|---|---|---|---|
| 1 | −35.4 | 2.0 | 22.8 |
| 2 | 16.2 | 16.3 | 8.8 |
| 3 | −133.6 | 72.8 | 17.1 |
| 4 | −157.0 | 42.1 | 17.2 |
| 5 | −68.4 | 132.8 | 19.6 |
| 6 | −107.9 | 106.8 | 22.9 |
| 7 | −106.3 | −2.6 | 18.8 |
| 8 | −12.6 | 42.6 | 33.0 |
| 9 | −38.8 | 99.6 | 34.0 |
| 10 | −151.1 | 99.0 | 29.5 |
| 11 | −86.9 | 84.3 | 29.5 |
| 12 | 37.1 | −21.9 | 35.1 |
| 13 | −69.4 | 104.5 | 36.3 |
| 14 | −28.4 | −113.4 | 38.8 |
| Water Layer/cm | Total Number of Coordinate Points/Piece | Number of Clusters (Including Noise Clusters)/Piece | Number of Noise Points/Piece | Number of Fish in This Layer/Piece |
|---|---|---|---|---|
| 0~40 | 439 | 2 | 0 | 2 |
| 25~65 | 0 | 0 | 0 | 0 |
| 50~90 | 792 | 4 | 0 | 4 |
| 75~115 | 1794 | 5 | 0 | 5 |
| 100~140 | 5384 | 16 | 1 | 16 |
| 125~165 | 11,242 | 31 | 1 | 31 |
| 150~190 | 15,273 | 19 | 1 | 19 |
| 175~215 | 15,431 | 18 | 2 | 19 |
| 200~240 | 11,861 | 40 | 2 | 41 |
| 225~265 | 4432 | 26 | 0 | 26 |
| 250~290 | 253 | 2 | 0 | 2 |
| Total | 66,901 | 162 | 7 | 164 |
| Noise | X/cm | Y/cm | Depth/cm |
|---|---|---|---|
| 1 | 16.4 | 40.4 | 11.9 |
| Data Number | 1 | 3 | 5 | 7 | 9 | 11 | 13 | 15 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Water Layer/cm | ||||||||||
| 0~40 | 3 | 4 | 6 | 3 | 5 | 5 | 5 | 12 | 5 | |
| 25~65 | 6 | 8 | 7 | 5 | 4 | 7 | 3 | 16 | 10 | |
| 50~90 | 2 | 13 | 9 | 7 | 13 | 8 | 9 | 15 | 19 | |
| 75~115 | 5 | 16 | 12 | 11 | 18 | 13 | 14 | 18 | 25 | |
| 100~140 | 12 | 14 | 11 | 12 | 22 | 20 | 31 | 26 | 30 | |
| 125~165 | 14 | 11 | 18 | 22 | 24 | 25 | 23 | 19 | 21 | |
| 150~190 | 16 | 10 | 13 | 22 | 21 | 28 | 14 | 17 | 22 | |
| 175~215 | 13 | 8 | 10 | 20 | 24 | 17 | 16 | 10 | 20 | |
| 200~240 | 10 | 3 | 4 | 23 | 20 | 10 | 35 | 27 | 26 | |
| 225~265 | 3 | 1 | 3 | 15 | 5 | 7 | 26 | 18 | 20 | |
| 250~290 | 2 | 0 | 0 | 4 | 2 | 3 | 7 | 2 | 7 | |
| Total | 86 | 88 | 93 | 144 | 158 | 143 | 183 | 180 | 205 | |
| Methods | Equipment Usage | Monitoring Content | Advantage | Disadvantage |
|---|---|---|---|---|
| IoT-based fish monitoring system | A pH sensor, a temperature sensor, and some other equipment | pH level, the water temperature, dissolved oxygen level, and ammonia level | Monitoring various indicators of water quality can provide a healthy breeding environment for fish. | Lack of estimation of the number and distribution of fish populations in aquaculture water bodies makes it impossible to comprehensively consider water issues. |
| Deep convolutional neural networks | Electronic monitoring systems (EMSs) | The number of the fish, the types and body lengths of the fish | Automated, high precision | High computational burden |
| Deep learning | Sonar (oculusM750d) | Fish counting and tracking | Real-time data | Data limitation |
| Species checklist data method | Bottom trawl nets | Differences in fish assemblages | Large scale, diverse species | High demand for data and difficulty in data analysis |
| Our method | omnidirectional scanning sonar | Distribution of fish | Convenient, low implementation difficulty | The estimated range is small, lack of applicability to large sample data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Hu, J.; Sun, P.; Zhu, G.; Sun, J.; Tao, Q.; Yuan, T.; Li, G.; Pang, G.; Huang, X. A Method for Estimating the Distribution of Trachinotus ovatus in Marine Cages Based on Omnidirectional Scanning Sonar. J. Mar. Sci. Eng. 2024, 12, 1571. https://doi.org/10.3390/jmse12091571
Hu Y, Hu J, Sun P, Zhu G, Sun J, Tao Q, Yuan T, Li G, Pang G, Huang X. A Method for Estimating the Distribution of Trachinotus ovatus in Marine Cages Based on Omnidirectional Scanning Sonar. Journal of Marine Science and Engineering. 2024; 12(9):1571. https://doi.org/10.3390/jmse12091571
Chicago/Turabian StyleHu, Yu, Jiazhen Hu, Pengqi Sun, Guohao Zhu, Jialong Sun, Qiyou Tao, Taiping Yuan, Gen Li, Guoliang Pang, and Xiaohua Huang. 2024. "A Method for Estimating the Distribution of Trachinotus ovatus in Marine Cages Based on Omnidirectional Scanning Sonar" Journal of Marine Science and Engineering 12, no. 9: 1571. https://doi.org/10.3390/jmse12091571
APA StyleHu, Y., Hu, J., Sun, P., Zhu, G., Sun, J., Tao, Q., Yuan, T., Li, G., Pang, G., & Huang, X. (2024). A Method for Estimating the Distribution of Trachinotus ovatus in Marine Cages Based on Omnidirectional Scanning Sonar. Journal of Marine Science and Engineering, 12(9), 1571. https://doi.org/10.3390/jmse12091571







