Responses of Different Morphological Cells of Phaeocystis globosa to UV-B Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Culture and Treatment with UV-B Radiation
2.2. Photosynthetic Activity
2.3. Damage Rate (k, min−1) and Repair Rate (r, min−1) of PSII
2.4. ROS Content and Antioxidant Enzyme Activity
2.5. Caspase-3-like Activity
2.6. PS Detected by Annexin V-FITC
2.7. TUNEL Detected by Fluorescence Microscopy
2.8. Statistical Analysis
3. Result
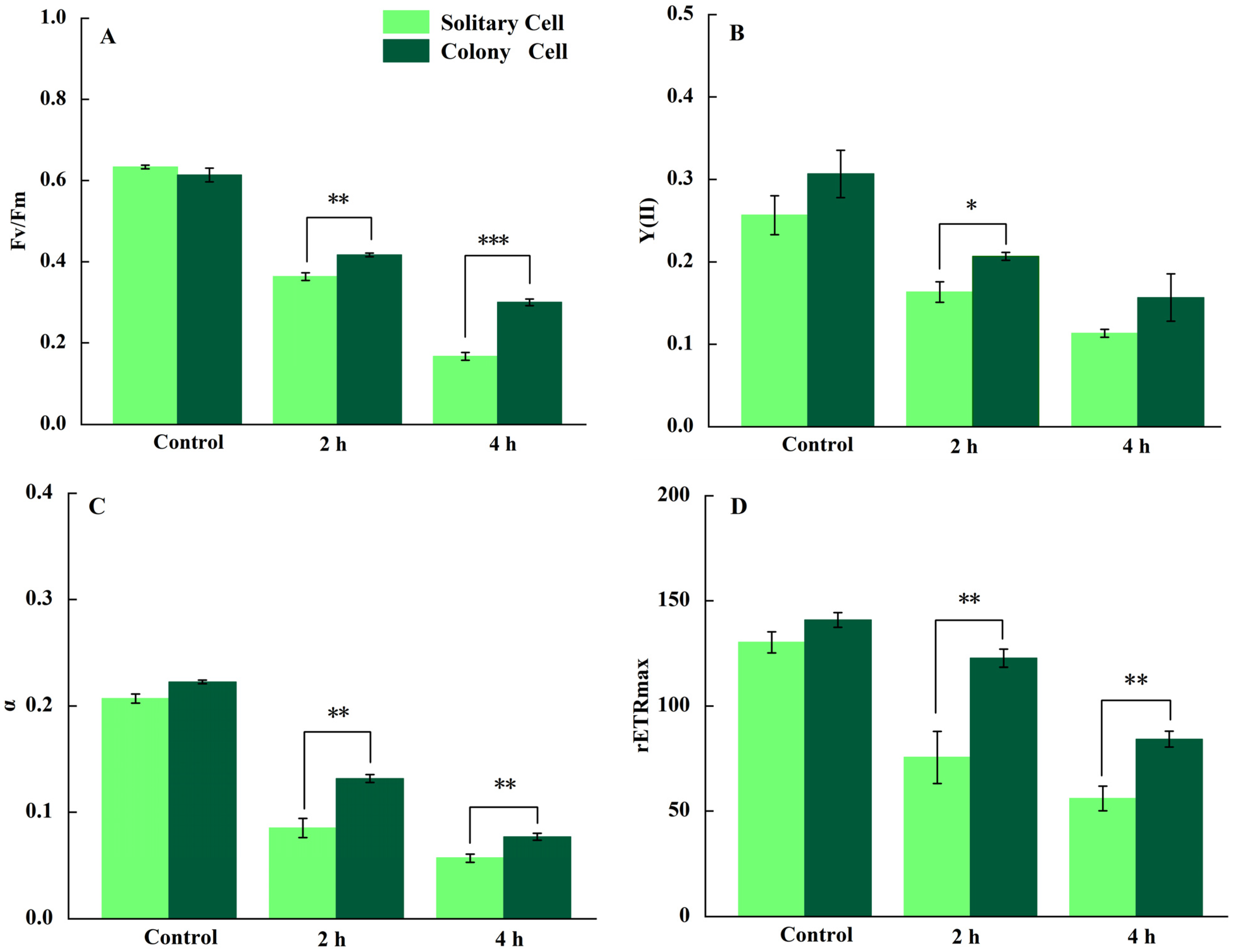
3.1. Photosynthetic Activity
3.2. Damage Rate and Repair Rate of PSII
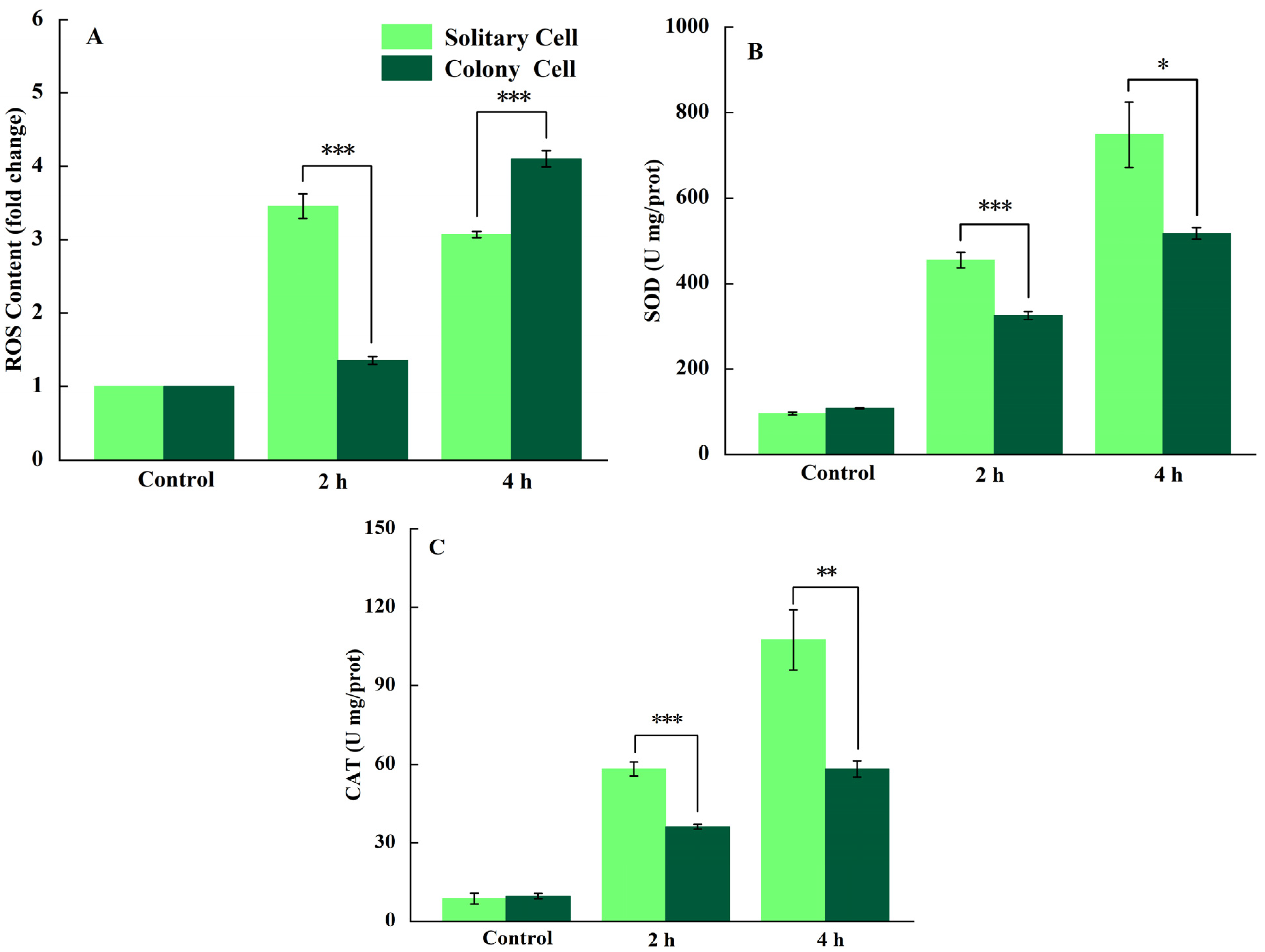
3.3. Determination of ROS and Antioxidant Enzyme Activities
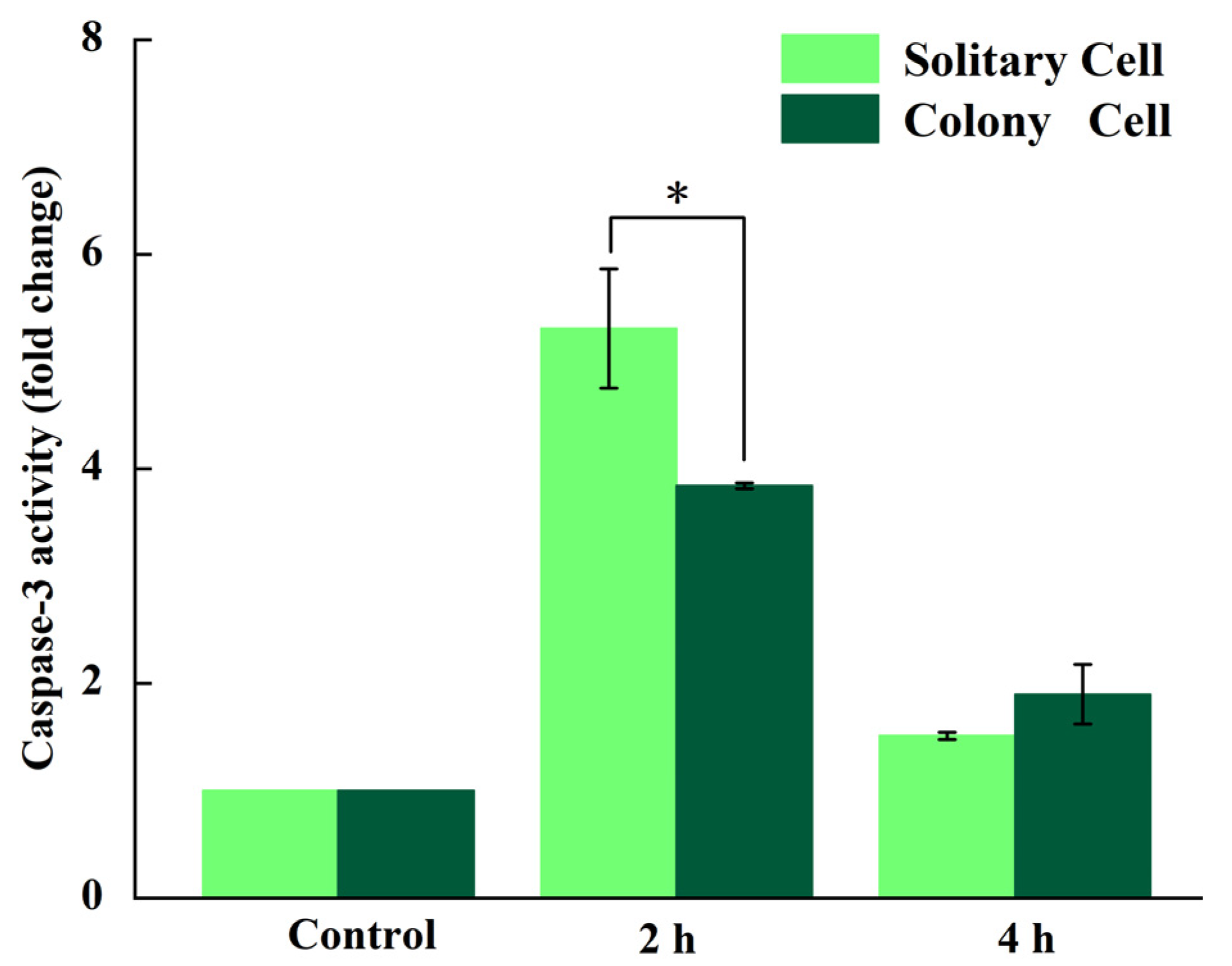
3.4. Measurement of Caspase-3-like Activity
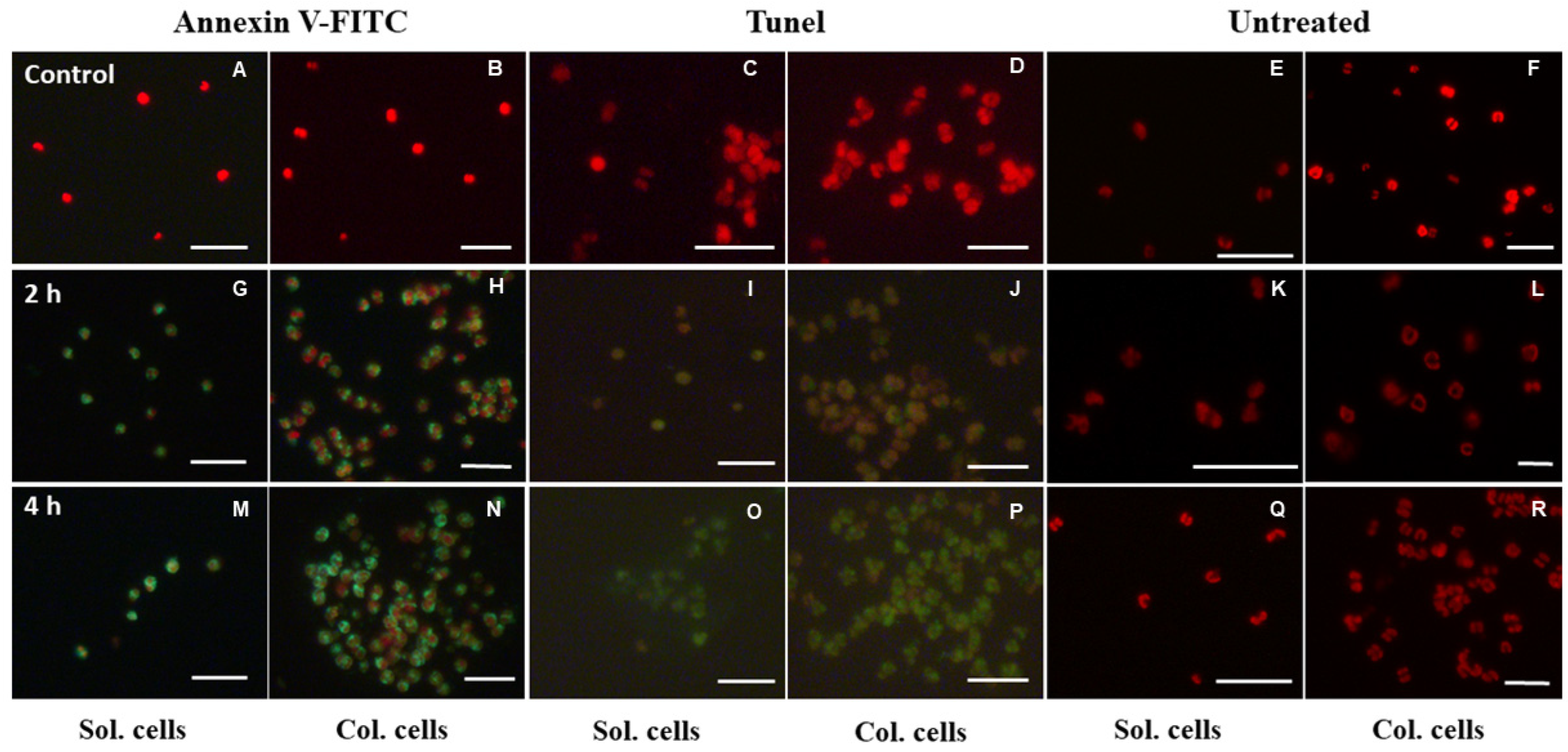
3.5. Determination of PS Externalization and DNA Fragmentation
4. Discussion
4.1. Effect of UV-B on the Photosynthesis of P. globosa
4.2. Response of P. globosa Solitary and Colony Cells to UV-B Radiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rousseau, V.; Chrétiennot Dinet, M.J.; Jacobsen, A.; Verity, P.; Whipple, S. The life cycle of Phaeocystis: State of knowledge and presumptive role in ecology. Biogeochemistry 2007, 83, 29–47. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Ou, L. The roles of light–dark cycles in the growth of Phaeocystis globosa from the South China Sea: The cost of colony enlargement. J. Sea Res. 2014, 85, 518–523. [Google Scholar] [CrossRef]
- Rousseau, V.; Vaulot, D.; Casotti, R.; Cariou, V.; Lenz, J.; Gunkel, J.; Baumann, M. The life cycle of Phaeocystis (Prymnesiophycaea): Evidence and hypotheses. J. Mar. Syst. 1994, 5, 23–39. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wang, N.; Zhang, P.; Zhou, H.; Qu, L. Molecular evidence identifies bloom-forming Phaeocystis (Prymnesiophyta) from coastal waters of southeast China as Phaeocystis globosa. Biochem. Syst. Ecol. 2002, 30, 15–22. [Google Scholar] [CrossRef]
- Schoemann, V.; Becquevort, S.; Stefels, J.; Rousseau, V.; Lancelot, C. Phaeocystis blooms in the global ocean and their controlling mechanisms: A review. J. Sea Res. 2005, 53, 43–66. [Google Scholar] [CrossRef]
- Peperzak, L.; van Wezel, R. Human fatalities related to a Phaeocystis harmful algal bloom in the North Sea. Harmful Algae 2023, 130, 102545. [Google Scholar] [CrossRef]
- Smith, W.O., Jr.; Trimborn, S. Phaeocystis: A Global Enigma. Ann. Rev. Mar. Sci. 2024, 16, 417–441. [Google Scholar] [CrossRef]
- Riegman, R.; Van Boekel, W. The ecophysiology of Phaeocystis globosa: A review. J. Sea Res. 1996, 35, 235–242. [Google Scholar] [CrossRef]
- Zhang, S.F.; Zhang, K.; Cheng, H.M.; Lin, L.; Wang, D.Z. Comparative transcriptomics reveals colony formation mechanism of a harmful algal bloom species Phaeocystis globosa. Sci. Total Environ. 2020, 719, 137454. [Google Scholar] [CrossRef]
- Wang, X.; Song, H.; Wang, Y.; Chen, N. Research on the biology and ecology of the harmful algal bloom species Phaeocystis globosa in China: Progresses in the last 20 years. Harmful Algae 2021, 107, 102057. [Google Scholar] [CrossRef]
- Wu, K.; Tang, S.; Wu, X.; Zhu, J.; Song, J.; Zhong, Y.; Zhou, J.; Cai, Z. Colony formation of Phaeocystis globosa: A case study of evolutionary strategy for competitive adaptation. Mar. Pollut. Bull. 2023, 186, 114453. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, K. Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophyceae). Hydrobiologia 2011, 675, 105–117. [Google Scholar] [CrossRef]
- Neale, P.J.; Williamson, C.E.; Banaszak, A.T.; Häder, D.P.; Hylander, S.; Ossola, R.; Rose, K.C.; Wängberg, S.Å.; Zepp, R. The response of aquatic ecosystems to the interactive effects of stratospheric ozone depletion, UV radiation, and climate change. Photochem. Photobiol. Sci. 2023, 22, 1093–1127. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Williamson, C.E.; Wängberg, S.-Å.; Rautio, M.; Rose, K.C.; Gao, K.; Helbling, E.W.; Sinha, R.P.; Worrest, R. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 108–126. [Google Scholar] [CrossRef]
- Holzinger, A.; Lütz, C. Algae and UV irradiation: Effects on ultrastructure and related metabolic functions. Micron 2006, 37, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Rúa, A.; Liebezeit, G. Effects of UV radiation on five marine microphytobenthic Wadden sea diatoms, isolated from the Solthrn tidal flat (Lower Saxony, southern North Sea)—Part II: Changes in carbohydrate, amino acid and fatty acid composition. Eur. J. Phycol. 2014, 49, 97–114. [Google Scholar] [CrossRef]
- Pessoa, M. Algae and aquatic macrophytes responses to cope to ultraviolet radiation—A Review. Emir. J. Food Agric. 2012, 24, 527–545. [Google Scholar] [CrossRef]
- Montero, O.; Klisch, M.; Häder, D.P.; Lubian, L.M. Comparative Sensitivity of Seven Marine Microalgae to Cumulative Exposure to Ultraviolet-B Radiation with Daily Increasing Doses. Bot. Mar. 2002, 45, 305–315. [Google Scholar] [CrossRef]
- Saber, H.; El Sheekh, M.M.; Ibrahim, A.; Alwaleed, E.A. Effect of UV-B radiation on amino acids profile, antioxidant enzymes and lipid peroxidation of some cyanobacteria and green algae. Int. J. Radiat. Biol. 2020, 96, 1192–1206. [Google Scholar] [CrossRef]
- Xue, S.; Zang, Y.; Chen, J.; Shang, S.; Gao, L.; Tang, X. Ultraviolet-B radiation stress triggers reactive oxygen species and regulates the antioxidant defense and photosynthesis systems of intertidal red algae Neoporphyra haitanensis. Front. Mar. Sci. 2022, 9, 1043462. [Google Scholar] [CrossRef]
- Lesser, M. Acclimation of phytoplankton to UV-B radiation: Oxidative stress and photoinhibition of photosynthesis are not prevented by UV-absorbing compounds in the dinoflagellate Prorocentrum micans. Mar. Ecol.-Prog. Ser. 1996, 132, 287–297. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar]
- Zhang, P.Y.; Yu, J.; Tang, X.X. UV-B Radiation Suppresses the Growth and Antioxidant Systems of Two Marine Microalgae, Platymonas subcordiformis (Wille) Hazen and Nitzschia closterium (Ehrenb.) W. Sm. J. Integr. Plant Biol. 2005, 47, 683–691. [Google Scholar] [CrossRef]
- Cai, X.; Hutchins, D.A.; Fu, F.; Gao, K. Effects of ultraviolet radiation on photosynthetic performance and N2 fixation in IMS 101. Biogeosciences 2017, 14, 4455–4466. [Google Scholar] [CrossRef]
- Gao, K.; Beardall, J.; Häder, D.P.; Hall Spencer, J.M.; Gao, G.; Hutchins, D.A. Effects of Ocean Acidification on Marine Photosynthetic Organisms Under the Concurrent Influences of Warming, UV Radiation, and Deoxygenation. Front. Mar. Sci. 2019, 6, 322. [Google Scholar] [CrossRef]
- Edreva, A.M.; Pouneva, I.D.; Gesheva, E.Z. UV-B radiation induces biphasic burst of hydrogen peroxide in mesophyll Chlorella vulgaris. Russ. J. Plant Physiol. 2015, 62, 219–223. [Google Scholar] [CrossRef]
- Tanouchi, Y.; Lee, A.J.; Meredith, H.; You, L. Programmed cell death in bacteria and implications for antibiotic therapy. Trends Microbiol. 2013, 21, 265–270. [Google Scholar] [CrossRef]
- Bidle, K.D. Programmed Cell Death in Unicellular Phytoplankton. Curr. Biol. 2016, 26, R594–R607. [Google Scholar] [CrossRef]
- Valandro, F.; Menguer, P.K.; Cabreira-Cagliari, C.; Margis-Pinheiro, M.; Cagliari, A. Programmed cell death (PCD) control in plants: New insights from the Arabidopsis thaliana deathosome. Plant Sci. 2020, 299, 110603. [Google Scholar] [CrossRef]
- Franklin, D.J.; Brussaard, C.P.D.; Berges, J.A. What is the role and nature of programmed cell death in phytoplankton ecology? Eur. J. Phycol. 2006, 41, 1–14. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Jurczak, T.; Poniedziałek, B. Oxidative Stress, Programmed Cell Death and Microcystin Release in Microcystis aeruginosa in Response to Daphnia Grazers. Front. Microbiol. 2020, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Orellana, M.V.; Pang, W.L.; Durand, P.M.; Whitehead, K.; Baliga, N.S. A Role for Programmed Cell Death in the Microbial Loop. PLoS ONE 2013, 8, e62595. [Google Scholar] [CrossRef] [PubMed]
- Rotari, V.; Gordon, A.; He, R.; Gallois, P. Caspase-like activities and UV-induced programmed cell death in Arabidopsis. BMC Plant Biol. 2005, 5, S18. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wen, F.; Xing, D. Sorting out the role of reactive oxygen species during plant programmed cell death induced by ultraviolet-C overexposure. Plant Signal. Behav. 2008, 3, 197–198. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Dao, G.H.; Hu, H.Y. Synergetic suppression effects upon the combination of UV-C irradiation and berberine on Microcystis aeruginosa and Scenedesmus obliquus in reclaimed water: Effectiveness and mechanisms. Sci. Total Environ. 2020, 744, 140937. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Capasso, J.M.; Edelstein, C.L.; Rivard, C.J.; Lucia, S.; Breusegem, S.; Berl, T.; Segovia, M. Different ways to die: Cell death modes of the unicellular chlorophyte Dunaliella viridis exposed to various environmental stresses are mediated by the caspase-like activity DEVDase. J. Exp. Bot. 2009, 60, 815–828. [Google Scholar] [CrossRef]
- Zheng, N.; Lin, X.; Huang, P.; Liu, Y.; Bartlam, M.; Wang, Y. Tea polyphenols inhibit blooms caused by eukaryotic and prokaryotic algae. Ecotoxicol. Environ. Saf. 2023, 265, 115531. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Alwaleed, E.A.; Ibrahim, A.; Saber, H. Detrimental effect of UV-B radiation on growth, photosynthetic pigments, metabolites and ultrastructure of some cyanobacteria and freshwater chlorophyta. Int. J. Radiat. Biol. 2021, 97, 265–275. [Google Scholar] [CrossRef]
- Moharikar, S.; D’Souza, J.; Kulkarni, A.; Rao, B. Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (Chlorophyceae) cells following UV irradiation: Detection and functional analyses. J. Phycol. 2006, 42, 423–433. [Google Scholar] [CrossRef]
- Dunn, S.R.; Bythell, J.C.; Le Tissier, M.D.A.; Burnett, W.J.; Thomason, J.C. Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. J. Exp. Mar. Biol. Ecol. 2002, 272, 29–53. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, H.; Zheng, J.; Teng, F.; Wang, X.; Lou, K.; Zhang, X.; Tao, Y. Suppression of water-bloom cyanobacterium Microcystis aeruginosa by algaecide hydrogen peroxide maximized through programmed cell death. J. Hazard. Mater. 2020, 393, 122394. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Zhuang, J.; Lu, J.; Cao, K.-F.; Li, J. Haploid Helps Phaeocystis globosa distribute to deeper dim water, as evidenced by growth and photosynthetic physiology. Front. Mar. Sci. 2022, 9, 902330. [Google Scholar] [CrossRef]
- Heraud, P.; Beardall, J. Changes in chlorophyll fluorescence during exposure of Dunaliella tertiolecta to UV radiation indicate a dynamic interaction between damage and repair processes. Photosynth. Res. 2000, 63, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Moejes, F.W.; Matuszyńska, A.; Adhikari, K.; Bassi, R.; Cariti, F.; Cogne, G.; Dikaios, I.; Falciatore, A.; Finazzi, G.; Flori, S.; et al. A systems-wide understanding of photosynthetic acclimation in algae and higher plants. J. Exp. Bot. 2017, 68, 2667–2681. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Wang, B.; Jia, J. Photoprotection mechanisms of Nannochloropsis oceanica in response to light stress. Algal Res. 2020, 46, 101784. [Google Scholar] [CrossRef]
- Campbell, D.; Eriksson, M.J.; Öquist, G.; Gustafsson, P.; Clarke, A.K. The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc. Natl. Acad. Sci. 1998, 95, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D.; Nakamoto, H.; Incharoensakdi, A. Resilience and self-regulation processes of microalgae under UV radiation stress. J. Photochem. Photobiol. C 2020, 43, 100322. [Google Scholar] [CrossRef]
- Abo-Shady, A.M.; El-Sheekh, M.M.; El-Naggar, A.H.; Abomohra, A.E.-F. Effect of UV-B radiation on growth, photosynthetic activity and metabolic activities of Chlorococcum sp. Ann. Microbiol. 2008, 58, 21–27. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Wang, M.; Zhang, W.; Zhou, B.; Wang, Y. ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J. Photochem. Photobiol. B Biol. 2017, 173, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, S.; Li, D. Differential responses of different phenotypes of Microcystis (Cyanophyceae) to UV-B radiation. Phycologia 2015, 54, 118–129. [Google Scholar] [CrossRef]
- Taira, H.; Taguchi, S. Cellular Mycosporine-like amino acids protect photosystem II of the Dinoflagellate Scrippsiella sweeneyae from ultraviolet radiation damage. J. Photochem. Photobiol. B Biol. 2017, 174, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Lee, D.B.; Kang, S.H.; Shin, K.H. Strategy of photo-protection in phytoplankton assemblages in the Kongsfjorden, Svalbard, Arctic. Chin. J. Oceanol. Limnol. 2016, 34, 1–12. [Google Scholar] [CrossRef]
- Marchant, H.J.; Davidson, A.T.; Kelly, G.J. UV-B protecting compounds in the marine alga Phaeocystis pouchetii from Antarctica. Mar. Biol. 1991, 109, 391–395. [Google Scholar] [CrossRef]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef]
- Xu, C.; Yu, S.; Hu, J.; Effiong, K.; Ge, Z.; Tang, T.; Xiao, X. Programmed cell death process in freshwater Microcystis aeruginosa and marine Phaeocystis globosa induced by a plant derived allelochemical. Sci. Total Environ. 2022, 838, 156055. [Google Scholar] [CrossRef]
- Zuppini, A.; Andreoli, C.; Baldan, B. Heat stress: An inducer of programmed cell death in Chlorella saccharophila. Plant Cell Physiol. 2007, 48, 1000–1009. [Google Scholar] [CrossRef]
- Malanga, G.; Susana, P. Oxidative stress and antioxidant content in Chlorella vulgaris after exposure to ultraviolet—B radiation. Physiol. Plant. 1995, 94, 672–679. [Google Scholar] [CrossRef]
- Sun, X.M.; Geng, L.J.; Ren, L.J.; Ji, X.J.; Hao, N.; Chen, K.Q.; Huang, H. Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae. Bioresour. Technol. 2018, 250, 868–876. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.S.; Liang, J.R.; Lin, Q.; Li, C.; Bowler, C.; Anderson, D.M.; Wang, P.; Wang, X.W.; Gao, Y.H. Cellular Responses Associated with ROS Production and Cell Fate Decision in Early Stress Response to Iron Limitation in the Diatom Thalassiosira pseudonana. J. Proteome Res. 2014, 13, 5510–5523. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Berman Frank, I.; Rozenberg, T.; Hadas, O.; Kaplan, A.; Levine, A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Curr. Biol. 1999, 9, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Spungin, D.; Bidle, K.D.; Berman Frank, I. Metacaspase involvement in programmed cell death of the marine cyanobacterium Trichodesmium. Environ. Microbiol. 2019, 21, 667–681. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, S.; Jiang, D.; Lu, J.; Huang, Z.; Liu, Z.; Zhou, H.; Zhuang, C.; Li, J. Initiation and Execution of Programmed Cell Death and Regulation of Reactive Oxygen Species in Plants. Int. J. Mol. Sci. 2021, 22, 12942. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Lee, T.M.; Shiu, C.T. Implications of mycosporine-like amino acid and antioxidant defenses in UV-B radiation tolerance for the algae species Ptercladiella capillacea and Gelidium amansii. Mar. Environ. Res. 2009, 67, 8–16. [Google Scholar] [CrossRef]
| Cell Morphology | r (×10−3 min−1) | k (×10−3 min−1) |
|---|---|---|
| Solitary cell | 5.62 ± 0.42 | 10.87 ± 0.252 |
| Colony cell | 16.10 ± 1.00 *** | 7.74 ± 0.157 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Li, J.; Lan, C.; Lai, J. Responses of Different Morphological Cells of Phaeocystis globosa to UV-B Radiation. J. Mar. Sci. Eng. 2024, 12, 1619. https://doi.org/10.3390/jmse12091619
Wei W, Li J, Lan C, Lai J. Responses of Different Morphological Cells of Phaeocystis globosa to UV-B Radiation. Journal of Marine Science and Engineering. 2024; 12(9):1619. https://doi.org/10.3390/jmse12091619
Chicago/Turabian StyleWei, Wei, Jie Li, Caibi Lan, and Junxiang Lai. 2024. "Responses of Different Morphological Cells of Phaeocystis globosa to UV-B Radiation" Journal of Marine Science and Engineering 12, no. 9: 1619. https://doi.org/10.3390/jmse12091619





