Abstract
Crossing between selected lines could combine the additive genetic variance accumulated within the lines and the non-additive genetic variance between the lines in the genetic improvement of aquatic animals, thus obtaining progeny with favorable traits. However, the value of this breeding strategy has not been investigated in the Fujian oyster Crassostrea angulata due to the lack of available sufficiently selected lines. In this study, a complete 3 × 3 diallel cross was established between the normal (N), golden (G), and black (B) shell lines of C. angulata. The growth (shell height and living weight) and survival of three purebred groups and six hybrid groups under three environments (Luoyu, Jiangkou, and Houhai) were comprehensively evaluated during the larval and grow-out stages. The general combining ability (GCA) of the parental lines and the specific combining ability (SCA) of the hybrid groups were also estimated. The shell heights of the hybrid groups were significantly lower than those of their parental groups on day 25, exhibiting negative heterosis (MPH: −13.01 to −1.45; HPH: −16.69 to −5.76). Meanwhile, the survival rates of the hybrids were significantly higher than those of the parental groups. A negative value of SCA was recorded for NG (N♀ × G♂) (−0.031), which was in line with its lower survival rate on day 25. Significant heterosis was observed in the growth and survival of each hybrid group during the grow-out stage. The shell height and living weight of the hybrid groups were significantly higher in Houhai than in Luoyu and Jiangkou, but the survival rate in Houhai was significantly lower than in the other two sites. BG (B♀ × G♂) and GB (G♀ × B♂) had higher SCA values than the other four groups, which was consistent with their superior traits. The heterosis of shell height, living weight, and survival rate of BG was significantly greater than in the other five hybrid groups, which could be used as potential parents for breeding high-quality diploid and triploid Fujian oysters. This study demonstrated that the traits of C. angulata could be significantly improved by crossing between different selected lines, providing a reference for evaluating the utilization value of non-additive genetic effects (heterosis) between selected lines in bivalves.
1. Introduction
Oysters, as an important aquaculture species, are being farmed extensively worldwide. In China, the production of oysters ranks first in the world (about 80%) [1]. Seven species of oysters of the genus Crassostrea are widely distributed along the stretched coastline of China, and three of them (the Fujian oyster C. angulata, the Pacific oyster C. gigas, and the Hong Kong oyster C. hongkongensis) are the main species of farmed oysters, which account for 98% of the total farmed oyster production. Among these three species, the Fujian oyster has the highest farmed production, which was about 2.41 million tons in 2022, accounting for 38.19% of the total farmed oyster production in China [2].
Due to the high fecundity of oysters, their relatively short propagation cycles, and the ample genetic variation in the population, additive genetic variance can be well accumulated and transferred during selective breeding, providing permanent and sustained genetic gains to the offspring [3]. Moreover, non-additive genetic variance exists between different lines [4,5]. Therefore, the additive genetic variance accumulated within lines during the selection process and the non-additive genetic variance between the lines can be combined by crossing different selected lines to obtain progeny with superior traits (rapid growth, high survival, disease resistance, etc.) [6]. This breeding strategy of inter-selected line hybridization has been applied in many aquatic animals, such as C. gigas [7,8], C. virginica [9], and Pleurotus pulmonarius [10]. However, due to the lack of available sufficiently selected lines, the utilized values of non-additive genetic variation between selected lines of the Fujian oyster C. angulata have not been studied and lack a clear understanding.
In order to improve and enrich the germplasm resources of Fujian oysters, a genetic improvement program started in 2022 [2]. By targeting traits determining shell color and growth, three C. angulata lines were created with normal (no visible shell color, with or without visible radial streaks), black (solid black on both left and right shells), and golden shell colors (solid golden on both left and right shells), respectively. These three lines with different shell colors could serve as appropriate materials for the study of non-additive variance among selected lines of C. angulata. In addition, the productive traits (growth, survival, thermotolerance, etc.) of the three parental lines have been previously studied [2]. However, the performance of their hybrid offspring remains poorly investigated. On another note, as the party providing the eggs, high-quality diploid parents are of great importance to the production and performance of triploid oysters. Therefore, in order to explore the application value of the additive and non-additive variance of C. angulata and cultivate diploid parents with excellent traits, it was necessary to conduct the inter-line crossing between the three lines of Fujian oyster mentioned above.
The aims of this study were (1) to select lines with favorable traits through heterosis and combining ability assessments to provide informative materials for the production of superior diploid and triploid Fujian oysters, as well as (2) to select suitable lines of C. angulata for different sea areas in order to facilitate industrialized production.
2. Materials and Methods
2.1. Parental Oyster Collection and Temporary Rearing
In August 2022, 5000 wild individuals of C. angulata were collected in Putian, Fujian Province, China (25.35° N, 119.06° E). Based on the shell color, shell height, and living weight, 85 and 100 oysters with pure black and/or golden shell colors were selected as the parents to establish the first generation of the black (B) and/or golden (G) shell lines of C. angulata, respectively. Additionally, another 94 individuals with no distinctive color on both the left and right shells, with or without radial stripes, were collected as parents for the normal shell color line, and, thus, the first generation of the normal (N) shell line of C. angulata was also established (Figure 1). In August 2023, one-year-old gonadally mature oysters from the normal, golden, and black shell lines of C. angulata were collected from Luoyu, Fujian Province, China (25.17 °N, 119.03 °E), and transferred to Haifa Seedling Ltd., Putian, Fujian Province, China (25.19° N, 119.22° E), where they were temporarily reared at a temperature of 27.00 ± 0.5 °C and a salinity of 30.00 ± 1 psu.

Figure 1.
Phenotypes of the normal, golden, and black shell lines of C. angulata.
2.2. Mating Strategies and Larval Rearing
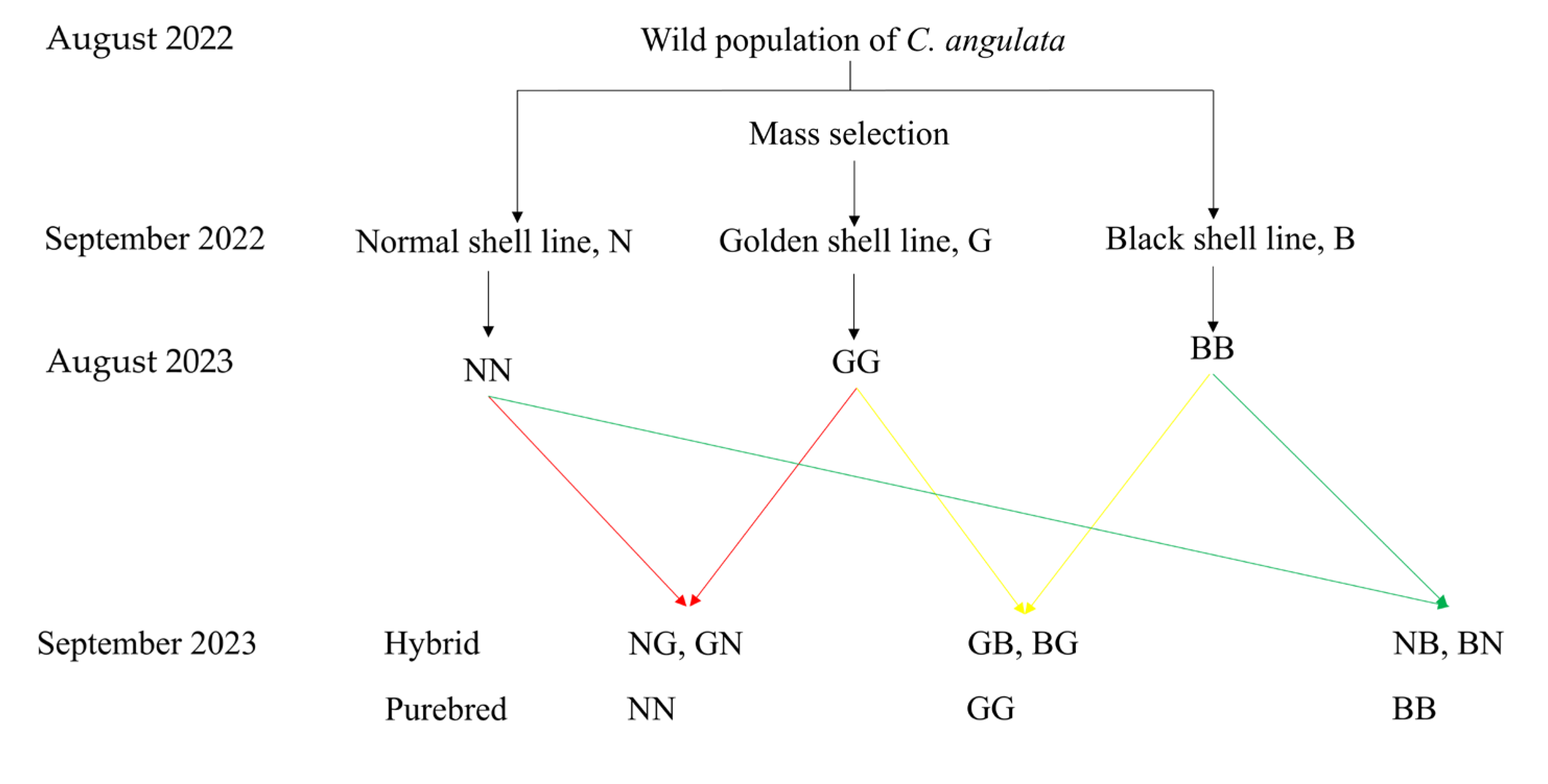
Three sires and three dams were selected from each of the normal (N), golden (G), and black (B) shell lines of C. angulata in August 2023 based on shell color, shell height (N: 61.72 ± 5.41 mm; B: 83.03 ± 10.41 mm; and G: 89.33 ± 5.04 mm) and living weight (N: 70.50 ± 7.36 g; B: 87.15 ± 13.47 g; and G: 90.80 ± 26.01 g). The eggs of every oyster from each line were collected by filtering through 300-mesh sieves and then placed in a 15 L polyethylene plastic bucket for 1 h at a temperature of 27.00 ± 0.5 °C and a salinity of 30.00 ± 1 psu for maturation. Later, the sperm of each male from each line was gathered and also placed in a 15 L polyethylene plastic bucket. Afterwards, the collected sperm and/or eggs were divided into three equal groups for fertilization. A 3 × 3 complete diallel cross was conducted between the normal (N), golden (G), and black (B) shell lines of C. angulata, with eggs from each oyster of each line completing fertilization with the sperm from both the home line and the other two lines, in turn. Therefore, three purebred groups (NN, N♀ × N♂, BB, B♀ × B♂, and GG, G♀ × G♂) and six hybrid groups (NG, N♀ × G♂, NB, N♀ × B♂, GN, G♀ × N♂, GB, G♀ × B♂, BN, B♀ × N♂, BG, and B♀ × G♂) were established (Figure 2). At least 50 spermatozoa were guaranteed to surround each egg during fertilization. Each group was obtained by fertilization with an independent one-pair parent, and three replications of each group were performed. Thus, 27 full-sib families were deployed for this study. All experimental apparatuses used during fertilization, such as sieve nets and buckets, were rinsed with fresh water to prevent any inter-contamination.
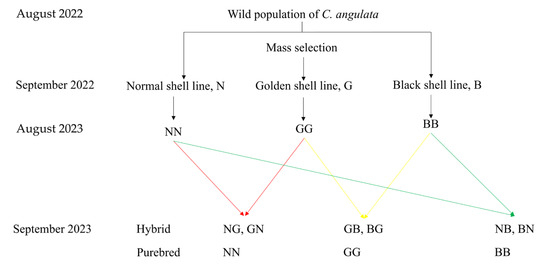
Figure 2.
The parental origin and mating strategy in this study.
After fertilization, each group was transferred to a 100 L polyethylene plastic bucket for incubation at a temperature of 27.00 ± 0.5 °C and a salinity of 30.00 ± 1 psu. An appropriate number of D-larvae were collected from each group after 24 h and re-located in a new 100 L polyethylene plastic bucket for rearing [11]. The initial density of larvae was set to 2 larvae per ml and gradually adjusted to 0.5 larvae per ml as the larvae grew. During the breeding period, fully aerated and filtered seawater was used to renew the water, with a water change of 1/3 of the total volume of the bucket every 2 days. In the early stages, the larvae were fed appropriate Isochrysis galbana, followed by Platymonas sp. to promote growth. After 30% of the larvae had developed eyespots, re-cleaned oyster strings were hung for larval attachment and metamorphosis. Subsequently, each group was transferred to Luoyu, Houhai (25.29° N, 119.19° E), and Jiangkou (25.49° N, 119.20° E), Fujian Province, China, respectively. The average annual temperatures of Jiangkou, Houhai, and Luoyu are 22.75 °C, 23.55 °C, and 24.13 °C, respectively. The mean annual salinity is 28, 30, and 28 psu, respectively. The mean annual pH is 8.18, 8.25, and 8.22, respectively. After one month of long-line culturing, each group was transferred to 10 layers of net cages with 30 individuals placed in each layer for trait measurement.
2.3. Trait Measuring
The shell heights and survival rates of each group during the larval stage were counted on days 5 and 25, respectively. At random, 30 larvae were collected and preserved with Lugo’s reagent. The shell height of the larvae was measured using the image analysis software (Image- pro Plus 6.0). The larval survival rate was the ratio of the number of surviving larvae to the total number of D-larvae on sampling day.
The shell height and survival rate of each group in the three environments during the grow-out stage were estimated on days 90 and 360, and the living weight was calculated on day 360. The shell height and living weight were measured using vernier calipers (accuracy 0.01 mm) and electronic scales (accuracy 0.01 g), respectively. The juvenile survival rates were calculated based on the total number of live oysters in each group on sampling day.
2.4. Statistical Analyses
Data are hereby presented as the mean ± standard deviation (SD). The growth parameters (shell height or living weight) were log-transformed based on 10, and the survival rates for each group were arc-transformed to improve normality and homoscedasticity. Differences in the characteristics between groups at the same site were analyzed by one-way ANOVA and Tukey’s test for multiple comparisons in SPSS 26.0. A value of p < 0.05 was accepted as a significant difference.
The heterosis of the hybrids (mid-parent heterosis, MPH, and high-parent heterosis, HPH) was calculated using the following equations, respectively [12,13]:
where XF1 and XMP represent the mean values of the shell height (survival rate or living weight) of one hybrid and their corresponding parental groups, respectively. XHP indicates the mean phenotypic value (shell height, survival rate, etc.) of its better parent. MPH and HPH indicate the mid-parent heterosis and high-parent heterosis of the hybrids, respectively.
MPH (%) = [(XF1 − XMP) × 100]/XMP
HPH (%) = (XF1 − XHP) ×100/XHP
The effects of the general combining ability (GCA) of the three purebred groups (NN, GG, and BB) and the specific combining ability (SCA) of the six hybrid groups (BG, GB, NB, BN, GN, and NG) for shell height, living weight, and survival rate were calculated and estimated using the best linear unbiased prediction of the ASReml-R software (3.0) [3]. The following statistical model was used to calculate the GCA and SCA for each group.
Yhijk = µ + Eh + Sirei + Damj + (Sire × Dam)ij + eijk
Here, Yhijk is the mean value of the kth hybrid group; μ is the overall mean; Eh is the effect of the hth environment; Sirei is the effect of the ith sire; Damj is the effect of the jth dam; (Sire × Dam)ij is the effect of the interaction between the ith sire and the jth dam; and eijk is the residual effect. The Eh was excluded from the above model when analyzing for traits in one specific environment.
To determine the effects of the genotypes (hybrid groups vs. purebred groups), environmental factors (Luoyu vs. Jiangkou vs. Houhai), egg origin (N vs. B vs. G dams), and mating strategy (intra- vs. inter-line crosses) on the growth and survival rate of C. angulata on day 360, two standalone two-factor ANOVAs were carried out.
3. Results
3.1. Growth, Survival, and Combining Ability During the Larval Stage
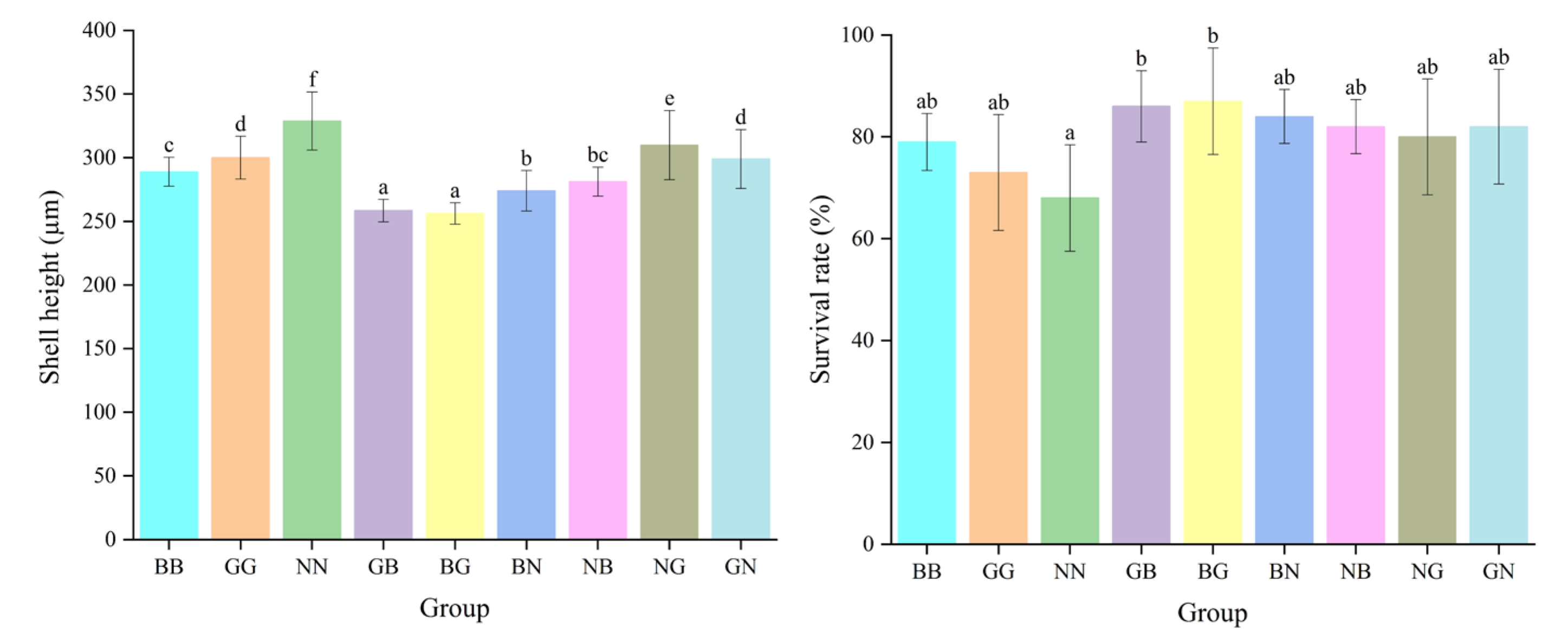
The shell height of each hybrid group was significantly greater than that of the purebred group on day 5, and the shell height of GB was significantly higher than that of the other hybrid groups (p < 0.05) (Figure 3). The mid-parent heterosis (MPH) and high-parent heterosis (HPH) of the shell height in each group ranged from 1.73% to 3.73% and from 3.37% to 5.61%, respectively (p < 0.05) (Table 1). However, the shell heights of the hybrid groups were significantly smaller than those of the purebred groups on day 25, while the survival rate was significantly greater than that of the purebred group (p < 0.05). The survival rates of GB and BG were significantly greater than those of their parental groups and other groups, with the high-parent heterosis at 17.81% and 19.18%, respectively (p < 0.05). The combining ability analysis revealed that the values of the general combining ability (GCA) whereby G acted as the dam and B as the sire was greater than in the other groups, with values of 7.564 and 2.313, respectively (Table 2). However, the values of GCA for shell height on day 25 were at their maximum when N worked as the dam and G as the sire, with values of 8.26 and 3.188, respectively (Table 3). The special combining ability (SCA) for the survival rate was greatest for BG and GB with values of 1.909 and 1.632, respectively, which was consistent with their actual trait performance. The SCA for NG and GN was the largest, with values of 12.796 and 11.472, respectively.
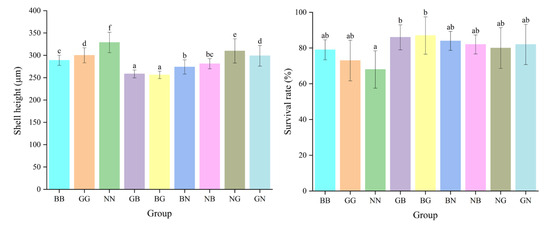
Figure 3.
The shell height and survival rate for the nine groups of C. angulata at the larval stage. Different superscript letters indicate significant difference (p < 0.05).

Table 1.
The mid-parent heterosis (MPH%) and high-parent heterosis (HPH%) for the shell height and survival rate of the six hybrid groups of C. angulata during the larval stage.

Table 2.
The general combining ability (GCA) and specific combining ability (SCA) for the survival rate of the three purebred groups and six hybrid groups of C. angulata at both larval and grow-out stages.

Table 3.
The general combining ability (GCA) and specific combining ability (SCA) for shell height and living weight of the three purebred groups and six hybrid groups of C. angulata at both larval and grow-out stages.
3.2. Growth, Survival, and Combining Ability During the Grow-Out Stage
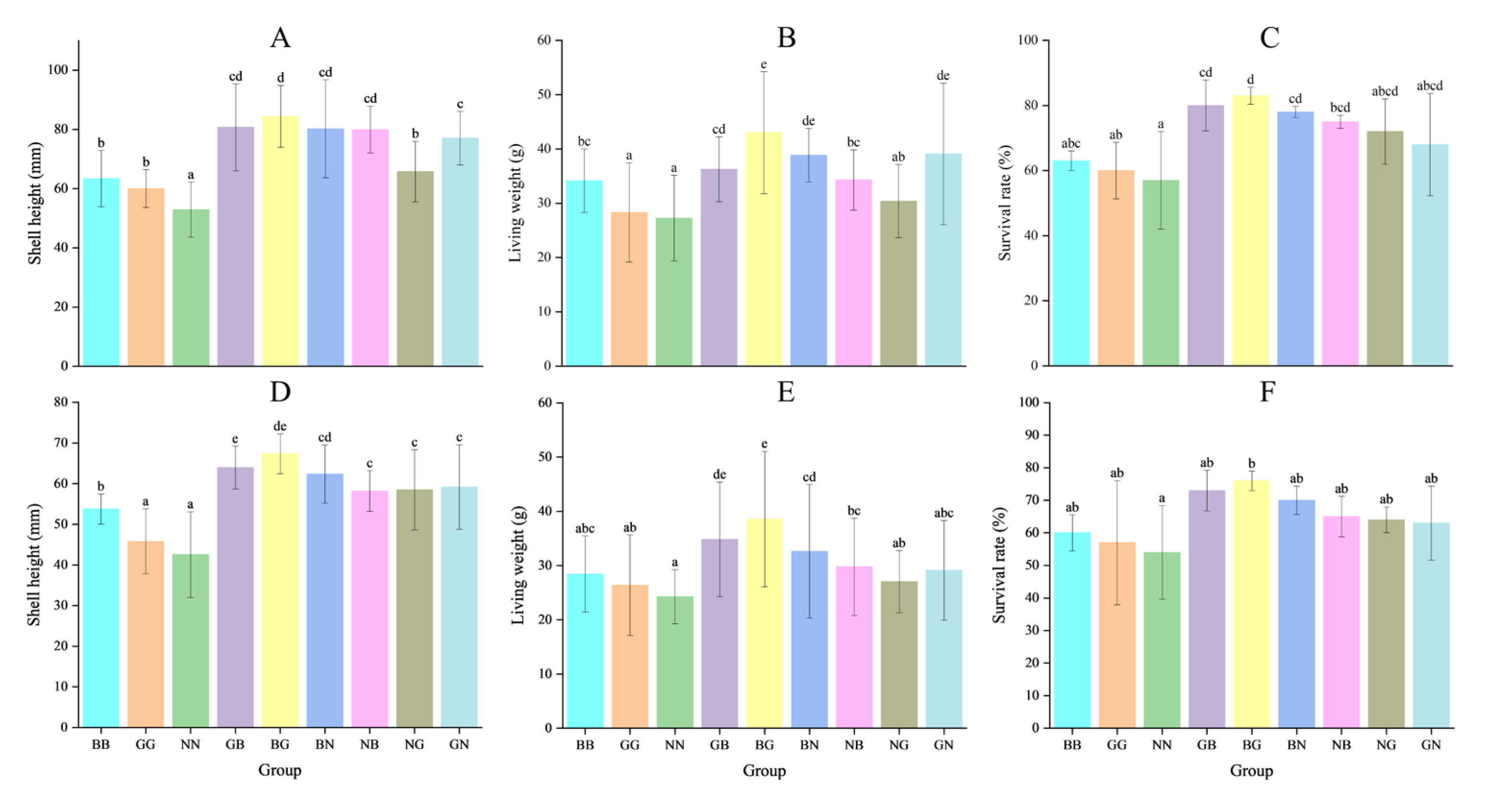
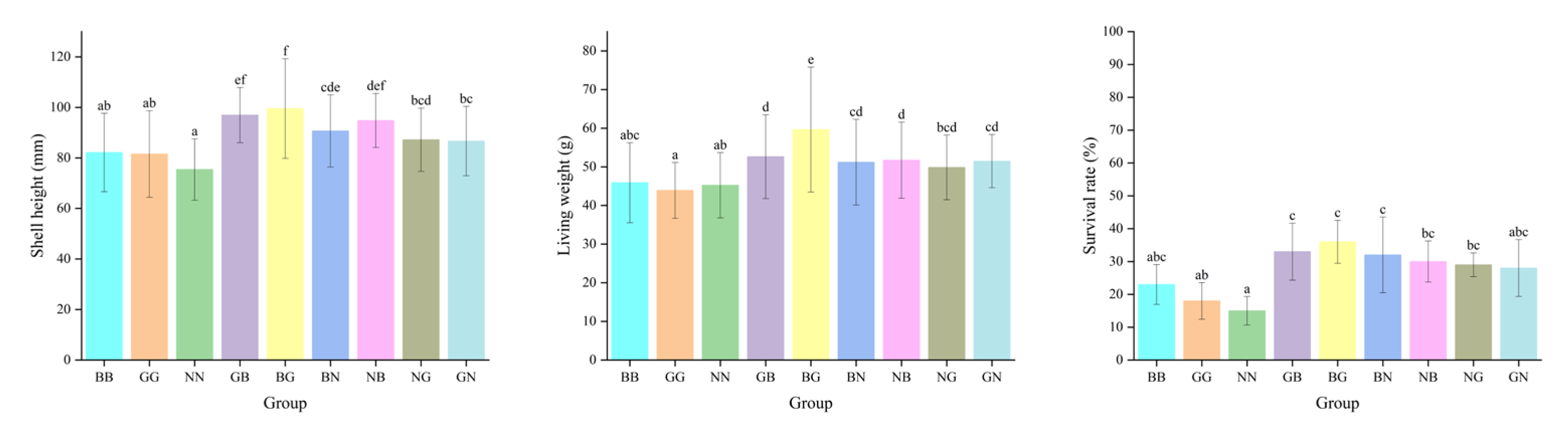
The two-way ANOVA showed that egg source and mating mode exerted significant effects on the growth and survival of Fujian oysters at all three sites (Table 4). The shell heights of GB and BG were significantly higher than those of the other groups in all three environments on day 90, with the greatest high-parent heterosis recorded in Houhai, with values of 39.09% and 39.04%, respectively (p < 0.05) (Table 5 and Table 6). However, the shell heights of NB, NG, and GN were smaller than their better parents, with negative high-parent heterosis (from −21.29% to −0.04%) found in Luoyu. The NG and GN had positive high-parent heterosis (9.92~20.46%) in Jiangkou and Houhai (Table 5). The ranking order of shell heights for the three parental lines was BB > GG > NN (Figure 4 and Figure 5). The survival rates of all hybrid groups were significantly higher than those of their parental groups, with the mid-parent heterosis and high-parent heterosis ranging from 5.69% to 23.38% and 3.17% to 21.79%, respectively (p < 0.05) (Table 5). The shell height, living weight, and survival rate of each hybrid group in the three environments were significantly higher than their parental groups on day 360. GB and BG were significantly greater than the other four hybrid groups (p < 0.05) (Figure 5). The shell height and living weight of BG were significantly larger than those of BB and GG in Luoyu, with high-parent heterosis of 33.13% and 25.97%, respectively. The shell heights and living weights of all groups were significantly smaller in Jiangkou and Luoyu than in Houhai (p < 0.05) (Figure 4 and Figure 5).

Table 4.
Two-factor analysis of variance (ANOVA) showing egg origin (N vs. B vs. G dams) and mating strategy (intra- vs. inter-line crosses) effects on the growth and survival rate of C. angulata on day 360.

Table 5.
The mid-parent heterosis (MPH%) and high-parent heterosis (HPH%) for the shell height, living weight, and survival rate of the six hybrid groups of C. angulata during the grow-out stage.

Table 6.
Two-factor analysis of variance (ANOVA) showing genotypes (hybrid groups vs. purebred groups) and environmental factors (Luoyu vs. Jiangkou vs. Houhai) effects for the growth and survival rate of the Fujian oyster Crassostrea angulata on day 360.
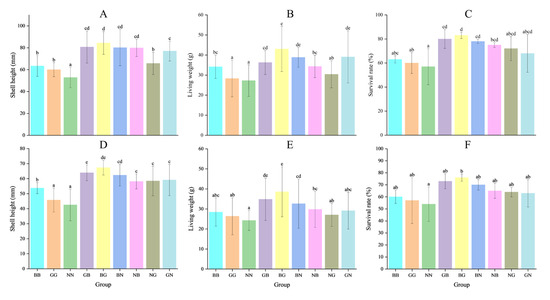
Figure 4.
The shell height, living weight, and survival rate for the nine groups of C. angulata in Luoyu (A–C) and Jiangkou (D–F) on day 360. Different superscript letters indicate significant difference (p < 0.05).
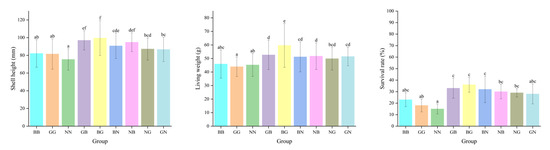
Figure 5.
The shell height, living weight, and survival rate for the nine groups of C. angulata in Houhai on day 360. Different superscript letters indicate significant difference (p < 0.05).
The survival rates of all groups in Houhai were significantly lower than under the other two environments (p < 0.05) (Figure 4 and Figure 5). The survival rate of NN was lowest in Houhai on day 360, with a value of 15.00% (Figure 5). All six hybrid groups still showed significant advantages in their survival rate, with BG exhibiting the greatest heterosis (M: 75.61%; H: 56.52%) (Table 4). The GCA of B as a dam was greater than that of G and N on day 360 in all three environments (Table 3). Furthermore, when B was used as the sire, its general combining ability was greater than that of G and N in the other two sites (except for Jiangkou). The SCA for the survival rate was the largest for BG (from 4.570 to 8.405) and the smallest for GN, with values from −1.817 to 0.580 on day 360. The SCA for the shell height and living weight of NG (except for the shell height in Jiangkou) showed negative values (from −5.670 to −0.271), which corresponded to its actual measured values of shell height and living weight, both of which were significantly smaller in NG than in the other hybrid groups (Table 3). The SCA of the shell height of BG was negative on day 25 and turned positive on day 360. It was greater than the other hybrid groups, with values ranging from 5.458 to 12.410. In addition, the SCA of the shell height of GN was negative in Houhai and became positive in Luoyu and Jiangkou (Table 3).
4. Discussion
There were significant differences in the traits between the hybrid groups. The ancestral parents of the three lines of C. angulata used in this study were not equally abundant in the wild population. Thus, genetic diversity must have varied considerably among the parental lines. Beyond this, the genetic diversity among the six hybrid groups also differed, which may have accounted for the differences in the performance of the different crosses in this study. Additionally, trait differences existed among the reciprocal hybrids in this study (e.g., the high-parent heterosis for the survival rate was two-fold higher in BN than in NB). The asymmetric trait performance of reciprocal hybrids has also been found in many other marine shellfish [13,14,15,16], possibly due to sex-linked genes, parental effects, cytoplasmic inheritance, etc. [17].
Moreover, when the three parental lines used in this study were artificially selected, the target traits were shell color and growth, meaning that survival was not purposefully included in the breeding objectives. The ancestors of the three lines were selected from the wild population rich in genetic variations, and a large amount of additive genetic variations for fast growth were intentionally retained during the selection process. However, the three lines were not intentionally selected for survival during the selection process, and, thus, the survival-related genes among the three lines might have been more diverse and complementary than the growth-related genes. As a result, there were significant differences in the heterosis of growth and survival among the different hybrid groups in this study. Of course, the genetic mechanisms underlying the differences in heterosis among various traits need to be further investigated.
Attention should be drawn to the fact that, theoretically, when the parental lines have been selected for several generations, the hybrid progeny will exhibit heterosis due to the accumulation of a substantial quantity of different non-additive genetic variations, and the higher the number of succeeding generations of the parents, the higher the heterosis [6]. Therefore, the combined employment of additive and non-additive genetic variations was vital in the breeding process applied in our study. This type of breeding had already been well utilized in C. gigas, producing progeny with excellent traits [7,8]. Furthermore, numerous crossbreeding studies (inter-line and/or population, inter- and/or intra-specific, etc.) have demonstrated the existence of abundant non-additive genetic variances (heterosis) in shellfish related to growth and survival [4,5,17,18,19]. The parents used in this study were selected for as few as two generations to obtain progeny with significantly improved traits. Therefore, it is necessary and valuable to re-evaluate hybridization within lines after multi-generation selection of parental lines.
Nevertheless, heterosis among different selected lines was not prevalent and consistent. Inter-line hybridization experiments in fish have also demonstrated that non-additive genetic variance varies significantly among lines and that the magnitude of non-additive effects can be significantly influenced by the environment [20], which was also proven in this study. The significant differences in traits among the six hybrid groups in the three environments in this study might have been due to the distinctive variations in the environments (algal abundance and species, temperature, salinity, tidal range, etc.). Therefore, evaluating the phenotypic traits of oysters in different environments would be valuable for maximizing heterosis [8].
During the process of genetic improvement in oysters, the superior traits of the parents are inherited by the offspring. This has been reported not only in diploids [21,22] but also in tetraploids [23]. In addition, due to the inability of triploids to produce normal gametes, genetic improvement for triploids is often achieved by improving their diploid and tetraploid parents, as reported in C. gigas [24,25] and their hybridization with other oysters of the genus Crassostrea [26,27,28]—C. angulata [2] and C. virginica [29,30,31]. Therefore, the progeny with excellent traits obtained in this study can be potentially employed as parents for breeding excellent diploids and triploids, or even tetraploids. Of course, this requires further study and validation. In summary, this study demonstrated the existence of substantial non-additive genetic effects of crosses between selected lines, as exemplified by crossing three shell color lines of Fujian oysters; this is crucial for breeding progeny with superior traits. More attention should be paid to the crosses between selected lines to utilize the additive effect within lines and the non-additive effect between lines in the subsequent breeding process of shellfish.
In this study, a large amount of non-additive genetic variation (SCA) was demonstrated, which is consistent with the results of hybridization in C. gigas [7]. The relatively high proportion of variation in the traits could be explained by SCA, which indicated the importance of non-additive genetic variation in the genetic improvement of oysters [4,5,7]. This may have also accounted for the extremely low heritability estimated in this study (Supplementary File: Table S1). On the other hand, the combining ability was assessed in this study through full-sib families. However, in general, the half-sib correlation and the regression of the offspring compared to the father are the most reliable methods of estimating heritability. Full-sibling correlations are the least reliable because they are subject to common environmental effects and dominant variance [32]. This may be another reason for the very small heritability estimates in this study. On the other hand, scholars are dubious about the correct interpretation of genetic parameters such as heritability in hybrids due to the possibility of genetic imbalance in hybrids [33]. It has been suggested that additive genetic components in purebreds and hybrids are theoretically not comparable [34]. Additionally, the heritability of hybrids for growth or survival traits in oysters is a theoretical value that often falls short of theoretical expectations in practice. In actuality, hybrids are more resistant and show significant survival hybrid advantage, but the heritability of survival traits is relatively low, as also demonstrated in C. hongkongensis [35].
5. Conclusions
In this study, a complete 3 × 3 diallel cross was conducted between the normal, golden, and black shell lines of C. angulata. Among them, BG (B♀ × G♂) showed significantly higher shell height, growth, and survival rate than the other hybrids. It can be used as a prospective candidate parent for the breeding of superior diploids and triploids of C. angulata. In summary, this study provided a reference for utilizing non-additive genetic effects (heterosis) among selected lines of C. angulata. It would be valuable and necessary to exploit the additive genetic effects within lines and the inter-line non-additive genetic effects through crosses between selected lines in subsequent breeding efforts in shellfish. On the other hand, whether crossbreeding would increase genetic diversity, improve nutritional quality, and enhance stress tolerance in hybrid progeny needs to be further investigated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse13020281/s1, Table S1: Estimates of sire variance, dam variance, (sire × dam) variance and residual variance of shell height, living weight and survival rate for C. angulata.
Author Contributions
Conceptualization, Y.L. and Q.L.; methodology, Y.L. and Q.L.; software, H.H.; validation, Q.L.; formal analysis, Y.L.; investigation, H.H. and Z.P.; resources, C.X.; data curation, Z.P.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L. and Q.L.; visualization, Y.L. and Q.L.; supervision, Q.L.; project administration, Q.L.; and funding acquisition, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fujian Province (2024N3003), Research on Breeding Technology of Candidate Species for Guangdong Modern Marine Ranching (2024-MRB-00-001), and the Laoshan Laboratory.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liang, Y.; Xu, C.; Li, Q. Heterosis and genetic diversity of intraspecific hybrids crosses between two selected lines of the Pacific oyster Crassostrea gigas. Aquaculture 2023, 569, 739369. [Google Scholar] [CrossRef]
- Liang, Y.; Hu, H.; Cheng, G.; Xu, C.; Li, Q. Growth, survival and thermotolerance of diploids, triploids and tetraploids of the Fujian oyster Crassostrea angulata with normal, golden and black shell colors. Aquaculture 2024, 591, 741131. [Google Scholar] [CrossRef]
- Mallet, A.L.; Haley, L.E. Growth rate and survival in pure population matings and crosses of the oyster Crassostrea virginica. Can. J. Fish. Aquat. Sci. 1983, 40, 948–954. [Google Scholar] [CrossRef]
- Hedgecock, D.; McGoldrick, D.J.; Bayne, B.L. Hybrid vigor in pacific oysters: An experimental approach using crosses among inbred lines. Aquaculture 1995, 137, 285–298. [Google Scholar] [CrossRef]
- Hedgecock, D.; Davis, J.P. Heterosis for yield and crossbreeding of the pacific oyster Crassostrea gigas. Aquaculture 2007, 272, S17–S29. [Google Scholar] [CrossRef]
- Sheridan, A.K. Genetic improvement of oyster production—A critique. Aquaculture 1997, 153, 165–179. [Google Scholar] [CrossRef]
- Han, Z.; Li, Q.; Liu, S.; Kong, L. Crossbreeding of three different shell color lines in the pacific oyster reveals high heterosis for survival but low heterosis for growth. Aquaculture 2020, 529, 735621. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, G.; Jiang, G.; Hu, Y.; Fang, J.; Chi, Y.; Xu, C.; Liu, W.; Liu, H.; Li, Q. Hybridization between “Haida No. 1” and orange-shell line of the pacific oyster reveals high heterosis in survival. Aquaculture 2022, 551, 737945. [Google Scholar] [CrossRef]
- Rawson, P.; Feindel, S. Growth and Survival for Genetically improved lines of eastern oysters (Crassostrea virginica) and interline hybrids in Maine, USA. Aquaculture 2012, 326–329, 61–67. [Google Scholar] [CrossRef]
- Avin, F.A.; Bhassu, S.; Rameeh, V.; Tan, Y.S.; Vikineswary, S. Genetics and hybrid breeding of pleurotus pulmonarius: Heterosis, heritability and combining ability. Euphytica 2016, 209, 85–102. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Liu, S.; Kong, L. Selection response and realized heritability for growth in three stocks of the pacific oyster Crassostrea gigas. Fish. Sci. 2011, 77, 643–648. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Filho, J.B.M. Heterosis. In Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010; pp. 477–529. ISBN 978-1-4419-0765-3. [Google Scholar]
- Wang, C.; Liu, B.; Li, J.; Liu, S.; Li, J.; Hu, L.; Fan, X.; Du, H.; Fang, H. Introduction of the peruvian scallop and its hybridization with the bay scallop in China. Aquaculture 2011, 310, 380–387. [Google Scholar] [CrossRef]
- Soletchnik, P. A Comparative field study of growth, survival and reproduction of Crassostrea gigas, C. angulata and their hybrids. Aquatic Living Resources 2002, 15, 243–250. [Google Scholar] [CrossRef]
- Xue, B.; Shen, B.; Li, H.; Meng, D.; Niu, D.; Li, J.; Shen, H. Heterosis analysis at early generations for complete diallel crosses in three different geographical culture populations of Sinonovacula Constricta (Lamarck 1818) in Zhejiang, China. Aquac. Res. 2020, 51, 1388–1397. [Google Scholar] [CrossRef]
- Militz, T.A.; Braley, R.D.; Schoeman, D.S.; Southgate, P.C. Larval and early juvenile culture of two giant clam (Tridacninae) hybrids. Aquaculture 2019, 500, 500–505. [Google Scholar] [CrossRef]
- Kong, L.; Song, S.; Li, Q. The effect of interstrain hybridization on the production performance in the pacific oyster Crassostrea gigas. Aquaculture 2017, 472, 44–49. [Google Scholar] [CrossRef]
- In, V.V.; Sang, V.V.; O’Connor, W.; Van, P.T.; Dove, M.; Knibb, W.; Nguyen, N.H. Are strain genetic effect and heterosis expression altered with culture system and rearing environment in the Portuguese oyster (Crassostrea angulata)? Aquac. Res. 2017, 48, 4058–4069. [Google Scholar] [CrossRef]
- Tan, K.; Liu, H.; Ye, T.; Ma, H.; Li, S.; Zheng, H. Growth, Survival and lipid composition of Crassostrea gigas, c. angulata and their reciprocal hybrids cultured in southern China. Aquaculture 2020, 516, 734524. [Google Scholar] [CrossRef]
- Chaivichoo, P.; Koonawootrittriron, S.; Chatchaiphan, S.; Srimai, W.; Na-Nakorn, U. Genetic components of growth traits of the hybrid between ♂North African catfish (Clarias gariepinus burchell, 1822) and ♀Bighead catfish (C. macrocephalus Günther, 1864). Aquaculture 2020, 521, 735082. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Q.; Xu, C.; Liu, S.; Kong, L.; Yu, H. Response to selection for growth in successive mass selected generations of iwagaki oyster Crassostrea nippona. Aquaculture 2022, 560, 738575. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Q.; Xu, C. Comparative study of growth, survival and yield of selected, inbreeding and wild populations in pacific oysters. Aquaculture 2023, 566, 739232. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Shi, G.; Li, S.; Liao, Q.; Ma, H.; Li, J.; Suo, A.; Ding, D.; Yu, Z.; et al. Genetic improvement of aquaculture performance for tetraploid pacific oysters, Crassostrea gigas: A case study of four consecutive generations of selective breeding. Aquaculture 2023, 563, 738910. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, G.; Xu, C.; Bai, X.; Li, Q. Growth, Survival and gonad development of diploids, triploids and tetraploids of ‘Haida No. 3’ line of the Pacific oyster Crassostrea gigas. Aquaculture 2023, 571, 739472. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, C.; Li, Q. Influence of Ploidy, Genetic and environment on production traits of the pacific oyster Crassostrea gigas. Aquaculture 2024, 586, 740756. [Google Scholar] [CrossRef]
- Yue, C.; Qin, Y.; Wan, W.; Shi, G.; Li, S.; Li, J.; Wang, Z.; Ma, H.; Li, J.; Yu, Z.; et al. Phenotypic traits of reciprocal tetraploid hybrids derived from tetraploid Crassostrea gigas and tetraploid Crassostrea angulata. Aquaculture 2024, 582, 740495. [Google Scholar] [CrossRef]
- Li, H.; Yu, R.; Li, Q.; Ma, P. Evaluation of advantages in the growth, survival and reproductive aspects of triploid hybrids derived from Crassostrea gigas tetraploids and C. ariakensis diploids in northern China. Aquaculture 2022, 548, 737675. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, C.; Li, Q. Growth, survival and reproductive traits of two genetically improved allotriploid oysters derived from Crassostrea gigas and C. angulata. Aquaculture 2024, 587, 740882. [Google Scholar] [CrossRef]
- Callam, B.R.; Allen, S.K.; Frank-Lawale, A. Genetic and environmental influence on triploid Crassostrea virginica grown in Chesapeake Bay: Growth. Aquaculture 2016, 452, 97–106. [Google Scholar] [CrossRef]
- Bodenstein, S.; Callam, B.R.; Walton, W.C.; Rikard, F.S.; Tiersch, T.R.; La Peyre, J.F. Survival and growth of triploid eastern oysters, Crassostrea virginica, produced from wild diploids collected from low-salinity areas. Aquaculture 2023, 564, 739032. [Google Scholar] [CrossRef]
- Matt, J.L.; Guévélou, E.; Small, J.M.; Allen, S.K. A field test investigating the influence of brood stock origin and ploidy on the susceptibility of Crassostrea virginica to “triploid mortality” in the Chesapeake Bay. Aquaculture 2020, 526, 735375. [Google Scholar] [CrossRef]
- Gjedrem, T. Selection and Breeding Programs in Aquaculture; Springer: Dordrecht, The Netherlands, 2005; pp. 122–132. [Google Scholar] [CrossRef]
- Gordon, I.L. Quantitative Genetics of Intraspecies Hybrids. Heredity 1999, 83, 757–764. [Google Scholar] [CrossRef]
- Volker, P.W.; Potts, B.M.; Borralho, N.M.G. Genetic Parameters of Intra- and Inter-Specific Hybrids of Eucalyptus Globulus and E. Nitens. Tree Genet. Genomes 2008, 4, 445–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Yu, Z.; Yan, X. Evaluation on cross-heritability of interspecific hybrids between female Crassostrea Hongongensis and male Crassostrea gigas. Oceanol. Et Limnol. Sin. 2014, 45, 1367–1373. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CJFD2014&filename=HYFZ201406029&dbcode=CJFD (accessed on 27 January 2025). (In Chinese).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).