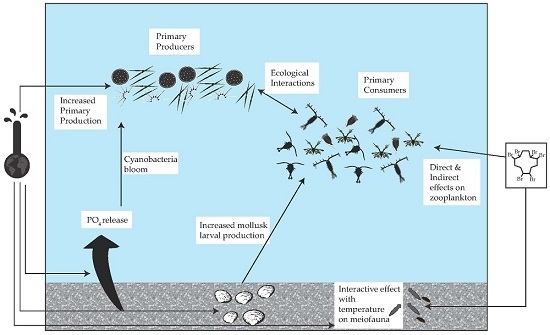
Coastal Ecosystem Effects of Increased Summer Temperature and Contamination by the Flame Retardant HBCDD
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Design
2.2. Collection of Components of the Experimental System
2.3. Spiking of Organic Material with HBCDD
2.4. Experimental Set-Up and Cosm Construction
2.5. Sampling
2.5.1. Whole System Metabolism
2.5.2. Pelagic Endpoints
2.5.3. Benthic Endpoints
2.5.4. CNP and HBCDD Sampling and Analysis
2.6. Data Handling and Statistics
3. Results
3.1. HBCDD Partitioning and Exposure of the Model Ecosystem
3.2. Pelagic Part of the Ecosystem
3.2.1. Phytoplankton
3.2.2. Zooplankton
3.3. Benthic Part of the Ecosystem
3.3.1. Meiofauna
3.3.2. Benthic-Pelagic Coupling
4. Discussion
4.1. Benthic-Pelagic Coupling: Nutrient Release from Sediment and Effects on the Plankton
4.2. Benthic-Pelagic Coupling: Benthic Species’ Larvae in the Zooplankton
4.3. HBCDD Effects
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darling, E.S.; Côté, I.M. Quantifying the evidence for ecological synergies. Ecol. Lett. 2008, 11, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Yletyinen, J.; Biggs, R.; Blenckner, T.; Peterson, G. Marine regime shifts: Drivers and impacts on ecosystems services. Philos. Trans. R. Soc. B Biol. Sci. 2014, 370, 20130273. [Google Scholar] [CrossRef]
- Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012, 76, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- UNEP-AMAP Climate Change and POPs: Predicting the Impacts; Report of the UnePAMAP Expert Group; Secretariat of the Stockholm Convention: Geneva, Switzerland, 2011; pp. 1–66.
- Schiedek, D.; Sundelin, B.; Readman, J.W.; Macdonald, R.W. Interactions between climate change and contaminants. Mar. Pollut. Bull. 2007, 54, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Fleeger, J.W.; Carman, K.R.; Nisbet, R.M. Indirect effects of contaminants in aquatic ecosystems. Sci. Total Environ. 2003, 317, 207–233. [Google Scholar] [CrossRef]
- Jutterström, S.; Andersson, H.C.; Omstedt, A.; Malmaeus, J.M. Multiple stressors threatening the future of the Baltic Sea-Kattegat marine ecosystem: Implications for policy and management actions. Mar. Pollut. Bull. 2014, 86, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Helsinki Commission (HELCOM). Red List of Baltic Sea underwater biotopes, habitats and biotope complexes. In Baltic Sea Environment Proceedings No. 138; HELCOM: Helsinki, Finland, 2013; pp. 1–74. [Google Scholar]
- Helsinki Commission (HELCOM). Climate Change in the Baltic Sea Area—HELCOM Thematic Assessment. In Baltic Sea Environment Proceedings No. 111; HELCOM: Helsinki, Finland, 2007; pp. 1–54. [Google Scholar]
- Helsinki Commission (HELCOM). Climate Change in the Baltic Sea Area Draft HELCOM Thematic Assessment. In Baltic Sea Environment Proceedings No. 137; HELCOM: Helsinki, Finland, 2013. [Google Scholar]
- BACC II Author Team (Ed.) Second Assessment of Climate Change for the Baltic Sea Basin. Regional Climate Studies; Springer: Cham, Germany, 2015. [Google Scholar]
- Helsinki Commission (HELCOM). HELCOM core indicators: Final report of the HELCOM CORESET Project. In Baltic Sea Environment Proceedings No. 136; HELCOM: Helsinki, Finland, 2013; pp. 1–74. [Google Scholar]
- Hansen, J. Benthic Vegetation in Shallow Inlets of the Baltic Sea: Analyses of Human Influences and Proposal of a Method for Assessment of Ecological Status; Report from the EU Central Baltic Interreg IVA Project NANNUT; Department of Botany, Stockholm University: Stockholm, Sweden, 2012. [Google Scholar]
- European Chemicals Agency ECHA (European Chemicals Agency). Risk Assessment: Hexabromocyclododecane; Final Report; ECB: Luxembourg, 2008. [Google Scholar]
- Bignert, A.; Danielsson, S.; Nyberg, E.; Asplund, L. Comments Concerning the National Swedish Contaminant Monitoring Programme in Marine Biota; Swedish Museum of Natural History: Stockholm, Sweden, 2016; Volume 5. [Google Scholar]
- Smolarz, K.; Berger, A. Long-term toxicity of hexabromocyclododecane (HBCDD) to the benthic clam Macoma balthica (L.) from the Baltic Sea. Aquat. Toxicol. 2009, 95, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, P.; Gustafsson, K.; Woin, P. Effects of the Brominated Flame Retardant HBCDD on the Structure of Baltic Sea plankton Communities—A Microcosm Study. In Gustafsson K (2004) Uptake and Toxicity of Brominated Flame Retardants and Pesticides. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2004, unpublished. [Google Scholar]
- Bradshaw, C.; Näslund, J.; Hansen, J.; Kozlowsky-Suzuki, B.; Sundström, B.; Gustafsson, K. Hexabromocyclododecane affects benthic-pelagic coupling in an experimental ecosystem. Environ. Pollut. 2015, 206, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.; Strid, A.; von Stedingk, H.; Gustafsson, K. Effects of benthos, temperature, and dose on the fate of hexabromocyclododecane in experimental coastal ecosystems. Environ. Toxicol. Chem. 2015, 34, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, A.M.; Christoffersen, K. Measurements of chlorophyll—A from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 1987, 109, 445–454. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org (accessed on 3 October 2016).
- Eklöf, J.S.; Havenhand, J.N.; Alsterberg, C.; Gamfeldt, L. Community-level effects of rapid experimental warming and consumer loss outweigh effects of rapid ocean acidification. Oikos 2015, 124, 1040–1049. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O'Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package version 2.4-1. Available online: https://CRAN.R-project.org/package=vegan (accessed on 3 October 2016).
- Boström, B.; Andersen, J.M.; Fleischer, S.; Jansson, M. Exchange of Phosphorus Across the Sediment—Water Interface. Hydrobiologia 1988, 170, 229–244. [Google Scholar] [CrossRef]
- Jensen, H.S.; Andersen, F. Importance of temp etc for PO4 release from lake seds. Limnol. Oceanogr. 1992, 37, 577–589. [Google Scholar] [CrossRef]
- Mortimer, C.H. Chemical Exchanges Between Sediments and Water in the Great Lakes-Speculations on Probable Regulatory Mechanisms. Limnol. Oceangr. 1971, 16, 387–404. [Google Scholar] [CrossRef]
- Lukkari, K.; Leivuori, M.; Kotilainen, A. The chemical character and behaviour of phosphorus in poorly oxygenated sediments from open sea to organic-rich inner bay in the Baltic Sea. Biogeochemistry 2009, 96, 25–48. [Google Scholar] [CrossRef]
- Sanz-Lázaro, C.; Valdemarsen, T.; Holmer, M. Effects of temperature and organic pollution on nutrient cycling in marine sediments. Biogeosciences 2015, 12, 4565–4575. [Google Scholar] [CrossRef]
- Viktorsson, L.; Almroth-Rosell, E.; Tengberg, A.; Vankevich, R.; Neelov, I.; Isaev, A.; Kravtsov, V.; Hall, P.O.J. Benthic Phosphorus Dynamics in the Gulf of Finland, Baltic Sea. Aquat. Geochem. 2012, 18, 543–564. [Google Scholar] [CrossRef]
- Gächter, R.; Meyer, J.S. The Role of Microorganisms in Mobilization and Fixation of Phosphorus in Sediments. Hydrobiologia 1993, 253, 103–121. [Google Scholar] [CrossRef]
- Holdren, G.C.; Armstrong, D.E. Factors Affecting Phosphorus Release From Intact Lake Sediment Cores. Environ. Sci. Technol. 1980, 14, 79–87. [Google Scholar] [CrossRef]
- Jensen, H.S.; Mortensen, P.B.; Andersen, F.O.; Rasmussen, E.; Jensen, A. Phosphorus Cycling in a Coastal Marine Sediment, Aarhus Bay, Denmark. Limnol. Oceangr. 1995, 40, 908–917. [Google Scholar] [CrossRef]
- Koop, K.; Boynton, W.R.; Wulff, F.; Carman, R. Sediment-Water Oxygen and Nutrient Exchanges Along a Depth Gradient in the Baltic Sea. Mar. Ecol. Prog. Ser. 1990, 63, 65–77. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Duberg, J.; Motwani, N.H.; Hogfors, H.; Klawonn, I.; Ploug, H.; Svedén, J.B.; Garbaras, A.; Sundelin, B.; Hajdu, S.; et al. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio 2015, 44, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Walve, J.; Larsson, U. Seasonal changes in Baltic Sea seston stoichiometry: The influence of diazotrophic cyanobacteria. Mar. Ecol. Prog. Ser. 2010, 407, 13–25. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Winder, M. Seasonal dynamics of zooplankton resource use revealed by carbon amino acid stable isotope values. Mar. Ecol. Prog. Ser. 2015, 531, 143–154. [Google Scholar] [CrossRef]
- Brandl, Z. Freshwater Copepods and Rotifers: Predators and their Prey. Hydrobiologia 2005, 546, 475–489. [Google Scholar] [CrossRef]
- Bonsdorff, E.; Norkko, A.; Boström, C. Recruitment and population maintenance of the bivalve Macoma balthica (L.)—factors affecting settling success and early survival on shallow sandy bottoms. In Biology and Ecology of Shallow Coastal Waters: Proceedings of the 28 EMBS Symposium; Olsen & Olsen: Fredensborg, Denmark, 1995; pp. 253–260. [Google Scholar]
- Caddy, J.F. Development of Mantle Organs, Feeding, and Locomotion in Postlarval Macoma balthica (L) (Lamellibranchiata). Can. J. Zool. 1969, 47, 609–617. [Google Scholar] [CrossRef]
- Drent, J. Temperature responses in larvae of Macoma balthica from a northerly and southerly population of the European distribution range. J. Exp. Mar. Biol. Ecol. 2002, 275, 117–129. [Google Scholar] [CrossRef]
- De Goeij, P.; Honkoop, P.J. Experimental effects of immersion time and water temperature on body condition, burying depth and timing of spawning of the tellinid bivalve Macoma balthica. Helgol. Mar. Res. 2003, 57, 20–26. [Google Scholar]
- Kennedy, V.S.; Mihursky, J.A. Upper temperature tolerances of some estuarine bivalves. Chesap. Sci. 1971, 12, 193–204. [Google Scholar] [CrossRef]
- Bos, O.G.; Philippart, C.; van der Meer, J. Effects of temporary food limitation on development and mortality of Macoma balthica larvae. Mar. Ecol. Prog. Ser. 2007, 330, 155–162. [Google Scholar] [CrossRef]
- Bos, O.G.; Hendriks, I.E.; Strasser, M.; Dolmer, P.; Kamermans, P. Estimation of food limitation of bivalve larvae in coastal waters of north-western Europe. J. Sea Res. 2006, 55, 191–206. [Google Scholar] [CrossRef]
- Bos, O.G.; Philippart, C.J.M.; Cadee, G.C.; van der Meer, J. Recruitment variation in Macoma balthica: A laboratory examination of the match/mismatch hypothesis. Mar. Ecol. Prog. Ser. 2006, 320, 207–214. [Google Scholar] [CrossRef]
- Raby, D.; Mingelbier, M.; Dodson, J.J.; Klein, B.; Lagadeuc, Y.; Legendre, L. Food-particle size and selection by bivalve larvae in a temperate embayment. Mar. Biol. 1997, 127, 665–672. [Google Scholar] [CrossRef]
- Lassen, H.H. Reproductive effort in Danish mudsnails (Hydrobiidae). Oecologia 1979, 40, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.D.; Fish, S. The effects of temperature and salinity on the embryonic development of Hydrobia ulvae (Pennant). J. Mar. Biol. Assoc. UK 1977, 57, 213–218. [Google Scholar] [CrossRef]
- Hagerthey, S.E.; Defew, E.C.; Paterson, D.M. Influence of Corophium volutator and Hydrobia ulvae on intertidal benthic diatom assemblages under different nutrient and temperature regimes. Mar. Ecol. Prog. Ser. 2002, 245, 47–59. [Google Scholar] [CrossRef]
- Blanchard, G.F.; Guarini, J.-M.; Provot, L.; Richard, P.; Sauriau, P.-G. Measurement of ingestion rate of Hydrobia ulvae (Pennant) on intertidal epipelic microalgae: The effect of mud snail density. J. Exp. Mar. Biol. Ecol. 2000, 255, 247–260. [Google Scholar] [CrossRef]
- De Troch, M.; Chepurnov, V.A.; Vincx, M.; Olafsson, E. The effect of Fucus vesiculosus on the grazing of harpacticoid copepods on diatom biofilms. J. Sea Res. 2008, 60, 139–143. [Google Scholar] [CrossRef]
- Nascimento, F.; Karlson, A. Settling blooms of filamentous cyanobacteria as food for meiofauna assemblages. Limnol. Oceanogr. 2008, 53, 2636–2643. [Google Scholar] [CrossRef]
- Olafsson, E.; Modig, H.; van de Bund, W.J. Species specific uptake of radio-labelled phyto-detritus by benthic meiofauna from the Baltic Sea. Mar. Ecol. Prog. Ser. 1999, 177, 63–72. [Google Scholar] [CrossRef]
- Martens, P.M.; Schockaert, E.R. The Importance of Turbellarians in the Marine Meiobenthos—A Review. Hydrobiologia 1986, 132, 295–303. [Google Scholar] [CrossRef]
- Nascimento, F.J.A.; Karlson, A.M.L.; Näslund, J.; Elmgren, R. Diversity of larger consumers enhances interference competition effects on smaller competitors. Oecologia 2011, 166, 337–347. [Google Scholar] [CrossRef] [PubMed]










| Sample | Mean | SE | n | % Present in the Dissolved Phase |
|---|---|---|---|---|
| Water (unfiltered) (μg L−1) | ||||
| t1, A+ | 2.12 × 10−3 | 1.14 × 10−5 | 2 | 83.7 |
| t1, W+ | 1.08 × 10−3 | 1.09 × 10−5 | 2 | 82.8 |
| t13, A+ | 2.92 × 10−5 | 1.66 × 10−6 | 2 | 66.5 |
| t13, W+ | 3.98 × 10−5 | 1 | 78.7 | |
| Surface sediment (μg gdw−1) | ||||
| t1, A+ | 2.46 × 10−1 | 7.16 × 10−2 | 4 | |
| t1, W+ | 1.29 × 10−1 | 3.87 × 10−2 | 4 | |
| t13, A+ | 3.82 × 10−1 | 1.46 × 10−1 | 3 | |
| t13, W+ | 1.71 × 10−1 | 3.02 × 10−2 | 3 | |
| Limecola (μg g lipid−1) | ||||
| t13, A+ | 1.18 × 102 | |||
| t13, W+ | 9.63 × 101 |
| t6 | t13 | ||||
|---|---|---|---|---|---|
| Correlated Variable | r | p | Correlated Variable | r | p |
| Benthic primary production | |||||
| Dolichospermum heterocysts | 0.670 | 0.001 | |||
| PO4 | 0.657 | 0.002 | no significant correlations | ||
| Melosira sp. | 0.627 | 0.003 | |||
| Dolichospermum filaments | 0.596 | 0.006 | |||
| Chlorophyll a | 0.478 | 0.033 | |||
| Bivalve larvae | |||||
| Pelagic PP | 0.694 | 0.002 | no significant correlations | ||
| PO4 | −0.631 | 0.007 | |||
| Dolichospermum heterocysts | 0.583 | 0.014 | |||
| Gastropod larvae | Gastropod larvae | ||||
| PO4 | 0.718 | 0.000 | Total meiofauna | 0.620 | 0.008 |
| Chlorophyll a | 0.511 | 0.021 | Chlorophyll a | −0.566 | 0.018 |
| Dolichospermum heterocysts | 0.501 | 0.024 | Dolichospermum heterocysts | −0.505 | 0.039 |
| PO4 | 0.475 | 0.054 | |||
| Hydrobiidae | no data available | ||||
| Total N | 0.449 | 0.047 | |||
| no data available | Total Limecola balthica biomass (gdw) | ||||
| Melosira sp. | −0.481 | 0.050 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradshaw, C.; Golz, A.-L.; Gustafsson, K. Coastal Ecosystem Effects of Increased Summer Temperature and Contamination by the Flame Retardant HBCDD. J. Mar. Sci. Eng. 2017, 5, 18. https://doi.org/10.3390/jmse5020018
Bradshaw C, Golz A-L, Gustafsson K. Coastal Ecosystem Effects of Increased Summer Temperature and Contamination by the Flame Retardant HBCDD. Journal of Marine Science and Engineering. 2017; 5(2):18. https://doi.org/10.3390/jmse5020018
Chicago/Turabian StyleBradshaw, Clare, Anna-Lea Golz, and Kerstin Gustafsson. 2017. "Coastal Ecosystem Effects of Increased Summer Temperature and Contamination by the Flame Retardant HBCDD" Journal of Marine Science and Engineering 5, no. 2: 18. https://doi.org/10.3390/jmse5020018
APA StyleBradshaw, C., Golz, A.-L., & Gustafsson, K. (2017). Coastal Ecosystem Effects of Increased Summer Temperature and Contamination by the Flame Retardant HBCDD. Journal of Marine Science and Engineering, 5(2), 18. https://doi.org/10.3390/jmse5020018