An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea)
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Field Work: Sampling and Processing
3. Results
3.1. Collector Colonization and Growth of Sabellid Worms
3.2. Growing of the Sponges
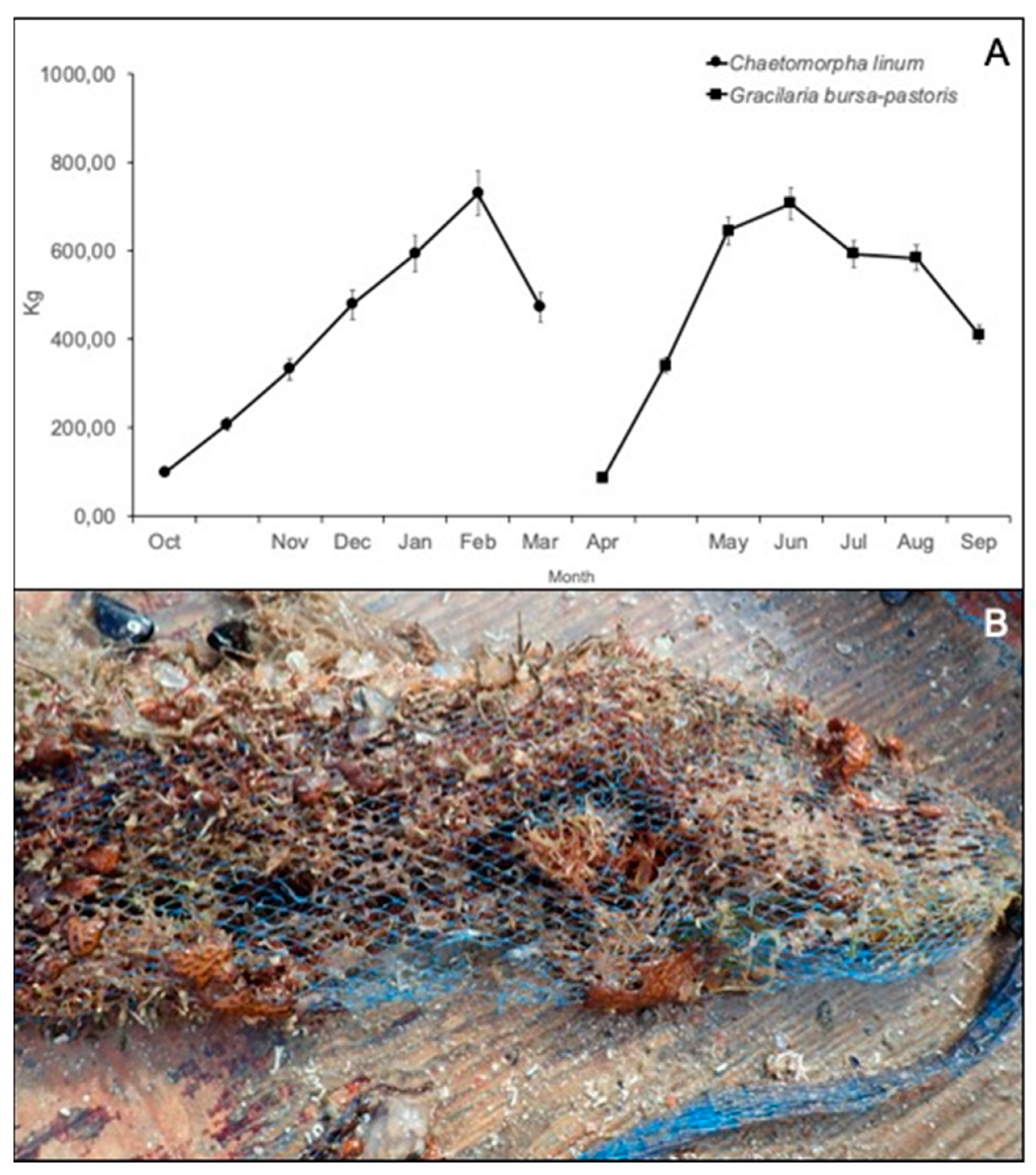
3.3. Growing of Macroalgae
4. Discussion and Conclusions
4.1. Performances of the Target Species
4.1.1. Polychaetes
4.1.2. Sponges
4.1.3. Macroalgae
4.2. Conclusive Remarks
Author Contributions
Funding
Aknowledgments
Conflicts of Interest
References
- Karakassis, I.; Tsapakis, M.; Hatziyanni, E.; Papadopoulou, K.N.; Plaiti, W. Impact of cage farming of fish on the seabed in three Mediterranean coastal areas. ICES J. Mar. Sci. 2000, 57, 1462–1471. [Google Scholar] [CrossRef]
- Mazzola, A.; Sarà, G. The effect of fish farming organic waste on food availability for bivalve molluscs (Gaeta Gulf, Central Tyrrhenian, MED): Stable carbon isotopic analysis. Aquaculture 2001, 192, 361–379. [Google Scholar] [CrossRef]
- Sarà, G.; Zenone, A.; Tomasello, A. Growth of Mytilus galloprovincialis (mollusca, bivalvia) close to fish farms: A case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 2009, 636, 129–136. [Google Scholar] [CrossRef]
- Moraitis, M.; Papageorgiou, N.; Dimitriou, P.D.; Petrou, A.; Karakassis, I. Effects of offshore tuna farming on benthic assemblages in the Eastern Mediterranean. Aquac. Environ. Interact. 2013, 4, 41–51. [Google Scholar] [CrossRef]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F.L. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges? A review. Rev. Aquac. 2016, 8, 283–300. [Google Scholar] [CrossRef]
- Hughes, A.D.; Black, K.D. Going beyond the search for solutions: Understanding trade-offs in European integrated multi-trophic aquaculture development. Aquac. Environ. Interact. 2016, 8, 191–199. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In Integrated Mariculture: A Global Review; Soto, D., Ed.; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2009; Volume 529, pp. 7–46. [Google Scholar]
- Fang, J.; Zhang, J.; Xiao, T.; Huang, D.; Liu, S. Integrated multi-trophic aquaculture (IMTA) in Sanggou Bay, China. Aquac. Environ. Interact. 2016, 8, 201–205. [Google Scholar] [CrossRef]
- Heilskov, A.C.; Alperin, M.; Holmer, M. Benthic fauna bio-irrigation effects on nutrient regeneration in fish farm sediments. J. Exp. Mar. Biol. Ecol. 2006, 339, 204–225. [Google Scholar] [CrossRef]
- Kinoshita, K.; Tamaki, S.; Yoshioka, M.; Srithonguthai, S.; Kunihiro, T.; Ohwada, K.; Tsutsumi, H. Bioremediation of organically enriched sediment deposited below fish farms with artificially mass-cultured colonies of a deposit-feeding polychaete Capitella sp. I. Fish. Sci. 2008, 74, 77–87. [Google Scholar] [CrossRef]
- Wang, X.; Olsen, L.M.; Reitan, K.I.; Olsen, Y. Discharge of nutrient wastes from salmon farms: Environmental effects, and potential for integrated multi-trophic aquaculture. Aquac. Environ. Interact. 2012, 2, 267–283. [Google Scholar] [CrossRef]
- Bergström, P.; Carlsson, M.S.; Lindegarth, M.; Petersen, J.K.; Lindegarth, S.; Holmer, M. Testing the potential for improving quality of sediments impacted by mussel farms using bioturbating polychaete worms. Aquac. Res. 2017, 48, 161–176. [Google Scholar] [CrossRef]
- Israel, D.; Lupatsch, I.; Angel, D.L. Testing the digestibility of seabream wastes in three candidates for integrated multi-trophic aquaculture: Grey mullet, sea urchin and sea cucumber. Aquaculture 2019, 510, 364–370. [Google Scholar] [CrossRef]
- Jansen, H.M.; Hansen, P.K.; Brennan, N.; Dahlgren, T.G.; Fang, J.; Nederlof, M.A.J.; Strohmeier, T.; Sveier, H.; Strand, Ø. Enhancing opportunistic polychaete communities under fish farms: An alternative concept for integrated aquaculture. Aquac. Environ. Interact. 2019, 11, 331–336. [Google Scholar] [CrossRef]
- Cromey, C.J.; Nickell, T.D.; Black, K.D. DEPOMOD—Modelling the deposition and biological effects of waste solids from marine cage farms. Aquaculture 2002, 214, 211–239. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Mazzola, A.; Modica, A. Effects of fish farming waste to sedimentary and particulate organic matter in a southern Mediterranean area (Gulf of Castellammare, Sicily): A multiple stable isotope study ($δ$13C and $δ$15N). Aquaculture 2004, 234, 199–213. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Milazzo, M.; Modica, A. Use of stable isotopes to investigate dispersal of waste from fish farms as a function of hydrodynamics. Mar. Ecol. Prog. Ser. 2006, 313, 261–270. [Google Scholar] [CrossRef]
- Sarà, G.; Lo Martire, M.; Buffa, G.; Mannino, A.M.; Badalamenti, F. The fouling community as an indicator of fish farming impact in Mediterranean. Aquac. Res. 2007, 38, 66–75. [Google Scholar] [CrossRef]
- Black, K.D. Sustainability of aquaculture. In Environmental Impacts of Aquaculture; Black, K.D., Ed.; Sheffield Academic Press: Sheffield, UK, 2001; pp. 199–212. [Google Scholar]
- Angel, D.L.; Eden, N.; Breitstein, S.; Yurman, A.; Katz, T.; Spanier, E. In situ biofiltration: A means to limit the dispersal of effluents from marine finfish cage aquaculture. Hydrobiologia 2002, 469, 1–10. [Google Scholar] [CrossRef]
- Angel, D.L.; Spanier, E. An application of artificial reefs to reduce organic enrichment caused by net-cage fish farming: Preliminary results. ICES J. Mar. Sci. 2002, 59, S324–S329. [Google Scholar] [CrossRef]
- Hughes, D.J.; Cook, E.J.; Sayer, M.D.J. Biofiltration and biofouling on artificial structures in Europe: The potential for mitigating organic impacts. Oceanogr. Mar. Biol. 2005, 43, 123. [Google Scholar]
- Chopin, T.; Robinson, S.M.C.; Troell, M.; Neori, A.; Buschmann, A.; Fang, J.G. Multi-trophic integration for sustainable marine aquaculture. In The Encyclopaedia of Ecology, 1st ed.; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Oxford, UK, 2008; Volume 3, pp. 2463–2475. [Google Scholar]
- Chopin, T.; Cooper, J.A.; Reid, G.; Cross, S.; Moore, C. Open-water integrated multi-trophic aquaculture: Environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquac. 2012, 4, 209–220. [Google Scholar] [CrossRef]
- Ren, J.S.; Stenton-Dozey, J.; Plew, D.R.; Fang, J.; Gall, M. An ecosystem model for optimising production in integrated multitrophic aquaculture systems. Ecol. Modell. 2012, 246, 34–46. [Google Scholar] [CrossRef]
- Woods, C.M.C.; Floerl, O.; Hayden, B.J. Biofouling on Greenshell mussel (Perna canaliculus) farms: A preliminary assessment and potential implications for sustainable aquaculture practices. Aquac. Int. 2012, 20, 537–557. [Google Scholar] [CrossRef]
- Gonzalez-Silvera, D.; Izquierdo-Gomez, D.; Fernandez-Gonzalez, V.; Martínez-López, F.J.; López-Jiménez, J.A.; Sanchez-Jerez, P. Mediterranean fouling communities assimilate the organic matter derived from coastal fish farms as a new trophic resource. Mar. Pollut. Bull. 2015, 91, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Montalto, V.; Rinaldi, A.; Ape, F.; Mangano, M.C.; Gristina, M.; Sarà, G.; Mirto, S. Functional role of biofouling linked to aquaculture facilities in Mediterranean enclosed locations. Aquac. Environ. Interact. 2020, 12, 11–22. [Google Scholar] [CrossRef]
- Navarrete-Mier, F.; Sanz-Lázaro, C.; Marín, A. Does bivalve mollusc polyculture reduce marine fin fish farming environmental impact? Aquaculture 2010, 306, 101–107. [Google Scholar] [CrossRef]
- Mahmood, T.; Fang, J.; Jiang, Z.; Zhang, J. Carbon and nitrogen flow, and trophic relationships, among the cultured species in an integrated multi-trophic aquaculture (IMTA) bay. Aquac. Environ. Interact. 2016, 8, 207–219. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, S.; Zhang, G.; Ning, X.; Li, R.; Jiang, Z.; Fang, J.; Zhang, J. Impacts of an integrated multi-trophic aquaculture system on benthic nutrient fluxes: A case study in Sanggou Bay, China. Aquac. Environ. Interact. 2016, 8, 221–232. [Google Scholar] [CrossRef]
- Sanz-Lazaro, C.; Sanchez-Jerez, P. Mussels do not directly assimilate fish farm wastes: Shifting the rationale of integrated multi-trophic aquaculture to a broader scale. J. Environ. Manag. 2017, 201, 82–88. [Google Scholar] [CrossRef]
- Giangrande, A.; Lezzi, M.; Del Pasqua, M.; Pierri, C.; Longo, C.; Gravina, M.F. Two cases study of fouling colonization patterns in the Mediterranean sea in the perspective of integrated aquaculture system. Aquac. Rep. 2020, in press. [Google Scholar] [CrossRef]
- Badalamenti, F.; D’anna, G.; Gristina, M.; Scalisi, M.; Tumbiolo, L. Remarks on a method to quantify the total biomass of a benthic community on artificial substrata. Rapp. Comm. Int. Mer Mediterr. 1992, 33, 377. [Google Scholar]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef]
- Giangrande, A.; Cavallo, A.; Licciano, M.; Mola, E.; Pierri, C.; Trianni, L. Utilization of the filter feeder polychaete Sabella spallanzanii Gmelin (Sabellidae) as bioremediator in aquaculture. Aquac. Int. 2005, 13, 129–136. [Google Scholar] [CrossRef]
- Licciano, M.; Stabili, L.; Giangrande, A. Clearance rates of Sabella spallanzanii and Branchiomma luctuosum (Annelida: Polychaeta) on a pure culture of Vibrio alginolyticus. Water Res. 2005, 39, 4375–4384. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Pusceddu, A.; Danovaro, R.; Giangrande, A. Particulate organic matter uptake rates of two benthic filter-feeders (Sabella spallanzanii and Branchiomma luctuosum) candidates for the clarification of aquaculture wastewaters. Mar. Pollut. Bull. 2007, 54, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Schirosi, R.; Licciano, M.; Mola, E.; Giangrande, A. Bioremediation of bacteria in aquaculture waste using the polychaete Sabella spallanzanii. New Biotechnol. 2010, 27, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Licciano, M.; Lezzi, M.; Giangrande, A. Microbiological accumulation by the Mediterranean invasive alien species Branchiomma bairdi (Annelida, Sabellidae): Potential tool for bioremediation. Mar. Pollut. Bull. 2014, 86, 325–331. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering activity of Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae) on bacterioplankton: Implications for bioremediation of polluted seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Longo, C.; Corriero, G.; Mercurio, M. Evaluation of microbiological accumulation capability of the commercial sponge Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae). Water Res. 2008, 42, 2499–2506. [Google Scholar] [CrossRef]
- Longo, C.; Corriero, G.; Licciano, M.; Stabili, L. Bacterial accumulation by the Demospongiae Hymeniacidon perlevis: A tool for the bioremediation of polluted seawater. Mar. Pollut. Bull. 2010, 60, 1182–1187. [Google Scholar] [CrossRef]
- Longo, C.; Cardone, F.; Corriero, G.; Licciano, M.; Pierri, C.; Stabili, L. The co-occurrence of the demosponge Hymeniacidon perlevis and the edible mussel Mytilus galloprovincialis as a new tool for bacterial load mitigation in aquaculture. Environ. Sci. Pollut. Res. 2016, 23, 3736–3746. [Google Scholar]
- Giangrande, A.; Cavallo, A.; Pierri, C. Ammonium uptake of Cladophora prolifera (Chlorophyta, Cladophorales) a candidate species for bioremediation of aquaculture wastes. Thalass. Salentina 2007, 30, 107–116. [Google Scholar]
- Alabiso, G.; Cecere, E.; Petrocelli, A.; Ricci, P. Ammonium uptake by Gracilaria dura (Rhodophyta, Gracilariales) from the Mar Piccolo of Taranto. Biol. Mar. Mediterr. 2007, 14, 268–269. [Google Scholar]
- Alabiso, G.; Ricci, P.; Belmonte, M.; Petrocelli, A.; Cecere, E. Ammonium uptake by Gracilaria gracilis (Gracilariales, Rhodophyta) (Stackhouse) Steentoft, Irvine and Farnham, from the Mar Piccolo of Taranto. Biol. Mar. Mediterr. 2009, 16, 246–247. [Google Scholar]
- Aquilino, F.; Paradiso, A.; Trani, R.; Longo, C.; Pierri, C.; Corriero, G.; De Pinto, M.C. Chaetomorpha linum in the bioremediation of aquaculture wastewater: Optimization of nutrient removal efficiency at the laboratory scale. Aquaculture 2020, 523, 735133. [Google Scholar]
- Stabili, L.; Acquaviva, M.I.; Angilè, F.; Cavallo, R.A.; Cecere, E.; Del Coco, L.; Fanizzi, F.P.; Gerardi, C.; Narracci, M.; Petrocelli, A. Screening of Chaetomorpha linum lipidic extract as a new potential source of bioactive compounds. Mar. Drugs 2019, 17, 313. [Google Scholar]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.-J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar]
- Pierri, C.; Fanelli, G.; Giangrande, A. Experimental co-culture of low food-chain organisms, Sabella spallanzanii (Polychaeta, Sabellidae) and Cladophora prolifera (Chlorophyta, Cladophorales), in Porto Cesareo area (Mediterranean Sea). Aquac. Res. 2006, 37, 966–974. [Google Scholar]
- Giangrande, A.; Pierri, C.; Fanelli, G.; Schirosi, R.; Licciano, M.; Stabili, L. Rearing experiences of the polychaete Sabella spallanzanii in the Gulf of Taranto (Mediterranean Sea, Italy). Aquac. Int. 2014, 22, 1677–1688. [Google Scholar]
- Pronzato, R.; Cerrano, C.; Cubeddu, T.; Lanza, S.; Magnino, G.; Manconi, R.; Pantelis, J.; Sara, A.; Sidri, M. Sustainable development in coastal areas: Role of sponge farming in integrated aquaculture. In Aquaculture and Water: Fish Culture, Shellfish Culture and Water Usage; Luiz, F., Aspeslagh, L., Van Craeynest, S., Eds.; Special Pubblication No. 26; European Aquaculture Society: Oostende, Belgium, 1998; pp. 231–232. [Google Scholar]
- Manconi, R.; Cubeddu, T.; Corriero, G.; Pronzato, R. Commercial sponges farming as natural control of floating cages pollution. In New Species for Mediterranean Aquaculture; Enne, G., Greppi, G.F., Eds.; Biofutur Elsevier: Paris, France, 1999; pp. 269–274. [Google Scholar]
- Corriero, G.; Longo, C.; Mercurio, M.; Marzano, C.N.; Lembo, G.; Spedicato, M.T. Rearing performance of Spongia officinalis on suspended ropes off the Southern Italian Coast (Central Mediterranean Sea). Aquaculture 2004, 238, 195–205. [Google Scholar]
- Gökalp, M.; Wijgerde, T.; Sarà, A.; De Goeij, J.M.; Osinga, R. Development of an integrated mariculture for the collagen-rich sponge Chondrosia reniformis. Mar. Drugs 2019, 17, 29. [Google Scholar] [CrossRef]
- Gökalp, M.; Kooistra, T.; Rocha, M.S.; Silva, T.H.; Osinga, R.; Murk, A.J.; Wijgerde, T. The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847). Mar. Drugs 2020, 18, 358. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Chopin, T. Marine aquaculture in Canada: Well-established monocultures of finfish and shellfish and an emerging integrated multi-trophic aquaculture (IMTA) approach including seaweeds, other invertebrates, and microbial communities. Fisheries 2015, 40, 28–31. [Google Scholar] [CrossRef]
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The Bioremediation Potential of Seaweeds: Recycling Nitrogen, Phosphorus, and Other Waste Products. In Blue Biotechnology: Production and Use of Marine Molecules; La Barre, S., Bates, S.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 1, pp. 217–239. [Google Scholar]
- Prazukin, A.V.; Anufriieva, E.V.; Shadrin, N.V. Is biomass of filamentous green algae Cladophora spp. (Chlorophyta, Ulvophyceae) an unlimited cheap and valuable resource for medicine and pharmacology? A review. In Reviews in Aquaculture; John Wiley & Sons, Ltd.: Queensland, Australia, 2020; Volume 1. [Google Scholar]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? New Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. New Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Freile-Pelegrín, Y.; Tasdemir, D. Seaweeds to the rescue of forgotten diseases: A review. Bot. Mar. 2019, 62, 211–226. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Ryther, J.H.; DeBoer, J.A.; Lapointe, B.E. Cultivation of seaweeds for hydrocolloids, waste treatment and biomass for energy conversion. In Proceedings of the Ninth Proc. Int. Seaweed Symp; SciencePress: Princeton, NJ, USA, 1977; Volume 9, pp. 1–16. [Google Scholar]
- Friedlander, M.; Zelikovitch, N. Growth rates, phycocolloid yield and quality of the red seaweeds, Gracilaria sp., Pterocladia capillacea, Hypnea musciformis, and Hypnea cornuta, in field studies in Israel. Aquaculture 1984, 40, 57–66. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M.; Guttman, L.; Israel, A. Development of polyculture and integrated multi-trophic aquaculture (IMTA) in Israel: A review. Isr. J. Aquac. 2017, 69, 1–19. [Google Scholar]
- Largo, D.B.; Diola, A.G.; Marababol, M.S. Development of an integrated multi-trophic aquaculture (IMTA) system for tropical marine species in southern Cebu, Central Philippines. Aquac. Rep. 2016, 3, 67–76. [Google Scholar] [CrossRef]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Kleitou, P.; Kletou, D.; David, J. Is Europe ready for integrated multi-trophic aquaculture? A survey on the perspectives of European farmers and scientists with IMTA experience. Aquaculture 2018, 490, 136–148. [Google Scholar] [CrossRef]
- Cecere, E.; Fanelli, G.; Petrocelli, A.; Portacci, G.; Saracino, O.D. Polyculture of Gracilaria dura (Rhodophyta, Gracilariales) with mussels and polychaetes in the Mar Piccolo of Taranto: Preliminary results. In Proceedings of the 18th International Seaweed Symposium, Bergen, Norway, 20–25 June 2004. [Google Scholar]
- Pierri, C.; Longo, C.; Giangrande, A. Variability of fouling communities in the Mar Piccolo of Taranto (Northern Ionian Sea, Mediterranean Sea). J. Mar. Biol. Assoc. UK 2010, 90, 159–167. [Google Scholar] [CrossRef]
- Lezzi, M.; Del Pasqua, M.; Pierri, C.; Giangrande, A. Seasonal non-indigenous species succession in a marine macrofouling invertebrate community. Biol. Invasions 2018, 20, 937–961. [Google Scholar] [CrossRef]
- Lezzi, M.; Giangrande, A. Seasonal and bathymetric effects on macrofouling invertebrates’ primary succession in a mediterraenan non-indigenous species hotspot area. Mediterr. Mar. Sci. 2018, 19, 572–588. [Google Scholar] [CrossRef]
- Del Pasqua, M.; Borghese, J.; Arduini, D.; Licciano, M.; Giangrande, A. Ex ante monitoring assessment of an aquaculture plan within the project remedia life: The use of polychaetes as indicators. Biol. Mar. Mediterr. 2019, 26, 53–56. [Google Scholar]
- Pierri, C.; Colangelo, P.; Del Pasqua, M.; Longo, C.; Giangrande, A. Consequences of the experimental removal of Sabella spallanzanii (Gmelin, 1791) from the fouling assemblage of a Mediterranean harbour. Mediterr. Mar. Sci. 2019, 20, 476–486. [Google Scholar] [CrossRef]
- Stabili, L.; Cecere, E.; Licciano, M.; Petrocelli, A.; Sicuro, B.; Giangrande, A. Integrated Multitrophic Aquaculture By-Products with Added Value: The Polychaete Sabella spallanzanii and the Seaweed Chaetomorpha linum as Potential Dietary Ingredients. Mar. Drugs 2019, 17, 677. [Google Scholar] [CrossRef]
- Abed, C.; Legrave, N.; Dufies, M.; Robert, G.; Guérineau, V.; Vacelet, J.; Auberger, P.; Amade, P.; Mehiri, M. A new hydroxylated nonaprenylhydroquinone from the Mediterranean marine sponge Sarcotragus spinosulus. Mar. Drugs 2011, 9, 1210–1219. [Google Scholar] [CrossRef]
- Mercurio, M.; Corriero, G.; Gherardi, M.; Baldacconi, R.; Gaino, E. Sexual reproduction in Sarcotragus spinosulus from two different shallow environments. Mar. Ecol. 2013, 34, 394–408. [Google Scholar]
- Perez-Lopez, P.; Ledda, F.D.; Bisio, A.; Feijoo, G.; Perino, E.; Pronzato, R.; Manconi, R.; Moreira, M.T. Life cycle assessment of in situ mariculture in the Mediterranean Sea for the production of bioactive compounds from the sponge Sarcotragus spinosulus. J. Clean. Prod. 2017, 142, 4356–4368. [Google Scholar] [CrossRef]
- Petrocelli, A.; Cecere, E.; Rubino, F. Successions of phytobenthos species in a Mediterranean transitional water system: The importance of long term observations. Nat. Conserv. 2019, 34, 217. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Yong, Y.S.; Yong, W.T.L.; Anton, A. Analysis of formulae for determination of seaweed growth rate. J. Appl. Phycol. 2013, 25, 1831–1834. [Google Scholar] [CrossRef]
- Carvalho, L.M.C.; Turra, A.; Alves, J.L.; De Almeida Marques, H.L. Capture of anemones and polychaetes in artificial collectors for ornamental purposes, effects of: Depth, deployment period, and time of immersion. Bol. Inst. Pesca 2019, 45. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M.; Pagliara, P.; Gambi, M.C. Gametogenesis and larval development in Sabella spallanzanii (Polychaeta: Sabellidae) from the Mediterranean Sea. Mar. Biol. 2000, 136, 847–861. [Google Scholar] [CrossRef]
- Longo, C.; Scalera-Liaci, L.; Manuel, M.; Corriero, G. Note sui poriferi del Mar Grande e del Mar Piccolo di Taranto (Mar Ionio). Biol. Mar. Mediterr. 2004, 11, 440–443. [Google Scholar]
- Scalera Liaci, L.; Mercurio, M.; Palladino, F.; Massari, S.; Corriero, G. La spongicoltura: Una forma di maricoltura costiera compatibile con i vincoli di tutela delle aree protette. In Proceedings of the 29° Congresso S.I.B.M., Ustica, Italy, 15–20 January 1998. [Google Scholar]
- Scalera Liaci, L.; Mercurio, M.; Palladino, F.; Massari, S.; Corriero, G. L’allevamento di spugne commerciali nella Riserva Marina di Porto Cesareo (LE). Biol. Mar. Mediterr. 1999, 6, 110–118. [Google Scholar]
- Mercurio, M.; Longo, C.; Nonnis Marzano, C.; Scalera Liaci, L.; Corriero, G. L’allevamento di spugne commerciali nella Riserva Naturale Marina ‘Isola di Ustica’. Biol. Mar. Mediterr. 2003, 10, 462–464. [Google Scholar]
- Van Treeck, P.; Eisinger, M.; Müller, J.; Paster, M.; Schuhmacher, H. Mariculture trials with Mediterranean sponge species: The exploitation of an old natural resource with sustainable and novel methods. Aquaculture 2003, 218, 439–455. [Google Scholar] [CrossRef]
- De Voogd, N.J. The mariculture potential of the Indonesian reef-dwelling sponge Callyspongia (Euplacella) biru: Growth, survival and bioactive compounds. Aquaculture 2007, 262, 54–64. [Google Scholar] [CrossRef]
- De Caralt, S.; Sánchez-Fontenla, J.; Uriz, M.J.; Wijffels, R.H. In situ aquaculture methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Mar. Drugs 2010, 8, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Sidri, M.; Cerig, E.; Gokalp, S.Z.; Gokalp, M. Sponge aquaculture trials in the East-Mediterranean Sea: New approaches to earlier ideas. Open Mar. Biol. J. 2010, 4, 74–81. [Google Scholar] [CrossRef]
- Nemoy, P.; Spanier, E.; Angel, D. Sustainable cultivation of sponges in the Eastern Mediterranean Sea: Integrated aquaculture with fish farms. In Proceedings of the 10th World Sponge Conference, NUI, Galway, Ireland, 25–30 June 2017; p. 89. [Google Scholar]
- Pronzato, R.; Manconi, R. Mediterranean commercial sponges: Over 5000 years of natural history and cultural heritage. Mar. Ecol. 2008, 29, 146–166. [Google Scholar] [CrossRef]
- Holdt, S.L.; Edwards, M.D. Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. J. Appl. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Pereira, R.; Yarish, C.; Critchley, A.T. Seaweed aquaculture for human foods in land-based and IMTA systems. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2013; pp. 9109–9128. [Google Scholar]
- Ge, S.; Champagne, P. Cultivation of the marine macroalgae Chaetomorpha linum in municipal wastewater for nutrient recovery and biomass production. Environ. Sci. Technol. 2017, 51, 3558–3566. [Google Scholar] [CrossRef]
- Korzen, L.; Abelson, A.; Israel, A. Growth, protein and carbohydrate contents in Ulva rigida and Gracilaria bursa-pastoris integrated with an offshore fish farm. J. Appl. Phycol. 2016, 28, 1835–1845. [Google Scholar] [CrossRef]
- Bermejo, R.; Cara, C.L.; Macías, M.; Sánchez-García, J.; Hernández, I. Growth rates of Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei (Rhodophyta) cultured in ropes: Implication for N biomitigation in Cadiz Bay (Southern Spain). J. Appl. Phycol. 2020, 32, 1–13. [Google Scholar] [CrossRef]
- De Casabianca, M.L.; Marinho-Soriano, E.; Laugier, T. Growth of Gracilaria bursa-pastoris in a Mediterranean lagoon: Thau, France. Bot. Mar. 1997, 40, 29–38. [Google Scholar] [CrossRef]
- Stévant, P.; Rebours, C.; Chapman, A. Seaweed aquaculture in Norway: Recent industrial developments and future perspectives. Aquac. Int. 2017, 25, 1373–1390. [Google Scholar] [CrossRef]
- Giaccone, G.; Princi, M.; Rizzi Longo, L. Risposte morfologiche e fisiologiche di alghe marine in coltura all’inquinamento da liquami urbani ed industriale. Ing. Ambient. 1976, 6, 572–582. [Google Scholar]
- Lavery, P.S.; McComb, A.J. The nutritional eco-physiology of Chaetomorpha linum and Ulva rigida in Peel Inlet, Western Australia. Bot. Mar. 1991, 34, 251–260. [Google Scholar] [CrossRef]
- Menéndez, M.; Herrera-Silveira, J.; Comín, F.A. Effect of nitrogen and phosphorus supply on growth, chlorophyll content and tissue composition of the macroalga Chaetomorpha linum (OF Mull), Kutz, in a Mediterranean Coastal Lagoon. Sci. Mar. 2002, 66, 355–364. [Google Scholar] [CrossRef]
- Beolchini, F.; Pennesi, C.; Testaferri, B.; Totti, C.; De Michelis, I.; Vegliò, F. Waste Biomass from Marine Environment as Arsenic and Lead Biosorbent. Adv. Mater. Res. 2009, 71–73, 597–600. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Gravina, M.F.; Giangrande, A. Filtering activity on a pure culture of Vibrio alginolyticus by the solitary ascidian Styela plicata and the colonial ascidian Polyandrocarpa zorritensis: A potential service to improve microbiological seawater quality economically. Sci. Total Environ. 2016, 573, 11–18. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.T.; Minale, L. Polyprenyl derivatives from the sponge Ircinia spinosula: 2-Polyprenylbenzoquinones, 2-polyprenylbenzoquinols, prenylated furans and a C-31 difuranoterpene. Tetrahedron 1972, 28, 1315–1324. [Google Scholar] [CrossRef]
- Yildiz, G.; Vatan, Ö.; Çelikler, S.; Dere, Ş. Determination of the phenolic compounds and antioxidative capacity in red algae Gracilaria bursa-pastoris. Int. J. Food Prop. 2011, 14, 496–502. [Google Scholar] [CrossRef]
- Ramdani, M.; Elasri, O.; Saidi, N.; Elkhiati, N.; Taybi, F.A.; Mostareh, M.; Zaraali, O.; Haloui, B.; Ramdani, M. Evaluation of antioxidant activity and total phenol content of Gracilaria bursa-pastoris harvested in Nador lagoon for an enhanced economic valorization. Chem. Biol. Technol. Agric. 2017, 4, 1–7. [Google Scholar]
- Janarthanan, M.; Senthil Kumar, M. The properties of bioactive substances obtained from seaweeds and their applications in textile industries. J. Ind. Text. 2018, 48, 361–401. [Google Scholar]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. https://doi.org/10.3390/jmse8100733
Giangrande A, Pierri C, Arduini D, Borghese J, Licciano M, Trani R, Corriero G, Basile G, Cecere E, Petrocelli A, et al. An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea). Journal of Marine Science and Engineering. 2020; 8(10):733. https://doi.org/10.3390/jmse8100733
Chicago/Turabian StyleGiangrande, Adriana, Cataldo Pierri, Daniele Arduini, Jacopo Borghese, Margherita Licciano, Roberta Trani, Giuseppe Corriero, Grazia Basile, Ester Cecere, Antonella Petrocelli, and et al. 2020. "An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea)" Journal of Marine Science and Engineering 8, no. 10: 733. https://doi.org/10.3390/jmse8100733
APA StyleGiangrande, A., Pierri, C., Arduini, D., Borghese, J., Licciano, M., Trani, R., Corriero, G., Basile, G., Cecere, E., Petrocelli, A., Stabili, L., & Longo, C. (2020). An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea). Journal of Marine Science and Engineering, 8(10), 733. https://doi.org/10.3390/jmse8100733









