Long-Term Stochastic Modeling of Sheet Pile Corrosion in Coastal Environment from On-Site Measurements
Abstract
:1. Introduction
2. Materials and Methods
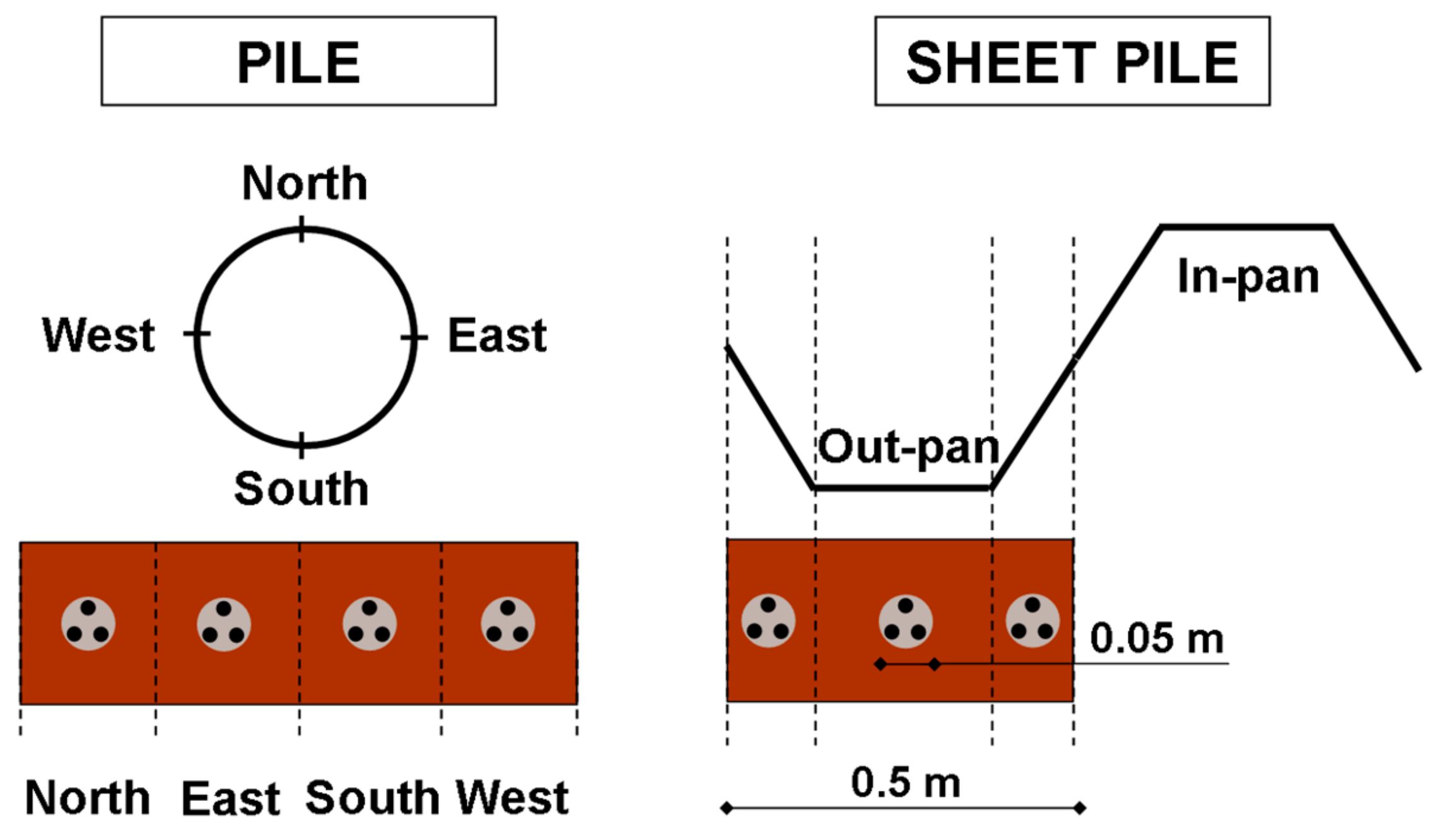
2.1. Corrosion Database from On-Site Measurements in France
2.2. Type and Age of Considered Dock Structures
2.3. Environmental Characteristics of Selected Structures
2.4. Crude Statistical Analysis and Selected Method
- -
- Most of the structures were built between the 50s and the 80s and inspections were carried out 10 to 40 years after when the environmental data was not available until 30 to 50 years after. Knowing the environmental data mainly affects the initial corrosion, this behavior cannot be captured from the database;
- -
- Environmental parameters were not carried out at the same place but in the same zone and cannot be simply compared since the evolution of these parameters with depth is high ([23] Appendix B 108–111);
- -
- Kinetics of redox reaction as a function of environmental parameters are non-linear and depend on the relative value and influence of each parameter. Table 1 shows besides that, the order of magnitude of environmental parameters is generally similar and their scatter in each location is high. Moreover, there is a competition of physical-chemical reactions at several scales of time and that cannot be captured due to the sampling of corrosion (one to three over 30 years) and environmental parameters (one to 10 per year);
- -
- The last point is tricky to analyze when the seasonal and multi-annual variations of these parameters are high. For instance, the Coefficient of Variation (CoV) of suspended materials exceeds 90% in all locations and reaches 200% in one location in the HA harbor and the CoV of temperature and dissolved O2 are respectively higher than 30% and 20% at a single location.
- -
- The gradient of the LoT in the immersion area is a well-known phenomenon since 1949 [24,25] and it was recently shown to be the case in harbors with regard to environmental parameters [23]; besides, it depends on the dock type (Figure 4). Knowing that the measurements of corrosion and environmental parameters were not carried out at the same depth, the relationship between parameters and corrosion is highly difficult to capture.
3. Results and Discussion
3.1. Modeling Corrosion Stochastic Process with Depth
- -
- Interpretation of the experimental data (over 35,000 measurements of sheet pile LoT) and the physico-chemical phenomena, which allows spatial corrosion to be treated essentially as a one-dimensional problem according to the depth.
- -
- Probabilistic modeling in each zone to account for the variability of the phenomena. Having taken the preceding points into consideration, the stochastic time-dependent corrosion model presented in this paper is based on the temporal evolution of parameters for a given probability density function of the random variable “steel LoT” in each exposure zone.
3.2. Definition of Exposure Zones in Relation to Environmental Conditions
- -
- Mean High Water (MHW);
- -
- Mean High Water Springs (MHWS);
- -
- Mean Low Water Springs (MLWS); the arithmetic mean of the low waters occurring at the time of the spring tides observed over a specific 19-year Metonic cycle;
- -
- Mean Low Water (MLW): the arithmetic mean of the low water heights observed each tidal day;
- -
- Splash zone (ZS): it is a wetland where a continuous water film is maintained on the surface of metal exposed to the atmosphere. This area is accessible at low tide. The corrosion in this area is particularly active and is manifested locally by densely pickled areas
- -
- Tidal zone (ZT): this zone is located between the average level of high tides and the average level of low tides and is alternately submerged and emergent. This area is only accessible at low tide at high tide coefficients. The moderate degradation of the metal in this area (corrosion rate in the order of that of the submerged area) is due to partial atmospheric corrosion, together with a cathodic depolarization caused by the highly oxygenated water film deposited by tides in relation to superficial water layers. Therefore, at the interface between the wetland and the submerged area, corrosion cells are produced by different concentrations of oxygen. The metal under the water is anodic and corrosion rates are intense because of short electrical paths
- -
- Low seawater level zone (ZL): the area of lower waters lies just below the average level of lowest tides. This is the area where corrosion is particularly active because of the differential ventilation battery that is established with the upper structure. The metal top, situated in the tidal zone, highly oxygenated, is a cathode. The metal found just below in the area constantly submerged, is the anode, the seat of a major dissolution of metal.
- -
- Immersion zone (ZI): this area is located between the area of lowest waters and the mud area. The metal is in permanent contact with seawater and macro-fouling will colonize and grow. This is a difficult area to access for maintenance without using cofferdams or under-water techniques.
- -
- Mud zone (ZM): this buried area corresponds to the buried parts of a metal structure. The feedback shows that this area usually requires very little maintenance. The top of this level can be inspected in most cases in harbors because sediments can be removed but the conditions of inspection are harsh.
3.3. Pricewise Modeling with Gamma Probability Density Functions
3.4. LoT Long Term Space Dependent Model (LoT-TS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter\Harbour | BO | HA | PL | SE | |
|---|---|---|---|---|---|
| Temperature (°C) | Mean (range) | 13.3 (NA) | 13.6 (13.3–13.8) | 15.5 (NA) | 16.1 (15.7–17) |
| Min (range) | 7.2 (NA) | 8 (6.6–8.7) | 5.7 (NA) | 10.3 (9.8–11.7) | |
| Max (range) | 19.5 (NA) | 20.5 (19.8–21) | 22.4 (NA) | 21.1 (20.4–24.2) | |
| pH | Mean (range) | 8 (NA) | 8 (7.9–8.1) | 8.2 (NA) | 8.1 (8.1–8.2) |
| Min (range) | 7.8 (NA) | 7.7 (7.5–7.9) | 7.8 (NA) | 7.9 (7.7–8.1) | |
| Max (range) | 8.1 (NA) | 8.4 (8.3–8.5) | 8.6 (NA) | NA | |
| Conductivity (ms/cm) | Mean (range) | 49 (NA) | 35.4 (29.6–47.6) | 50.3 (NA) | NA |
| Min (range) | 46.8 (NA) | 31.4 (26.7–38.2) | 33.4 (NA) | NA | |
| Max (range) | 50.5 (NA) | 40.3 (34.3–47.1) | 57.4 (NA) | NA | |
| Salinity (g/L) | Mean (range) | 32.9 (NA) | 23.8 (19.2–28.6) | 33 (NA) | 37.5 (35.2–41.2) |
| Min (range) | 31.5 (NA) | 21.5 (17.1–25.9) | 24.3 (NA) | 34.2 (31.6–35.7) | |
| Max (range) | 3.7 (NA) | 26.7 (22.2–31.6) | 37.9 (NA) | 40.4 (38.2–46.6) | |
| Dissolved O2 (mg/L) | Mean (range) | 8.7 (NA) | 8.3 (7.8–8.7) | 9.2 (NA) | 6.8 (5.6–8.2) |
| Min (range) | 6.4 (NA) | 6.6 (6.1–7.1) | 6 (NA) | 5.6 (4.4–6.9) | |
| Max (range) | 1.2 (NA) | 10.4 (9.8–11) | 1.8 (NA) | 8.3 (6.6–11.4) | |
| Suspended Materials (mg/L) | Mean (range) | 9.9 (NA) | 11.5 (8–19.5) | 34.8 (NA) | NA |
| Min (range) | 4.7 (NA) | 5 (2.8–10.2) | 10 (NA) | NA | |
| Max (range) | 17.7 (NA) | 21.8 (12.6–35.4) | 130 (NA) | NA |
References
- Southwell, C.R.; Bultman, J.D.; Hummer, C.W. Estimating Service Life of Steel in Seawater; Schumacher, M., Ed.; Noyes Data Corporation: Park Ridge, NJ, USA, 1979; pp. 374–387. [Google Scholar]
- Melchers, R.E. Probabilistic Modelling of Marine Corrosion of Steel Specimens. In Proceedings of the Fifth International Offshore and Polar Engineering Conference, International Society of Offshore and Polar Engineers, ISOPE-I-95-311, The Hague, The Netherlands, 11–16 June 1995. [Google Scholar]
- Melchers, R.E. Probabilistic modelling of immersion marine corrosion. Struct. Saf. Reliab. 1998, 3, 1143–1149. [Google Scholar]
- Paik, J.K.; Kim, S.K.; Lee, S.K. Probabilistic corrosion rate estimation model for longitudinal strength members of bulk carriers. Ocean Eng. 1998, 25, 837–860. [Google Scholar] [CrossRef]
- Soares, C.G.; Garbatov, Y. Reliability of maintained, corrosion protected plates subjected to non-linear corrosion and compressive loads. Mar. Struct. 1999, 12, 425–445. [Google Scholar] [CrossRef]
- Memet, J.B. La Corrosion Marine des Structures Métalliques Portuaires: Étude des Mécanismes D’amorçage et de Croissance des Produits de Corrosion. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2000; p. 164. [Google Scholar]
- Chernov, B.B.; Ponomarenko, S.A. Physico-Chemical Modelling for the Prediction of Seawater Metal Corrosion. In Proceedings of the 10th International Congress on Marine Corrosion and Fouling, University of Melbourne, Parkville, Australia, February 1999; p. 222. [Google Scholar]
- Schoefs, F. Sensitivity approach for modelling the environmental loading of marine structures through a matrix response surface. Reliab. Eng. Syst. Saf. 2008, 93, 1004–1017. [Google Scholar] [CrossRef] [Green Version]
- Paik, J.K.; Thayamballi, A.K. Ultimate strength of ageing ships. J. Eng. Marit. Environ. 2002, 216, 57–77. [Google Scholar] [CrossRef]
- Paik, J.K.; Thayamballi, A.K. Ultimate Limit State Design of Steel-Plated Structures; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Qin, S.; Cui, W. Effect of corrosion models on the time-dependent reliability of steel plated elements. Mar. Struct. 2003, 16, 15–34. [Google Scholar] [CrossRef]
- Van der Toorn, A.; Leatimia, F.; Jongbloed, P.; de Beijer, P.; Louwen, P. Corrosion Aspects in the Port of Rotterdam. In Proceedings of the International Maritime-Port Technology and Development Conference, Singapore, 26–28 September 2008; pp. 1–7. [Google Scholar]
- Jeffrey, R.; Melchers, R.E. Corrosion Tests of Mild Steel in Temperate Seawater; Research Report; Department of Civil, Surveying and Environmental Engineering, University of Newcastle: Callaghan, Australia, 2001. [Google Scholar]
- Melchers, R.E. Modelling of marine immersion corrosion for mild and low-alloy steels-part 1: Phenomenological model. Corrosion 2003, 59, 319–334. [Google Scholar] [CrossRef]
- Melchers, R.E.; Wells, T. Models for the anaerobic phases of marine immersion corrosion. Corros. Sci. 2006, 48, 1791–1811. [Google Scholar] [CrossRef]
- Melchers, R.E. Modeling and Prediction of Long-Term Corrosion of Steel in Marine Environments. Int. J. Offshore Polar Eng. 2012, 22, 257–263. [Google Scholar]
- Melchers, R.E. Modelling long term corrosion of steel infrastructure in natural marine environments. Underst. Biocorros. Fundam. Appl. 2014, 66, 213. [Google Scholar]
- Boéro, J.; Schoefs, F.; Capra, B.; Rouxel, N. Technical management of French harbour structures-Part 1: Description of built assets. PARALIA 2009, 2, 6.1–6.11. [Google Scholar]
- Boéro, J.; Schoefs, F.; Yañez-Godoy, H.; Capra, B. Time-function reliability of harbour infrastructures from stochastic modelling of corrosion. Eur. J. Environ. Civil Eng. 2012, 16, 1187–1201. [Google Scholar] [CrossRef] [Green Version]
- Schoefs, F.; Clément, A.; Nouy, A. Assessment of spatially dependent ROC curves for inspection of random fields of defects. Struct. Saf. 2009, 31, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Boéro, J.; Schoefs, F.; Melchers, R.; Capra, B. Statistical Analysis of Corrosion Process along French Coast. In Proceedings of the 10th Internation Conference on Structural Safety and Reliability, San Francisco, CA, USA, August 31–September 4 2009. [Google Scholar]
- Schoefs, F.; Le, K.T.; Lanata, F. Response surface meta-models for the assessment of embankment frictional angle stochastic properties from monitoring: Application to harbour structures. Comput. Geotech. 2013, 53, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Boéro, J. Port Infrastructure Reliability: Innovative Approach of Analysis and Probabilistic Modeling of Inspection Data—Application to the Corrosion of Metallic Structures/Fiabilité des Infrastructures Portuaires: Approche Innovante D’analyse et de Modélisation Probabiliste des Données D’inspection. Application à la Corrosion des Structures Métalliques. Ph.D. Thesis, Université de Nantes, Nantes, France, 26 October 2010. Available online: https://www.researchgate.net/publication/338396402_Fiabilite_des_infrastructures_portuaires_approche_innovante_d%27analyse_et_de_modelisation_probabiliste_des_donnees_d%27inspection_Application_a_la_corrosion_des_structures_metalliques (accessed on 22 January 2020).
- Humble, H.A. Cathodic Protection of Steel Piling In Sea Water. Corrosion 1949, 5, 292–302. [Google Scholar] [CrossRef]
- Edwards, W.E. Marine Corrosion: Its Cause and Care. In Proceedings of the Eighth Annual Appalachian Underground Corrosion Short Course; Technical Bulletin; West Virginia University Bulletin: Morgantown, WV, USA, 1963; Volume 69, p. 486. [Google Scholar]
- Songa, T. SP1—Travaux Réalisés Dans le Domaine de la Corrosion Marine par le Comité Exécutif « Corrosion et Protection de Surface » de la C.C.E . In Proceedings of the Conférence Internationale “L’acier Dans les Structures Marines—Sessions Spéciales et Plénières”, Paris, France, 5–8 October 1981. [Google Scholar]
- Bourreau, L.; Bouteiller, V.; Gaillet, L.; Schoefs, F.; Thauvin, B.; Schneider, J.; Naar, S. Spatial identification of exposure zones of concrete structures exposed to a marine environment with respect to reinforcement corrosion. Struct. Infrastruct. Eng. 2019, 16, 346–354. [Google Scholar] [CrossRef]
- Hawchar, L.; El Soueidy, C.P.; Schoefs, F. Polynomial Chaos Expansions for Time-Variant Reliability Problems. Reliab Eng. Syst. Saf. 2017, 167, 406–416. [Google Scholar] [CrossRef]













| Harbour | Bounds of Each Corrosion Zone (m) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ZS | ZT | ZL | ZI | ZM | ||||||
| Upper Bound MHWS | Lower Bound MHW | Upper Bound MHW | Lower Bound MLW | Upper Bound MLW | Lower Bound MLWS | Upper Bound MLWS | Lower Bound Soil +0.5 m | Upper Bound Soil +0.5 m | Lower Bound Soil −0.5 m | |
| BO | 8.85 | 8.00 | 8.00 | 1.86 | 1.86 | 1.10 | 1.10 | Depends on each structure | Depends on each structure | Depends on each structure |
| HA | 7.90 | 7.23 | 7.239 | 2.01 | 2.01 | 1.20 | 1.20 | |||
| PL | 0.62 | 0.59 | 0.59 | 0.20 | 0.20 | 0.16 | 0.16 | |||
| SE | 0.62 | 0.59 | 0.59 | 0.20 | 0.20 | 0.16 | 0.16 | |||
| zN | 0.50 | 0.25 | 0.25 | 0.00 | 0.00 | −0.20 | −0.20 | −0.80 | −0.80 | −1.20 |
| Harbour | Duration of Exposure (year) | Average Corrosion Rate (mm/year) | |||
|---|---|---|---|---|---|
| ZS | ZT | ZL | ZM | ||
| BO | 25 | - | 0.10 | 0.11 | 0.06 |
| HA | 10 to 65 | 0.03–0.11 | 0.03–0.08 | 0.04–0.29 | 0.01–0.15 |
| PL | 34 | - | - | 0.06 | 0.03 |
| SE | 53 to 63 | - | - | 0.07–0.11 | 0.07–0.11 |
| Exposure Zone ZE | t = 10 years | t = 25 years | t = 50 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | s | Cov (%) | 95 % Fractie | m | s | Cov (%) | 95 % Fractile | m | s | Cov (%) | 95 % Fractile | |
| ZT | 1.54 | 0.93 | 60 | 3.32 | 2.46 | 1.53 | 62 | 5.40 | 2.82 | 1.81 | 64 | 6.30 |
| ZL | 1.67 | 1.15 | 69 | 3.90 | 2.87 | 1.81 | 63 | 6.34 | 3.50 | 2.06 | 59 | 7.42 |
| ZI | 1.18 | 1.19 | 101 | 3.55 | 2.37 | 1.46 | 62 | 5.17 | 3.42 | 1.49 | 44 | 6.17 |
| ZM | 0.72 | 0.73 | 101 | 2.18 | 1.68 | 1.22 | 72 | 4.05 | 3.00 | 1.44 | 48 | 5.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoefs, F.; Boéro, J.; Capra, B. Long-Term Stochastic Modeling of Sheet Pile Corrosion in Coastal Environment from On-Site Measurements. J. Mar. Sci. Eng. 2020, 8, 70. https://doi.org/10.3390/jmse8020070
Schoefs F, Boéro J, Capra B. Long-Term Stochastic Modeling of Sheet Pile Corrosion in Coastal Environment from On-Site Measurements. Journal of Marine Science and Engineering. 2020; 8(2):70. https://doi.org/10.3390/jmse8020070
Chicago/Turabian StyleSchoefs, Franck, Jérôme Boéro, and Bruno Capra. 2020. "Long-Term Stochastic Modeling of Sheet Pile Corrosion in Coastal Environment from On-Site Measurements" Journal of Marine Science and Engineering 8, no. 2: 70. https://doi.org/10.3390/jmse8020070
APA StyleSchoefs, F., Boéro, J., & Capra, B. (2020). Long-Term Stochastic Modeling of Sheet Pile Corrosion in Coastal Environment from On-Site Measurements. Journal of Marine Science and Engineering, 8(2), 70. https://doi.org/10.3390/jmse8020070






