Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment
Abstract
:1. Introduction and General Context
2. Frameworks of Theory and Computations
3. Results and Discussions
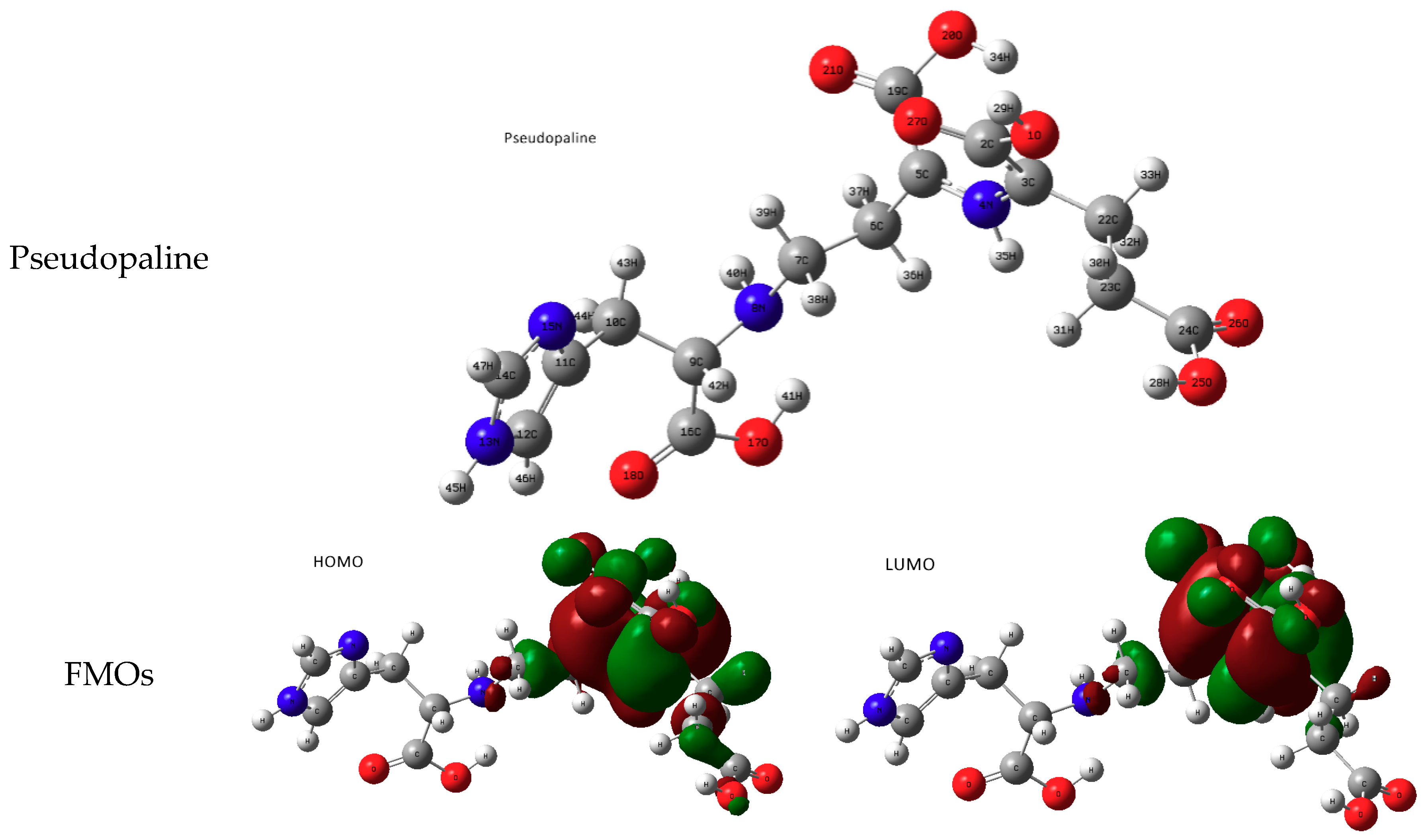
3.1. Molecular Study of Both Metallophores: Pseudopaline and Staphylopine
3.1.1. IR Spectra
3.1.2. Geometry of the Complex
3.2. Chelating Metal Ions Using Pseudopaline and Staphylopine
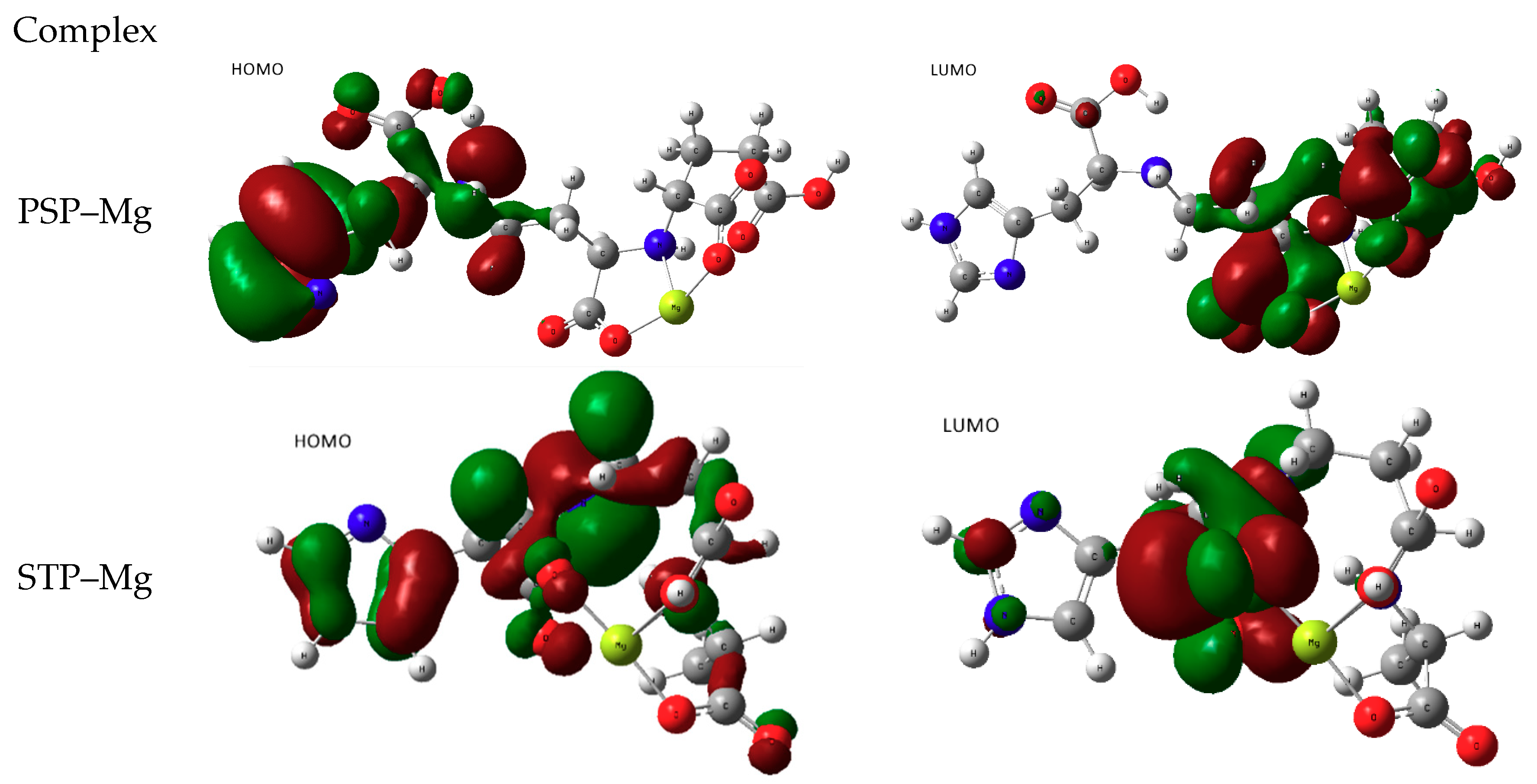
3.2.1. Attempts of Chelation with Alkaline Earth Mg2+
3.2.2. Chelation with Transition-Metal Ions (TM++)
Pseudopaline with Divalent Ni and Zn
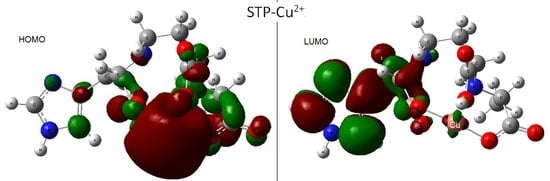
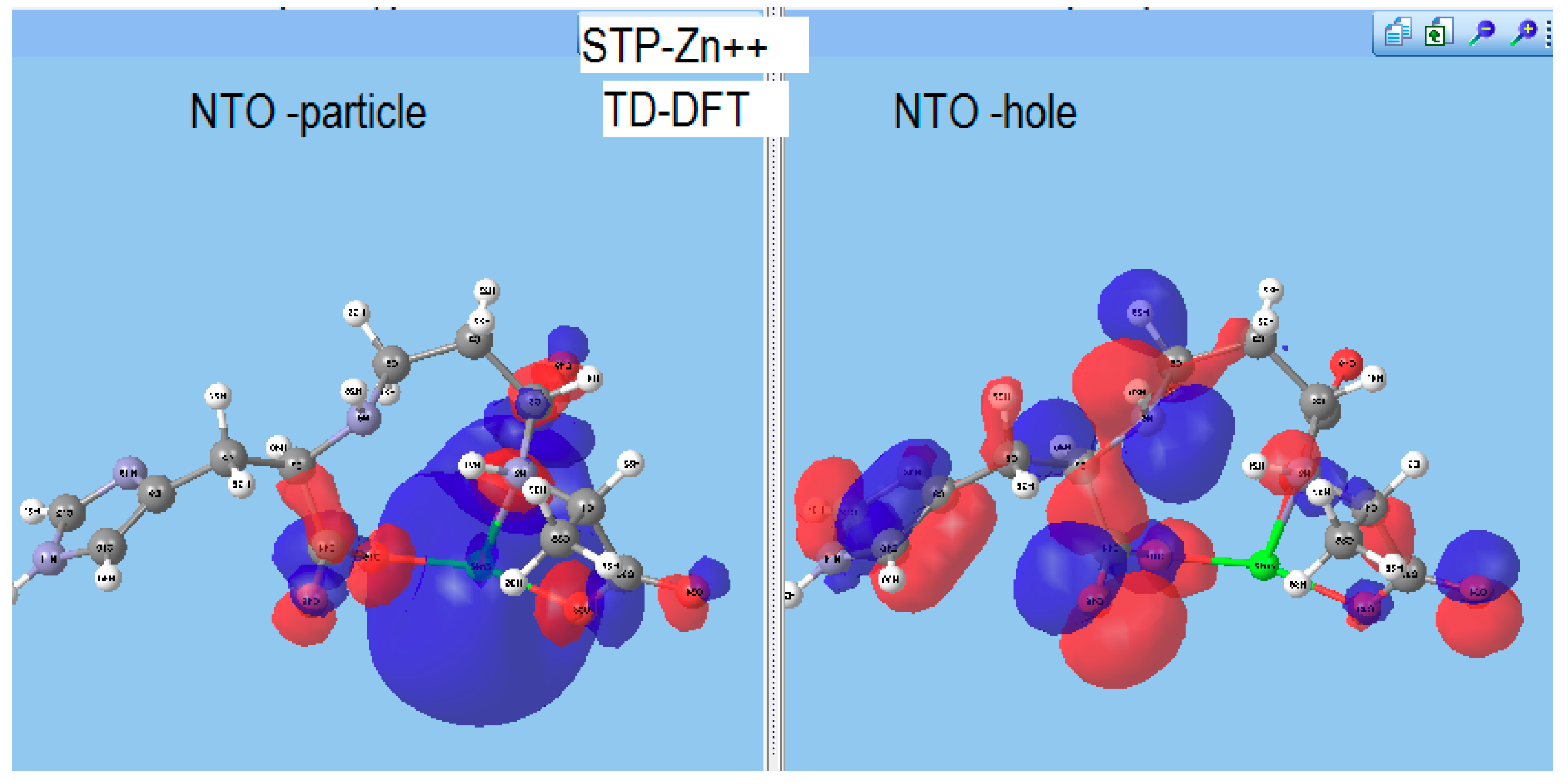
Staphylopine with Divalent Ni, Cu, and Zn
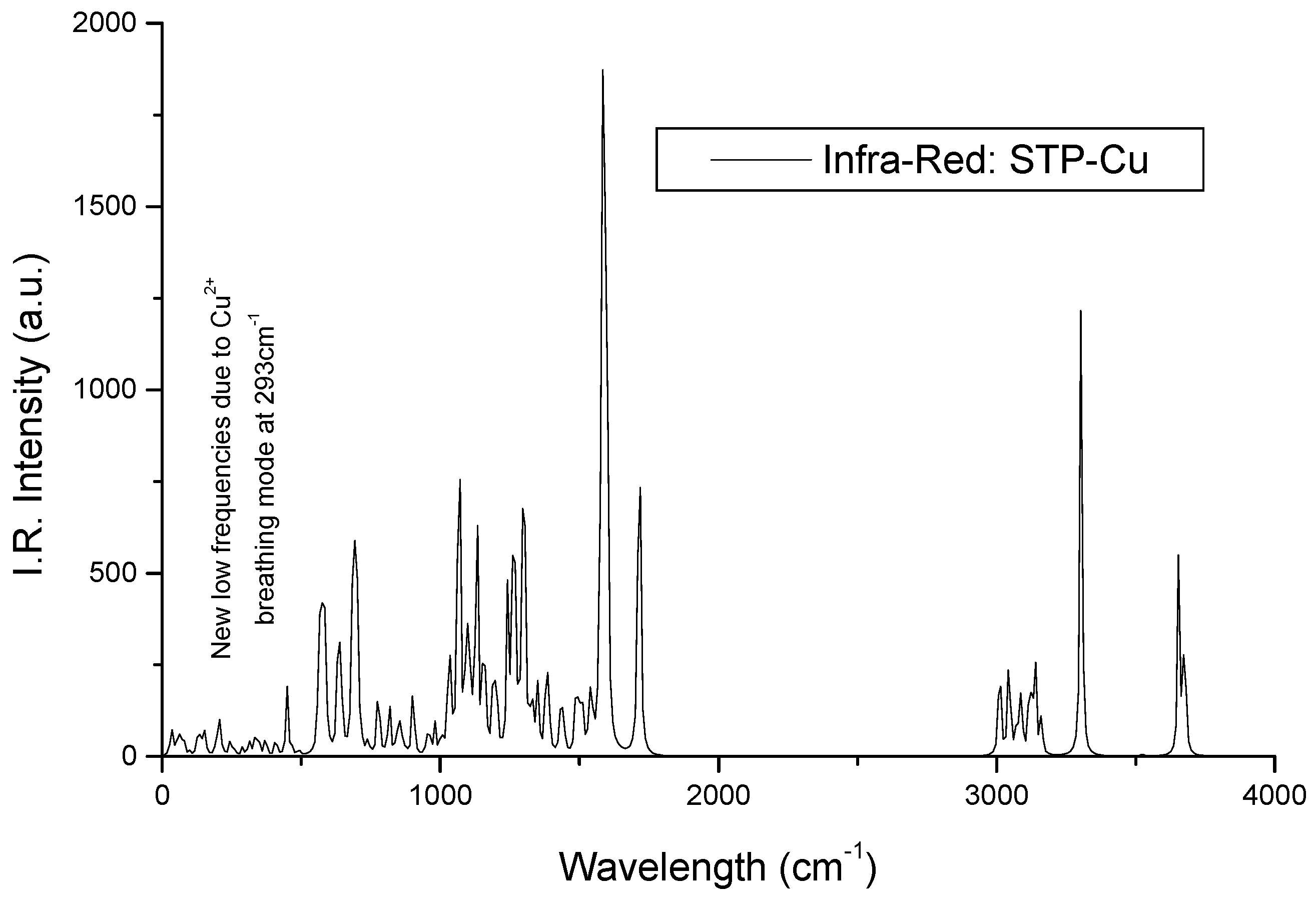
Infrared Signature of the Complex
4. Concluding Notes and Future Perspectives
- two metallophores (pseudopaline and staphylopine);
- metal-chelated metallophores, mainly reporting on the charge transfers in frontier orbitals (HOMO to LUMO) where MLCT (metal-to-ligand charge transfer) mechanisms were identified, firstly inferred from FMOs, and further supported by NTOs from TD-DFT calculations.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, A.R. New advances in iron chelation therapy. Am. Soc. Hematol. 2006, 2006, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Finkenstedt, A.; Wolf, E.; Höfner, E.; Isasi Gasser, B.; Bösch, S.; Bakry, R.; Creus, M.; Kremser Ch Schocke, M.; Theur, M.; Moser, P.; et al. Hepatic but not brain iron is rapidly chelated by Deferasirox in aceruloplasminemia due to a novel gene mutation. J. Hepatol. 2010, 53, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Reniere, M.L.; Pishchany, G.; Skaar, E.P. Iron Uptake and Homeostasis in Microorganisms; Cornelis, P., Andrews, S.C., Eds.; Caister Academic Press: Norwich, UK, 2010; pp. 264–274. [Google Scholar]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Budzikiewicz, A.G. Siderophores from bacteria and from fungi. In Iron Uptake and Homeostasis in Microorganisms; Corneis, P., Andrews, S.C., Eds.; Caister Academic Press: Norwich, UK, 2010; pp. 1–16. [Google Scholar]
- Cornelissen, C.N.; Hollander, A. TonB-Dependent Transporters Expressed by Neisseria gonorrhoeae. Front. Microbiol. 2011, 2, 117. [Google Scholar] [CrossRef] [PubMed]
- Minandri, F.; Imperi, F.; Frangipani, E.; Bonchi, C.; Visaggio, D.; Facchini, M.; Pasquali, P.; Bragonzi, A.; Visca, P. Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect. Immun. 2016, 84, 2324–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Graham, D.W.; DiSpirito, A.A.; Alterman, M.A.; Galeva, N.; Larive, C.K.; Asunskis, D.; Sherwood, P.M. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 2004, 305, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Dassama, L.M.K.; Kenney, G.E.; Ro, S.Y.; Zielazinski, E.L.; Rosenzweig, A.C. Methanobactin transport machinery. Proc. Natl. Acad. Sci. USA 2016, 113, 13027–13032. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Zhao, C.; Burkinshaw, B.; Zhang, B.; Wei, D.; Wang, Y.; Dong, T.G.; Shen, X. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. USA 2017, 114, E2233–E2242. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.V.; Cavazza, C.; Bochot, C.; Lemaire, D.; Fontecilla-Camps, J.C. Structural characterization of a putative endogenous metal chelator in the periplasmic nickel transporter NikA. Biochemistry (Mosc.) 2008, 47, 9937–9943. [Google Scholar] [CrossRef] [PubMed]
- Lebrette, H.; Borezée-Durant, E.; Martin, L.; Richaud, P.; Boeri Erba, E.; Cavazza, C. Novel insights into nickel import in Staphylococcus aureus: The positive role of free histidine and structural characterization of a new thiazolidine-type nickel chelator. Metallomics 2015, 7, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.M.; Cendron, L.; Salamina, M.; Ruzzene, M.; Zanotti, G. Helicobacter pylori periplasmic receptor CeuE (HP1561) modulates its nickel affinity via organic metallophores. Mol. Microbiol. 2014, 91, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, A.G.; Kirillina, O.; Fetherston, J.D.; Miller, M.C.; Burlison, J.A.; Perry, R.D. The Yersinia pestis Siderophore, Yersiniabactin, and the ZnuABC system both contribute to Zinc acquisition and the development of lethal septicemic plague in mice. Mol. Microbiol. 2014, 93, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, A.G.; Kirillina, O.; Fosso, M.Y.; Fetherston, J.D.; Miller, M.C.; VanCleave, T.T.; Burlison, J.A.; Arnold, W.K.; Lawrenz, M.B.; Garneau-Tsodikova, S.; et al. Zinc transporters YbtX and ZnuABC are required for the virulence of Yersinia pestis in bubonic and pneumonic plague in mice. Met. Integr. Biomet. Sci. 2017, 9, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Kallifidas, D.; Pascoe, B.; Owen, G.A.; Strain-Damerell, C.M.; Hong, H.J.; Paget, M.S. The zinc-responsive regulator Zur controls expression of the coelibactin gene cluster in Streptomyces coelicolor. J. Bacteriol. 2010, 192, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Brutesco, C.; Ouerdane, L.; Fojcik, C.; Izaute, A.; Wang, S.; Hajjar, C.; Lobinski, R.; Lemaire, D.; Richaud, P.; et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science 2016, 352, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Lhospice, S.; Gomez, N.O.; Ouerdane, L.; Brutesco, C.; Ghssein, G.; Hajjar, C.; Liratni, A.; Wang, S.; Richaud, P.; Bleves, S.; et al. Pseudomonas aeruginosa zincuptake in chelating environmentis primarily mediated by the metallophore pseudopaline. Sci. Rep. 2017, 7, 17123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Bai, Y.; Guo, Q.; Zhou, J.; Lei, X. Total Syntheses of Natural Metallophores Staphylopine and Aspergillomarasmine A. J. Org. Chem. 2017, 82, 13643–13648. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722. [Google Scholar] [CrossRef]
- Atkins, P.W.; Friedman, R. Molecular Quantum Mechanics, 4th ed.; Oxford University Press: New York, NY, USA, 2005; ISBN 978-0-19-927498-7. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. B 1964, 136, 864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. A 1965, 140, 1133. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Reiher, M.; Salomonn, O.; Hess, B.A. Reparameterization of hybrid functionals based on energy differences of states of different multiplicity. Theor. Chem. Acc. 2001, 107, 48–55. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. J. Chem. Phys. 1971, 54, 724. [Google Scholar] [CrossRef]
- Gaussian09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775. [Google Scholar] [CrossRef]
- Zerdane, S.; Collet, E.; Dong, X.; Matar, S.F.; Wang, H.F.; Desplanches, C.; Chastanet, G.; Chollet, M.; Glownia, J.M.; Lemke, H.; et al. Electronic and Structural Dynamics During the Switching of the Photomagnetic Complex [Fe(L222N5)(CN)2]. Chem. Eur. J. 2018, 24, 5064. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, Y.; Chen, W.; Gu, T.; Zhang, S.-Y.; Ji, Q. Mechanistic insights into staphylopine-mediated metal acquisition. Proc. Natl. Acad. Sci. USA 2018, 115, 3942–3947. [Google Scholar] [CrossRef] [PubMed]
- Matar, S.F.; Guionneau, P.; Chastanet, G. Multiscale Experimental and Theoretical Investigations of Spin Crossover FeII Complexes: Examples of [Fe(phen)2(NCS)2] and [Fe(PM-BiA)2 (NCS)2]. Int. J. Mol. Sci. 2015, 16, 4007–4027. [Google Scholar] [CrossRef] [PubMed]






| Designation | Infrared Wave Number (cm−1) | |
|---|---|---|
| Pseudopaline | Staphylopine | |
| Whole molecule | 9.27 | 11.62 |
| Stretching all molecule | 170.71 | 157.03 |
| Imidazole torsion | 649.81 | 643.43 |
| COOH stretching torsion | 773.97 | 745.85 |
| Imidazol in-plane distortion | 954.73 | 962.07 |
| C–C stretching | 1035.72 | 1057.10 |
| C–N–C stretching | 1125.66 | 1144.20 |
| C–O–H angular torsion | 1216.88 | 1196.49 |
| CH2/NH dangling | 1325, …, 1515 | 1315, …, 1556 |
| C=O stretching | 1718.45: 19C–21 & 2C–27OO | 1705.68: 17C–19O |
| 1776.65: 16C–18O | 1726.27: 14C–16O | |
| 1783.79: 24C–26O | 1751.66: 20C–33O | |
| C–H stretching | 3027,…, 3225 | 2953–3525 |
| N–H stretching | 3225, …, 3439 | 3525, …., 3566 |
| O–H stretching | 3548, …, 3633 | 3587, …, 3635 |
| Imidazol–H stretch | 3680.12 | 3672.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghssein, G.; Matar, S.F. Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment. Computation 2018, 6, 56. https://doi.org/10.3390/computation6040056
Ghssein G, Matar SF. Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment. Computation. 2018; 6(4):56. https://doi.org/10.3390/computation6040056
Chicago/Turabian StyleGhssein, Ghassan, and Samir F. Matar. 2018. "Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment" Computation 6, no. 4: 56. https://doi.org/10.3390/computation6040056
APA StyleGhssein, G., & Matar, S. F. (2018). Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment. Computation, 6(4), 56. https://doi.org/10.3390/computation6040056