Biomechanical Properties and Biocompatibility of a Non-Absorbable Elastic Thread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Rationale
2.2. Experimental Materials and Setting
2.3. Experimental Procedures
2.3.1. Characterization of Surface Properties
2.3.2. Measurement of Tensile Strength and Elongation
2.3.3. Quantification of Release of Bovine Serum Albumin (BSA)
2.3.4. Assessment of in Vivo Stability
2.3.5. Measurement of the Degree of Frictional Strength within the Tissue
2.4. Statistical Analysis
3. Results
3.1. Tensile Strength and Elongation
3.2. The Degree of BSA Release
3.3. In Vitro Cytotoxicity
3.4. Ex Vivo Frictional Properties
4. Discussion
5. Conclusions
- The degree of tensile strength and elongation of Si threads was significantly higher in both NAT-R and -S as compared with Elasticum® (p < 0.05). Moreover, the degree of tensile strength and elongation of PET threads was significantly lower in both NAT-R and -S as compared with Elasticum® (p < 0.05). Furthermore, the degree of tensile strength and elongation of braided Si/PET threads was significantly lower in NAT-S as compared with NAT-R and Elasticum® (p < 0.05).
- The degree of BSA release was significantly higher in NAT-R as compared with Elasticum® and NAT-S throughout a 2-h period in the descending order (p < 0.05).
- The degree of cell viability was significantly higher in both NAT-R and -S as compared with Elasticum® (p < 0.05).
- The degree of coefficient of friction as well as the frictional force and strength was significantly higher in NAT-R as compared with NAT-S and Elasticum® (p < 0.05).
Author Contributions
Funding
Conflicts of Interest
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Nuschke, A.; Yates, C.C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 2016, 49, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, P.; Worthington, H.; Esposito, M.; Elst, M.; Waes, O.J. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst. Rev. 2010, CD004287. [Google Scholar] [CrossRef]
- Selvi, F.; Cakarer, S.; Can, T.; Kirli, T.S.İ.; Palancioglu, A.; Keskin, B.; Bilgic, B.; Yaltirik, M.; Keskin, C. Effects of different suture materials on tissue healing. J. Istanb. Univ. Fac. Dent. 2016, 50, 35–42. [Google Scholar] [CrossRef]
- Al-Mubarak, L.; Al-Haddab, M. Cutaneous wound closure materials: An overview and update. J. Cutan. Aesthet. Surg. 2013, 6, 178–188. [Google Scholar] [CrossRef]
- Pillai, C.K.; Sharma, C.P. Review paper: Absorbable polymeric surgical sutures: Chemistry, production, properties, biodegradability, and performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef]
- Luck, R.P.; Flood, R.; Eyal, D.; Saludades, J.; Hayes, C.; Gaughan, J. Cosmetic outcomes of absorbable versus nonabsorbable sutures in pediatric facial lacerations. Pediatr. Emerg. Care 2008, 24, 137–142. [Google Scholar] [CrossRef]
- Parell, G.J.; Becker, G.D. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch. Facial Plast. Surg. 2003, 5, 488–490. [Google Scholar] [CrossRef]
- Najibi, S.; Banglmeier, R.; Matta, J.; Tannast, M. Material properties of common suture materials in orthopaedic surgery. Iowa Orthop. J. 2010, 30, 84–88. [Google Scholar]
- Jost, B.; Zumstein, M.; Pfirrmann, C.W.; Gerber, C. Long-Term outcome after structural failure of rotator cuff repairs. J. Bone Jt. Surg. Am. 2006, 88, 472–479. [Google Scholar]
- Rettig, A.C.; Liotta, F.J.; Klootwyk, T.E.; Porter, D.A.; Mieling, P. Potential risk of rerupture in primary achilles tendon repair in athletes younger than 30 years of age. Am. J. Sports Med. 2005, 33, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Vince, K.G.; Abdeen, A. Wound problems in total knee arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.B.; Budoff, J.E.; Yeh, M.L.; Kelm, Z.S.; Luo, Z.P. Strength of damaged suture: An in vitro study. Arthroscopy 2006, 22, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Herbert, M.A.; Richards, D.P. Sutures and suture anchors: Update 2003. Arthroscopy 2003, 19, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Herbert, M.A.; Coons, D.A.; Boothby, M.H. Sutures and suture anchors—Update 2006. Arthroscopy 2006, 22, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kim, S.H.; Nam, M.S.; Park, E.S. Evaluation of Elastic Lift for Neck Rejuvenation. Arch. Aesthet. Plast. Surg. 2016, 22, 68–73. [Google Scholar] [CrossRef]
- Oh, C.H.; Jang, S.B.; Kang, C.M.; Shim, J.S. Buttock Lifting Using Elastic Thread (Elasticum®) with a New Classification of Gluteal Ptosis. Aesthetic Plast. Surg. 2018, 42, 1050–1058. [Google Scholar] [CrossRef]
- Da Silva, M.C.; da Silva, H.N.; Alves Leal Cruz, R.C.; Sagoe Amoah, S.K.; de Lima Silva, S.M.; Lia Fook, M.V. N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials. Materials 2019, 12, 1807. [Google Scholar] [CrossRef]
- Marchant, L.H.; Knapp, S.; Apter, J.T. Effect of elongation rate on tensile strength of surgical suture materials. Surg. Gynecol. Obstet. 1974, 139, 231–233. [Google Scholar]
- Von Fraunhofer, J.A.; Storey, R.J.; Masterson, B.J. Tensile properties of suture materials. Biomaterials. 1988, 9, 324–327. [Google Scholar]
- Silva, M.C.; Leal, R.D.; Silva, H.N.; Fook, M.V. Biodegradable suture threads as controlled drug delivery systems. Mater. Res. Innov. 2019. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Giamporcaro, A.; De, C.S.; Tranquillo, E.; Catauro, M. Threads Made with Blended Biopolymers: Mechanical, Physical and Biological Features. Polymers 2019, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Abellán, D.; Nart, J.; Pascual, A.; Cohen, R.E.; Sanz-Moliner, J.D. Physical and Mechanical Evaluation of Five Suture Materials on Three Knot Configurations: An in Vitro Study. Polymers 2016, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Suchenski, M.; McCarthy, M.B.; Chowaniec, D.; Hansen, D.; McKinnon, W.; Apostolakos, J.; Arciero, R.; Mazzocca, A.D. Material properties and composition of soft-tissue fixation. Arthroscopy 2010, 26, 821–831. [Google Scholar] [CrossRef] [PubMed]
- McFarland, E.G.; Park, H.B.; Keyurapan, E.; Gill, H.S.; Selhi, H.S. Suture anchors and tacks for shoulder surgery, part 1: Biology and biomechanics. Am. J. Sports Med. 2005, 33, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Khiste, S.V.; Ranganath, V.; Nichani, A.S. Evaluation of tensile strength of surgical synthetic absorbable suture materials: An in vitro study. J. Periodontal Implant. Sci. 2013, 43, 130–135. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A.; Beavis, R.C. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy 2009, 25, 192–199. [Google Scholar] [CrossRef]
- Carofino, B.C.; Santangelo, S.A.; Kabadi, M.; Mazzocca, A.D.; Browner, B.D. Olecranon fractures repaired with FiberWire or metal wire tension banding: A biomechanical comparison. J. Arthrosc. Relat. Surg. 2007, 23, 964–970. [Google Scholar] [CrossRef]
- Harrell, R.M.; Tong, J.; Weinhold, P.S.; Dahners, L.E. Comparison of the mechanical properties of different tension band materials and suture techniques. J. Orthop. Trauma 2003, 17, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, O.A.; Younas, S.A.; Ho, D.M.; Noble, P.C. Security of knots tied with ethibond, fiberwire, orthocord, or ultrabraid. Am. J. Sports Med. 2008, 36, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Sileo, M.J.; Lee, S.J.; Kremenic, I.J.; Orishimo, K.; Ben-Avi, S.; McHugh, M.; Nicholas, S.J. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy 2009, 25, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Faten, D.; Saber, B.A. Effect of Braiding and Hot Stretching Conditions on Sutures Performances. J. Appl. Sci. 2011, 11, 3276–3284. [Google Scholar]
- Rajendran, S.; Anand, S. Developments in Medical Textile. Text. Prog. 2002, 32, 1–37. [Google Scholar] [CrossRef]
- Saber, B.A.; Faten, D.; Hanen, J.; Saber, E.; Sofiene, M. Tensile and Knot Performance of Polyester Braided Sutures. Text. Res. J. 2009, 79, 247–252. [Google Scholar]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharm. 2017, 524, 454–466. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Sun, J. Layer-by-layer deposition of polymeric microgel films on surgical sutures for loading and release of ibuprofen. Langmuir 2009, 25, 7990–7994. [Google Scholar] [CrossRef]
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.Y.; Manivasagam, G. Suture materials—Current and emerging trends. J. Biomed. Mater. Res. Part A 2016, 104, 1544–1559. [Google Scholar] [CrossRef]
- Weldon, C.B.; Tsui, J.H.; Shankarappa, S.A.; Nguyen, V.T.; Ma, M.; Anderson, D.G.; Kohane, D.S. Electrospun drug-eluting sutures for local anesthesia. J. Control Release 2012, 161, 903–909. [Google Scholar] [CrossRef]
- Guan, J.; Stankus, J.J.; Wagner, W.R. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. J. Control Release 2007, 120, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers. Materials 2017, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, B.; Park, M.; Park, S.J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Bouillot, P.; Ubrich, N.; Sommer, F.; Duc, T.M.; Loeffler, J.P.; Dellacherie, E. Protein encapsulation in biodegradable amphiphilic microspheres. Int. J. Pharm. 1999, 181, 159–172. [Google Scholar] [CrossRef]
- Carrasquillo, K.G.; Carro, J.C.; Alejandro, A.; Toro, D.D.; Griebenow, K. Reduction of structural perturbations in bovine serum albumin by non-aqueous microencapsulation. J. Pharm. Pharmacol. 2001, 53, 115–120. [Google Scholar] [CrossRef]
- Jiang, W.; Schwendeman, S.P. Stabilization and controlled release of bovine serum albumin encapsulated in poly(D, L-lactide) and poly(ethylene glycol) microsphere blends. Pharm. Res. 2001, 18, 878–885. [Google Scholar] [CrossRef]
- Kim, M.S.; Seo, K.S.; Hyun, H.; Kim, S.K.; Khang, G.; Lee, H.B. Sustained release of bovine serum albumin using implantable wafers prepared by MPEG-PLGA diblock copolymers. Int. J. Pharm. 2005, 304, 165–177. [Google Scholar] [CrossRef]
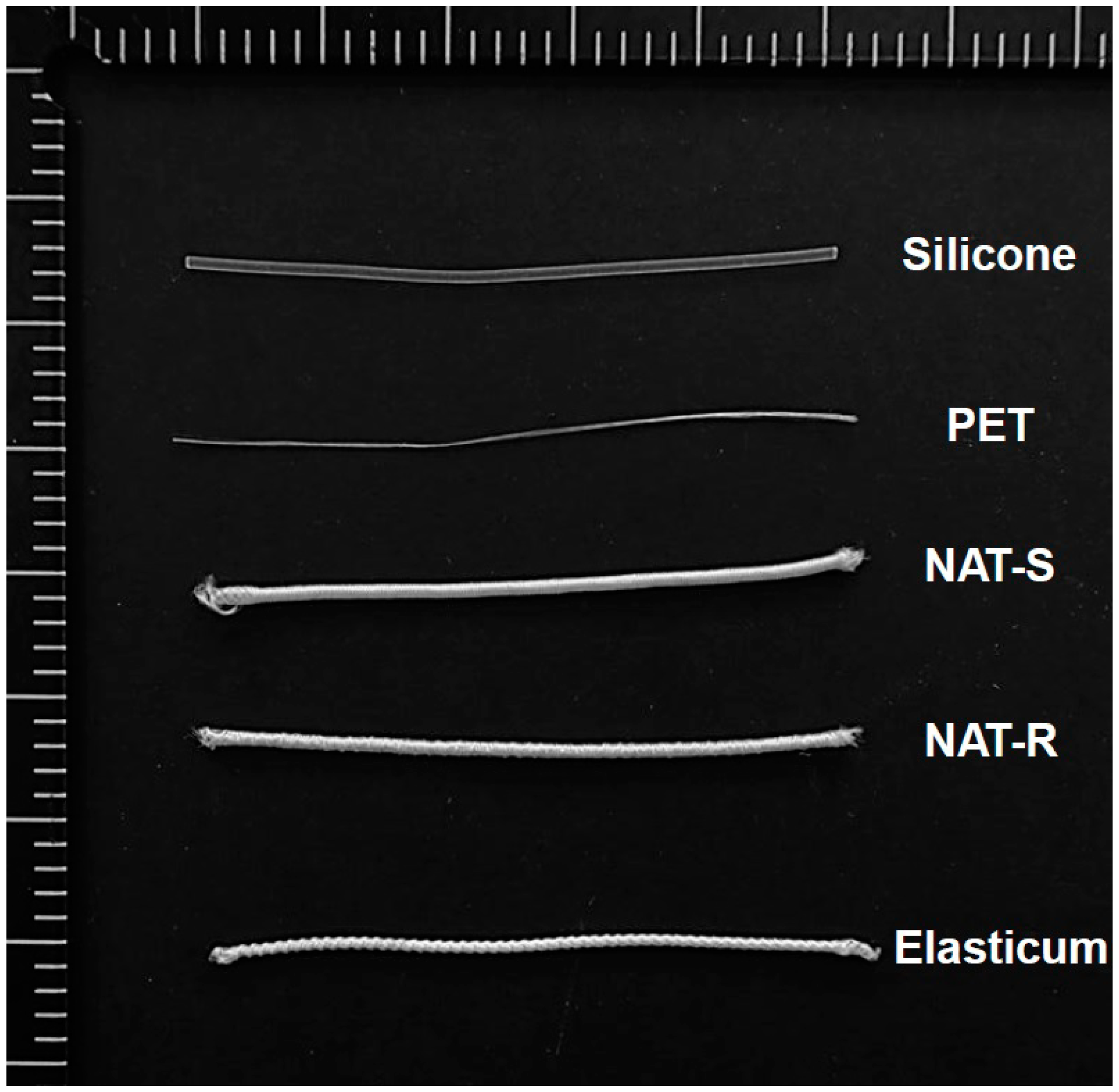






| Si | PET | Diameter(mm) | |||
|---|---|---|---|---|---|
| Diameter (mm) | EA | Diameter (mm) | EA | ||
| NAT-R | 1.00 | 1 | 0.05 | 8 | 1.20 |
| NAT-S | 1.00 | 1 | 0.05 | 8 | 1.20 |
| Elasticum® | 0.52 | 2 | 0.20 | 2 | 1.10 |
| Variables | Values | |||
|---|---|---|---|---|
| NAT | Elasticum® | |||
| Si | PET | Si | PET | |
| Tensile strength (N) | 8.68 ± 0.41 * | 12.26 ± 0.23 ** | 4.84 ± 0.46 * | 23.56 ± 0.97 ** |
| Elongation (mm/mm) | 19.07 ± 0.16 * | 0.39 ± 0.01 ** | 14.26 ± 0.44 * | 1.18 ± 0.11 ** |
| Variables | Values | ||
|---|---|---|---|
| NAT-S | NAT-R | Elasticum® | |
| Tensile strength (N) | 43.89 ± 0.87 ab | 50.83 ± 0.89 a | 49.97 ± 0.01 b |
| Elongation (mm/mm) | 6.10 ± 0.31 a | 5.53 ± 0.17 bc | 7.16 ± 0.01 abc |
| Experimental Materials | Time Points | |||
|---|---|---|---|---|
| 0 h | 0.5 h | 1.0 h | 2.0 h | |
| NAT-R | 0 | 92.31 | 102.83 | 102.83 |
| NAT-S | 0 | 67.50 | 71.16 | 71.16 |
| Elasticum® | 0 | 75.61 | 94.71 | 95.29 |
| Variables | Values | ||
|---|---|---|---|
| NAT-R | NAT-S | Elasticum® | |
| Cell viability (%) | 72.19 ± 5.39 a | 71.67 ± 0.68 b | 55.52 ± 2.91 ab |
| Variables | Values | ||
|---|---|---|---|
| NAT-R | NAT-S | Elasticum® | |
| Frictional force(N) | 0.62 ± 0.21 ab | 0.37 ± 0.14 b | 0.35 ± 0.12 a |
| Frictional strength (MPa) | 0.55 ± 0.18 ab | 0.32 ± 0.12 a | 0.31 ± 0.11 b |
| Coefficient of friction | 0.30 ± 0.08 ab | 0.15 ± 0.05 a | 0.15 ± 0.04 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Kang, M.; Choi, M.S.; Kim Song, J.; Lih, E.; Lee, D.; Jung, H.-H. Biomechanical Properties and Biocompatibility of a Non-Absorbable Elastic Thread. J. Funct. Biomater. 2019, 10, 51. https://doi.org/10.3390/jfb10040051
Choi Y, Kang M, Choi MS, Kim Song J, Lih E, Lee D, Jung H-H. Biomechanical Properties and Biocompatibility of a Non-Absorbable Elastic Thread. Journal of Functional Biomaterials. 2019; 10(4):51. https://doi.org/10.3390/jfb10040051
Chicago/Turabian StyleChoi, Yeji, Moonseok Kang, Moon Seop Choi, Jennifer Kim Song, Eugene Lih, Deahyung Lee, and Hong-Hee Jung. 2019. "Biomechanical Properties and Biocompatibility of a Non-Absorbable Elastic Thread" Journal of Functional Biomaterials 10, no. 4: 51. https://doi.org/10.3390/jfb10040051
APA StyleChoi, Y., Kang, M., Choi, M. S., Kim Song, J., Lih, E., Lee, D., & Jung, H.-H. (2019). Biomechanical Properties and Biocompatibility of a Non-Absorbable Elastic Thread. Journal of Functional Biomaterials, 10(4), 51. https://doi.org/10.3390/jfb10040051




