Intrinsic Cellular Responses of Human Wharton’s Jelly Mesenchymal Stem Cells Influenced by O2-Plasma-Modified and Unmodified Surface of Alkaline-Hydrolyzed 2D and 3D PCL Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plasma Modified 2D and 3D PCL Scaffolds
2.2. PCL Scaffold Characterization
2.3. Standard Cell Culture
2.4. Indirect Cell Viability Assay
2.5. Cell Proliferation and Attachment Assays by ELISA
2.6. Observation of Cellular Attachment under Scanning Electron Microscopy (SEM)
2.7. Gene Expression by Semi-Quantitative Polymerase Chain Reaction (PCR)
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Properties of 2D and 3D PCL Scaffolds
3.2. Differences in Cell Viability, Attachment, and Proliferation on 2D and 3D Scaffolds
3.3. Attached-Cell Morphology on Different Substrates
3.4. Shifts in Apoptosis/Necrosis-Related and Pluripotency/Differentiation-Related Gene Expression on Different Substrates
3.4.1. Apoptosis/Necrosis-Related Genes
3.4.2. Pluripotency/Differentiation-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbasi, N.; Soudi, S.; Hayati-Roodbari, N.; Dodel, M.; Soleimani, M. The effects of plasma treated electrospun nanofibrous poly (ε-caprolactone) Scaffolds with different orientations on mouse embryonic stem cell proliferation. Cell J. 2014, 16, 245–254. [Google Scholar] [PubMed]
- Düzyer, S.; Koral Koç, S.; Hockenberger, A.; Evke, E.; Kahveci, Z.; Uǧuz, A. Effects of different sterilization methods on polyester surfaces. Tekst. Konfeksiyon 2013, 23, 319–324. [Google Scholar] [CrossRef]
- Augustine, R.; Saha, A.; Jayachandran, V.P.; Thomas, S.; Kalarikkal, N. Dose-dependent effects of gamma irradiation on the materials properties and cell proliferation of electrospun polycaprolactone tissue engineering scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 526–533. [Google Scholar] [CrossRef]
- Zhu, W.; Castro, N.J.; Cheng, X.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma modified electrospun scaffolds with embedded microspheres for improved cartilage regeneration. PLoS ONE 2015, 10, e0134729. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Wu, R.X.; Xu, X.Y.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl. Mater. Today 2018, 11, 144–165. [Google Scholar] [CrossRef]
- Chen, F.M.; Wu, L.A.; Zhang, M.; Zhang, R.; Sun, H.H. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 2011, 32, 3189–3209. [Google Scholar] [CrossRef]
- Pappa, A.M.; Karagkiozaki, V.; Krol, S.; Kassavetis, S.; Konstantinou, D.; Pitsalidis, C.; Tzounis, L.; Pliatsikas, N.; Logothetidis, S. Oxygen-plasma-modified biomimetic nanofibrous scaffolds for enhanced compatibility of cardiovascular implants. Beilstein J. Nanotechnol. 2015, 6, 254–262. [Google Scholar] [CrossRef]
- Gilbert, P.M.; Blau, H.M. Engineering a stem cell house into a home. Stem Cell Res. Ther. 2011, 2, 3. [Google Scholar] [CrossRef]
- Liu, X.; Lee, A.; Ii, M.; Fundora, K.A.; Yaszemski, M.J.; Lu, L. Poly(ε-caprolactone) Dendrimer Cross-Linked via Metal-Free Click Chemistry: Injectable Hydrophobic Platform for Tissue Engineering. ACS Macro Lett. 2016, 5, 1261–1265. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Portolés, M.T.; Vallet-Regí, M.; Izquierdo, I.; Galletti, L.; Comas, J.V.; Pagani, R. Vascular endothelial and smooth muscle cell culture on NaOH-Treated Poly (ε-caprolactone) films: A preliminary study for vascular graft development. Macromol. Biosci. 2005, 5, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Teoh, S.H. Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials 2004, 25, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Shen, J. Surface modification of polycaprolactone with poly (methacrylic acid) and gelatin covalent immobilization for promoting its cytocompatibility. Biomaterials 2002, 23, 4889–4895. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.M.; Lee, S.J.; Lee, S.G.; Jeong, Y.K.; Kim, S.E.; Lee, S.C. Surface hydrolysis of fibrous poly (ε-caprolactone) scaffolds for enhanced osteoblast adhesion and proliferation. Macromol. Res. 2007, 15, 424–429. [Google Scholar] [CrossRef]
- Gao, J.; Niklason, L.; Langer, R. Surface hydrolysis of poly (glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 42, 417–424. [Google Scholar] [CrossRef]
- Lee, H.U.; Kang, Y.H.; Jeong, S.Y.; Koh, K.; Kim, J.P.; Bae, J.S.; Cho, C.R. Long-term aging characteristics of atmospheric-plasma-treated poly (ɛ-caprolactone) films and fibres. Polym. Degrad. Stab. 2011, 96, 1204–1209. [Google Scholar] [CrossRef]
- Şaşmazel, H.T.; Manolache, S.; Gümüşderelioğlu, M. Water/O2-plasma-assisted treatment of PCL membranes for biosignal immobilization. J. Biomater. Sci. Polym. Ed. 2009, 20, 1137–1162. [Google Scholar] [CrossRef]
- Kosorn, W.; Thavornyutikarn, B.; Uppanan, P.; Kaewkong, P. Surface modification of polycaprolactone scaffolds by plasma treatment for chondrocyte culture. IPCBEE 2012, 43, 44–48. [Google Scholar] [CrossRef]
- Inthanon, K.; Daranarong, D.; Techaikool, P.; Punyodom, W.; Khaniyao, V.; Bernstein, A.M.; Wongkham, W. Biocompatibility assessment of PLCL-sericin copolymer membranes using Wharton’s jelly mesenchymal stem cells. Stem Cells Int. 2016, 2016, 5309484. [Google Scholar] [CrossRef]
- Zhang, Y. The Effect of Surface Roughness Parameters on Contact and Wettability of Solid Surfaces. Theses and Dissertations. 2007. Available online: https://lib.dr.iastate.edu/rtd/15934 (accessed on 24 June 2018).
- Monjo, M.; Petzold, C.; Ramis, J.M.; Lyngstadaas, S.P.; Ellingsen, J.E. In vitro osteogenic properties of two dental implant surfaces. Int. J. Biomater. 2012, 2012, 181024. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Liu, X.Y.; Song, H.; Yarema, K.J.; Mao, H.Q. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 2010, 31, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Von Recum, A.F. Handbook of Biomaterials Evaluation: Scientific, Technical and Clinical Testing of Implant Materials, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 418–419. [Google Scholar]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells. In Assay Guidance Manual [Internet]; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2019. [Google Scholar]
- Chiarugi, P. Reactive oxygen species as mediators of cell adhesion. Ital. J. Biochem. 2003, 52, 28–32. [Google Scholar] [PubMed]
- Hauck, C.R.; Hsia, D.A.; Schlaepfer, D.D. The focal adhesion kinase—A regulator of cell migration and invasion. IUBMB Life 2002, 53, 115–119. [Google Scholar] [CrossRef]
- Pirone, D.M.; Liu, W.F.; Ruiz, S.A.; Gao, L.; Raghavan, S.; Lemmon, C.A.; Romer, L.H.; Chen, C.S. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006, 174, 277–288. [Google Scholar] [CrossRef]
- Gérard, C.; Goldbeter, A. The balance between cell cycle arrest and cell proliferation: Control by the extracellular matrix and by contact inhibition. Interface Focus 2014, 4, 20130075. [Google Scholar] [CrossRef]
- Tomakidi, P.; Schulz, S.; Proksch, S.; Weber, W.; Steinberg, T. Focal adhesion kinase (FAK) perspectives in mechanobiology: Implications for cell behaviour. Cell Tissue Res. 2014, 357, 515–526. [Google Scholar] [CrossRef]
- Yam, C.H.; Fung, T.K.; Poon, R.Y.C. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef]
- Hochegger, H.; Takeda, S.; Hunt, T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat. Rev. Mol. Cell Biol. 2008, 9, 910–916. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnubhotla, R.; Bharadwaj, S.; Sun, S.; Metlushko, V.; Glover, S.C. Treatment with Y-27632, a ROCK inhibitor, increases the proinvasive nature of SW620 cells on 3d collagen type 1 matrix. Int. J. Cell Biol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Egger, D.; Tripisciano, C.; Weber, V.; Dominici, M.; Kasper, C. Dynamic cultivation of mesenchymal stem cell aggregates. Bioengineering 2018, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, T.V.; Vasudev, U.; Kumar, A.; Menon, B. Cell surface interactions in the study of biocompatibility. Trends Biomater. Artif. Organs 2001, 15, 37–41. [Google Scholar]
- Michaelis, S.; Robelek, R.; Wegener, J. Studying cell–surface interactions in vitro: A survey of experimental approaches and techniques. Adv. Biochem. Eng. Biotechnol. 2012, 126, 33–66. [Google Scholar] [CrossRef]
- You, R.; Li, X.; Liu, Y.; Liu, G.; Lu, S.; Li, M. Response of filopodia and lamellipodia to surface topography on micropatterned silk fibroin films. J. Biomed. Mater. Res. Part A 2014, 102, 4206–4212. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.Y.; Panayotov, I.; Secret, E.; Cunin, F.; Gergely, C.; Cuisinier, F.; Martin, M. Initial stem cell adhesion on porous silicon surface: Molecular architecture of actin cytoskeleton and filopodial growth. Nanoscale Res. Lett. 2014, 9, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inthanon, K.; Saranwong, N.; Wongkham, W.; Wanichapichart, P.; Prakrajang, K.; Suwannakachorn, D.; Yu, L.D. PIII-induced enhancement and inhibition of human cell attachment on chitosan membranes. Surf. Coat. Technol. 2013, 229, 112–119. [Google Scholar] [CrossRef]
- Vignesh, S.N.; Bhuminathan, M. Comparative evaluation of the three different surface treatments—Conventional, laser and nano technology methods in enhancing the surface characteristics of commercially pure titanium discs and their effects on cell adhesion: An in vitro study. J. Pharm. Bioallied Sci. 2015, 7, S87–S91. [Google Scholar] [CrossRef]
- Thies, E.; Davenport, R.W. Independent roles of Rho-GTPases in growth cone and aX°nal behavior. J. Neurobiol. 2003, 54, 358–369. [Google Scholar] [CrossRef]
- Bergert, M.; Chandradoss, S.D.; Desai, R.A.; Paluch, E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc. Natl. Acad. Sci. USA 2012, 109, 14434–14439. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Milner, K.R.; Siedlecki, C.A. Fibroblast response is enhanced by poly(L-lactic acid) nanotopography edge density and proximity. Int. J. Nanomed. 2007, 2, 201–211. [Google Scholar]
- Wang, K.; Bruce, A.; Mezan, R.; Kadiyala, A.; Wang, L.; Dawson, J.; Rojanasakul, Y.; Yang, Y. Nanotopographical modulation of cell function through nuclear deformation. ACS Appl. Mater. Interfaces 2016, 8, 5082–5092. [Google Scholar] [CrossRef] [Green Version]
- Hsuuw, Y.; Chan, W. Dose-Dependently Induces Apoptosis or Necrosis in Human MCF-7 Cells. Ann. N. Y. Acad. Sci. 2007, 440, 428–440. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, M.; Armeanu, S.; Prinz, F.; Ungerechts, G.; Wybranietz, W.; Spiegel, M.; Bernlöhr, C.; Cecconi, F.; Gregor, M.; Neubert, W.J.; et al. Caspase-8 and Apaf-1-independent caspase-9 activation in Sendai virus-infected cells. J. Biol. Chem. 2002, 277, 29817–29824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegh, A.H.; Barnhart, B.C.; Volkland, J.; Algeciras-Schimnich, A.; Ke, N.; Reed, J.C.; Peter, M.E. Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: Role for the bifunctional apoptosis regulator protein. J. Biol. Chem. 2002, 277, 4351–4360. [Google Scholar] [CrossRef] [Green Version]
- Sosna, J.; Voigt, S.; Mathieu, S.; Lange, A.; Thon, L.; Davarnia, P.; Herdegen, T.; Linkermann, A.; Rittger, A.; Chan, F.K.M.; et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell. Mol. Life Sci. 2014, 71, 331–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kracikova, M.; Akiri, G.; George, A.; Sachidanandam, R.; Aaronson, S.A. A threshold mechanism mediates p53 cell fate decision between growth arrest and apoptosis. Cell Death Differ. 2013, 20, 576–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; Fisher, J.C.; Dillon, C.P.; Kriwacki, R.W.; Kuwana, T.; Green, D.R. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 20327–20332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2011, 21, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Fryer, B.; Nelson, S.; Nielsen, V.; Marwood, T.K.; Brevig, T. Methods, Surface Modified Plates and Compositions for Cell Attachment, Cultivation and Detachment. U.S. Patent Application NO. 12/388,930, 27 August 2009. [Google Scholar]
- Buxton, A.N.; Zhu, J.; Marchant, R.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef]
- Noisa, P.; Ramasamy, T.S.; Lamont, F.R.; Yu, J.S.L.; Sheldon, M.J.; Russell, A.; Jin, X.; Cui, W. Identification and characterisation of the early differentiating cells in neural differentiation of human embryonic stem cells. PLoS ONE 2012, 7, e37129. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.; Mitchell, K.; Hix, J.; Medicetty, S.; El-Zarkouny, S.; Grieger, D.; Troyer, D. Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp. Neurol. 2003, 182, 288–299. [Google Scholar] [CrossRef]
- Gharibi, B.; Hughes, F.J. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 771–782. [Google Scholar] [CrossRef]
- Wolfe, M.; Tucker, A.; Reger, R.L.; Prockop, D.J. Multipotent Stromal Cells (hMSCs). In Human Adult Stem Cells; Masters, J.R., Palsson, B.Ø., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 45–72. ISBN 978-90-481-2269-1. [Google Scholar]
- De Bartolo, L.; Rende, M.; Morelli, S.; Giusi, G.; Salerno, S.; Piscioneri, A.; Gordano, A.; Di Vito, A.; Canonaco, M.; Drioli, E. Influence of membrane surface properties on the growth of neuronal cells isolated from hippocampus. J. Membr. Sci. 2008, 325, 139–149. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Madihally, S.V. Cell colonization in degradable 3D porous matrices. Cell Adhes. Migr. 2008, 2, 9–16. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, Y.; Wang, S.; Wu, Y. Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. J. Cell. Mol. Med. 2014, 18, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Chen, S.Y.; Li, J.R.; Young, T.H. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl. Med. 2013, 2, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part C 2009, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E. Biomaterials for cartilage tissue engineering. J. Tissue Sci. Eng. 2017, 8, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Quarto, N.; Longaker, M.T. FGF-2 Inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006, 12, 1405–1418. [Google Scholar] [CrossRef]
- Webb, P.R.; Doyle, C.; Anderson, N.G. Protein kinase C-ε promotes adipogenic commitment and is essential for terminal differentiation of 3T3-F442A preadipocytes. Cell. Mol. Life Sci. 2003, 60, 1504–1512. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Tanaka, M.; Naiki, T.; Chemistry, A.M.J. Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J. Biol. Chem. 2006, 281, 37913–37920. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow–derived and adipose tissue–derived stromal cells. Arthritis Rheum. 2003, 48, 418–429. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]


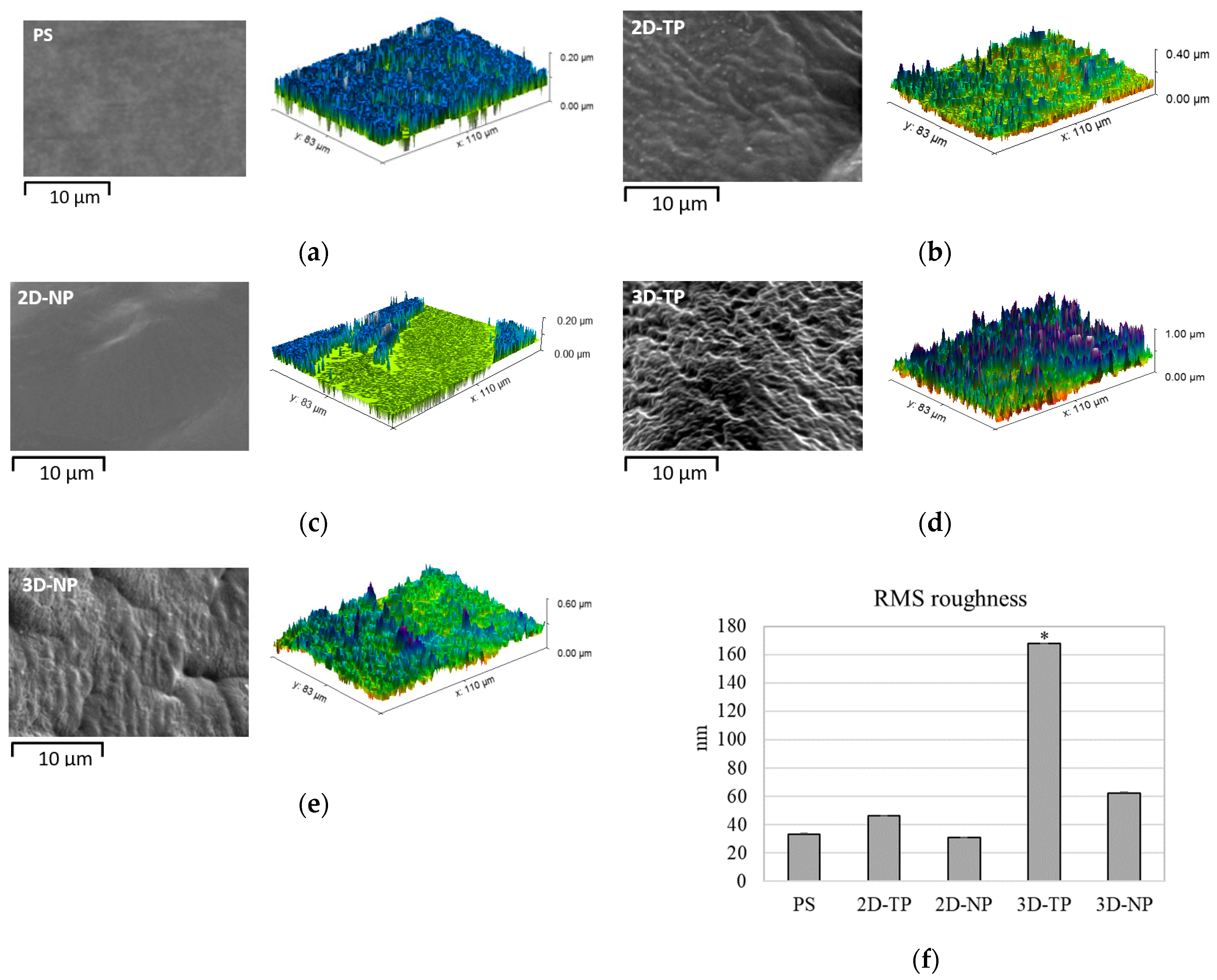
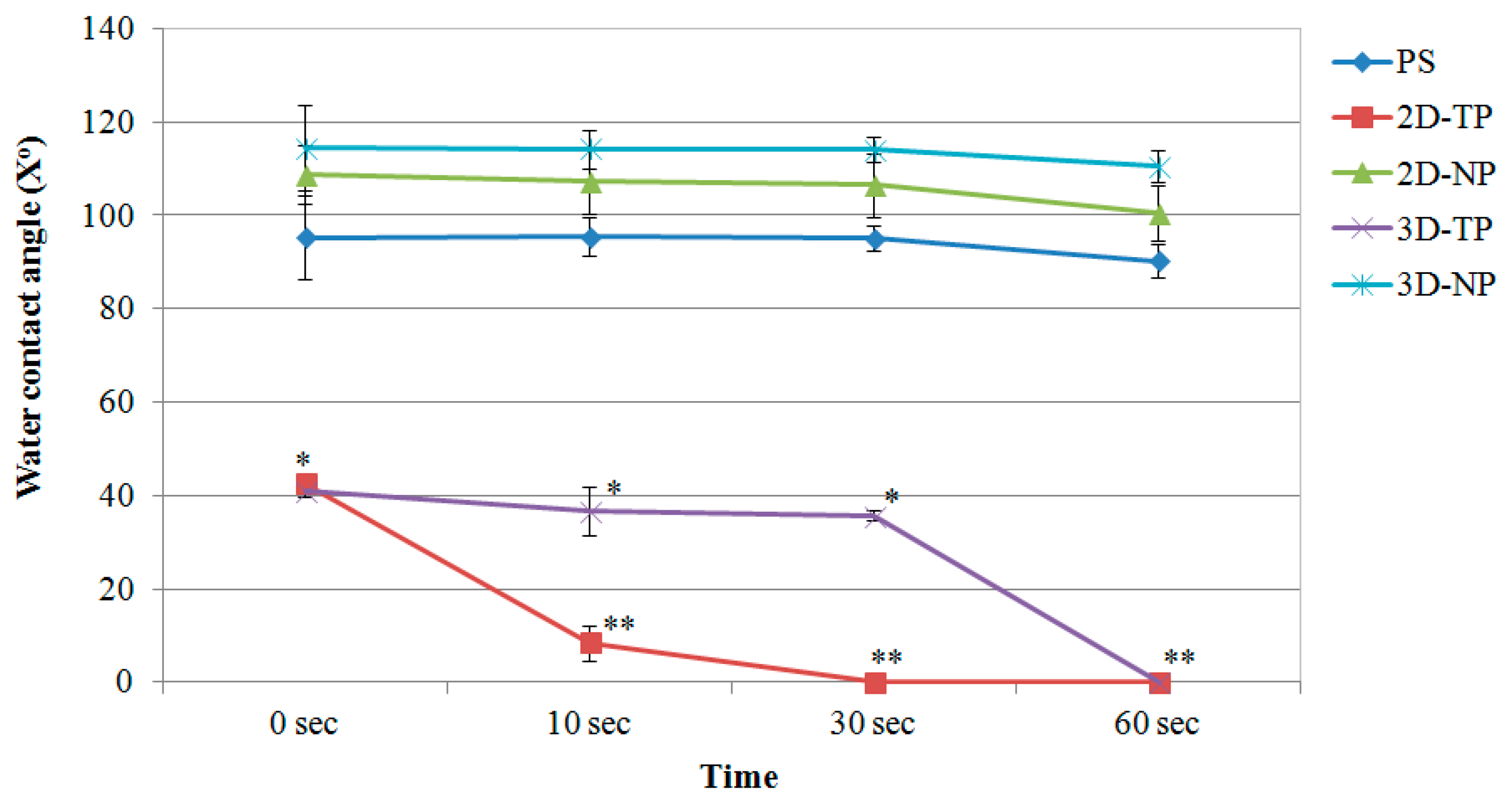


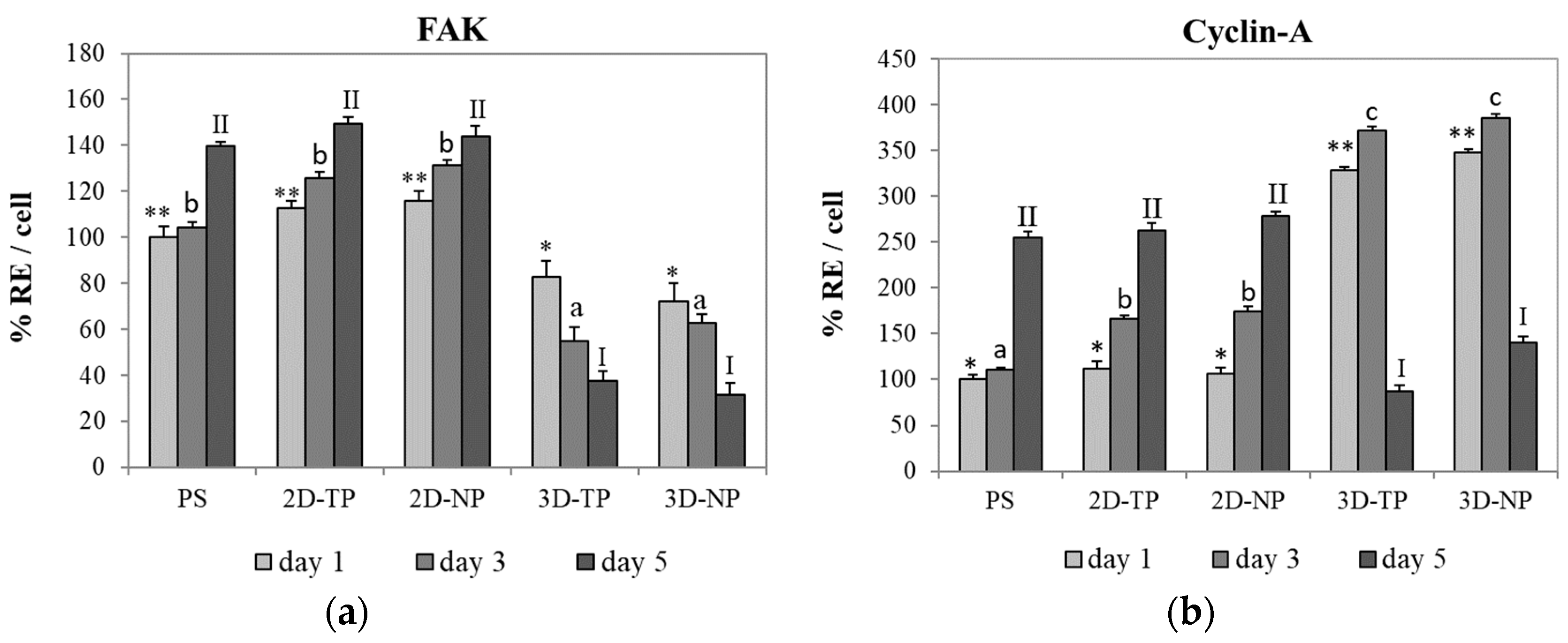



| Gene | Accession Number | Sequence |
|---|---|---|
| caspase 8 | NM_001228.4 | F: 5′-TGCAGGGGCTTTGACCACGA-3′ R: 5′-TGGGGGCCTCCTGTCCATCA-3′ |
| bcl-2 | NM_000633.2 | F: 5′-TGTGGCCTTCTTTGAGTTCG-3′ R: 5′-TCACTTGTGGCTCAGATAGG-3′ |
| Apaf-1 | NM_013229.2 | F: 5′-TGGCCAGTGCCAAGATGCACA-3′ R: 5′-CGACCTCCTGCTTGGCCTGC-3′ |
| PARP-1 | NM_017915.3 | F: 5′-AATCTCCAGGGGGTAGAACT-3′ R: 5′-CAGAGCCTGTTGAAGTTGTG-3′ |
| SSEA-4 | NM_203289.4 | F: 5′-GCCCTAGAACTCCAATCACA-3′ R: 5′-CCCAGATGGTATTGGACACA-3′ |
| NES | NM_006617.1 | F: 5′-TCCTGCTCGCTCTCTACTTT-3′ R: 5′-CCCAGATGGTATTGGACACA-3′ |
| COL2A1 | NM_001844.4 | F: 5′-CCCATTGGTCCTTGCATTAC-3′ R: 5′-GTCCTCTGCGACGACATAAT-3′ |
| PPAR-2 | NM_138712.3 | F: 5′-GCATTATGAGACATCCCCACT-3′ R: 5′-CCTATTGACCCAGAAAGCGAT-3′ |
| GAPDH | NM_002046.4 | F: 5′-TGCTGGCGCTGAGTACGTCG-3′ R: 5′-TGACCTTGGCCAGGGGTGCT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inthanon, K.; Janvikul, W.; Ongchai, S.; Chomdej, S. Intrinsic Cellular Responses of Human Wharton’s Jelly Mesenchymal Stem Cells Influenced by O2-Plasma-Modified and Unmodified Surface of Alkaline-Hydrolyzed 2D and 3D PCL Scaffolds. J. Funct. Biomater. 2019, 10, 52. https://doi.org/10.3390/jfb10040052
Inthanon K, Janvikul W, Ongchai S, Chomdej S. Intrinsic Cellular Responses of Human Wharton’s Jelly Mesenchymal Stem Cells Influenced by O2-Plasma-Modified and Unmodified Surface of Alkaline-Hydrolyzed 2D and 3D PCL Scaffolds. Journal of Functional Biomaterials. 2019; 10(4):52. https://doi.org/10.3390/jfb10040052
Chicago/Turabian StyleInthanon, Kewalin, Wanida Janvikul, Siriwan Ongchai, and Siriwadee Chomdej. 2019. "Intrinsic Cellular Responses of Human Wharton’s Jelly Mesenchymal Stem Cells Influenced by O2-Plasma-Modified and Unmodified Surface of Alkaline-Hydrolyzed 2D and 3D PCL Scaffolds" Journal of Functional Biomaterials 10, no. 4: 52. https://doi.org/10.3390/jfb10040052
APA StyleInthanon, K., Janvikul, W., Ongchai, S., & Chomdej, S. (2019). Intrinsic Cellular Responses of Human Wharton’s Jelly Mesenchymal Stem Cells Influenced by O2-Plasma-Modified and Unmodified Surface of Alkaline-Hydrolyzed 2D and 3D PCL Scaffolds. Journal of Functional Biomaterials, 10(4), 52. https://doi.org/10.3390/jfb10040052





